Introduction
Bacterial keratitis is a common cause of blindness.
It activates pattern recognition receptors in the cornea causing
inflammation. Leukocytes, predominantly neutrophils, infiltrate the
cornea and the anterior chamber of the eye (1,2). The
infection and inflammation often lead to corneal ulcers and
consecutive blindness. The estimated prevalence varies from 6.3 to
710 per 100,000 (1). The high
incidence is due not only to poor hygiene standards and a greater
risk of infection in developing countries, but also to the
widespread use of contact lenses with associated complications in
industrial nations (3,4). The two primary gram-positive and
gram-negative bacterial strains responsible for causing bacterial
keratitis are Staphylococcus aureus and Pseudomonas
aeruginosa (1). The first-line
treatment is primarily performed using fluoroquinolones (1). Due to the resistance of bacteria to
antibiotics, the effectiveness of the treatment of bacterial
keratitis is decreasing. These circumstances have made research
into the development of novel therapy approaches essential.
Photodynamic inactivation (PDI) was rarely used as a treatment
option for ocular infections, although other photodynamic therapy
procedures including corneal crosslinking with riboflavin and
ultraviolet light (UV-crosslinking), are established as a treatment
for specific corneal diseases such as keratoconus (5–10).
UV-crosslinking is a photochemical crosslinking of fibers within
the corneal tissue.
Chlorin e6 [Ce6; C34H36N4O6 in full: (17S,
18S)-18-(2-carboxyethyl)-20-(carboxymethyl)-12-ethenyl-7-ethyl-3,
8,13,17-tetramethyl-17,18,22,23-tetrahydroporphyrin-2-carboxylic
acid] and PDI have previously been investigated in vitro and
appear to be promising in the treatment of microbial keratitis
(11,12). PDI functions on the principle that
the accumulation of a specific photosensitizer in the relevant
tissue induces the generation of reactive oxygen species (ROS) when
exposed to light of a specific wavelength. These ROS may kill
bacteria and be the initial step in healing bacterial keratitis
(13–17). The histopathology of bacterial
infected mice indicates focal cellular infiltrates within the
corneal stroma. Concomitant, heavy cellular infiltrates are
identified in the anterior chamber, which is also called the
hypopyon. Typically, in the later stages corneal ulcers occur
(18). Corneal ulcers are estimated
to cause approximately two million new cases of monocular blindness
every year (1).
The purpose of the present study was to establish
experimental infectious keratitis in mice using pseudomonades and
to investigate the effect of treatment with Ce6 on corneal
inflammation.
Materials and methods
Animals
In the present study, a total of 30 C57BL6N mice
(Janvier Labs, Saint-Berthevin Cedex; weight, 29.1±3.8 g; 24
females:6 males) were used. Mice used were 8–11 months old as older
mice are more susceptible to experimental infection (19). The housing conditions were as
follows: temperature, 22°C; humidity, 60%; and a 12 h light/dark
cycle. The animals had access to chow and water ad libitum.
The animal experiments were approved by the Government of the
Saarland (Landesamt für Soziales, Gesundheit und
Verbraucherschutz). The Ethical Committee for Animal Experiments of
the Saarland approved the present study. One eye of each animal was
infected or treated, the other one was untouched. All experiments
were performed in accordance with the Guide for the Care and Use of
Laboratory Animals (20).
Induction of keratitis
Under anesthesia (0.05 mg/kg fentanyl, Hameln Pharma
Plus GmbH, Hameln, Germany; 5 mg/kg midazolam, Hameln Pharma Plus
GmbH; and 0.5 mg/kg medetomidine hydrochloride, Orion Corporation,
Espoo, Finland), three scratches of ~2 mm in length were applied to
the center of the cornea using a 27-gauge needle. Subsequently, 5
µl inoculum was pipetted onto the cornea and was left on the eye
for 20 min. Analgesia was applied daily by intraperitoneal
injection with 4 mg/kg carprofen (Zoetis Schweiz GmbH, Zuerich,
Schweiz). The inoculum consisted of multi-resistant Pseudomonas
aeruginosa, strain 54, which had been cultured on blood agar
plates (trypticase soy agar II, 5% sheep blood; BD GmbH,
Heidelberg, Germany) for 24 h at 37°C, then suspended in 10 ml 2%
BD Difco Luria Bertani broth (LB broth; BD GmbH) for an overnight
culture at 37°C. From the overnight culture, 100 µl was diluted in
10 ml 2% LB broth for another 3 h (37°C) of culturing and finally
the suspension was adjusted to an optical density of 10 for
inoculation using routine procedures (densitometer). Post-infection
treatment was performed for one pilot animal at 12 h and for the
rest at 24 h under anesthesia as described above.
