Introduction
Acquired immune deficiency syndrome (AIDS), is
caused by the human immunodeficiency virus type 1 (HIV-1)
infection. The virus damages the cell mediated immune system in the
infected individuals. The Joint United Nations Programme on
HIV/AIDS (UNAIDS) reported that mortality due to AIDS is more than
40 million patients to date, and prevalence of the infection is 35
million worldwide (1). Although
drugs have been developed to control the replication of the virus
and the deterioration of the disease, but so far, no treatment
strategy that can completely cure AIDS due to the high endurance
and mutation rate of HIV-1 virus, other factors are changed
cellular immune function after infection, and inhibited apoptosis
of infected cells (2,3). The main target of HIV-1 is the
CD4+ T cells of the human immune system, that is, helper
T cells (Th). These cells play a significant role in assisting
humoral immunity and cellular immunity (4).
On invasion of the body by antigen T lymphocytes
after perceiving the signal activate and proliferate into effector
T cells, which can play roles in immune response by releasing
lymphatic factors. T cell activation is a very complex process that
requires two kinds of signals from the extracellular stimulus. The
first signal is a specific binding of the T cell surface receptor
(TCR) to the MHC-antigen peptide complex on the surface of the
antigen presenting cell (APC), and the second signal is derived
from the interactions between co-stimulatory molecules in APC and
corresponding receptor molecules on the surface of T cells, the two
signals are both essential. CD4+ T cells and
CD8+ T cells (cytotoxic T cells, Tc) can combine with
MHC-II and MHC class I molecules, respectively (5). CD4+ T cells can be activated
into effector T cells and memory T cells, and the former can
secrete cytokine to regulate or assist the immune response
(6).
MicroRNA is a non-coding RNA with a length of ~22
nt. In recent years, microRNA has been reported to have a role in
immune response elicited by pathogenic infection (7). MicroRNA binds to the 3′-untranslated
region (3′-UTR) of the target genes by base pairing to silence the
expression of target gene at the post-transcriptional level.
MicroRNAs also play an important role in regulating metabolism,
cell differentiation, necrosis and cancer formation (8). With the development of high-throughput
sequencing and computational biology, up to hundreds of microRNA
families have been identified and reported, among which the miR-124
family has been shown to be able to inhibit tumorigenesis, and one
of the members of miR-124 family-miR-124a can promote the
differentiation of specific tissues and inhibit the expression of
target gene SIRT1 (histone deacetylase), thus indirectly control
the transcription factor Foxp3, so as to regulate the development
and functionalization of regulatory T cells (Tregs) (9,10).
The aim of this study was to investigate the role of
miR-124a in the regulation of T cell activation and immunity in
patients with AIDS, and to provide new insights and therapeutic
targets for the diagnosis, alleviation and treatment of AIDS.
Patients and methods
Patients
The study was conducted on 15 confirmed AIDS
patients (12 males and 3 females), peripheral blood samples of 15
patients with confirmed AIDS were collected. Those patients include
12 males and 3 females with an average age of of 25±4.74 years. Out
of the total, 11 cases were in pre-AIDS stage and 4 cases were in
symptomatic HIV infection stage. In addition, peripheral blood
samples were also collected from 15 healthy people, including 12
males and 3 females with an average age of 27±5.23 years. All the
patients signed the informed consent. The Ethics Committee of
Qingdao No. 6 People's Hospital approved this study.
Confidentiality agreement was also signed. Informed consents were
signed by the patients and/or guardians.
Methods
Materials and suppliers
PRIR-REPORT luciferase reporter vector, miR-124a
mimic/control and miR-124a inhibitor/control (Ambion, New York, NY,
USA); lymphocyte isolation liquid (Solarbio, New York, NY, USA);
Human T Cell Nuclear Transfection kit (Lonza, Bern, Switzerland);
MACSRCD4 MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany);
QuickChange Lighting Mutagenesis kit (Stratagene, La Jolla, CA,
USA); TRIzol reagent, PrimeScript® RT reagent kit with
gDNA Eraser and SYBR® Premix Ex Taq™ II (Takara
Biotechnology Co., Ltd., Dalian, China); protease inhibitors and
PVDF membranes (Roche Diagnostics, Basel, Switzerland);
Dual-Luciferase® Reporter (DLR™) Assay kit (Promega,
Madison, WI, USA); ELISA kit (R&D Systems, Minneapolis, MN,
USA); SIRT1, β-actin mouse monoclonal antibody, goat anti-mouse HRP
antibody (Cell Signaling Technology, Inc., Boston, MA, USA);
modified BCA assay kit (Sangon, Shanghai, China). All the primers
were synthesized by Sangon.
Separation of peripheral blood monocytes (PBMs)
and CD4+ T cells of by magnetic microBeads
The peripheral blood samples (PBS) (30 ml, heparin
was added for anticoagulation) of both groups were mixed with the
same volume of PBS. After adding the lymphocyte isolation liquid,
capillary was used to collect the monocyte layer cells. To the cell
layer 7 times volume of PBS was added, the mixture was centrifuged
at low-speed for 15 min, the supernatant was discarded and 1 ml PBS
was added after washing the cells to resuspend the cells. According
to the instruction of MACSRCD4 MicroBeads CD4+ T Cell
Isolation kit, CD4+ T cells were isolated from the
monocytes. The purity of the CD4+ T cells was detected
by flow cytometry, after that, the cells were frozen and stored at
−80°C.
