Introduction
Heat stroke is a life-threatening acute condition
characterized by a rapidly increasing core body temperature and
central nervous system injury. Brain damage is a common
manifestation of heat stroke and the high neurological morbidity
observed in heat stroke has been considered secondary to multiple
organ dysfunction syndrome (MODS) (1,2). The
mechanism is complex and involves a series of peptidergic nerve
reactions (3). Calcitonin
gene-related peptide (CGRP) is a novel endogenous neural active
peptide, which was identified by a gene recombination technique. It
not only has an important role in vasodilation and nerve
conduction, but is also closely associated with the occurrence and
development of brain injury (4,5).
Previous studies have demonstrated that CGRP significantly
increases the cerebral blood flow and reduces injury of ischemic
neurons in hypoxic-ischemic brain injury (6). After scald and cold stimulation,
ultraviolet irradiation and water immersion stress, the expression
of CGRP was also significantly increased (7–9).
Therefore, the present study hypothesized that CGRP also has a
protective role in brain injury induced by heat stroke. However, to
date, only few results have been reported in this field. The
present study aimed to explore the effects of CGRP on brain injury
induced by heat stroke by constructing a model of heat stroke and
injecting CGRP and its antagonist through the carotid artery.
Materials and methods
Animals
Male Wistar rats (n=20, weight, 200–230 g; age,
10–12 weeks; Southern Medical University, Guangzhou, China) were
maintained under controlled environmental conditions (12-h
light/dark cycle; humidity, 35±5%; temperature, 25°C) at the
Experimental Animal Center of Southern Medical University
(Guangzhou, China) and were given free access to standard
laboratory chow and water. Animal procedures were approved by the
Animal Care and Use Committee of Southern Medical University
(Guangzhou, China) and the experiment was performed according to
the Guidelines for Animal Care of Southern Medical University
(Guangzhou, China).
Preparation of the heat stroke model
and intervention
A total of 20 rats, housed for 6 h at an ambient
temperature of 25±0.5°C with a humidity of 35±5%, were randomly
divided into four groups of 5 animals each: Control group; HS
group, heat stroke; HS+CGRP group, heat stroke and injection of
CGRP (Abcam, Cambridge, MA, USA); HS+CGRP 8–37 group, heat stroke
and injection of CGRP8-37 (Sigma-Aldrich; Merck KGaA; Darmstadt,
Germany), a specific antagonist of CGRP receptor. Prior to
establishment of the model, an intraperitoneal injection of sodium
pentobarbital (50 mg/kg; Sigma-Aldrich; Merck KGaA) was applied to
abolish the corneal reflex and pain reflex. The carotid artery of
the rat was cannulated with a trocar (24-gauge) for drug injection.
A unipolar lead with scalp acupuncture was used for
electroencephalogram (EEG) examination. Acupuncture needles were
inserted in the epicranium of rat's bilateral temples. Rats in the
control group were maintained at 25±0.5°C and a humidity of 35±5%.
Rats from the other three groups were placed in a pre-warmed
incubator at 35.5±0.5°C and a relative humidity of 60±5% in the
absence of food and water. The rectal core temperature (Tc) was
continuously monitored with a rectal thermometer. Rats were removed
from the incubator and allowed to cool at an ambient temperature of
25±0.5°C when the Tc reached 41°C. Each rat in the HS+CGRP group
received a bolus injection of CGRP (2 µg/ml, 0.5 ml; Abcam) through
carotid artery trocar after heat stress. In the HS+CGRP8-37 group,
each rat received a bolus injection of CGRP8-37 (30 nmol/kg, 0.5
ml; Sigma-Aldrich; Merck KGaA) after heat stress. Rats in the HS
group and control group received a bolus injection of normal saline
(0.5 ml). At 2 h after heat stress, all rats received scalp EEG
examination (duration, 30 mm/s; gain, 0.5 cm=50 µV) and were then
subjected to further histopathological analysis and index
detection.
