Introduction
Depression disorders are the most severe psychiatric
disorders worldwide. The symptoms include retardation of thinking,
hypopraxia and downcast mood (1).
Many factors are considered to be related with pathogenesis of
depression disorder, including internal secretion, nervous system
and immune system. Earlier studies presented that liver-qi
depression may be related to signal transduction after receptors
related to synaptic plasticity, gene transcriptional regulation and
target gene expression alteration (2).
Signal transduction system plays a key role in
directing signal into cells, and is the target of anti-depression
drugs. The pathways involved in depression disorder include cAMP
pathway, mitogen-activated protein kinase (MAPK) pathway and CaMK
pathway (3–5), of which MAPK pathway plays a key role
in the brain-derived neurotrophic factor (BDNF) signal
transduction. Extracellular signal-regulated protein kinase
(ERK1/2), a number of MAPK family, transmits extracellular stimulus
signals into cells, such as neurotransmitter, neurotrophic factors
and nerve growth factor (6,7). Besides it involves in pathophysiology
of various nervous system diseases by regulating gene expression,
synaptic reorganization, axon growth and excitability of neure
(8). Phosphorylation of ERK could
activate cyclic AMP response element binding protein (CREB), which
is an endonuclear regulatory factor, regulating transcription by
self-phosphorylation, and playing a key role in regulating and
maintaining emotion and memory (9,10). BDNF
is a downstream target of CREB, and is a representative number of
neurotrophic factors, functioning in hippocampus and frontal lobe
tissues by regulating neuroplasticity (11,12). In
an earlier study, we found a significant difference of ERK, CREB
and BDNF between liver-qi depression rats and normal rats,
indicating that ERK-CREB-BDNF pathway may participate in the
pathogenesis of liver-qi depression (13). It is more likely a putative treatment
target for liver-qi depression.
The anti-depression drugs can be divided into five
groups, including monoamine oxidase inhibitor, tricyclic
antidepressant, selective noradrenaline reuptake inhibitor and
selective 5-HT reuptake inhibitor. But long-term intake may induce
toxic and side-effect and untoward effect. While Chinese medicine
in treating depression showed good curative effect and relative
lower recurrence rate. Thus, treatment of depression disorder by
using Chinese medicine attracts increased attention.
Shuyu capsule is a kind of Chinese medicine
developed by our research group, mainly containing active principle
from Radix Bupleuri, rhizoma cyperi, Radix Paeoniae Alba and
liquorice. It has been studied in a rat model of premenstrual
syndrome depression that Shuyu capsules rectified abnormal
5-HT3AR and 5-HT3BR expression and
5-HT3 channel current changes in a rat model (14). But no pathway alterations was
revealed related to depression. Thus in this study, we aimed to
analyze the influence on ERK-CREB-BDNF signal pathway of Shuyu
capsule and its component.
Materials and methods
Ethics statement
All animal experiments were approved by the Ethics
Committee of Taishan Medical University (Tai'an, China).
Animals and reagents
Healthy Wistar male rats (n=48, 150±20 g) were
obtained from Beijing Vital River Laboratory Animal Technology Co.,
Ltd. (Beijing, China). Rats were housed in animal room at 22±2°C.
Sterilized diet and water were freely accessed, with an adaptive
breeding for 2 weeks. Rats were divided into six groups according
to cardinal number of sugar water preference test and open-field
test, including control group, model group, Shuyu group, Radix
Bupleuri group, fluoxetine group and Radix Paeoniae Alba group, 8
rats in each group.
Shuyu capsule was obtained from Qingdao Haichuang
Center for Innovative Biomedical Research (Qingdao, China).
Clinical batch no. 2008 L11169. Fluoxetine capsule was obtained
from Eli Lilly Suzhou Pharmaceutical Co., Ltd. (Suzhou, China).
Clinical batch no. J20100016. Radix Bupleuri extraction and Radix
Paeoniae Alba were obtained from Qingdao Haichuang Center for
Innovative Biomedical Research.
Sucrose preference test
Sucrose preference test was performed according to
Moriyama et al (15). Two
drinking spouts (water and 1% sucrose) were provided to rats after
water starvation for 24 h. Bottles were exchanged every 1 h.
Sucrose preference was calculated as: Sucrose preference = [sucrose
consumption (ml)/water consumption (ml) + sucrose consumption (ml)]
× 100%.
Open-field test
Open-field test was performed according to Zhu et
al (16). Before the
experiments, rats were allowed an addaptive activity. Rats were
placed in the center of the arena, and the activity of rats was
recorded, including the number of vertical activity, total journey,
the time spend in the center of arena. The total journey was
considered as an index of this test.
Stress design
Liver-qi depression rats were prepared based
on chronic unpredictable mild stress (CUMS) and delayed constraint
(17,18). Depression rats were prepared by
providing 2–3 random types of stimulus every day, lasting for 4
weeks. The stimulus included cage inclining (45°), continuous
illumination, dirty cage (pouring 200 ml water in cage), empty
bottle, limiting food, white noise, strobe light, smell (mothball),
foreign matter (block, a strip of cloth), rotation and constraint
(four legs restrained by adhesive plaster).
Rats model
Control group rats were prepared without any
stimulus, and given a gavage of normal saline (1 ml/200 g). Model
group were prepared by giving a gavage of normal saline (1 ml/200
g) to depression rats. Shuyu group was prepared by giving a gavage
of Shuyu capsule (4.08 mg/kg, about 8-fold dosage of human) to
depression rats. Radix Bupleuri group was prepared by giving a
gavage of Radix Bupleuri capsule (4.08 mg/kg) to depression rats.
Fluoxetine group was prepared by giving a gavage of fluoxetine
(0.72 mg/kg/day) to depression rats. Radix Paeoniae Alba group was
prepared by giving a gavage of Radix Paeoniae Alba (36 mg/kg/day)
to depression rats.
Model evaluation and sampling
Rats were anesthetized using 3% chloral hydrate (0.3
ml/100 g). Blood was obtained from ostcava, half was stored in Ep
tubes treated with heparin in advance. The rest blood was
centrifuged at 1200 × g, 4°C for separating serum. All samples were
stored at −70°C.
Western blot analysis and ELISA
Blood was obtained from ostcava after rats were
anesthetized using 3% chloral hydrate (0.3 ml/100 g). Hippocampus
and frontal lobe tissues were obtained from head after rats were
sacrificed. All samples were stored at −70°C.
Western blot analysis was performed
according to standard protocols
Total protein was extracted using RIPA lysate.
Protein concentration was measured with BCA protein assay kit
(Pierce, Bonn, Germany) according to the manufacturer's
instructions. Total cellular proteins were separated by 10%
SDS-PAGE and transferred onto nitrocellulose membranes (EMD
Millipore, Billerica, MA, USA). After blocking in 5% skim milk for
1 h, the membranes were incubated with primary antibodies (β-actin,
A1978, 1:2,000; Sigma-Aldrich, St. Louis, MO, USA) (ERK, cat. no.