RNA extraction, reverse transcription, and
RT-qPCR
The total RNA in CD4+ T cells was
extracted and checked according to the instructions of the kit.
After reverse transcription, the cDNA was used to prepare the
reaction system of qRT-qPCR. The PCR reaction was performed using
the CFX-96 Real-Time PCR Detection system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The data were processed using
2−ΔΔCt method. The healthy people were used as a control
and β-actin was used as the endogenous control for the PCR
reaction. The primer sequences are shown in Table I.
 | Table I.The sequence of the primers used for
RT-qPCR and primers used for plasmid construction. |
Table I.
The sequence of the primers used for
RT-qPCR and primers used for plasmid construction.
| Primers | Sequence (5′-3′) |
|---|
| miR-124a | F:
GTGCAGGGGTCCGAGGT |
| miR-124a | R:
AAGGCACGCGGTGAATGC |
| ACTB | F:
CAGGGCGTGATGGTGGGCA |
| ACTB | R:
CAAACATCATCTGGGTCATCTTCTC |
| SIRT1-3′UTR | F:
AAGCTTCTGTGAAACAGGAAGTAACAGACA |
| SIRT1-3′UTR | R:
ACTAGTTGGCAGTAATGGTCCTAGCTG |
Construction and transfection of dual-luciferase
reporter plasmids
The 3′-UTR of SIRT1 was amplified by primer
SIRT1-3′UTR-F/R using the human CD4+ T cell genome as a
template. This region contained a potential miR-124a binding site.
After gel running, the amplified product was recovered. The
pMIR-REPORT luciferase reporter vector was digested with
HindIII and SpeI and the target fragment was also
recovered after gel running. Those two fragments were ligated
together according to the instruction of the kit. After the
construction of plasmid pMIR-REPORT-WT, the recombinant plasmid was
transfected and the recombinant plasmid was isolated from the
transfected cells to verify the transfection. In order to verify
that SIRT1 is the target gene of miR-124a, it is necessary to
construct SIRT1-3′-UTR mutant reporter plasmid pMIR-REPORT-MU. To
do this, the SIRT1-3′-UTR of the recombinant plasmid was mutated by
QuikChange Lightning Mutagenesis kit. The primers used for plasmid
construction are shown in Table
I.
The combination of miR-124a mimic or its control
with pMIR-REPORT-WT, and the combination of miR-124a mimic or its
control with pMIR-REPORT-MU were co-transfected into Jurkat cells
with the intraperitoneal luciferase endogenous control plasmid
pRenilla-IR, respectively. After 48 h, the cells were collected and
the luciferase activity was analyzed by the dual luciferase
reporter assay kit according to the instructions.
Western blot analysis
The total protein was extracted from CD4+
T cells of study and the control group. After quantification by BCA
method, 50 µg protein was subjected to SDS-PAGE gel running and
then transferred to PVDF membrane. The membrane was bleached and
rinsed in PBST solution, after that, the membrane was blocked in
block solution for 1 h. Then mouse anti-human SIRT1 and β-actin
primary antibody (1:1,000) were added respectively and incubated
overnight at 4°C. After washing, HRP goat anti-mouse secondary
antibody (1:5,000) was added and incubated for 1 h at room
temperature. Chemiluminescence reaction was performed and gray
scale analysis was performed using ImageJ software (National
Institutes of Health, Bethesda, MD, USA).
Transfection
Human CD4+ T cells were cultured in T
cell culture medium (10% FBS, 5% streptomycin). After collection,
the cells were resuspended in transfection solution. miR-124a mimic
and its control were transfected into CD4+ T cells of
the health people, respectively, and miR-124a inhibitor and its
control were transfected into CD4+ T cells of patients
with AIDS, respectively. After 48 h, the expression levels of SIRT1
protein and cytokines were detected. All the operations of
transfection were performed according to the instructions of Lonza
human T cell transfection kit.
ELISA to detect cytokine expression levels
The levels of interferon (IFN)-γ, interleukin
(IL)-10, transforming growth factor (TGF)-β, IL-2, IL-4 and IL-6
secreted by Th cells after transfection with miR-124a
mimic/inhibitor were detected using to ELISA kit according to the
instructions.
