Introduction
Immunosuppressive drugs are used for the treatment
of undesirable or abnormal activation of T lymphocytes and the
immune system associated with organ transplantation and autoimmune
diseases. T lymphocytes play a pivotal role in the pathogenesis of
cell-mediated autoimmune diseases and chronic inflammatory
disorders (1–3). Activation of T lymphocytes requires
stimulation of T-cell receptors and costimulatory signals.
Ca2+ influx is crucial for T-cell activation upon
antigen stimulation (4). The best
known costimulatory signals are those of NF-κB (nuclear factor
kappa-light-chain-enhancer of activated B cells). NF-κB is
activated by Ca2+/calmodulin dependent protein kinase
(CaMK II) (5,6). NF-κB translocates to the nucleus and
turns on transcription of specific genes, generally related to
inflammatory or immune responses, cell survival responses, or cell
proliferation.
Recently, fruits and vegetables have been recognized
as natural sources of various bioactive compounds (7–9). Natural
antioxidants often exist together in different combinations in
nature, and consequently researchers have been investigating the
additive and synergistic effects of different antioxidants
(10,11).
One such vegetable where a variety of antioxidants
can be found is the pepper. The pepper belongs to the genus
Capsicum, which contains >200 varieties, with Capsicum
annuum, Capsicum baccatum, Capsicum chinense,
Capsicum frutescens, and Capsicum pubescens being the
main five species (12,13). Peppers are consumed worldwide and
their importance has gradually increased to place them among the
most consumed spice crops in the world (14). They also have a significant role in
traditional medicine (15,16). It has been reported that the red
pepper fruit of Capsicum baccatum shows anti-inflammatory
activity via nitric oxide scavenging activity (17). These findings indicate that the bell
pepper has a potential immunosuppressive function through its
effects on cells. However, the anti-inflammatory and underlying
immunosuppressive mechanisms of the bell pepper (Capsicum annuum
L. var. grossum), one of the species of the genus
Capsicum, are still largely unknown and require further
investigation. In this study, we investigated the in vitro
anti-inflammatory effect of water extract from bell pepper leaves
(WEBP) on mouse spleen cells, and explored the potential mechanism
underlying this effect. We found that WEBP significantly inhibited
Con-A-stimulated spleen cell proliferation, cytokine production,
and expression of inflammatory proteins. The data also showed that
WEBP exhibited an immunosuppressive effect, via inhibition of
T-cell activation through the NF-κB pathway.
The results indicate that some bioactive components
are present in WEBP, which can exert anti-inflammatory and
immunosuppressive effects. The study of the anti-inflammatory
mechanism of WEBP has provided some useful information on its
potential for therapeutic application.
Materials and methods
Animals
ICR mice (male, 5 weeks of age) weighing 18–22 g
were purchased from the Kyudo Laboratory Animal Center Co., Ltd.,
(Fukuoka, Japan) and were housed in polypropylene cages with
sawdust bedding. The temperature was maintained at 24±1°C, with
humidity of 50±10% and a 12 h light/dark cycle. Food and water were
available ad libitum. The procedures used for the animals
and their care followed the internationally accepted Guidelines for
Keeping Experimental Animals, issued by the Government of Japan.
The researchers received ethical training from the Fukuoka
University Ethics Committee.
Preparation of water extract from bell
pepper (Capsicum annuum L. var. grossum) leaves
WEBP was obtained as follows. Bell pepper
(Capsicum annuum L. var. grossum) leaves were
collected in Fukuoka at harvest just before. This bell pepper's
seed was purchased from TAKII SEED CO., LTD (Kyoto, Japan). A total
of 5 g of wet bell pepper leaves were cut finely with scissors.
Bell pepper leaves were stored at −80°C in vacuum storage before
use. The leaves cut finely were homogenized, and were extracted
using a closed tissue grinder (Kimble Chase, Vineland, NJ, USA) in
5 ml of deionized water. The obtained extract was centrifuged
(3,000 × g, 25°C, 10 min). The supernatant of the extract was used
in this study as WEBP group. The fraction including components over
3 kDa was separated as >3 kDa group by ultrafiltration
(Ultracel® YM-3, Merck Millipore Ltd, Darmstadt,
Germany) from the original extract (WEBP). Meanwhile, the fraction
including components below 3 kDa was used as <3 kDa group. WEBP
and its fractions were used as diluted to 50, 150 and 450 times in
the water.
Chromatographic conditions
WEBP and its fractions were analyzed by using a
150×4.6 mm reversed-phase C18 5-µm column (CAPCELLPACK C18 UG120,
SHISEIDO Co. Ltd., Tokyo, Japan) maintained at a constant
temperature (30°C). The mobile phase was acetonitrile-water (70:
30) with a flow rate of 1 ml/min, and the detection wavelength was
at 280 nm. The sample injection was 5 µl. The Agilent 1220 Infinity
LC System (Agilent Technologies, Tokyo, Japan) was used as HPLC
system.
Spleen cell culture
Spleen cells were prepared as described in a
previous study (18,19). Briefly, the spleen was removed from
the mice, placed in phosphate buffered saline (PBS), minced, and
passed through a nylon mesh to yield a homogeneous cell suspension.
After 10 min of centrifugation at 400 × g at 4°C, the cell pellets
were washed twice with PBS and resuspended in RPMI 1640 medium
containing 10% heat-inactivated fetal calf serum (FCS). The spleen
cells were seeded in a 96-well flat-bottom plate (Nunc) at a
concentration of 5.0×106 cells/ml. Subsequently, Con-A
(5 µg/ml) and WEBP, or 3 kDa fraction in medium was added to
provide a final volume of 200 µl. Plates were incubated for 72 h at
37°C in a humidified atmosphere with 5% CO2. The
supernatants of this each well were used ELISA to measure the level
of cytokine.
Meanwhile, the cell survival/proliferetaion of WEBP
under non Con-A stimulation in mouse spleen cells was assessed
using the WST-8 assay kit (Nacalai Tesque Inc., Kyoto, Japan),
according to the manual. WST-8 reagents
(2-(2-methpxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium,
monosodium salt) were added to the culture. After 3 h of
incubation, the absorbance was determined at 450 nm using a
microplate reader.
Furthermore, the cytotoxicity of WEBP under non
Con-A stimulation in mouse spleen cells was assessed by analyzing
the membrane integrity, using a CellTox Green kit, according to the
manual (Promega Corporation, Madison, WI, USA).