9102, 1:2,000; p-ERK, cat. no. 9101, 1:2,000; CREB, cat. no. 9197,
1:150; P-CREB, cat. no. 9191, 1:150; all from Cell Signaling
Technology, Inc., Danvers, MA, USA) (BDNF, cat. no. ab108383,
1:1,200; Abcam, Cambridge, MA, USA) overnight at 4°C, followed by
the incubation with goat anti-rabbit secondary antibodies (ERK,
1:2,000; CREB 1:150; BDNF 1:1,200) for 2 h at room temperature.
Protein bands were visualized on X-ray film using enhanced
chemiluminescence ECL substrate (Pierce).
The levels of BDNF were detected with enzyme-linked
immunosorbent assay (ELISA) according to the manufacturer's
instruction.
Quantitative-real time PCR
Total RNA was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA). The cDNA was synthesized from RNA
samples using a RevertAid First-Strand cDNA Synthesis kit (Takara,
Dalian, China). qRT-PCR was performed with SYBR Premix ExTaq
(Takara) with a LightCycler 480 apparatus (Roche Molecular Systems,
Inc., Pleasanton, CA, USA). β-actin was used for internal control.
The relative expression of genes was calculated by
2−ΔΔCq method. The sequences of primers were: ERK
forward, 5′-GTGAAGTTCATTTCCAATCCGC-3′ and reverse,
5′-GGGACATCACCCTCACTTAC-3′; CREB forward,
5′-CCATCCACTCCTGTGTCATCT-3′ and reverse,
5′-CCTTGTAAATCCTCTTCCATCA-3′; BDNF forward,
5′-CACCCGCGAGTACAACCTTC-3′ and reverse,
5′-CCCATACCCACCATCACACC-3′.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5 (GraphPad Software, San Diego, CA, USA). All data are
presented as mean ± SD. A value with P<0.05 indicated
statistically significant difference.
Results
Body weight analysis
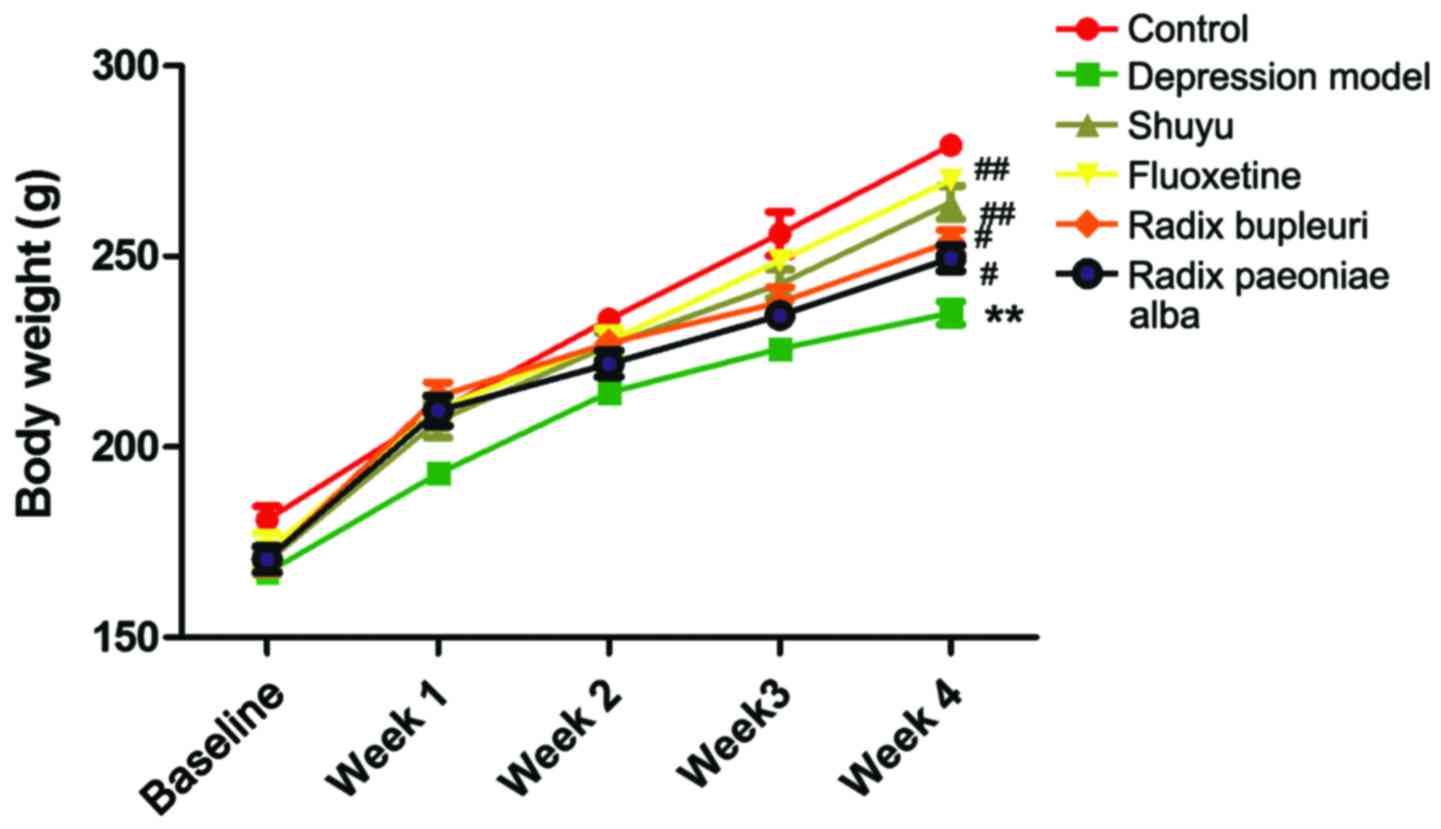
Body weight was measured once a week after model
rats were made, as shown in Fig. 1.
In week 4, body weights were significantly decreased in
liver-qi depression model rats (P<0.01) in comparison
with control group, but no significance were found in drug group
(Table I). Compared with depression
model group, body weights were significantly increased in Shuyu
group and fluoxetine group (P<0.01), and no significant
difference was found in Radix Paeoniae Alba group and Radix
Bupleuri group.
 | Table I.Comparison of weight (mean ± SD) in
rats in week 4. |
Table I.