Statistical analysis. Statistical analysis was
performed using SPSS 17.0 statistical software (SPSS, Inc.,
Chicago, IL, USA). All experiments were repeated three times and
the data were expressed as mean ± SD. Single factor analysis of
variance (ANOVA) and two-tailed t-test were performed for the
comparisons between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
There was no significant difference in the
distribution of age and sex between the patient group and the
normal group (p>0.05)
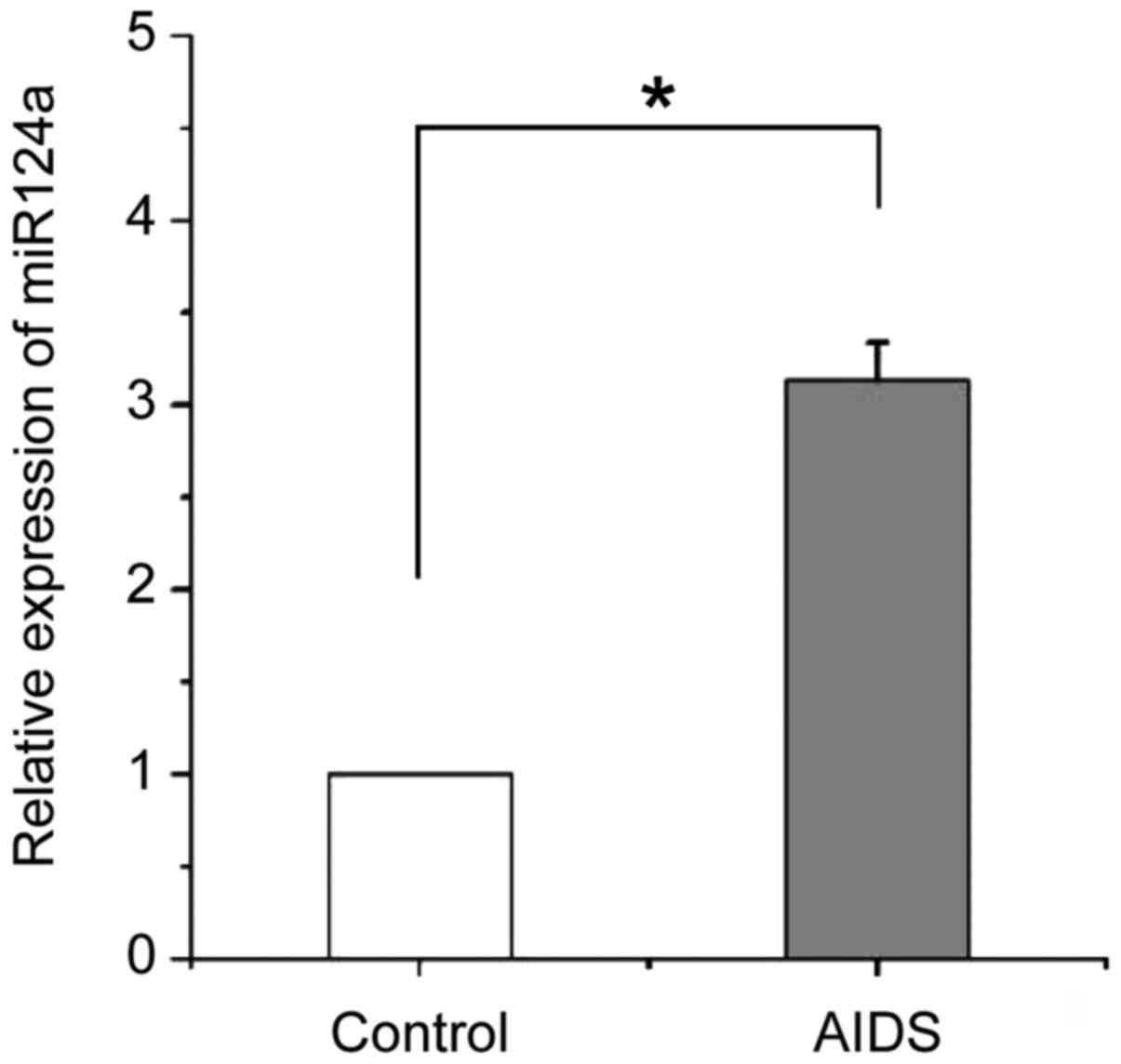
miR-124a is upregulated in
CD4+ T cells of patients with AIDS
As shown in Fig. 1,
quantitative and statistical analysis of the expression of miR-124a
in peripheral CD4+ T cells of patients with AIDS and
healthy people. It showed that miR-124a was significantly
(p<0.05) upregulated in CD4+ T cells of patients with
AIDS compared with healthy people.