Treatment
For PDI, the infected eyes were incubated with a
photosensitizer gel containing 0.1 % Ce6 or only the gel base
(hydroxypropyl methylcellulose) for the sham-treated group (both
APOCARE Pharma GmbH, Bielefeld, Germany) for 30 min. From the PDI
group, one mouse was treated once for 15 min and one pilot mouse
was treated twice, at 12 and 24 h, with a high Ce6 concentration
(2.0%) for 30 min. In six randomly selected animals the epithelium
of the cornea was removed with a hockey knife prior to PDI. The
procedure was completed in darkness to avoid premature activation
of the photosensitizer. The ointment was removed using a cotton
swab and the animals were subsequently exposed to red light of a
wavelength of 670 nm for 6 min.
Morphology
All animals were sacrificed by neck dissection
whilst under deep anesthesia as described above 3 days' post
infection and the globes were enucleated and histologically
processed as follows: Fixation was performed using
Davidson-Solution, (AppliChem GmbH, Darmstadt, Germany) for 6 h.
Subsequently, samples were left 12 h in isopropanol (30 %, in aqua
dest distilled water) on a shaker. The samples were embedded in
paraffin using routine procedures. Sections were performed and
mounted on glass slides using routine procedures and the mounted
sections were stained with hematoxylin and eosin. For evaluation,
five sections of 5 µm thickness were obtained from the center
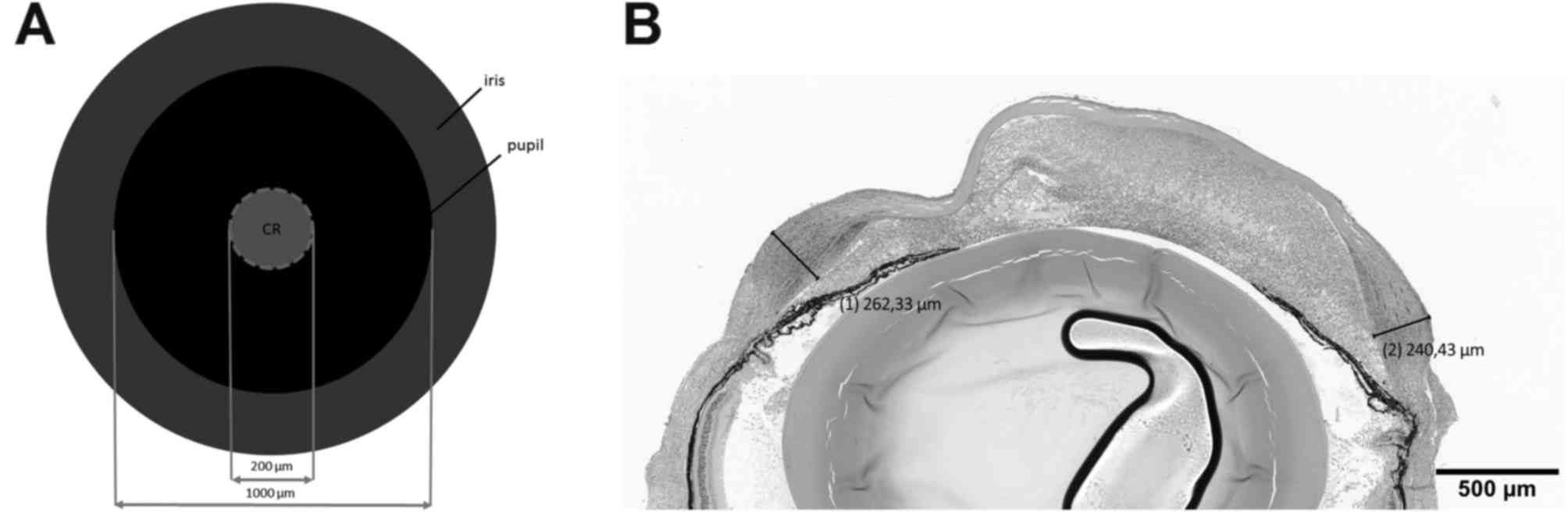
region (with a distance of 50 µm from each other; Fig. 1A). The location of the maximum
corneal thickness was selected and measured using cellSens Standard
software (version 1.8.1; Olympus Deutschland GmbH, Düsseldorf,
Germany). Fig. 1B indicates a
representative measurement of the maximal corneal thickness. Mean
values per group were calculated. In the data figures, each symbol
represents one animal. For evaluation, a light microscope was used
(magnification, ×100; Axiophot, Zeiss, Jena, Germany).