Comparison of weight (mean ± SD) in
rats in week 4.
| Group | Before
modeling | After modeling |
|---|
| Control group |
172.7±2.215 |
258.7±4.030 |
| Depression model
group |
170.1±2.493 |
225.7±3.290a |
| Shuyu group |
174.2±1.914 |
251.3±4.882b |
| Fluoxetine
group |
175.0±2.270 |
252.1±5.692b |
| Radix Bupleuri
group |
170.5±3.312 |
245.3±4.393c |
| Radix Paeoniae Alba
group |
169.8±4.704 |
244.3±6.218c |
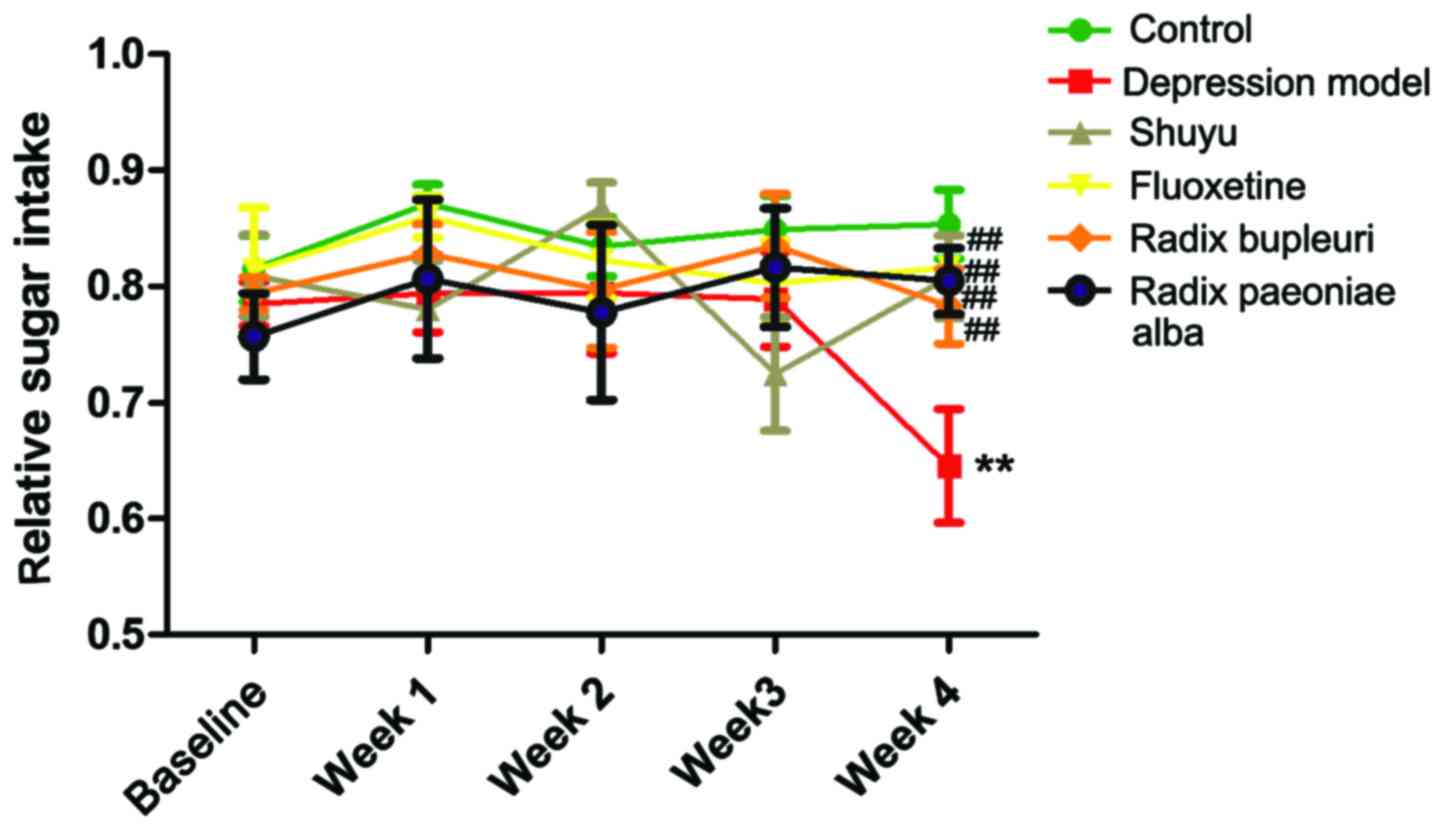
Sugar preference test
Sugar consumption was tested once a week in the six
groups (Fig. 2). In week 4, sugar
consumption was significantly decreased in depression group in
comparison with normal control (P<0.05), and significant
increase was found in drug groups in comparison with depression
group (P<0.01), indicating that depression rats lacked pleasant
sensation, which could be improved by fluoxetine, Shuyu capsule,
Radix Bupleuri extraction and Radix Paeoniae Alba extraction
(Table II).
 | Table II.Sugar consumption was tested in week
4 (mean ± SD). |
Table II.
Sugar consumption was tested in week
4 (mean ± SD).
| Groups | Before
modeling | After modeling |
|---|
| Control group |
0.8137±0.02404 |
0.8755±0.02270 |
| Depression model
group |
0.7822±0.04440 |
0.5507±0.04922a |
| Shuyu group |
0.7992±0.01608 |
0.8182±0.04202b |
| Fluoxetine
group |
0.7734±0.03438 |
0.8329±0.02393b |
| Radix Bupleuri
group |
0.7902±0.02464 |
0.7652±0.02632b |
| Radix Paeoniae Alba
group |
0.7760±0.04704 |
0.7930±0.02401b |
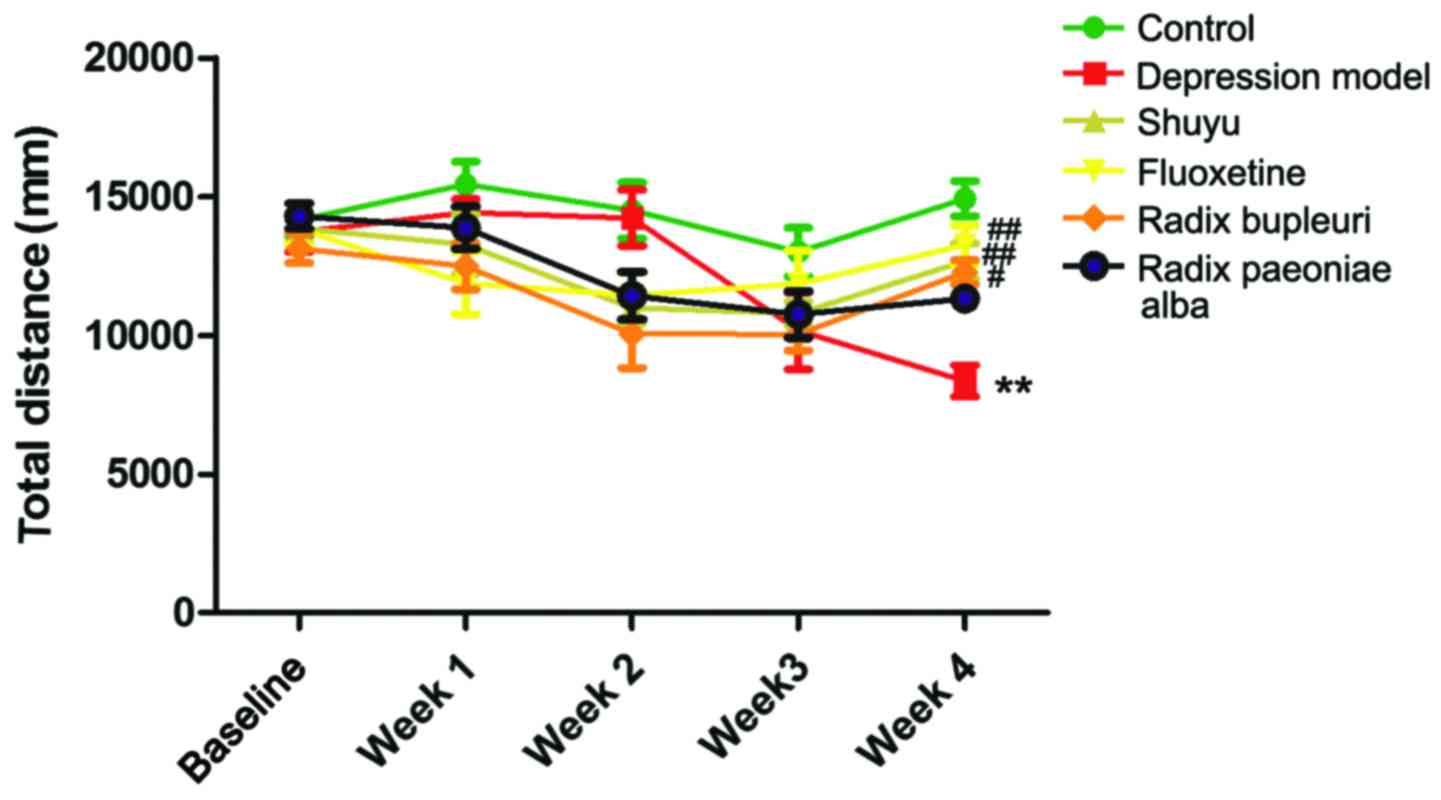
Open-field test
Total journey of rats in open-field was recorded
once a week (Fig. 3). In week 4,
total journey in depression group was significantly decreased
compared with control group (P<0.01), and no significant
difference were found in drug groups (Table III). Compared with depression
group, total journey in fluoxetine group (P<0.01), Shuyu group
(P<0.01) and Radix Bupleuri group (P<0.05) was significantly
increased, while no significant increase was found in Radix
Paeoniae Alba group.