Measurement of cytokines using
enzyme-linked immunosorbent assay (ELISA)
Cytokine levels in the collected sterilized
supernatants of the Con-A-stimulated mouse spleen cell cultures at
72 h were measured using ELISA. Briefly, 96 well plates were coated
with monoclonal antibodies [0.5 µg/ml anti-mouse interferon-γ
(IFN-γ), purified; 1.0 µg/ml anti-mouse interleukin 6 (IL-6),
purified; and 2.0 µg/ml anti-mouse tumor necrosis factor α (TNF-α),
purified] overnight at 4°C and washed with PBS. Blocking One
solution (Nacalai Tesque, Inc.) was diluted 5-fold with Tris
buffered saline containing 0.05 (v/v) % Tween-20 (TBST) and 250
µl/well of blocking solution were added to the plates. After 1 h of
incubation at room temperature (RT), the collected sterilized
supernatants of Con-A-stimulated mouse spleen cell cultures were
added to the plates in a 1:10 dilution in RPMI medium containing
10% FCS. The plates were further incubated for 1 h at RT and then
washed with PBS. Biotinylated antibodies (1.0 µg/ml biotinylated
anti-mouse IFN-γ; 1.0 µg/ml biotinylated anti-mouse IL-6; and 0.8
µg/ml biotinylated anti-mouse TNF-α) were added and the plates were
incubated for 1 h at RT. Streptavidin horseradish peroxidase (SNN
1004; Biosource International, Inc., Camarillo, CA, USA; 1:10,000)
was then added and the plates were incubated for 1 h at RT. After
the plates had been washed with TBST thoroughly, 100 µl of
peroxidase substrate solution consisting of equal volumes of
3,3′,5,5′-tetramethylbenzidine (TMB) peroxidase substrate and
peroxidase substrate solution B (TMB Microwell Peroxidase Substrate
System; KPL Inc., Gaithersburg, MD, USA) was added to each well and
incubated for 10 min, followed by an equal volume of stop solution.
Absorbance was measured at 450 nm with an ELISA reader (Bio-Rad
Model 680 Microplate Reader; Bio-Rad, Hercules, CA, USA). All
anti-cytokine antibodies were purchased from eBioscience (San
Diego, CA, USA).
Immunoblot analysis
For the detection of inflammatory proteins, the
cells were lysed in a lysis buffer. The total protein concentration
was measured using a BCA assay (Pierce Biotechnology, Rockford, IL,
USA). Each cell lysate (containing equal amounts of protein) was
subjected to SDS-PAGE (Bio-Rad) and the proteins were then
transferred onto polyvinylidene difluoride membranes (Bio-Rad). The
membranes were blocked with Blocking One (Nacalai Tesque, Inc.)
overnight at 4°C, and were then incubated for 1 h at RT with an
anti-iNOS (induced nitric oxide synthase) antibody, an anti-NF-κB
antibody, an anti-phosphorylated NF-κB antibody (Cell Signaling
Technology Inc., Beverly, MA, USA), or an anti-β-actin antibody
(Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) at a 1:500
dilution in blocking solution. After washing three times, the
membranes were incubated for 1 h at RT with a secondary antibody (a
horseradish peroxidase-conjugated species-specific antibody).
Immunoreactive bands were visualized using ImmunoStar®
LD (Wako Pure Chemical Industries Ltd., Osaka, Japan).
Statistical analysis
The results are expressed as mean ± SD (n=3-4). The
data were evaluated for statistical significance using the
Bonferroni test for differences between the groups. The overall
significance was determined using a one-way ANOVA (repeated
measures). P<0.05 was considered to indicate a statistically
significant difference.
Results
HPLC chromatography
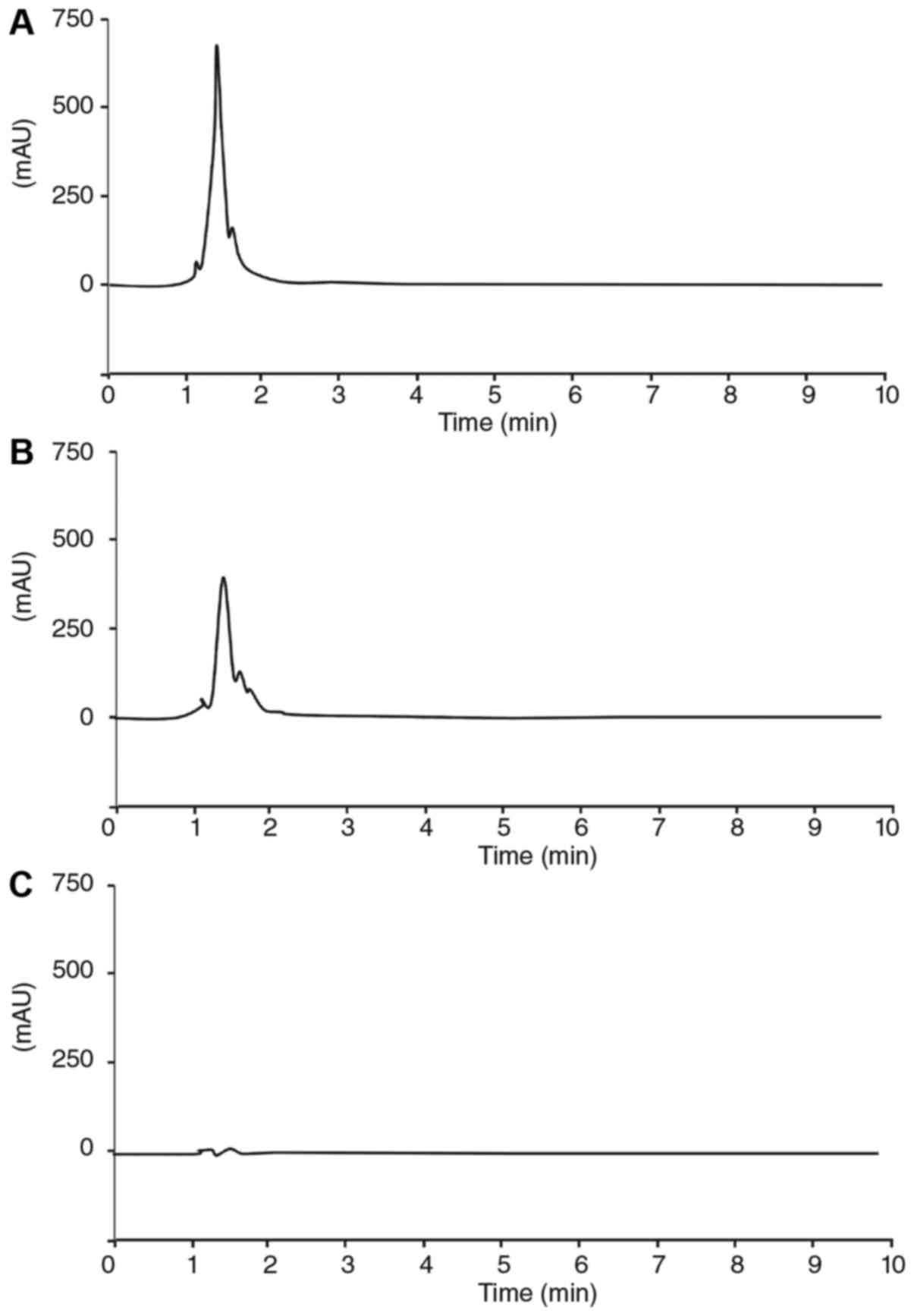
The one sharp peak of WEBP under 280 nm was appeared
at 1.345 min as shown in Fig. 1A.