Brain tissue sampling
Rats were anesthetized by intraperitoneal injection
of urethane and then sacrificed by decollation. Brain tissues were
obtained during immediate autopsy in all animals. Tissues for
hematoxylin and eosin (H&E) staining and terminal
deoxynucleotidyltransferase-mediated deoxyuridine triphosphate nick
end labelling (TUNEL) were fixed in 10% formalin and embedded into
paraffin blocks. Brain tissue sections (3 mm) were collected for
western blot analysis.
Histopathological analysis
Paraffin-embedded tissues were sectioned at 3-µm
thickness and stained with H&E for microscopic evaluation at a
magnification of ×400. The extent of brain injury was evaluated by
two certified pathologists in a blinded manner.
TUNEL assay
Paraffin-embedded tissues were sliced into frozen
sections using a freezing microtome (Leica Microsystems, Wetzlar,
Germany). The sections on the slides were conventionally de-waxed
with xylene, rehydrated with graded ethanol and incubated in 3%
hydrogen peroxide methanol at room temperature for 20 min, washed
with PBS for 30 min, permeabilized with 0.1% Triton X-100 for 2 min
at 4°C, washed twice with PBS, and then incubated with TUNEL
staining mixture (Nanjing KeyGen Biotech, Nanjing, China) at 37°C
in the dark for 1 h. Subsequently, samples were incubated with
3,3′-diaminobenzidine in the dark for 2 min. The samples were then
washed three times with PBS, counterstained with hematoxylin and
mounted with glycerol. Apoptosis was observed using a microscope
(Olympus, Tokyo, Japan) and three visual fields of view were
randomly selected to count the cells with positive staining.
Western blot analysis
Brain sections were lysed with
radioimmunoprecipitation assay buffer, which contained 50 mM
Tris-HCl, 150 mM NaCl, 1% Nonidet P-40, 0.25% Na-deoxycholate and 1
mM EDTA. Protein concentrations were determined using the
bicinchoninic acid protein assay and samples were diluted to a
concentration of 2 µg/µl. Protein samples (40 µg) were then
separated by 12% SDS-PAGE. The separated proteins were transferred
onto polyvinylidene difluoride membranes and then blocked with
confining liquid (5% skimmed milk powder and 0.1% Tween-20 in PBS)
at room temperature for 2 h, and incubated overnight at 4°C with
primary antibody: Rabbit anti-caspase-3 and β-actin antibodies
(004F and ab8226, 1:1,000 dilution; Abcam) in hybridization
solution. The following day, the membranes were incubated with
secondary antibody (goat-anti-rabbit immunoglobulin G, S001F,
1:2,000 dilution; Abcam) in hybridization solution at room
temperature for 2 h. Blots were visualized by enhanced
chemoluminescence (EMD Millipore, Billerica, MA, USA) was used for
signal detection. Images were quantified using the Quantity One
software (v4.62; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Values are expressed as the optical density ratio of the target
protein to β-actin.
Statistical analysis
Values are expressed as the mean ± standard
deviation and were analyzed using SPSS 19.0 statistical software
(IBM Corp., Armonk, NY, USA). The data were analyzed by one-way
ANOVA and further analyzed by Dunnett's T3 test for multiple
comparisons. A two-tailed P<0.05 was considered to indicate a
statistically significant difference.