 | Table III.Total journey of rats in open-field
test. |
Table III.
Total journey of rats in open-field
test.
| Groups | Before
modeling | After modeling |
|---|
| Control group |
13,327±756.5 |
9,786±654.8 |
| Depression model
group |
12,585±801.2 |
5,447±356.4a |
| Shuyu group |
12,732±669.1 |
9,320±531.9b |
| Fluoxetine
group |
13,073±810.2 |
9,646±718.6b |
| Radix Bupleuri
group |
12,336±980.8 |
9,057±643.0c |
| Radix Paeoniae Alba
group |
13,097±794.3 |
9,024±589.4 |
Detection of ERK, BDNF and CREB in
hippocampus and frontal lobe tissues
Expression levels of BDNF, ERK and CREB in
hippocampus and frontal lobe tissues were detected at both mRNA and
protein levels.
ERK expression
ERK expression was detected in hippocampus and
frontal lobe tissues in all groups. In hippocampus tissues
(Fig. 4A and B), ERK expression was
significantly decreased compared with control group (P<0.05) at
both mRNA and protein level. After gavaged with Shuyu capsule or
fluoxetine, ERK expression was significantly increased (P<0.01),
nearly to the normal group. Although in Radix Bupleuri group and
Radix Paeoniae Alba group ERK expression was increased, it was
still less than it in control group (P<0.05). In frontal lobe
tissues (Fig. 4C and D), similar
results were obtained, except for the Radix Bupleuri group and
Radix Paeoniae Alba group. No significant difference was found in
the two groups compared with depression model group.
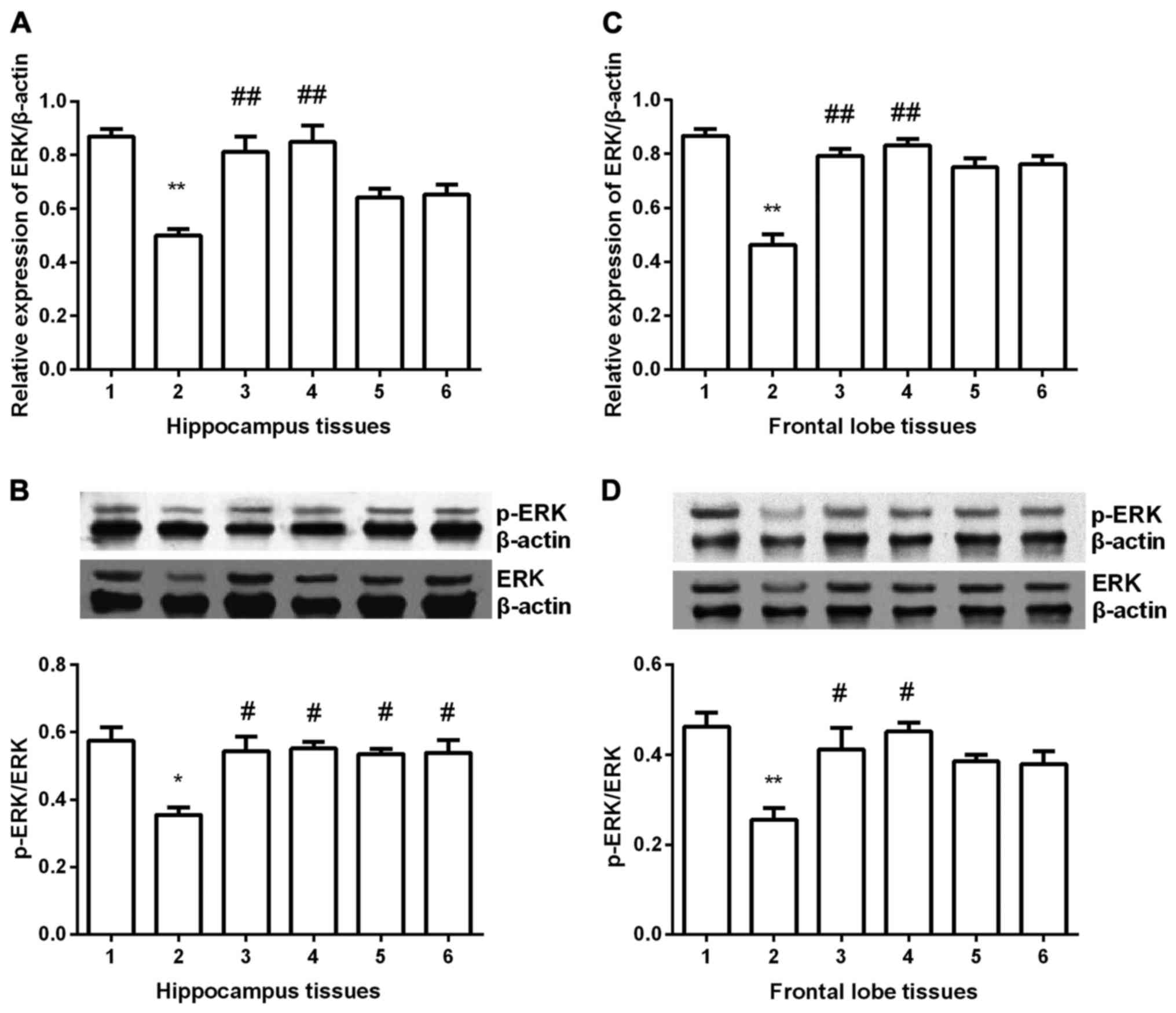 | Figure 4.Expression of ERK in hippocampus and
prefrontal cortex tissues. ERK expression was significantly
decreased in depression group (P<0.01) and Shuyu capsule and
fluoxetine significantly increased ERK expression at mRNA level (A
and C). Western blot analysis showed that p-ERK/ERK was
significantly decreased and Shuyu capsule and fluoxetine
significantly increased p-ERK/ERK in both tissues (P<0.05) (B
and D). Radix Bupleuri and Radix Paeoniae Alba significantly
increased p-ERK/ERK only in hippocampus tissues (P<0.05) (B).