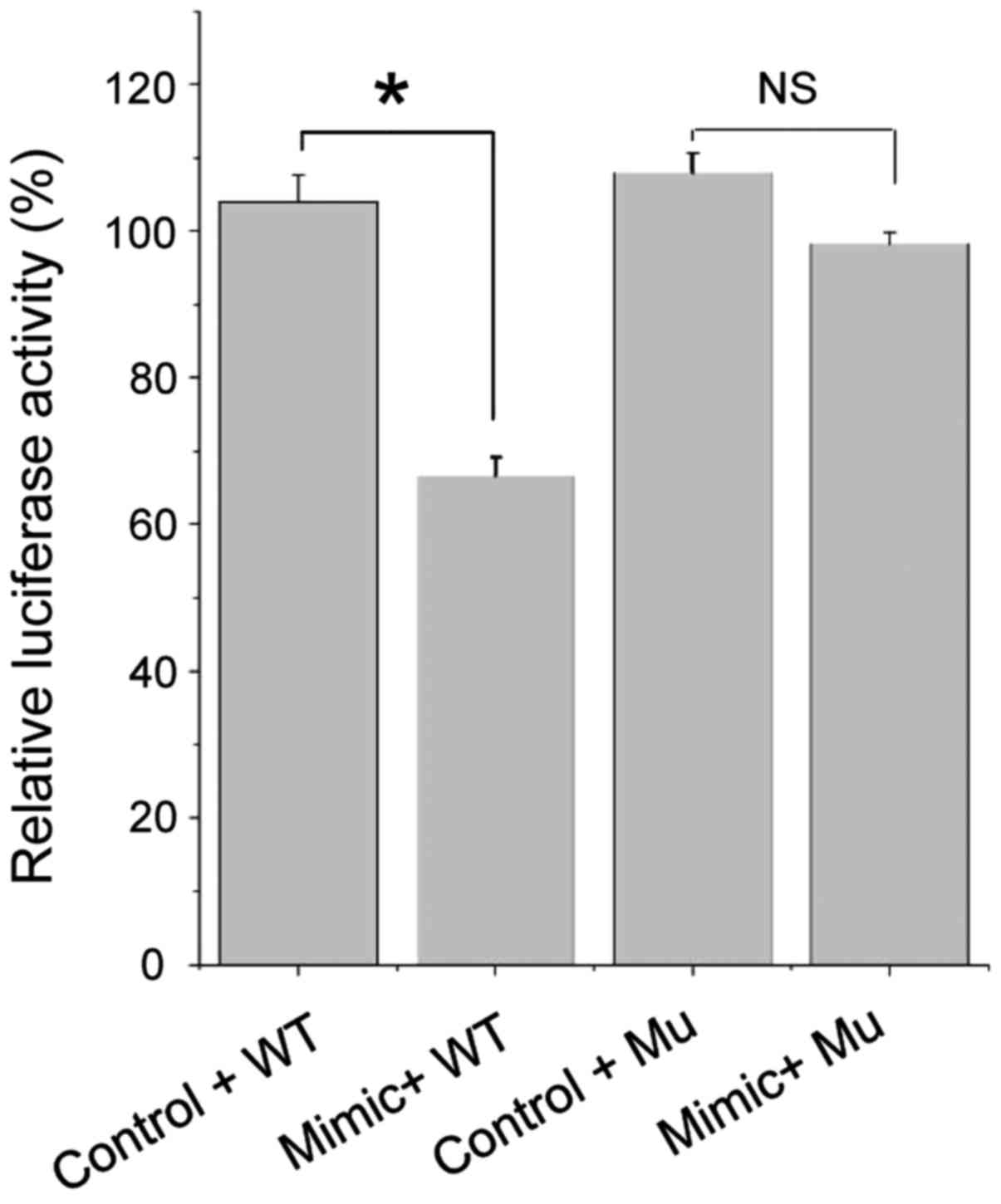
SIRT1 is the target gene of
miR-124a
Based on the prediction using MicroRNA target gene
prediction software (TargetScan, http://www.targetscan.org/) and the findings in
related studies, we initially identified SIRT1 as the target gene
of miR-124a. To demonstrate the direct regulatory relationship
between miR-124a and SIRT1, we constructed the dual-luciferase
reporter vector pMIR-REPORT-WT containing SIRT1 3′-UTR (containing
the predicted miR-124a binding site). This reporter vector was
co-transfected with miR-124a mimics or its negative control into
Jurkat cells to detect the relative activity of the firefly
luciferase.
The results of luciferase activity test (Fig. 2) showed that the luciferase activity
after miR-124a mimic and pWT co-transfection were significantly
lower than that after miR-124a negative control and pWT
co-transfection (p<0.05), while miR-124a mimic and pMu
co-transfection caused no significant change in luciferase activity
compared with the miR-124a negative control and pMu co-transfection
(p>0.05).
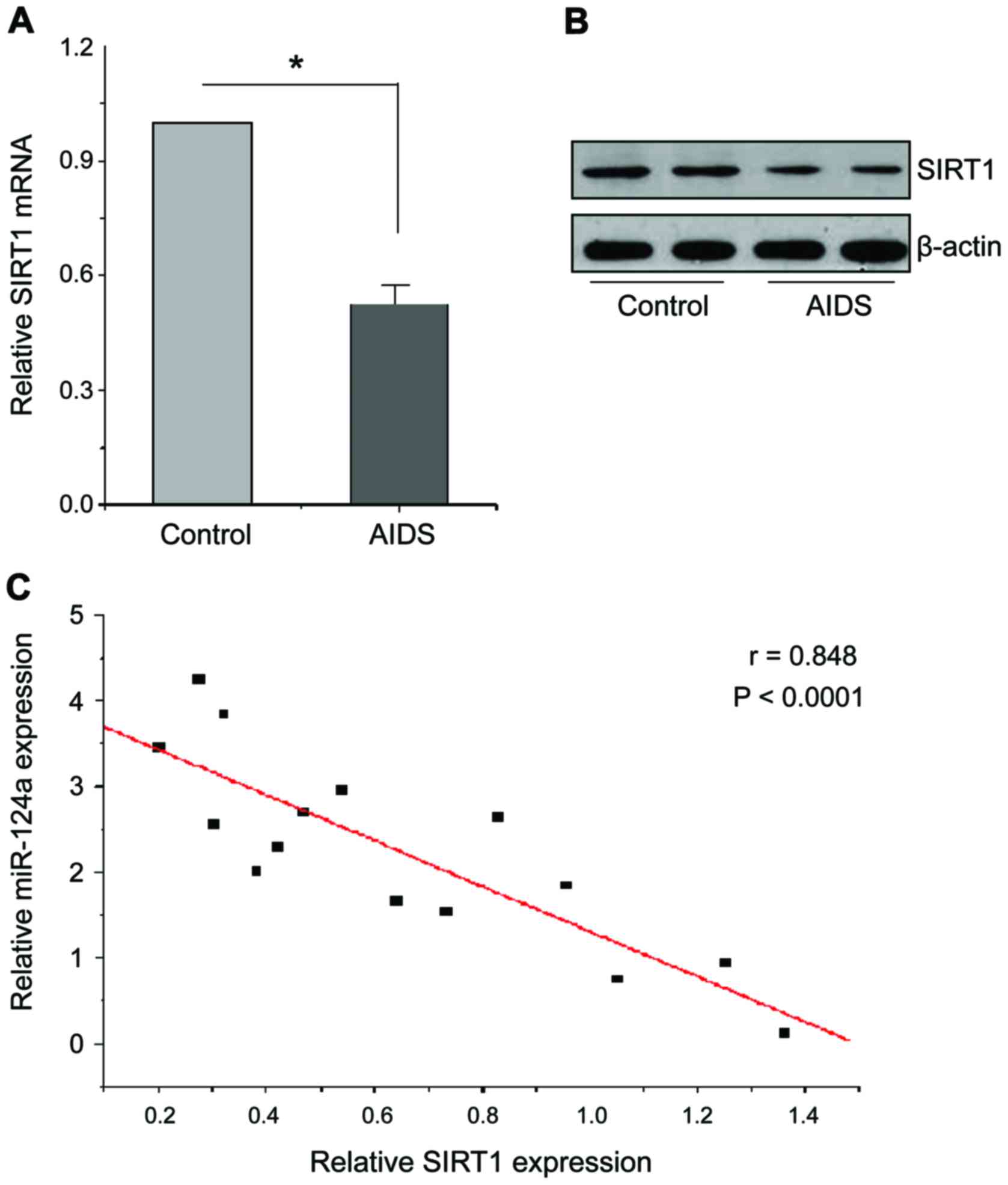
miR-124a regulated the expression of
target gene SIRT1
In order to investigate the regulatory relationship
between miR-124a and target gene SIRT1, we analyzed the levels of
SIRT1 mRNA and protein in CD4+ T cells in both groups of
people. As shown in Fig. 3A and B,
RT-qPCR results showed that SIRT1 mRNA levels in CD4+ T
cells of patients with AIDS were significantly reduced compared
with the healthy people, western blot results also showed that
SIRT1 protein levels in CD4+ T cells of patients with
AIDS were also significantly reduced. Therefore, miR-124a can bind
to 3′-UTR of SIRT1 and inhibit its expression. In order to verify
the negative regulation relationship between them, the expression