Evaluation
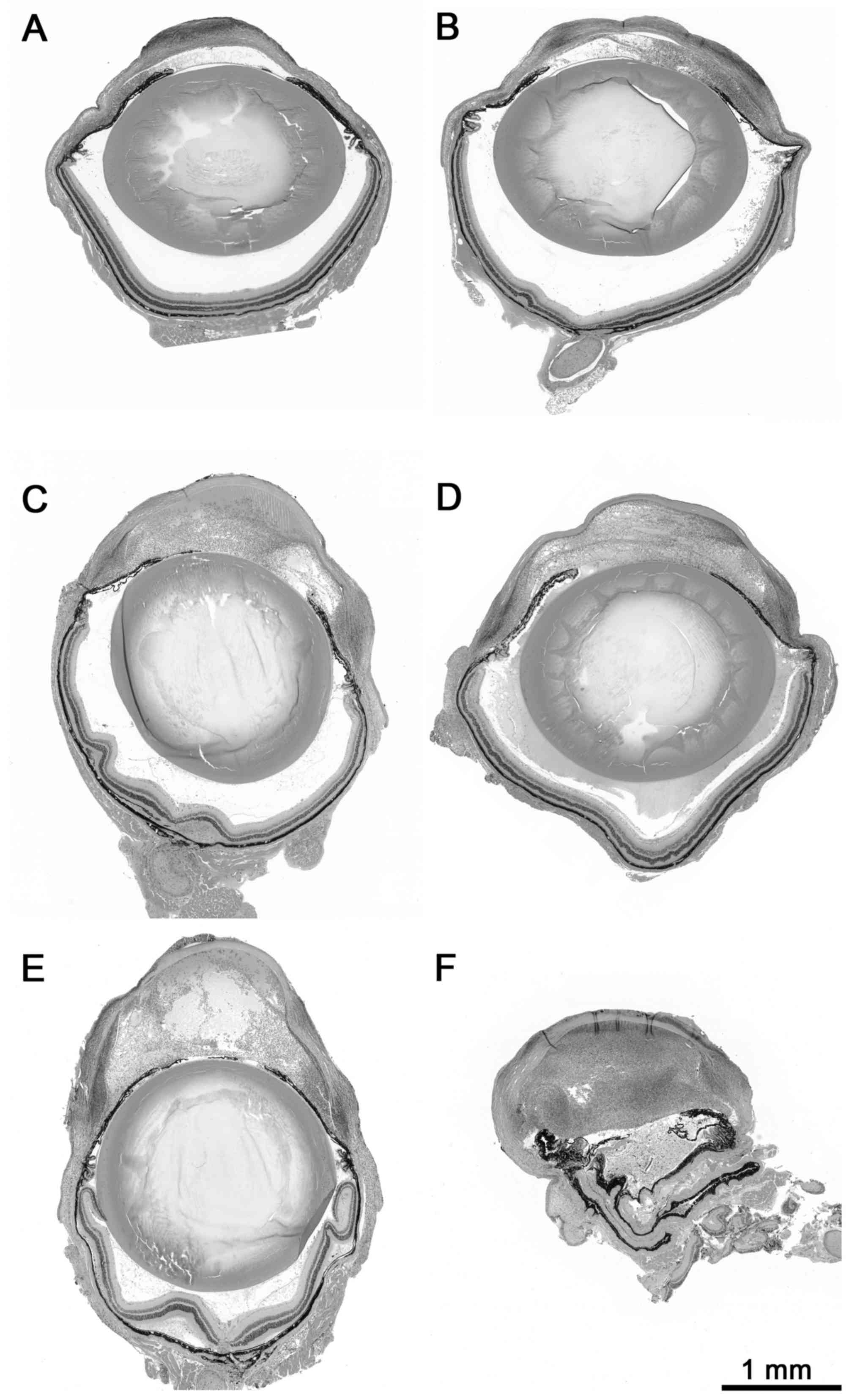
The severity of hypopyon was evaluated in a blinded
manner using a novel semi-quantitative scoring from 0–3 as follows:
0 points, no hypopyon (no cellular infiltrate within the anterior
eye chamber); 1 point, mild hypopyon (cellular infiltrate partly
filling the anterior eye chamber) 2 points, moderate hypopyon
(cellular infiltrate filling the anterior eye chamber); and 3
points; severe hypopyon (heavy cellular infiltrate filling and
distorting the anterior eye chamber), as presented in Fig. 2. In the preliminary study the final
duration of the treatment was set at 30 min. The pilot mice were
included within the analyses of the experimental groups as no