**Compared with control group, P<0.01. #Compared with
depression models, P<0.05. ##Compared with depression
models, P<0.01. ERK, extracellular signal-regulated protein
kinase; p-, phosphorylated; 1, control group; 2, depression group;
3, Shuyu group; 4, fluoxetine group; 5, Radix Bupleuri; 6, Radix
Paeoniae Alba. |
BDNF expression
BDNF expression was detected in hippocampus and
frontal lobe tissues in all groups. In hippocampus tissues
(Fig. 5A and B), BDNF expression was
significantly decreased compared with control group (P<0.01) at
both mRNA and protein level. After gavaged with Shuyu capsule or
fluoxetine, BDNF expression was significantly increased
(P<0.01), and in Radix Bupleuri group and Radix Paeoniae Alba
group, it was also increased (P<0.05). In frontal lobe tissues
(Fig. 5C and D), similar results
were obtained, except for the Radix Bupleuri group and Radix
Paeoniae Alba group. No significant difference was found in the two
groups compared with depression model group.
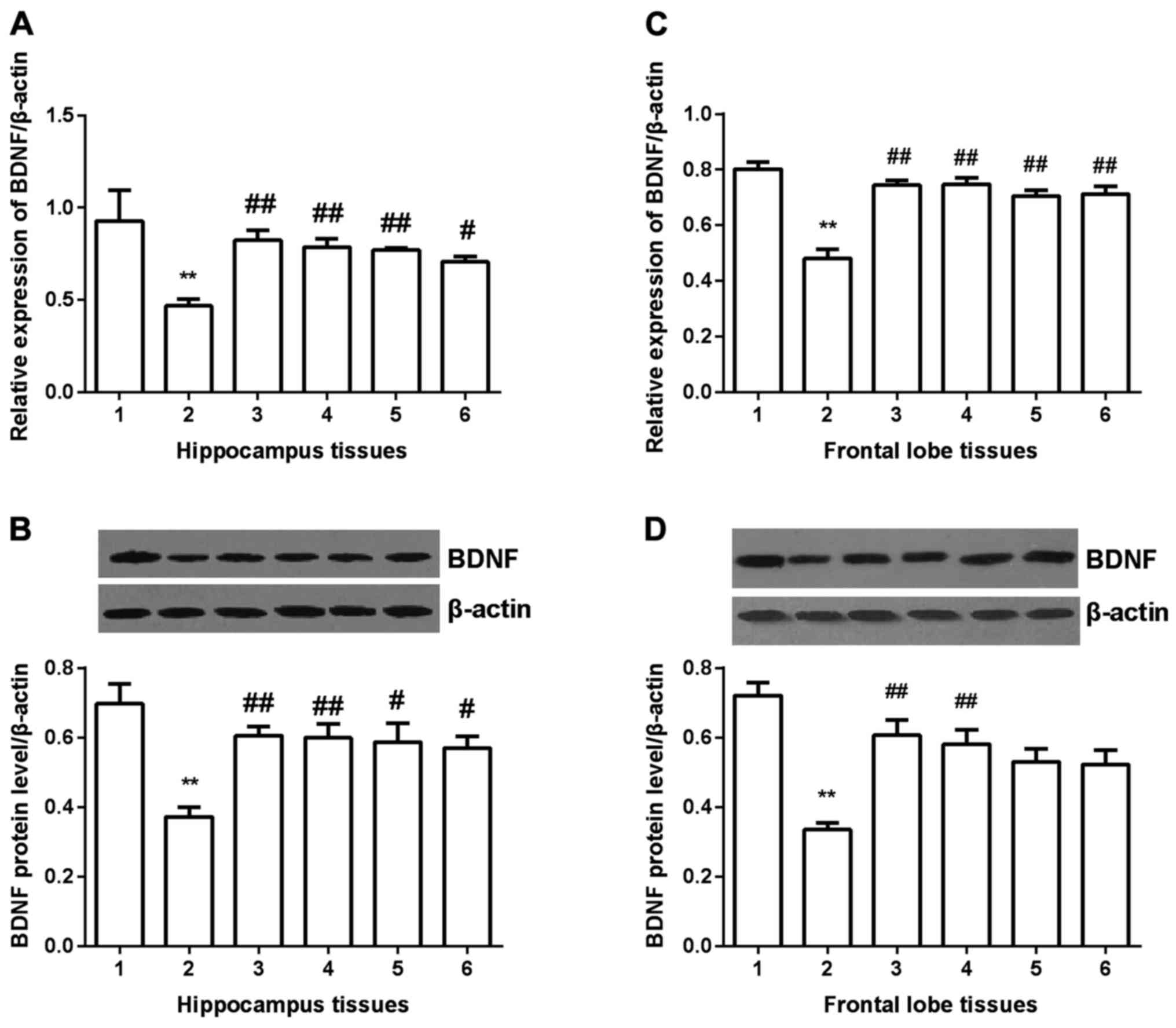 | Figure 5.Expression of BDNF in hippocampus and
prefrontal cortex tissues. BDNF expression was significantly
decreased at both mRNA and protein level. All the drugs
significantly increased BDNF expression at mRNA level (A and C). At
protein level, in hippocampus tissues, all the four drugs
significantly increased BDNF expression (B), but in prefrontal
cortex tissues, only Shuyu capsule and fluoxetinem significantly
increased BDNF expression (D). **Compared with control group,
P<0.01. #Compared with depression models, P<0.05.