The sharp peak of fraction (>3 kDa) was also appeared at 1.365
min as shown in Fig. 1B. Meanwhile,
the peak of fraction (<3 kDa) were not shown in Fig. 1C.
Effect of WEBP on inflammatory
cytokines
We used Con-A as a stimulus to promote cytokine
secretion in mouse spleen cells. Then, the effects of WEBP on the
production of the IFN-γ, IL-6, and TNF-α cytokines were
examined.
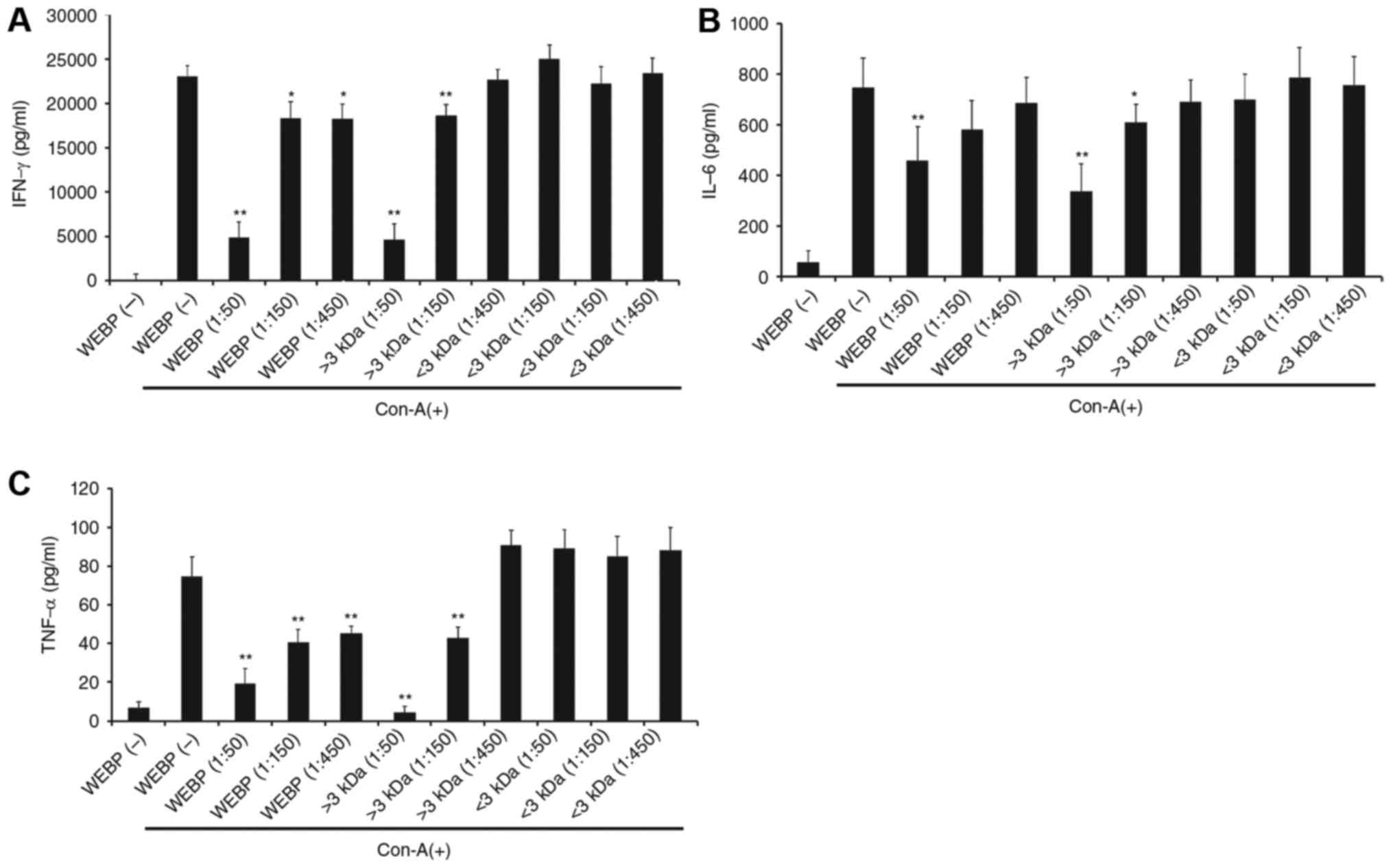
The level of IFN-γ, IL-6, and TNF-α production
increased in mouse spleen cells stimulated by Con-A. Meanwhile,
this increased production was suppressed by the addition of WEBP
(1:50, 1:150, 1:450 dilution of extract in water) as shown in
Fig. 2A, B and C, respectively. The
Con-A-stimulated increase in the level of IFN-γ, IL-6, and TNF-α
production was also suppressed by the addition of the fraction
containing extract component over 3 kDa (1:50, 1:150, 1:450
dilution of fraction in water) as shown in Fig. 2A, B and C, respectively. However,
addition of the fraction containing extract components below 3 kDa
produced no significant change in the level of IFN-γ, IL-6, and
TNF-α production.