levels of miR-124a and SIRT1 in 15 AIDS patients and 15 healthy
people were quantitatively analyzed, and the linear regression
equation was constructed, as shown in Fig. 3C, there is a significant negative
relationship between miR-124a and SIRT1 (p<0.0001).
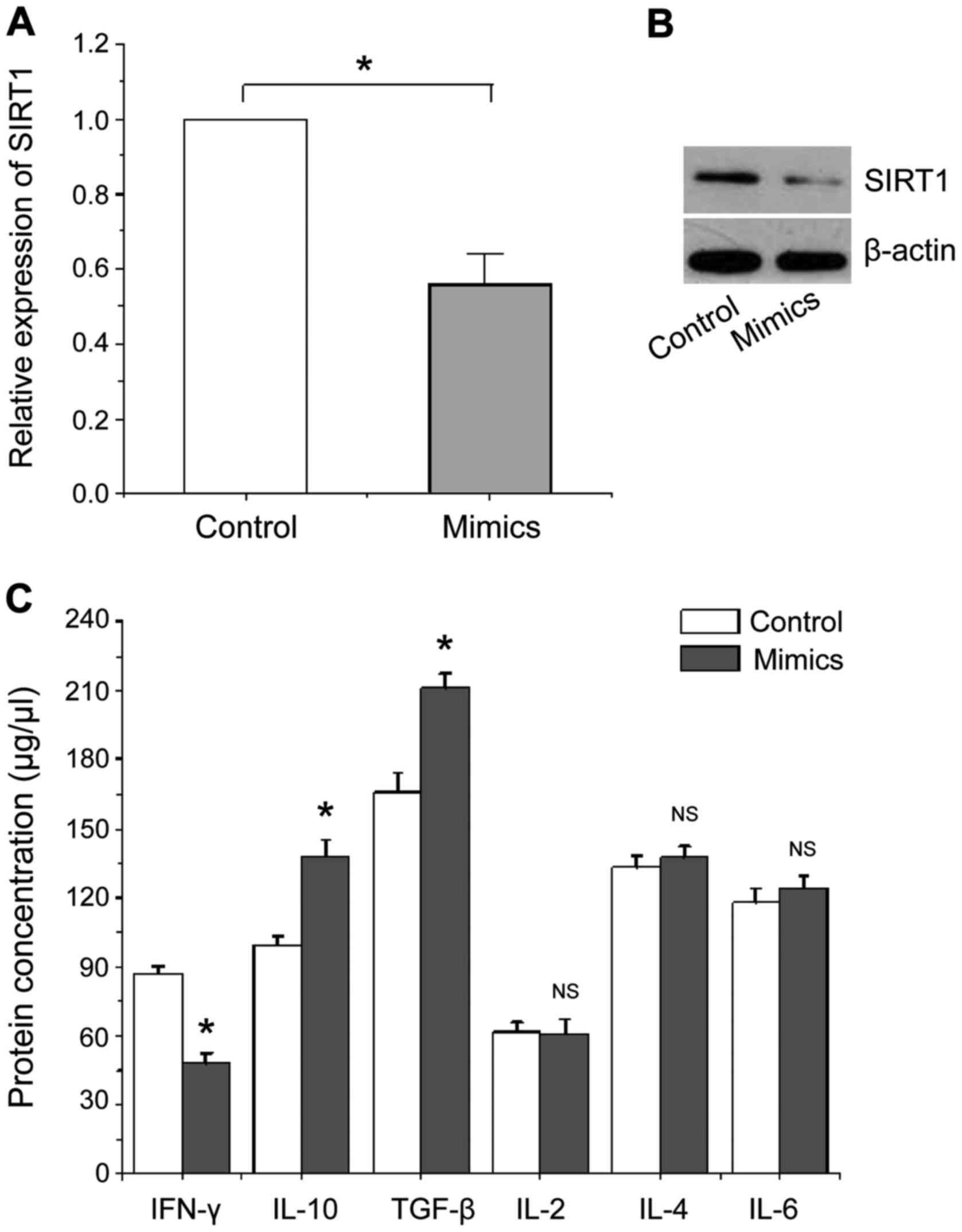
The effects of miR-124a overexpression
on the expression of SIRT1 and cytokine
We transfected miR-124a mimics and its negative
control into CD4+ T cells of healthy people,
respectively. RT-qPCR, western blot analysis and ELISA were used to
detect the expression of SIRT1 and related cytokines at 48 h after
transfection, the results are shown in Fig. 4. Fig. 4A
and B show that the expression levels of SIRT1 mRNA and protein
were significantly reduced in human CD4+ T cells with
miR-124a overexpression compared with the negative control, which
is consistent with the above mentioned experimental results.
Fig. 4C shows that the level of
cytokine IFN-γ protein secreted by Th1 type CD4+ T cells
is significantly lower than that of the negative control, while the
levels of cytokines IL-10 and TGF-β secreted by Th2 type
CD4+ T cells were significantly increased compared with
the negative control, no significant changes were found in other
cytokines. Our data suggest that overexpression of miR-124a can
activate Th2 type CD4+ T cells to secrete cytokines to
assist or mediate immune responses.
The effects of miR-124a expression
inhibition on the levels of SIRT1 and related cytokines
We transfected miR-124a inhibitor and its negative
control into CD4+ T cells of patients with AIDS. The
indicators were detected using the same method as described in the
section ‘The effects of miR-124a overexpression on the expression
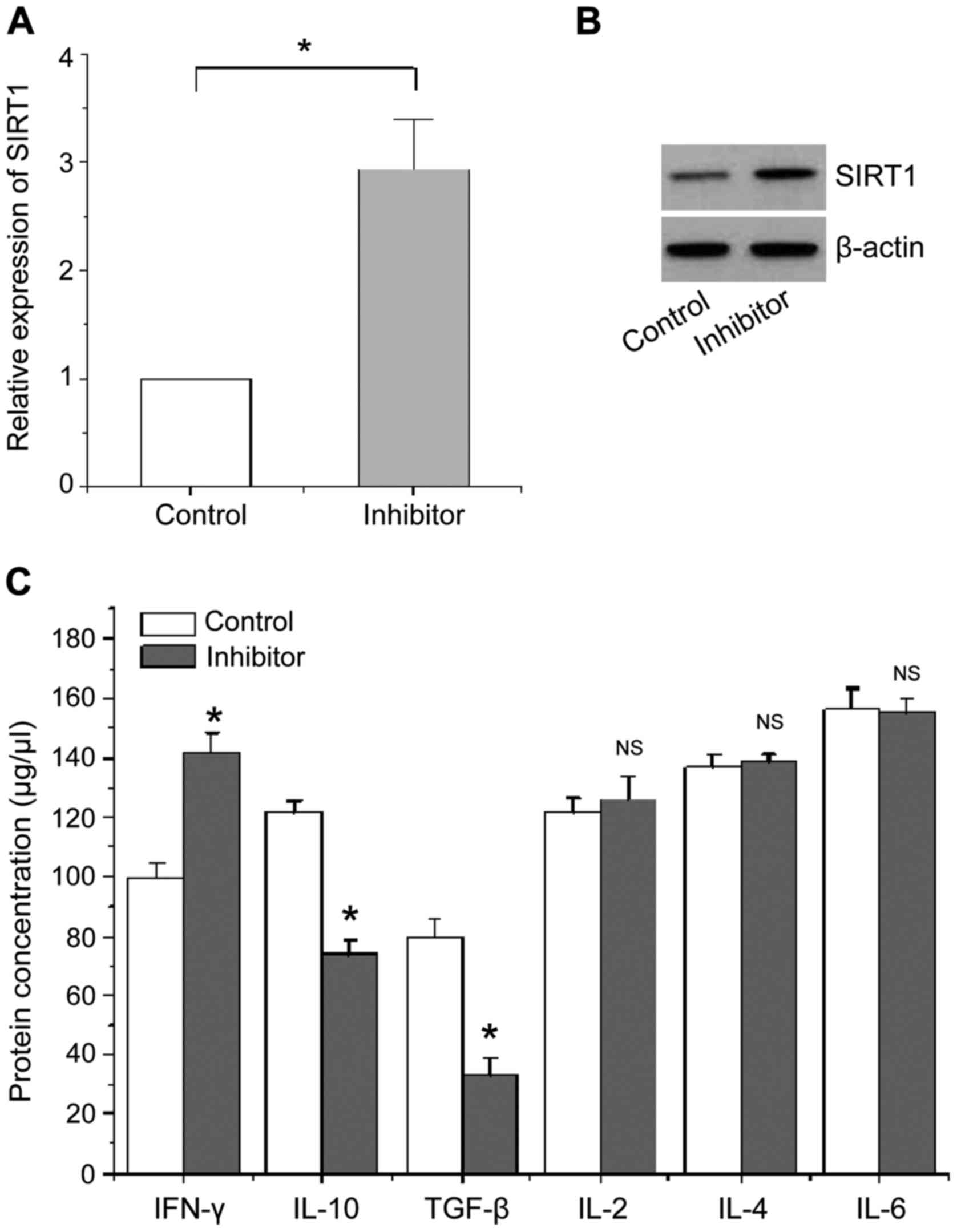
of SIRT1 and cytokine’. The results are shown in Fig. 5. Fig. 5A
and B show that the expression levels of SIRT1 mRNA and protein