marked differences to mice within the same experimental group were
observed. In summary, the groups for analyses were as follows:
Uninfected/untreated (n=6), infected/untreated (n=4),
infected/sham-treated (n=8) and infected/PDI-treated (n=12).
Statistical analyses were performed using Prism 5 software
(GraphPad Software Inc., La Jolla, CA, USA). Mann-Whitney U test
for two independent samples was used for median comparison. Data
are presented as mean ± standard error of the mean and the
two-sided level of significance was defined as P<0.05.
Results
Corneal inflammation
The present study identified focal cellular
infiltrates and inflammatory oedema within the corneal stroma in
the infected non-treated, but also-to a lesser extent-in the
infected-treated animals. No corneal ulcerations were observed
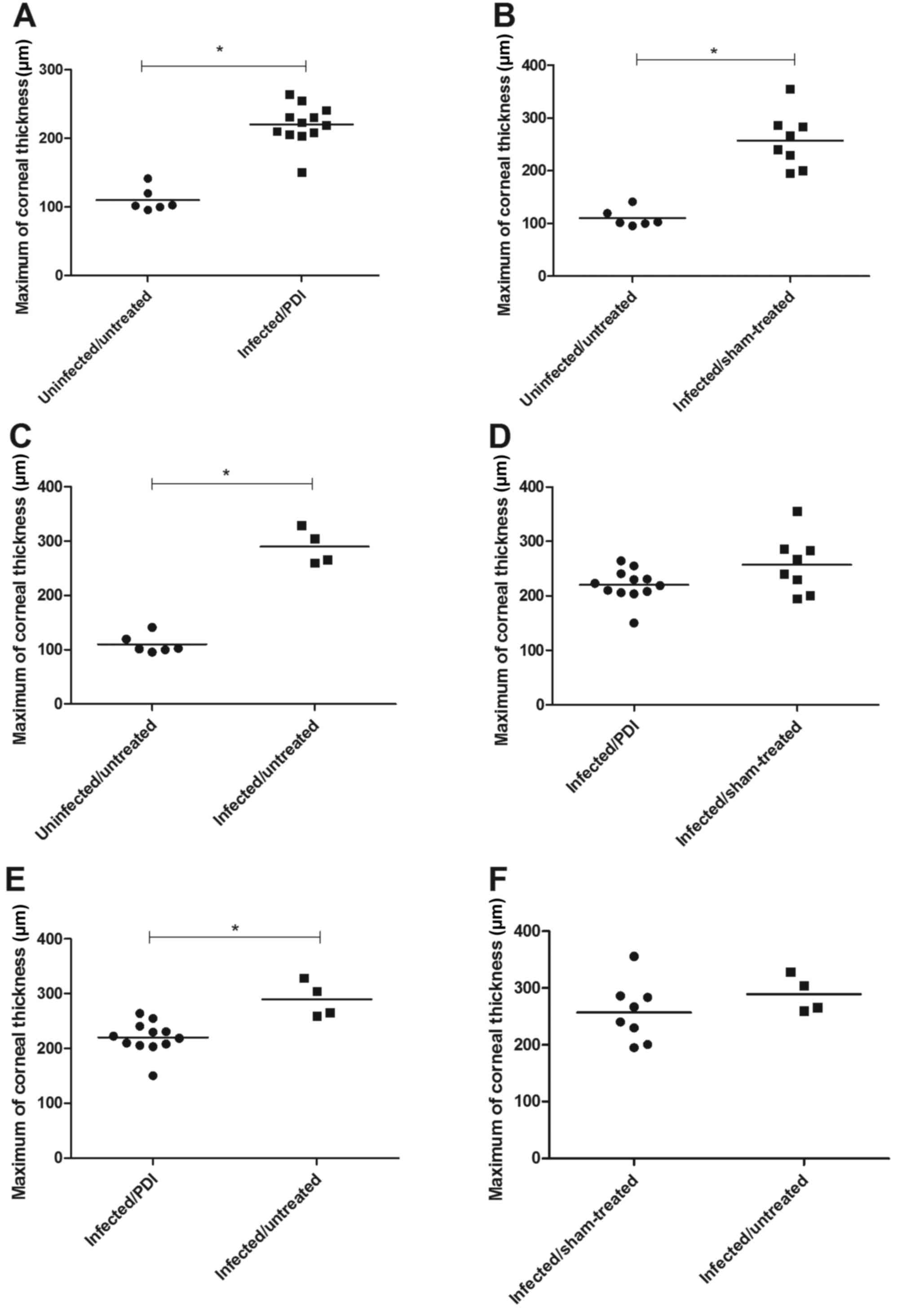
within any of the experimental groups. The maximal corneal
thickness of normal eyes of C57BL6N mice (uninfected/untreated) was
110±7 µm. The maximal corneal thickness of eyes from the
infected/PDI-treated group was 220±8 µm, whereas 257±19 µm was
indicated for eyes of the infected/sham-treated group and 290±16 µm
for eyes of the infected/untreated group, all of which were
significantly increased compared with the uninfected/untreated
group (all P<0.05). In addition, the maximal corneal thickness
of the infected/untreated group was significantly increased
compared with the infected/PDI-treated group (P<0.05; Fig. 3).