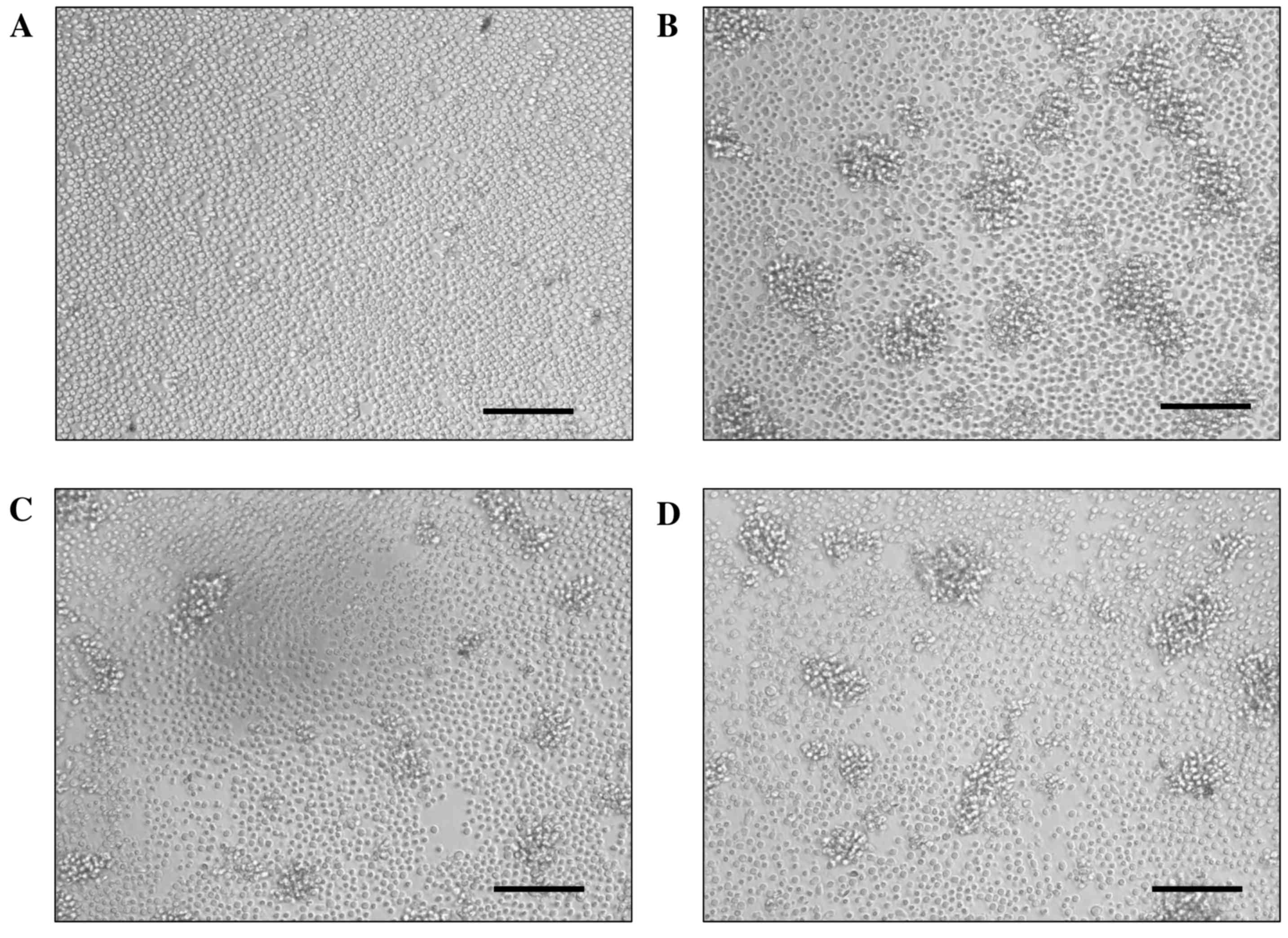
Cell clusters were induced in mouse spleen cell
cultures stimulated by Con-A, as shown in Fig. 3. Cell clusters induced by Con-A mean
activation of spleen cells but not proliferation. The number and
size of the clusters were reduced by treatment with WEBP, in a
dose-dependent manner, compared with the non-treatment group (Con-A
(+)). These results indicate that WEBP strongly suppressed T-cell
activation stimulated by Con-A.
Effect of WEBP on cell
survival/proliferation
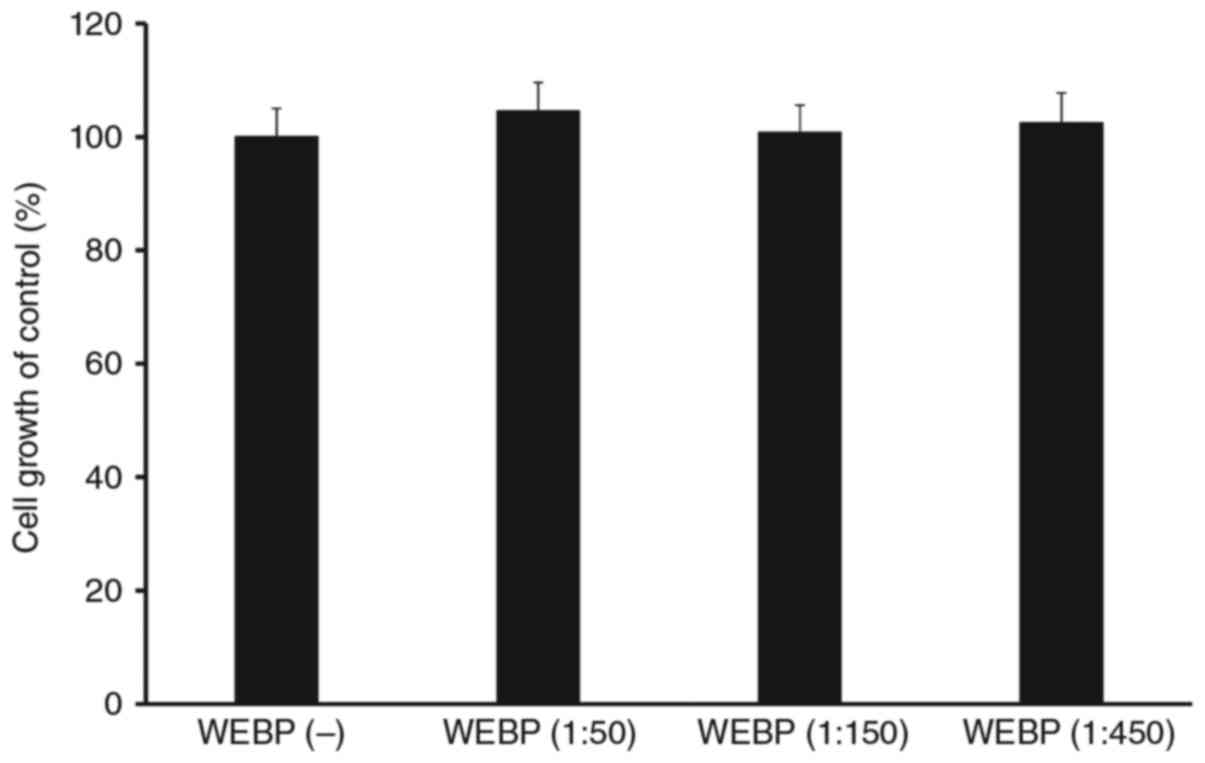
Spleen cells without Con-A were treated with
different doses of WEBP (1:50, 1:150, 1:450 dilution of extract in
water) and cell survival/proliferation were assessed using a WST-8
assay. As shown in Fig. 4, there was
no significant differences in cell growth of control (%) between
WEBP (−) and various concentration of WEBP treatment group.