were significantly increased in CD4+ T cells of patients
with AIDS compared with the negative control when the expression of
miR-124a was inhibited. Fig. 5C
shows that the level of cytokine IFN-γ protein secreted by Th1-type
CD4+ T cells is significantly higher than that of
negative control, whereas the levels of cytokines IL-10 and TGF-β
protein mainly secreted by Th2 type CD4+ T cells are
significantly reduced compared with the negative control, no
significant changes were found in other cytokines.
Discussion
Most of the previous studies on microRNAs focused on
their roles in the regulation of autoimmune diseases, such as
systemic lupus erythematous (11)
and psoriasis vulgaris (12). Many
studies have reported upregulation of immune response event of a
foreign body invasion. Despite this, the recognition of intrinsic
or external antigens by body's immune system begins with the
specific binding of the MHC-antigen peptide complex on the APC
surface to TCR, thereby activating T cell proliferation and
differentiation to generate effector T cells, thereby secreting
lymphokines to assist the regulation of cellular and humoral
immunity (5,6).
miR-124a is mainly expressed in the central nervous
system, but miR-124a can also be temporarily and spatially
expression in a variety of cells. The abnormal regulation of
miR-124a expression has been shown to participate in the regulation
of a variety of neurological immune diseases (13). Recent studies have shown that
miR-124a may be involved in macrophage polarization, which can
affect the occurrence of a series of diseases (14). In addition, miR-124 has a role in the
carcinogenesis and tumorigenesis. In the study of glioma and
endometrial cancer, miR-124 was found to be able to improve T
cell-mediated immune clearance and inhibit tumorigenesis by