Hypopyon
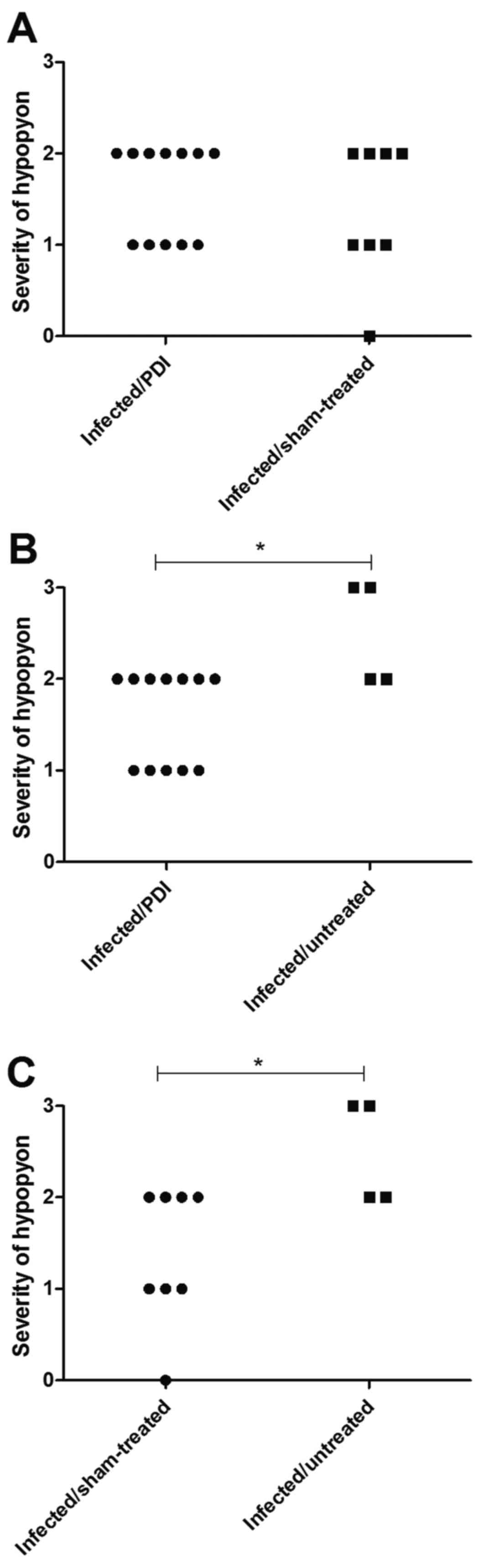
Concomitant infiltrates of neutrophils
(hypopyon/hypopya) were identified in the anterior chamber of the
infected mice. The severity of hypopyon was significantly higher in
the eyes of the infected/untreated group compared with those of the
infected/PDI-treated group (P<0.05). Furthermore, hypopyon in
the infected/sham-treated group was significantly less severe than
in infected/untreated mice (Fig.
4).
Discussion
The determination of the dimension of the induced
keratitis was difficult as a result of the inhomogeneous
infiltration and inflammation. Due to the focal infiltrates and
edema, a general increase in the thickness of the cornea was
observed, but particularly at the sites of the focal infiltrates.
As other methods, including counting the infiltrating cells or the
determination of the whole corneal area on the sections failed, the
authors of the current study selected the maximal corneal thickness
as a measure for the extent of inflammation, following an
evaluation of >1,000 sections, which indicated that the maximal
thickness of the cornea represents the dimension of the
inflammation. This indicates a drawback of the presented
histological approach: The production of numerous sections and
staining and even the number of h spent investigating and measuring
the slides is a limiting factor in those study designs. The maximal
corneal thickness or diameter is the most accurate measurement of
inflammation in the view of the authors. The determination of
colony forming units (CFU) would be an alternative and quantitative
method but may also be unsatisfactory, as the number of CFU alone
does not define keratitis. In addition, it would not be possible to
isolate the cornea of the eyeball in the mouse, due to its small
size. Most likely the eyeball would be homogenized and all
compartments of the eye would be mixed together and the specific
situation of the cornea would be uncertain. In the present study,
the maximal corneal thickness in mice with keratitis was ~20% less
following PDI when compared with untreated mice. This indicated a
beneficial effect of treatment using PDI that may be beneficial for
the treated mice. However, the maximum corneal thickness following
PDI therapy remained almost double that of uninfected animals. The
disease course following treatment with PDI was not investigated in
the present study. One PDI treatment may lead to healing or quicker
healing of the keratitis. It is likely that a repetitive treatment
using PDI may be necessary, and this should be determined in future
experiments. Notably, the infected and sham-treated mice did not
differ significantly from the animals undergoing treatment using
PDI. This is difficult to understand; however, a possible
explanation is that the sham treatment included a light exposure
without the active drug. This exposure itself may inhibit the
bacterial growth within the cornea. Another explanation would be
that the paste (solvent) without the drug had antibacterial
effects. However, the comparison between the sham-treated animals
and untreated animals did not reveal a significant difference.