Results
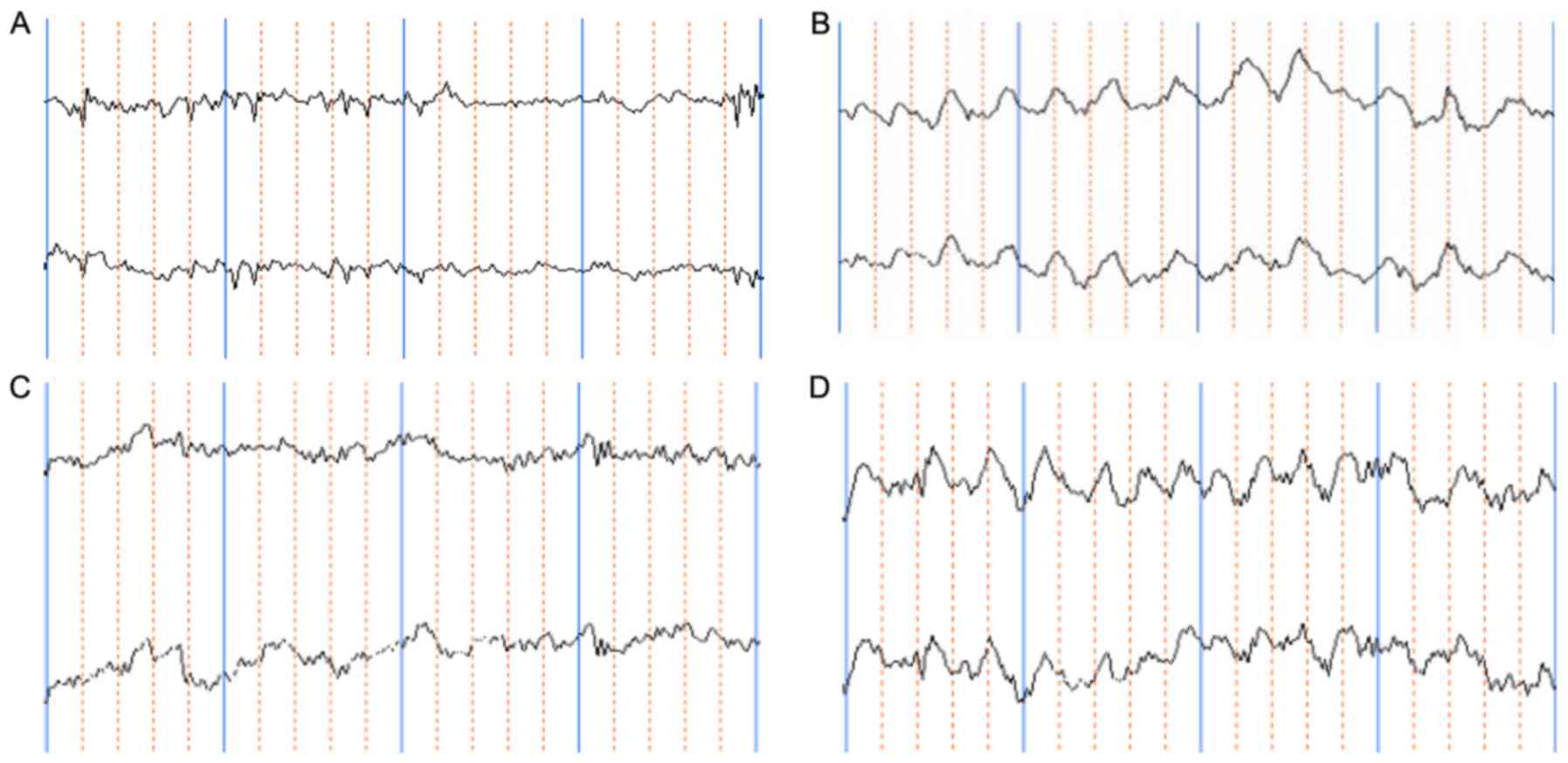
CGRP affects EEG in rats after heat
stroke
Compared with the control group, a rapid suppression
of the rat EEG was observed in the HS group. A representative slow
wave pattern appeared. The amplitude of the θ-wave increased
significantly (P<0.05) and the amplitude of the α-wave decreased
significantly (P<0.05). Administration of CGRP produced a rapid
recovery of the EEG. The EEG was characterized by short- to
long-term α-waves and low-voltage β-waves as well as a large amount
of intermittent δ- and θ-waves. Compared with that in the HS group,
the θ-wave decreased significantly (P<0.05) while the α-wave
increased significantly in the CGRP group (P<0.05). CGRP8-38
produced a great suppression in the duration of the EEG. The
characteristics of the EEG were a long-term frequency θ-wave,
inclusion of a partial δ-wave and a significant reduction in the
α-wave. Compared with those in the control group, the θ- and δ-wave
increased significantly (P<0.05), while the α-wave decreased
significantly (P<0.05). However, these differences did not
achieve any statistical significance when compared with the HS
group (Table I; Fig. 1).
 | Table I.Comparison of electroencephalogram
parameters (µV) for rats in different group. |
Table I.