##Compared with depression models, P<0.01. BDNF,
brain-derived neurotrophic factor; 1, control group; 2, depression
group; 3, Shuyu group; 4, fluoxetine group; 5, Radix Bupleuri; 6,
Radix Paeoniae Alba. |
CREB expression
CREB expression was detected in hippocampus and
frontal lobe tissues in all groups. In hippocampus tissues
(Fig. 6A and B), CREB expression was
significantly decreased compared with control group (P<0.01) at
both mRNA and protein level. After gavaged with Shuyu capsule
(P<0.01) fluoxetine (P<0.01), Radix Bupleuri (P<0.05) or
Radix Paeoniae Alba (P<0.05), BDNF expression was significantly
increased at mRNA level. At protein level (Fig. 6B), Shuyu capsule (P<0.01)
fluoxetine (P<0.01), Radix Bupleuri (P<0.05) showed a
significant increase of p-CREB/CREB. In frontal lobe group, all the
drug groups showed significant increase of CREB compared with
depression model group at mRNA level (Fig. 6C). At protein level, only Radix
Paeoniae Alba group didn't present significant increase of
p-CREB/CREB (Fig. 6D).
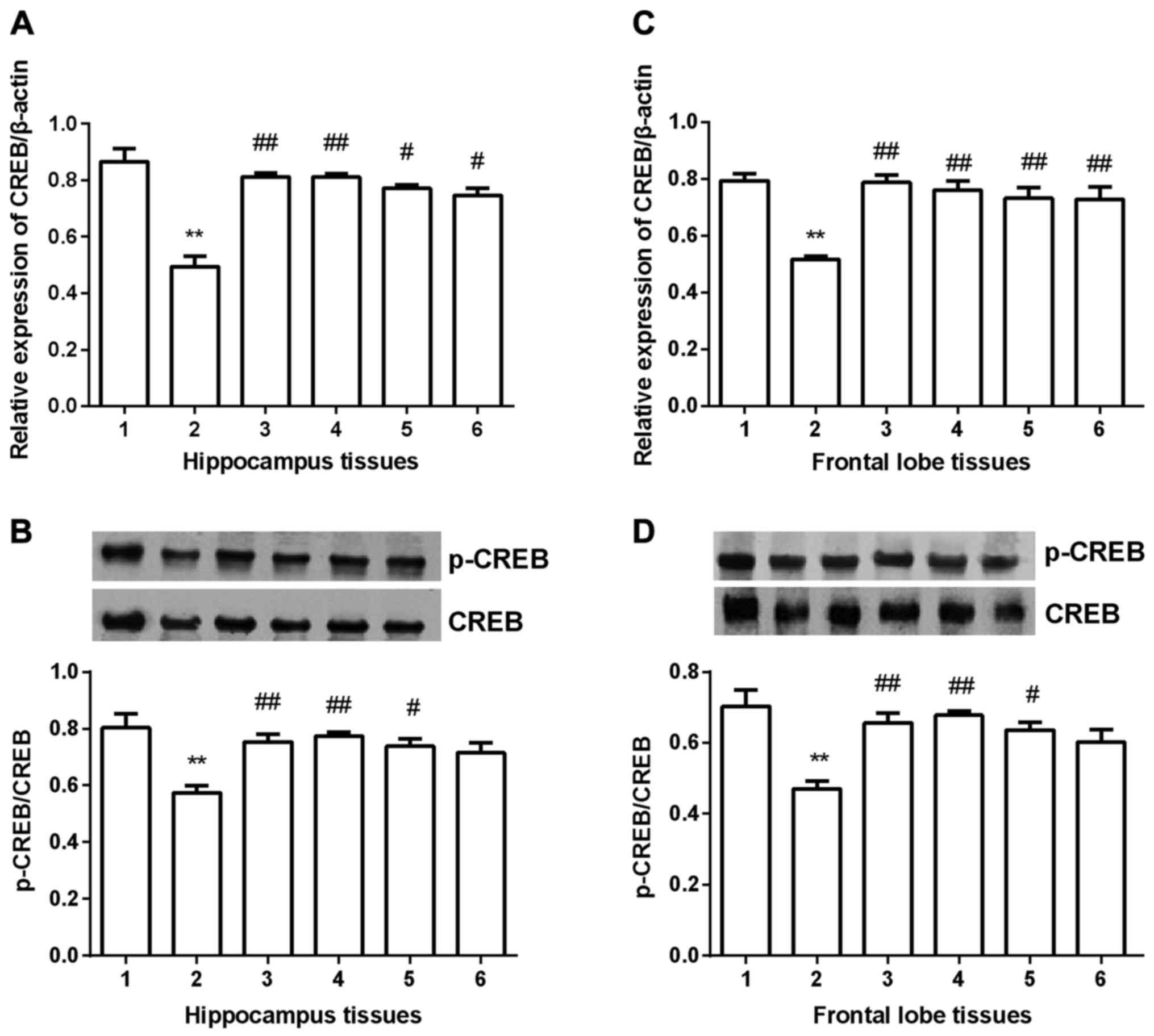 | Figure 6.Expression of CREB in hippocampus and
prefrontal cortex tissues. At mRNA level, CREB expression was
significantly decreased in depression group compared with
depression group and all the four drugs significantly increased its
expression (A and C). Western blot analysis showed that p-CREB/CREB
was significantly decreased in depression group compared with
control group, and only Radix Paeoniae Alba did not significantly
increase p-CREB/CREB (B and D). **Compared with control group,
P<0.01. #Compared with depression models, P<0.05.
##Compared with depression models, P<0.01. CREB,
cyclic AMP response element binding protein; p-, phosphorylated; 1,
control group; 2, depression group; 3, Shuyu group; 4, fluoxetine
group; 5, Radix Bupleuri; 6, Radix Paeoniae Alba. |
BDNF concentration in peripheral
blood
BDNF concentration was detected in peripheral blood
of all groups using ELISA. Compared with normal control, BDNF
concentration was significantly decreased in depression rats
(P<0.05), and it was significantly increased in the four drug
groups compared with depression model group (P<0.05) (Table IV).
 | Table IV.Concentration of BDNF in peripheral
blood. |
Table IV.
Concentration of BDNF in peripheral
blood.
| Groups | Concentration
(ng/ml) |
|---|
| Control group |
162.3±14.54 |
| Depression model
group |
116.5±4.627a |
| Shuyu group |
161.6±8.025b |
| Fluoxetine
group |
161.34±8.286b |
| Radix Bupleuri
group |
158.8±6.206b |
| Radix Paeoniae Alba
group |
161.1±6.764b |
Discussion
CUMS is a typical method to construct liver-qi
depression models (19). In this
study, we constructed liver-qi depression rat models by combining
CUMS and delayed constraint stimulus. In the procedures, rats were
randomly given several stimuli, which was similar with human
depression formation mechanism (20).