The hypopyon that occurred following the
experimental infection varied in shape and extent. The score used
for the evaluation was semi-quantitative. A weakness of this score
is that there is not a well-defined distinction between score 1 and
2 or between score 2 and 3. Notably, PDI-treated infected eyes and
sham-treated infected eyes had a less severe hypopyon score when
compared with the infected but untreated eyes. This supported the
hypothesis that the paste or the red light possessed
anti-inflammatory or antibacterial effects. This may have accounted
for an improvement of 1 point using the scoring system. However,
the present results of the hypopyon indicated a reduction of
hypopya after the use of therapy. It remains unclear, how the PDI
influenced the hypopyon; however, the hypothesis is that Ce6 and
the phototherapy reached the cornea and the anterior chamber,
leading to inactivation of pseudomonades in both compartments. By
contrast, the application of red light alone may have mediated the
effect since less severe hypopyon was observed following sham
treatment. Taken together, the present study presents preliminary
data that using Ce6 and PDI may be an important treatment approach
of severe bacterial keratitis.
Previously, it has been demonstrated in vitro
that Ce6 and PDI are a potent threat to leishmania species
(21). Experimental keratitis
depends on species and age of the experimental animals in addition
to the bacteria used (22). In
principal, it is challenging to use staphylococci in the murine
keratitis model due to the difficulties in establishing infection.
Even when using pseudomonades, the susceptibility varies in
different age groups. In general, older mice are more susceptible
than postnatal or young mice. Therefore, primarily older mice were
selected for the present study.
Effects of Ce6 treatment and PDI with regard to the
appropriate time point and duration of incubation time should be
repeated with higher numbers of animals. The present study was, to
the best of our knowledge, the first to assess the feasibility and
usefulness of PDI-therapy. Beyond the inflammation of the corneal
tissue, the burden of living bacterial cells would be a noteworthy
parameter. At the time of the present experiment, measurement of
the bacterial burden of infected cornea was not completed. As
mentioned, it is difficult to determine CFU within the isolated
cornea. In a previous study on the use of a high mobility group
box-1 inhibitor in a murine model of keratitis, the determination
of CFU in isolated cornea was established (22). However, the CFU numbers determined in
homogenates of whole eyeballs would also provide valuable
information.
The results presented in the current study may serve
as a promising basis for further investigations. The potential of
this approach should be investigated with the aim of further
development and optimization in ongoing studies in order to achieve
the integration of this therapy in future standard clinical
practice. In conclusion, Ce6 and PDI may be a potential approach
for treatment of patients suffering from severe bacterial or
acanthamoebiasis-keratitis, which is resistant against conventional
therapy.
Acknowledgements
The authors of the present study would like to thank
Mrs. Ann Soether for language editing and Mrs. Tina Wiesen-Philipps
for help with the manuscript.
References
|
1
|
Ong HS and Corbett MC: Corneal infections
in the 21st century. Postgrad Med J. 91:565–571. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Taube MA, del Mar Cendra M, Elsahn A,
Christodoulides M and Hossain P: Pattern recognition receptors in
microbial keratitis. Eye (Lond). 29:1399–1415. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Young G, Young AG and Lakkis C: Review of
complications associated with contact lenses from unregulated
sources of supply. Eye Contact Lens. 40:58–64. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lorenzo-Morales J, Khan NA and Walochnik
J: An update on Acanthamoeba keratitis: Diagnosis, pathogenesis and
treatment. Parasite. 22:102015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Makdoumi K, Mortensen J, Sorkhabi O,
Malmvall BE and Crafoord S: UVA-riboflavin photochemical therapy of
bacterial keratitis: A pilot study. Graefes Arch Clin Exp
Ophthalmol. 250:95–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stachon T, Wang J, Eppig T, Langenbucher
A, Bischoff M, Seitz B and Szentmáry N: KGF, FGFb, VEGF, HGF and
TGFβ1 secretion of human keratocytes following photodynamic
inactivation (PDI) in vitro. Graefes Arch Clin Exp Ophthalmol.