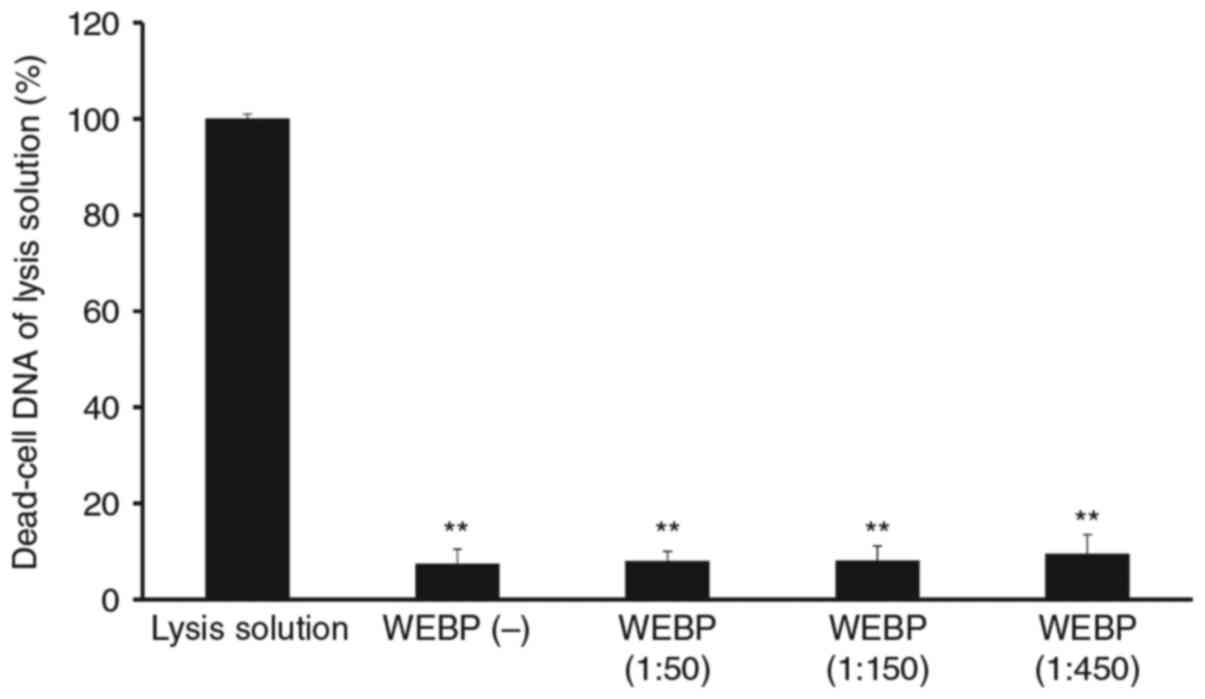
Cytotoxic activity of WEBP against
Con-A stimulated mouse spleen cell cultures
Spleen cells without Con-A were treated with
different doses of WEBP (1:50, 1:150, 1:450 dilution of extract in
water) and cytotoxicity was assessed using the CellTox Green assay.
There was no significant cytotoxicity produced by WEBP in mouse
spleen cells after 72 h of incubation as shown in Fig. 5.
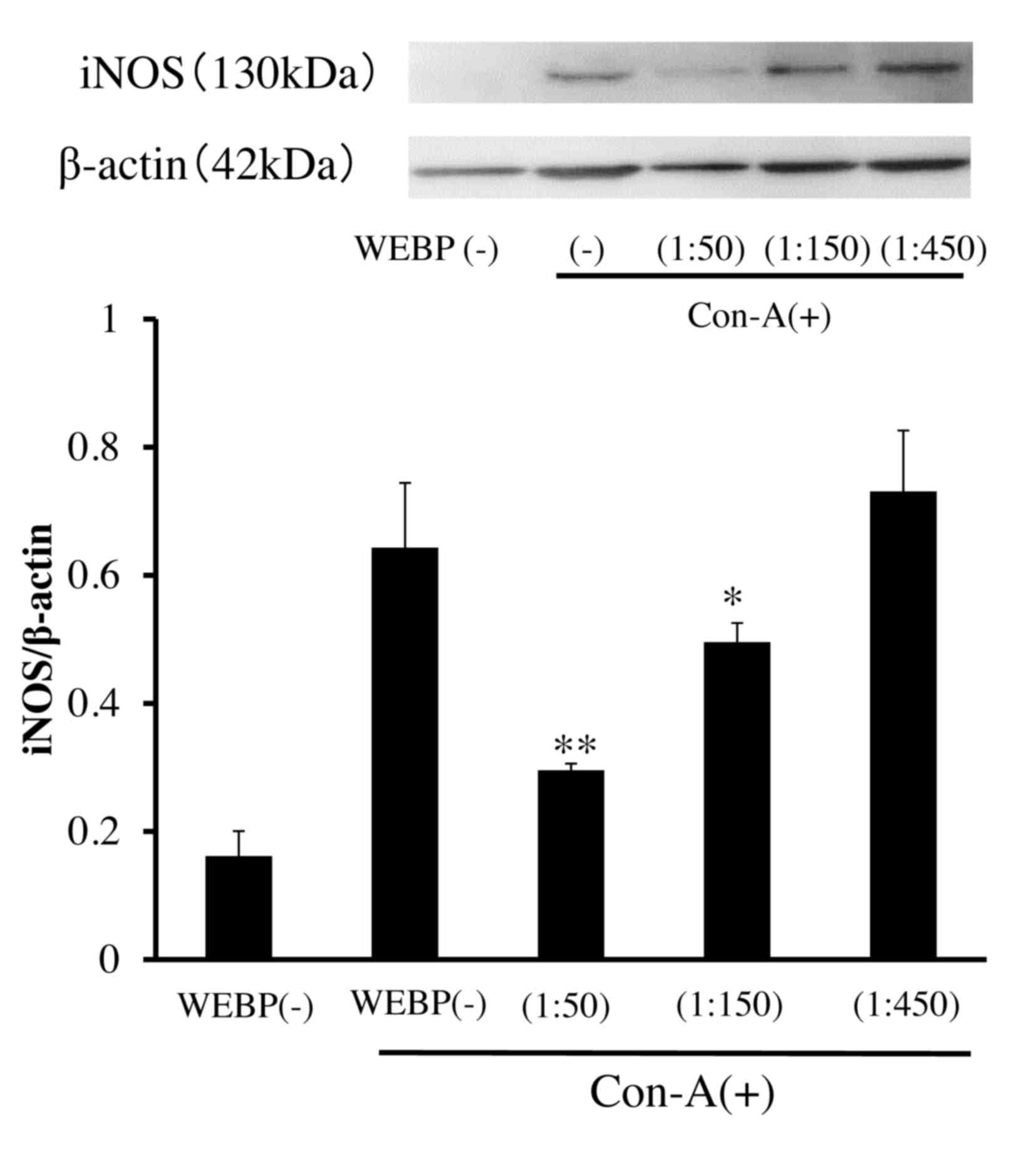
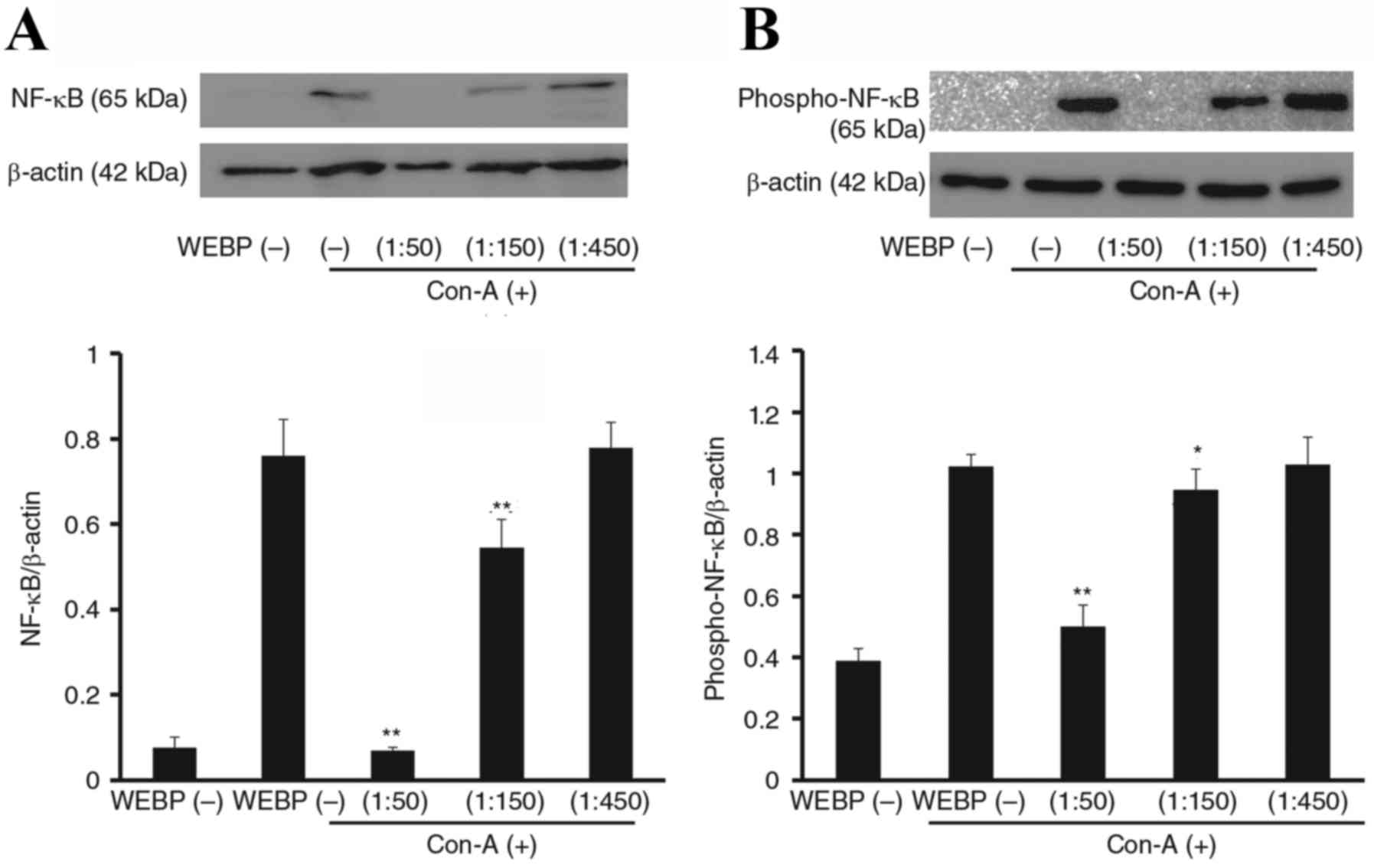
Inhibitory effect of WEBP on
expression of inflammatory proteins (iNOS, NF-κB) in Con-A
stimulated mouse spleen cells
To investigate the mechanism of WEBP, we examined
its effect on the expression of inflammatory proteins, as assessed
by western blotting. As shown in Fig.
6, expression levels of iNOS in mouse spleen cells stimulated
by Con-A were significantly inhibited by WEBP in a dose-dependent
manner. Furthermore, expression levels of NF-κB and phospho-NF-κB
in mouse spleen cells stimulated by Con-A were also significantly
inhibited by WEBP as shown in Fig.
7. The inhibition was detected 72 h after stimulation with
Con-A in mouse spleen cell cultures treated with WEBP (1:50, 1:150,
1:450 dilution of extract in water).
Discussion
Immunosuppressive agents are an important drug class
that is valuable for the treatment of various human diseases,
including autoimmune diseases. Although there are a variety of
immunosuppressive drugs such as cyclosporine A, tacrolimus (FK506),
methotrexate, azathioprine, and rituximab, their toxicity is a
major obstacle to their widespread use (20–22).