Comparison of electroencephalogram
parameters (µV) for rats in different group.
| Group | δ-wave | θ-wave | α-wave | β-wave |
|---|
| Control |
44.40±12.50 |
30.20±3.49 |
35.60±6.84 |
8.80±3.56 |
| HS |
60.80±6.90 |
54.00±10.86a |
17.40±4.45a |
10.80±4.15 |
| HS+CGRP |
52.00±8.37 |
35.50±5.70b |
28.80±4.82b |
9.00±2.24 |
| HS+CGRP8-37 |
65.00±7.91a |
70.00±11.73a |
20.40±2.61a |
10.00±2.45 |
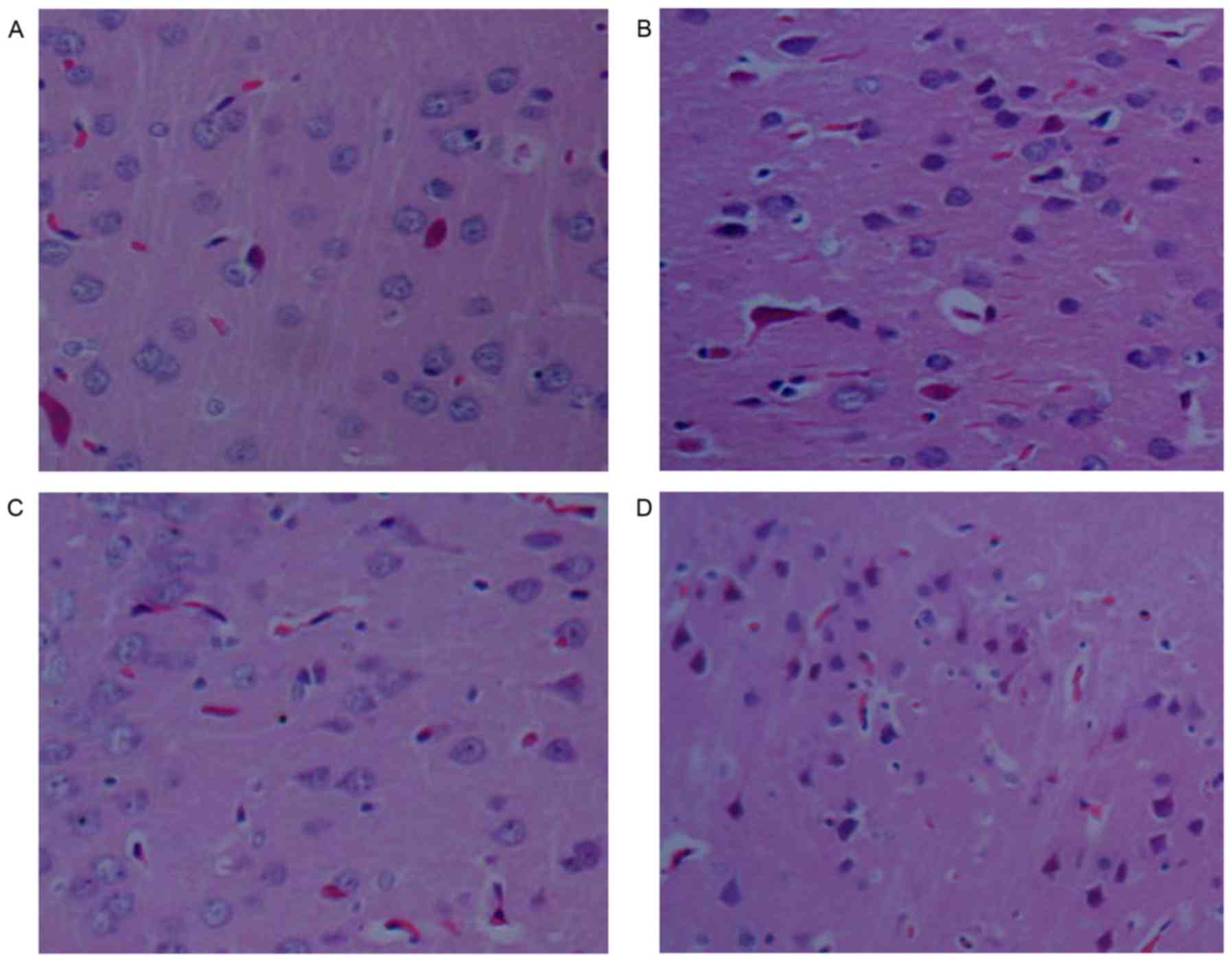
CGRP alleviates brain tissue damage
after heat stroke
Histopathological investigation revealed that no
significant abnormalities were present in the brains of control
rats. By contrast, the brain exhibited moderate edema,
characterized by vacant spaces surrounding the neurons and