In this study, we applied open-field test, weight
and sucrose preference test to evaluate depression rat models, of
which open-field test is the most important method to detect rats'
excitability and environmental adaptation (21). Open-field test showed that total
journal of depression models were significantly decreased compared
with control group, and it was significantly increased in all the
drug groups. These findings indicated that Shuyu capsule and its
component may effectively increased adaptive capacity and
excitability in depression rats. Although fluoxetine dramatically
increased adaptive capacity and excitability in rats, long-term
medication may induce overexcitation in central nervous system
(22).
Food-intake and digestion of rats were detected by
weight. Many depression patients showed decreased appetite and
digestion ability, thus it is an important index in evaluating
depression rats. In this study, weight increment in depression
models was significantly decreased compared to other groups
(P<0.05), indicating that depression rats showed obviously
decreased appetite, but fluoxetine and Shuyu capsule effectively
improved the situation.
Sucrose preference test was used to detect pleasant
sensation of rats. The preference coefficient is low when pleasant
sensation to environment is lost (23). In the present study, sucrose
preference in drug groups was significantly increased compared with
depression rats, indicating that capsules and components showed
effective performance in increasing of pleasant sensation.
The mechanism of depression is closely associated to
the central nervous system. In this study, we chose hippocampus and
frontal lobe as subject due to the two districts regulating motion
and behavioristics via variety of pathways (24). According to the posthumous autopsy
report of depression patients, hippocampus was atrophy to some
extent (25). Earlier study showed
that hippocampus and fascia dentata volume was much smaller in
patients with Alzheimer disease compared with normal samples, which
suggested that the shrinking of hippocampus induced cognitive
function obstacle, resulting in depression (26). Prefrontal cortex functions
importantly in the emotion process as it is found that left
prefrontal cortex compensatory conducting emotion stimulus in the
analysis of magnetoencephalogram in depression patients. Magnetic
stimulation interference across cranium showed that prefrontal lobe
provided attention in the coding and extracting memory (27). Thus prefrontal lobe performs
important roles in regulation of emotion and cognitive
function.
ERK belongs to the MAPK family, which participates
in the physiology activity, including signal transmission and
recognition, cell growth and development, and proliferation
(28). Signals are transmitted
through MAPK signal pathway via three kinase cascades. ERK pathway
is the classical MAPK signal pathway, and the reaction chain mainly
consists of three protein kinases. ERK pathway regulates cell
proliferation, differentiation or apoptosis through the
Ras/Raf/MEK/ERK pathway (29,30). In
this study, we found that expression of ERK was significantly lower
than other groups at both mRNA and protein level, which suggested
that liver-qi depression disorder may be related to the
lower expression of ERK.
CREB is widely distributed in hippocampus and
cerebral cortex (31).
Dephosphorylation of CREB means it has no transcriptional activity.
CREB is phosphorylated if signals stimulate and recognize the
corresponding site of CREB and then activate target gene
transcription. CREB-defective rats showed dysfunction in neure
plasticity in mice, mainly in the memory function related with
auditory sense (32). Dysmnesia is
improved after endogenous CREB expression is increased, indicating
that CREB could obviously promote nerve excitability caused by
cerebral injury (33). In this
study, CREB expression was significantly decreased in depression
group. It is increased in Shuyu group, fluoxetine grop and Radix
Bupleuri group nearly to the normal level. It is suggested that
these three drugs increased CREB expression and showed
anti-depression effect by improving depression, emotion and
behavior.
BDNF is a member of neurotrophic factor family.
Earlier study showed that long-term chronic stress could reduce
BDNF level in cerebrum limbic system, and further induce atrophy of
hippocampus and prefrontal cortex (34). BDNF functions in the nerve-protective
and anti-depression mechanism by promoting hippocampus
proliferation and differentiation via TrkB regulation, and by
increasing antioxidase and scavenging free radical (35). BDNF is also found in peripheral
nervous system, which may be related to the ability of across
hemato-encephalic barrier. In depression patients and rats, BDNF
expression is decreased and can be rescued by anti-depression
drugs, indicating that BDNF may be the target of anti-depression
drugs, but it is uncertain. In this study, we identified BDNF
expression was significantly decreased in depression rats compared
with normal control. The expression was rescued when rats were
gavaged with drugs. Thus we suggested that anti-depression drugs
exerts anti-depression function by increasing BDNF expression at
both mRNA and protein level.
In this study, fluoxetine, Shuyu capsule, Radix
Paeoniae Alba and Radix Bupleuri were used to detect the effect
on depression rats. Radix Paeoniae Alba and Radix Bupleuri are two
mainly components in capsule. Radix Bupleuri showed effective
promotion on the expression of ERK, CREB and BDNF, indicating that
it is the main effective constituent.
There are some limitations in this study: i) In
addition to hippocampus and prefrontal cortex tissues, inferior
colliculus, amygdaloid nucleus and corpus striatum also involved in
the regulation of depression. Further study is needed to
investigate on these encephalic regions. ii) We prepared
liver-qi depression models by combining CUMS and delayed
constraint. Classic evaluation method can be used on depression
model, but there is still controversy in evaluating liver-qi
depression model. iii) To investigate the active composition, we
analyzed the effect of Radix Bupleuri and Radix Paeoniae Alba on
depression rats, but did not extract the effective monomer, which
still needs more research.
Acknowledgements
The present study was supported by the National
Natural Youth Foundation of China (81202620).
References
|
1
|
Zandio M, Ferrín M and Cuesta MJ:
Neurobiology of depression. An Sist Sanit Navar. 25 Suppl 3:43–62.
2002.(In Spanish). PubMed/NCBI
|
|
2
|
Bremner JD, Vythilingam M, Vermetten E,
Vaccarino V and Charney DS: Deficits in hippocampal and anterior
cingulate functioning during verbal declarative memory encoding in
midlife major depression. Am J Psychiatry. 161:637–645. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marsden WN: Synaptic plasticity in
depression: Molecular, cellular and functional correlates. Prog
Neuropsychopharmacol Biol Psychiatry. 43:168–184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kratz AL, Ehde DM and Bombardier CH:
Affective mediators of a physical activity intervention for
depression in multiple sclerosis. Rehabil Psychol. 59:57–67. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qi X, Lin W, Li J, Li H, Wang W, Wang D
and Sun M: Fluoxetine increases the activity of the ERK-CREB signal
system and alleviates the depressive-like behavior in rats exposed
to chronic forced swim stress. Neurobiol Dis. 31:278–285. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alboni S, Benatti C, Capone G, Corsini D,
Caggia F, Tascedda F, Mendlewicz J and Brunello N: Time-dependent
effects of escitalopram on brain derived neurotrophic factor (BDNF)
and neuroplasticity related targets in the central nervous system
of rats. Eur J Pharmacol. 643:180–187. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kumamaru E, Numakawa T, Adachi N, Yagasaki
Y, Izumi A, Niyaz M, Kudo M and Kunugi H: Glucocorticoid prevents
brain-derived neurotrophic factor-mediated maturation of synaptic
function in developing hippocampal neurons through reduction in the
activity of mitogen-activated protein kinase. Mol Endocrinol.