Therefore, new and safer immunosuppressive drugs against acute and
chronic rejection of transplants, and for the treatment of other
autoimmune diseases, are eagerly awaited. There has been an
increasing interest in exploring phytochemicals with therapeutic
potential in autoimmune disease, in that they can be purified,
synthesized, and chemically modified to design new drugs and often
have low toxicity. In this study, we report that WEBP has potent
immunosuppressive activity in vitro.
The fraction (>3 kDa) with sharp peak in the HPLC
chromatogram showed anti-inflammatory activity as shown in Fig. 2. Meanwhile, the fraction (<3 kDa)
without peak did not show anti-inflammatory activity as shown in
Fig. 2. Thus, there were correlation
between existence of peak and anti-inflammatory activity. In the
previous study, capsaicin included in other Capsicum plants has
been known to show anti-inflammatory activity (23,24). In
this study, we tried to measure of HPLC chromatogram of capsaicin
in the same HPLC condition. The peak of capsaicin appeared at 6.400
min (data not shown). Furthermore, capsaicin did not suppress the
level of IL-6 and expression level of NF-κB in spleen cell culture
stimulated by Con-A. From these result, the active ingredient
including WEBP was not capsaicin at least.
In Fig. 2, IFN-γ,
IL-6 and TNF-α secretion after Con-A stimulation with WEBP
treatment has shown a dose-dependent manner. Furthermore, IFN-γ
levels treated with WEBP diluted at 1:50, 1:75 and 1:150 in water
were determined to confirm about a concentration-response between
1:50 and 1:150. The IFN-γ levels treated with WEBP diluted at 1:50,
1:75 and 1:150 in water were 5,283±116 (mean ± SD), 12,223±238 and
18,870±326 pg/ml, respectively. IFN-γ levels were suppressed
linearly by treatment with WEBP under 1:150 dilution.
Cytokines are important modulators and effectors in
the immune system. In particular, multiple proinflammatory
cytokines have been reported to be closely associated with many
autoimmune diseases. It has been shown that Th1 cells produce IL-2,
IFN-γ, IL-12, and other cytokines when stimulated; and Th2 cells
produce IL-4, IL-5, IL-6, and IL-10 (25). Overactivation of either response
could lead to Th1 or Th2 polarization. The imbalance of Th1/Th2
could lead to immunological diseases, such as rheumatoid arthritis,
type-1 diabetes mellitus, and multiple sclerosis (26). In this study, we used Con-A as a
activator of T-cell proliferation, and selected IFN-γ as a Th1
cytokine and IL-6 as a Th2 cytokine to test the effect of WEBP on
modulating the levels of Th1 and Th2 cytokines. The results showed
that WEBP could effectively rectify the Th1 and Th2 polarization in
mouse T lymphocytes.
Inhibition of important components of the
Ca2+ signaling pathway revealed that Con-A-induced NF-κB
activation depends on a Ca2+/calmodulin/CaMK II pathway
(27,28). It has been reported that daphnetin
inhibited p-CaMK II and NF-κB activation in mouse spleen cell
cultures stimulated by Con-A (29).
To determine the influence of WEBP on NF-κB activation, expression
of iNOS, NF-κB, and phospho-NF-κB were examined. The results showed
that expression of iNOS, NF-κB, and phospho-NF-κB were
significantly inhibited by treatment with WEBP. These results
indicate that immunosuppressive components present in WEBP can
exert an influence on the Ca2+ signaling pathway.
Furthermore, the WEBP inhibited expression of both NF-κB and
phospho-NF-κB to a similar degree, and the ratio of phosphorylated
NF-κB to non-phosphorylated NF-κB was not significantly altered,
indicating that WEBP likely had no direct effects on NF-κB
phosphorylation.
In conclusion, our results demonstrated that the
WEBP had anti-inflammatory activity, mediated as immunosuppressive
activity against T-cell activation in vitro. The
anti-inflammatory effects might be associated with NF-κB
translocation. These findings extend our understanding of the
immunomodulatory effects of WEBP and suggest that it has potential
as a new and effective phytochemical compound for the treatment of
T-cell-mediated immune diseases. Because WEBP produced no
cytotoxicity in mouse spleen cells, our study suggests the
possibility of using bioactive compounds present in WEBP as novel
safe immunosuppressants. Furthermore, our results pertaining to the
suppression of cytokine production suggest that these bioactive
compounds possess a molecular weight of over 3 kDa.
In the present study, the molecular size of fraction
was decided at 3 kDa, which is the lower limit in the column of
FPLC or Gel filtration chromatography to purify the crude extract
sample in the next step to identify the bioactive compounds. In
general, the molecular weight of monomeric polyphenol or saponins
might be below 3 kDa, the so-called low-molecular-weight compound.
However, its molecular weight might be over 3 kDa when the monomer
forms the multimetric compound. Furthermore, there is possibility
that the bioactive compounds might be amino acid, peptide or
protein because the peak was detected at 280 nm in the HPLC
profile.
We are planning to prepare the further fraction
using over 3 kDa extract by FPLC or Gel filtration chromatography
or ultrafiltration. Furthermore, the bioactive compounds including
further fraction will be separated by SDS-PAGE. The band derived
for bioactive compounds will be identified by LC/MS/MS or
N-terminal amino acid analysis. Finally, we are planning to
determine its anti-inflammatory activity mechanism of bioactive
compounds and further functional analysis.
Acknowledgements
The present study was supported by funds (gran no.
161045) from the Central Research Institute of Fukuoka
University.
References
|
1
|
Abbas AK, Mulphy KM and Sher A: Functional
diversity of helper T lymphocytes. Nature. 383:787–793. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Snell GI, Westall GP and Paraskeva MA:
Immunosuppression and allograft rejection following lung
transplantation: Evidence to date. Drugs. 73:1793–1813. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Sandwijk MS, Bemelman FJ and Ten Berg
IJ: Immunosupressive drugs after solid organ transplantation. Neth
J Med. 71:281–289. 2013.PubMed/NCBI
|
|
4
|
Pecarce EL: Metabolism in T cell
activation and differentiation. Curr Opin Immunol. 22:314–320.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma W, Mishra S, Gee K, Mishra JP, Nandan
D, Reiner NE, Angel JB and Kumar A: Cyclosporin A and FK506 inhibit
IL-12p40 production through the calmodulin/calmodulin-dependent
protein kinase-activated phosphoinositide 3-kinase in
lipopolysaccharide stimulated human monocytic cells. J Biol Chem.