inhibiting STAT3 signaling (15,16).
With the rapid development of bioinformatics and the refinement of
the microRNA database, the target genes that microRNAs can directly
act on have been able to be accurately predicted (17). In a recent study of neuropathic pain
and anti-inflammatory processes, miR-124a and miR-155 were found to
be able to inhibit the expression of target gene SIRT1, thereby
activating the expression of transcription factor Foxp3, which in
turn enhance the differentiation of CD4+ T into Tregs
cells, so SIRT1 and Foxp3 play important roles in the development,
differentiation, and functionalization of Tregs cells (18).
AIDS virus attack the most important CD4+
T cells in the human immune system severely reducing levels of
CD4+ T cells during the onset of the disease, which in
turn damage the immune system. The replication of HIV is faster and
the survivability is high, and the process of systemic infection of
this virus is usually accompanied by the formation of malignant
tumors (2). The above experimental
findings have aroused our attention. We speculated that miR-124a
also had a regulatory role in AIDS possibly by regulating the
expression of target genes in the process of T cell activation. We
also speculated that miR-124a was involved in the cellular immune
response after HIV-1 infection. To test this hypothesis, we
designed and performed experiments, resulting in the following: the
expression of miR-124a in CD4+ T cells of patients with
AIDS is abnormally upregulated compared with that of healthy
individuals. The upregulated miR-124a can silence the expression of
target gene SIRT1 to regulate the activation of Th2 type
CD4+ T cells, and the activated Th2 type CD4+
T cells can secret IL-10 and TGF-β cytokines to participate in
immune response, which in turn enhance the immunity of
patients.