22:546–558. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu H, Richardson Steven J and Li XM:
Dose-related effects of chronic antidepressants on neuroprotective
proteins BDNF, Bcl-2 and Cu/Zn-SOD in rat hippocampus.
Neuropsychopharmacology. 28:53–62. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bilecki W, Wawrzczak-Bargiela A and
Przewlocki R: Activation of AP-1 and CRE-dependent gene expression
via mu-opioid receptor. J Neurochem. 90:874–882. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Walters CL, Cleck JN, Kuo YC and Blendy
JA: Mu-opioid receptor and CREB activation are required for
nicotine reward. Neuron. 46:933–943. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ligeza A, Wawrzczak-Bargiela A, Kaminska
D, Korostynski M and Przewlocki R: Regulation of ERK1/2
phosphorylation by acute and chronic morphine - implications for
the role of cAMP-responsive element binding factor (CREB)-dependent
and Ets-like protein-1 (Elk-1)-dependent transcription; small
interfering RNA-based strategy. FEBS J. 275:3836–3849. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tiraboschi E, Tardito D, Kasahara J,
Moraschi S, Pruneri P, Gennarelli M, Racagni G and Popoli M:
Selective phosphorylation of nuclear CREB by fluoxetine is linked
to activation of CaM kinase IV and MAP kinase cascades.
Neuropsychopharmacology. 29:1831–1840. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Enjeti AK, D'Crus A, Melville K, Verrills
NM and Rowlings P: A systematic evaluation of the safety and
toxicity of fingolimod for its potential use in the treatment of
acute myeloid leukaemia. Anticancer Drugs. 27:560–568. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li F, Feng J, Gao D, Wang J, Song C, Wei S
and Qiao M: Shuyu capsules relieve premenstrual syndrome depression
by reducing 5-HT3AR and 5-HT3BR expression in the rat brain. Neural
Plast. 2016:79507812016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moriyama Y, Takagi N and Tanonaka K:
Intravenous injection of neural progenitor cells improved
depression-like behavior after cerebral ischemia. Transl
Psychiatry. 1:e292011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu X, Li T, Peng S, Ma X, Chen X and
Zhang X: Maternal deprivation-caused behavioral abnormalities in
adult rats relate to a non-methylation-regulated D2 receptor levels
in the nucleus accumbens. Behav Brain Res. 209:281–288. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang L, Luo J, Zhang M, Yao W, Ma X and
Yu SY: Effects of curcumin on chronic, unpredictable, mild,
stress-induced depressive-like behaviour and structural plasticity
in the lateral amygdala of rats. Int J Neuropsychopharmacol.
17:793–806. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang SS, Wang YG, Chen HY, Wu ZP and Xie
HG: Expression of genes encoding cytokines and corticotropin
releasing factor are altered by citalopram in the hypothalamus of
post-stroke depression rats. Neuro Endocrinol Lett. 34:773–779.
2013.PubMed/NCBI
|
|
19
|
Banasr M, Valentine GW, Li XY, Gourley SL,
Taylor JR and Duman RS: Chronic unpredictable stress decreases cell
proliferation in the cerebral cortex of the adult rat. Biol
Psychiatry. 62:496–504. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Taksande BG, Faldu DS, Dixit MP, Sakaria
JN, Aglawe MM, Umekar MJ and Kotagale NR: Agmatine attenuates
chronic unpredictable mild stress induced behavioral alteration in
mice. Eur J Pharmacol. 720:115–120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsunoda M, Sugaya C, Sugiura Y, Nagai Y
and Sakanishi K: Safety evaluation of self-assembling peptide gel
after intracranial administration to rats using the open field
test. Biol Pharm Bull. 39:1419–1423. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Szasz BK, Mike A, Karoly R, Gerevich Z,
Illes P, Vizi ES and Kiss JP: Direct inhibitory effect of
fluoxetine on N-methyl-D-aspartate receptors in the central nervous
system. Biol Psychiatry. 62:1303–1309. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang HN, Wang L, Zhang RG, Chen YC, Liu L,
Gao F, Nie H, Hou WG, Peng ZW and Tan Q: Anti-depressive mechanism
of repetitive transcranial magnetic stimulation in rat: The role of
the endocannabinoid system. J Psychiatr Res. 51:79–87. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu HF, Zhu CH and Guo JY: Effect of
ginsenoside Rg1 on behaviors and hippocampal amino acids in
depressive-like rats. Zhongguo Zhong Yao Za Zhi. 37:3117–3121.
2012.(In Chinese). PubMed/NCBI
|
|
25
|
McEwen BS: Stress and hippocampal
plasticity. Annu Rev Neurosci. 22:105–122. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fu AK, Hung KW, Huang H, Gu S, Shen Y,
Cheng EY, Ip FC, Huang X, Fu WY and Ip NY: Blockade of EphA4
signaling ameliorates hippocampal synaptic dysfunctions in mouse
models of Alzheimer's disease. Proc Natl Acad Sci USA.
111:9959–9964. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dudkin KN, Chueva IV and Makarov FN: The
role of the prefrontal and parietal cortex in learning and memory
in monkeys. Ross Fiziol Zh Im I M Sechenova. 86:1458–1470. 2000.(In
Russian). PubMed/NCBI
|
|
28
|
Drevets WC: Functional neuroimaging
studies of depression: The anatomy of melancholia. Annu Rev Med.
49:341–361. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M,
Tafuri A, et al: Roles of the Raf/MEK/ERK pathway in cell growth,
malignant transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang F, Steelman LS, Lee JT, Shelton JG,
Navolanic PM, Blalock WL, Franklin RA and McCubrey JA: Signal
transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine
receptors to transcription factors: Potential targeting for
therapeutic intervention. Leukemia. 17:1263–1293. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mantamadiotis T, Lemberger T, Bleckmann
SC, Kern H, Kretz O, Villalba Martin A, Tronche F, Kellendonk C,
Gau D, Kapfhammer J, et al: Disruption of CREB function in brain
leads to neurodegeneration. Nat Genet. 31:47–54. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lonze BE and Ginty DD: Function and
regulation of CREB family transcription factors in the nervous
system. Neuron. 35:605–623. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yiu AP, Mercaldo V, Yan C, Richards B,
Rashid AJ, Hsiang HL, Pressey J, Mahadevan V, Tran MM, Kushner SA,
et al: Neurons are recruited to a memory trace based on relative
neuronal excitability immediately before training. Neuron.
83:722–735. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hetman M and Gozdz A: Role of
extracellular signal regulated kinases 1 and 2 in neuronal
survival. Eur J Biochem. 271:2050–2055. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun ZG, Huang QZ, Xu CY and Chen LP:
Effects of shuyu ningxln recipe on the praxiology and the
expressions of hippocampal BDNF and trkB of model rats with chronic
stress-induced depression. Zhongguo Zhong Xi Yi Jie He Za Zhi.
33:370–375. 2013.(In Chinese). PubMed/NCBI
|