282:13351–13362. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hughes K, Edin S, Antonsson A and
Grundström T: Calmodulin-dependent kinase II mediaters T cell
receptor/CD3-and phorbol ester-induced activation of Ikappaβ
kinase. J Biol Chem. 276:36008–36013. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pennington JAT and Fisher RA: Food
component profiles for fruit and vegetable subgroups. J Food
Composition Analysis. 23:411–418. 2010. View Article : Google Scholar
|
|
8
|
Dembitsky VM, Poovarodom S, Leontowicz H,
Leontowicz M, Vearasilp S, Trakhtenberg S and Gorinstein S: The
multiple nutrition properties of some exotic fruits: Biological
activity and active metabolites. Food Res Int. 44:1671–1701. 2011.
View Article : Google Scholar
|
|
9
|
Ayala-Zavala JF, Vega-Vega V,
Rosas-Domínguez C, Palafox-Carlos H, Villa-Rodriguez JA, Wasim
Siddiqui Md, Dávila-Aviña JE and González-Aguilar GA:
Agro-industrial potential of exotic fruit by products as a source
of food additives. Food Res Int. 44:1866–1874. 2011. View Article : Google Scholar
|
|
10
|
Liu DH, Shi J, Ibarra AC, Kakuda Y and Xue
SJ: The scavenging capacity and synergistic effects of lycopene,
vitamin E, vitamin C, and β-carotene mixtures on the DPPH free
radical. Food Sci Technol. 41:1344–1349. 2008.
|
|
11
|
Müller L, Fröhlich K and Böhm V:
Comparative antioxidant activities of carotenoids measured by
ferric reducing antioxidant power (FRAP), ABTS bleaching assay
(αTEAC), DPPH assay and peroxyl radical scavenging assay. Food
Chem. 129:139–148. 2011. View Article : Google Scholar
|
|
12
|
Zimmer AR, Leonardi B, Miron D, Schapoval
E, Oliveira JR and Gosmann G: Antioxidant and anti-inflammatory
properties of Capsicum baccatum: From traditional use to scientific
approach. J Ethnopharmacol. 139:228–233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Menichini F, Tundis R, Bonesi M, Loizzo
MR, Conforti F, Stattti G, Cindio BD, Houghton PJ and Menichini F:
The influence of fruit ripening on the phytochemical content and
biological activity of Capsicum chinense Jacq. cv Habanero.
Food Chem. 114:553–560. 2009. View Article : Google Scholar
|
|
14
|
Bown D: Encyclopedia of Herbs and Their
Uses, Kindersley Dorling, London. Herb society of America; London,
UK: 2001
|
|
15
|
Meghvansi MK, Siddiqui S, Khan MH, Gupta
VK, Vairale MG, Gogoi HK and Singh L: Naga chili: A potential
source of capsaicinoids with broad-spectrum ethdopharmacological
applications. J Ethnopharmacol. 132:1–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wyk BEV and Wink M: Medicinal Plants of
the World: An Illustrated Scientific Guide to Important Medicinal
Plants and Their Uses. Timber Press; Portland, Ore, USA: 2004
|
|
17
|
Antonious GF, Kochhar TS, Jarret RL and
Snyder JC: Antioxidants in hot pepper: Variation among accessions.
J Environ Sci Health B. 41:1237–1243. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song X, Bao S, Wu L and Hu S: Ginseng
stem-leaf saponins (GSLS) and mineral oil act synergistically to
enhance the immune responses to vaccination against foot-and-mouth
disease in mice. Vaccine. 27:51–55. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie F, Li Y, Su F and Hu S: Adjuvant
effect of Atractylodis macrophalae Koidz. polysaccharides on the
immune response to foot-and-mouth disease vaccine. Carbohydr Polym.
87:1713–1719. 2012. View Article : Google Scholar
|
|
20
|
Strauss G, Osen W and Debatin KM:
Induction of apoptosis and modulation of activation and effector
function in T cells by immunosuppressive drugs. Clin Exp Immunol.
128:255–266. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sakuma S, Kato Y, Nishigaki F, Magari K,
Miyata S, Ohkubo Y and Goto T: Effects of FK506 and other
immunosuppressive anti-rheumatic agents on T cell activation
mediated IL-6 and IgM production in vitro. Int Immunopharmacol.
1:749–757. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sakuma S, Kato Y, Nishigaki F, Sasakawa T,
Magari K, Miyata S, Ohkubo Y and Goto T: FK506 potently inhibits T
cell activation induced TNF-alpha and IL-1beta production in vitro
by human peripheral blood mononuclear cells. Br J Pharmacol.
130:1655–1663. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Toyoda T, Shi L, Takasu S, Cho YM,
Kiriyama Y, Nishikawa A, Ogawa K, Tatematsu M and Tsukamoto T:
Anti-inflammatory effects of capsaicin and piperine on
helicobacter pylori-induced chronic gastritis in Mongolian
gerbils. Helicobacter. 21:131–142. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang J, Luo K, Li Y, Chen Q, Tang D, Wang
D and Xiao J: Capsaicin attenuates LPS-induced inflammatory
cytokine production by upregulation of LXRα. Int Immunopharmacol.
28:264–269. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dujovny N, Varghese A, Shen J, Yin D, Ji
S, Ma L, Finnegan A and Chong AS: Acute xenograft rejection
mediated by antibodies produced independently of TH1/TH2 cytokine
profiles. Am J Transplant. 2:526–534. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Di Renzo M, Rubegni P, De Aloe G, Paulesu
L, Pasqui AL, Andreassi L, Auteri A and Fimiani M: Extracorporeal
photochemotherapy restores Th1/Th2 imbalance in patients with early
stage cutaneous T-cell lymphoma. Immunology. 92:99–103. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ishiguro K, Green T, Rapley J, Wachtel H,
Giallourakis C, Landry A, Cao Z, Lu N, Takafumi A, Goto H, et al:
Ca2+/calmodulin-dependent protein kinase II is a
modulator of CARMA1-mediated NF-kappaB activation. Mol Cell Biol.
26:5497–5508. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Quesada AJ and Redondo JM:
CA++/calcineurin/NFAT signaling in endothelial activation and
angiogenesis: Effects od cyclosporine A. Nefrologia. 23 (Suppl
3):S44–S48. 2003.(In Spanish).
|
|
29
|
Lederer JA, Liou JS, Kim S, Rice N and
Lichtman AH: Regulation of NF-kappa B activation in T helper 1 and
T helper 2 cells. J Immunol. 156:56–63. 1996.PubMed/NCBI
|