The level of CD4+ T cell and the load of
HIV were the main indicators for the evaluation of the progression
of AIDS, so T cell activation may also be related to the virus
replication level, and T cell activation and virus replication can
promote each other. On the one hand, the deterioration of the
disease can increased the replication rate of virus, resulting in
increased production of antigen, which will provide more and
stronger stimulating signals for the activation of T cells. On the
other hand, the abnormal activation of T cells also provides more
host cells for viral replication, which in turn accelerate viral
replication (19). The pathogenesis
of AIDS and the in vivo immune response are both complex
processes, and it is of great value to understand the pathogenesis
of AIDS from the angle of microRNA. The microRNA based studies will
provide new insights for the diagnosis, alleviation and treatment
of AIDS, and microRNAs can potential to be used as a new target for
drug design in future (20).
References
|
1
|
Barré-Sinoussi F, Chermann JC, Rey F,
Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C,
Vézinet-Brun F, Rouzioux C, et al: Isolation of a T-lymphotropic
retrovirus from a patient at risk for acquired immune deficiency
syndrome (AIDS). Science. 220:868–871. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Klase Z, Winograd R, Davis J, Carpio L,
Hildreth R, Heydarian M, Fu S, McCaffrey T, Meiri E,
Ayash-Rashkovsky M, et al: HIV-1 TAR miRNA protects against
apoptosis by altering cellular gene expression. Retrovirology.
6:182009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Strebel K: HIV accessory proteins versus
host restriction factors. Curr Opin Virol. 3:692–699. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weiss RA: How does HIV cause AIDS?
Science. 260:1273–1279. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smith-Garvin JE, Koretzky GA and Jordan
MS: T cell activation. Annu Rev Immunol. 27:591–619. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
von Essen MR, Kongsbak M and Geisler C:
Mechanisms behind functional avidity maturation in T cells. Clin
Dev Immunol. 2012:1634532012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang J, Wang F, Argyris E, Chen K, Liang
Z, Tian H, Huang W, Squires K, Verlinghieri G and Zhang H: Cellular
microRNAs contribute to HIV-1 latency in resting primary CD4+ T
lymphocytes. Nat Med. 13:1241–1247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim VN, Han J and Siomi MC: Biogenesis of
small RNAs in animals. Nat Rev Mol Cell Biol. 10:126–139. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi XB, Xue L, Ma AH, Tepper CG,
Gandour-Edwards R, Kung HJ and deVere White RW: Tumor suppressive
miR-124 targets androgen receptor and inhibits proliferation of
prostate cancer cells. Oncogene. 32:4130–4138. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heyn J, Luchting B, Hinske LC, Hübner M,
Azad SC and Kreth S: miR-124a and miR-155 enhance differentiation
of regulatory T cells in patients with neuropathic pain. J
Neuroinflammation. 13:2482016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amr KS, Bayoumi FS, Elgengehy FT, Abdallah
SO, Ahmed HH and Eissa E: The role of microRNA-31 and microRNA-21
as regulatory biomarkers in the activation of T lymphocytes of
Egyptian lupus patients. Rheumatol Int. 36:1617–1625. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stagakis E, Bertsias G, Verginis P, Nakou
M, Hatziapostolou M, Kritikos H, Iliopoulos D and Boumpas DT:
Identification of novel microRNA signatures linked to human lupus
disease activity and pathogenesis: miR-21 regulates aberrant T cell
responses through regulation of PDCD4 expression. Ann Rheum Dis.
70:1496–1506. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ponomarev ED, Veremeyko T, Barteneva N,
Krichevsky AM and Weiner HL: MicroRNA-124 promotes microglia
quiescence and suppresses EAE by deactivating macrophages via the
C/EBP-α-PU.1 pathway. Nat Med. 17:64–70. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Willemen HL, Huo XJ, Mao-Ying QL, Zijlstra
J, Heijnen CJ and Kavelaars A: MicroRNA-124 as a novel treatment
for persistent hyperalgesia. J Neuroinflammation. 9:1432012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei J, Wang F, Kong LY, Xu S, Doucette T,
Ferguson SD, Yang Y, McEnery K, Jethwa K, Gjyshi O, et al: miR-124
inhibits STAT3 signaling to enhance T cell-mediated immune
clearance of glioma. Cancer Res. 73:3913–3926. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Zhang Z, Liu X, Huang T, He W, Shen
Y, Liu X, Hong K and Cao Q: miR-124 functions as a tumor suppressor
in the endometrial carcinoma cell line HEC-1B partly by suppressing
STAT3. Mol Cell Biochem. 388:219–231. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
eLife. 4:e050052015. View Article : Google Scholar
|
|
18
|
Beier UH, Akimova T, Liu Y, Wang L and
Hancock WW: Histone/protein deacetylases control Foxp3 expression
and the heat shock response of T-regulatory cells. Curr Opin
Immunol. 23:670–678. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hunt PW, Martin JN, Sinclair E, Bredt B,
Hagos E, Lampiris H and Deeks SG: T cell activation is associated
with lower CD4+ T cell gains in human immunodeficiency
virus-infected patients with sustained viral suppression during
antiretroviral therapy. J Infect Dis. 187:1534–1543. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Swaminathan S, Murray DD and Kelleher AD:
The role of microRNAs in HIV-1 pathogenesis and therapy. AIDS.
26:1325–1334. 2012. View Article : Google Scholar : PubMed/NCBI
|