251:1987–1993. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stachon T, Wang J, Song X, Langenbucher A,
Seitz B and Szentmáry N: Impact of
crosslinking/riboflavin-UVA-photodynamic inactivation on viability,
apoptosis and activation of human keratocytes in vitro. J Biomed
Res. 29:321–325. 2015.PubMed/NCBI
|
|
8
|
Wu MF, Stachon T, Wang J, Song X, Colanesi
S, Seitz B, Wagenpfeil S, Langenbucher A and Szentmáry N: Effect of
keratocyte supernatant on epithelial cell migration and
proliferation after corneal crosslinking (CXL). Curr Eye Res.
41:466–473. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Randleman JB, Khandelwal SS and Hafezi F:
Corneal cross-linking. Surv Ophthalmol. 60:509–523. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Papaioannou L, Miligkos M and
Papathanassiou M: Corneal collagen cross-linking for infectious
keratitis: A systematic review and meta-analysis. Cornea. 35:62–71.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang J, Stachon T, Eppig T, Langenbucher
A, Seitz B and Szentmáry N: Impact of photodynamic inactivation
(PDI) using the photosensitizer chlorin e6 on viability, apoptosis
and proliferation of human keratocytes in vitro. Graefes Arch Clin
Exp Ophthalmol. 251:2725–2731. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang J, Stachon T, Eppig T, Langenbucher
A, Seitz B and Szentmáry N: Impact of photodynamic inactivation
(PDI) using the photosensitizer chlorin e6 on viability, apoptosis,
and proliferation of human corneal endothelial cells. Graefes Arch
Clin Exp Ophthalmol. 251:1199–1204. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rovaldi CR, Pievsky A, Sole NA, Friden PM,
Rothstein DM and Spacciapoli P: Photoactive porphyrin derivative
with broad-spectrum activity against oral pathogens In vitro.
Antimicrob Agents Chemother. 44:3364–3367. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gad F, Zahra T, Francis KP, Hasan T and
Hamblin MR: Targeted photodynamic therapy of established
soft-tissue infections in mice. Photochem Photobiol Sci. 3:451–458.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park JH, Moon YH, Bang IS, Kim YC, Kim SA,
Ahn SG and Yoon JH: Antimicrobial effect of photodynamic therapy
using a highly pure chlorin e6. Lasers Med Sci. 25:705–710. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martins SA, Combs JC, Noguera G, Camacho
W, Wittmann P, Walther R, Cano M, Dick J and Behrens A:
Antimicrobial efficacy of riboflavin/UVA combination in vitro for
bacterial and fungal isolates: A potential new treatment for
infectious keratitis. Invest Ophthalmol Vis Sci. 49:3402–3408.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khan YA, Kashiwabuchi RT, Martins SA,
Castro-Combs JM, Kalyani S, Stanley P, Flikier D and Behrens A:
Riboflavin and ultraviolet light a therapy as an adjuvant treatment
for medically refractive Acanthamoeba keratitis: Report of 3 cases.
Ophthalmology. 118:324–331. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ekanayaka SA, McClellan SA, Barrett RP,
Kharotia S and Hazlett LD: Glycyrrhizin reduces HMGB1 and bacterial
load in Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis
Sci. 57:5799–5809. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Girgis DO, Sloop GD, Reed JM and
O'Callaghan RJ: Susceptibility of aged mice to Staphylococcus
aureus keratitis. Curr Eye Res. 29:269–275. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
AnimalsGuide for the Care and Use of Laboratory Animals. 8th.
National Academies Press (US); Washington (DC): 2011, PubMed/NCBI
|
|
21
|
Pinto JG, Pereira AH, de Oliveira MA,
Kurachi C, Raniero LJ and Ferreira-Strixino J: Chlorin E6
phototoxicity in L. major and L. braziliensis promastigotes-in
vitro study. Photodiagnosis Photodyn Ther. 15:19–24. 2016.(In
Press). View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marquart ME: Animal models of bacterial
keratitis. J Biomed Biotechnol. 2011:6806422011. View Article : Google Scholar : PubMed/NCBI
|