capillaries in HS rats. Pathological aberrations in the brains of
rats in the HS+CGRP group were significantly alleviated. Nerve
cells only exhibited slight swelling and the gaps between the
nerves and blood vessels were displayed. Severe damage was observed
in the HS+CGRP8-37 group. Neural cell shrinkage, volume reduction,
hyperchromatic nuclei, nuclear pyknosis, disappearance of part of
the nuclear membrane and cell necrosis were observed in the
HS+CGRP8-37 group (Fig. 2).
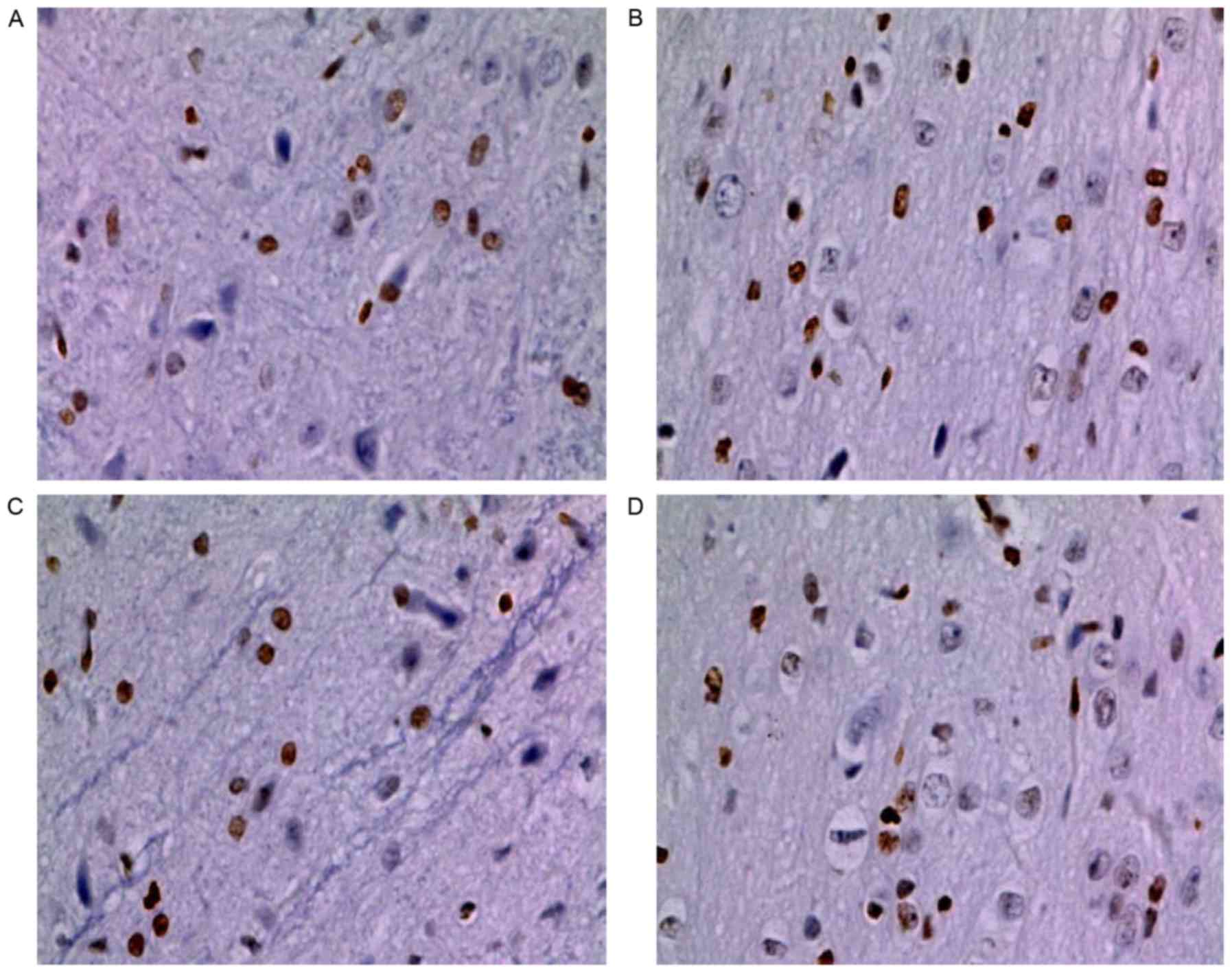
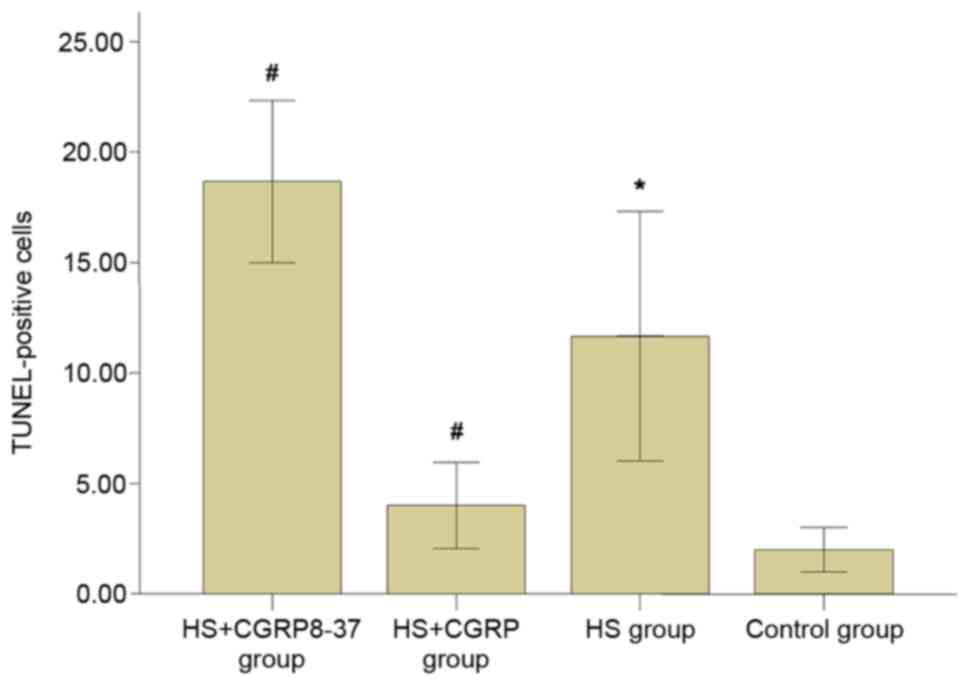
CGRP reduces heat stroke-induced
neuronal cell apoptosis
As presented in Figs.
3 and 4, heat stroke
significantly enhanced neuronal cell apoptosis as compared with
that in the control group (P<0.05). The heatstroke-induced
upregulation of cell apoptosis was significantly weakened after
administration of CGRP. The number of TUNEL-positive cells in the
HS+CGRP group was significantly lower than that in the HS group
(P<0.05). By contrast, CGRP8-37 enhanced neuronal cell
apoptosis. The results indicated a significantly enhanced number of
TUNEL-positive cells in the CGRP8-37 group compared with that in
the HS group (P<0.05). As indicated by these data, GRP and
CGRP8-37 affected neuronal cell apoptosis. It may be deduced that
CGRP contributed to the inhibitory effects of neuronal cell
apoptosis induced by heat stroke.
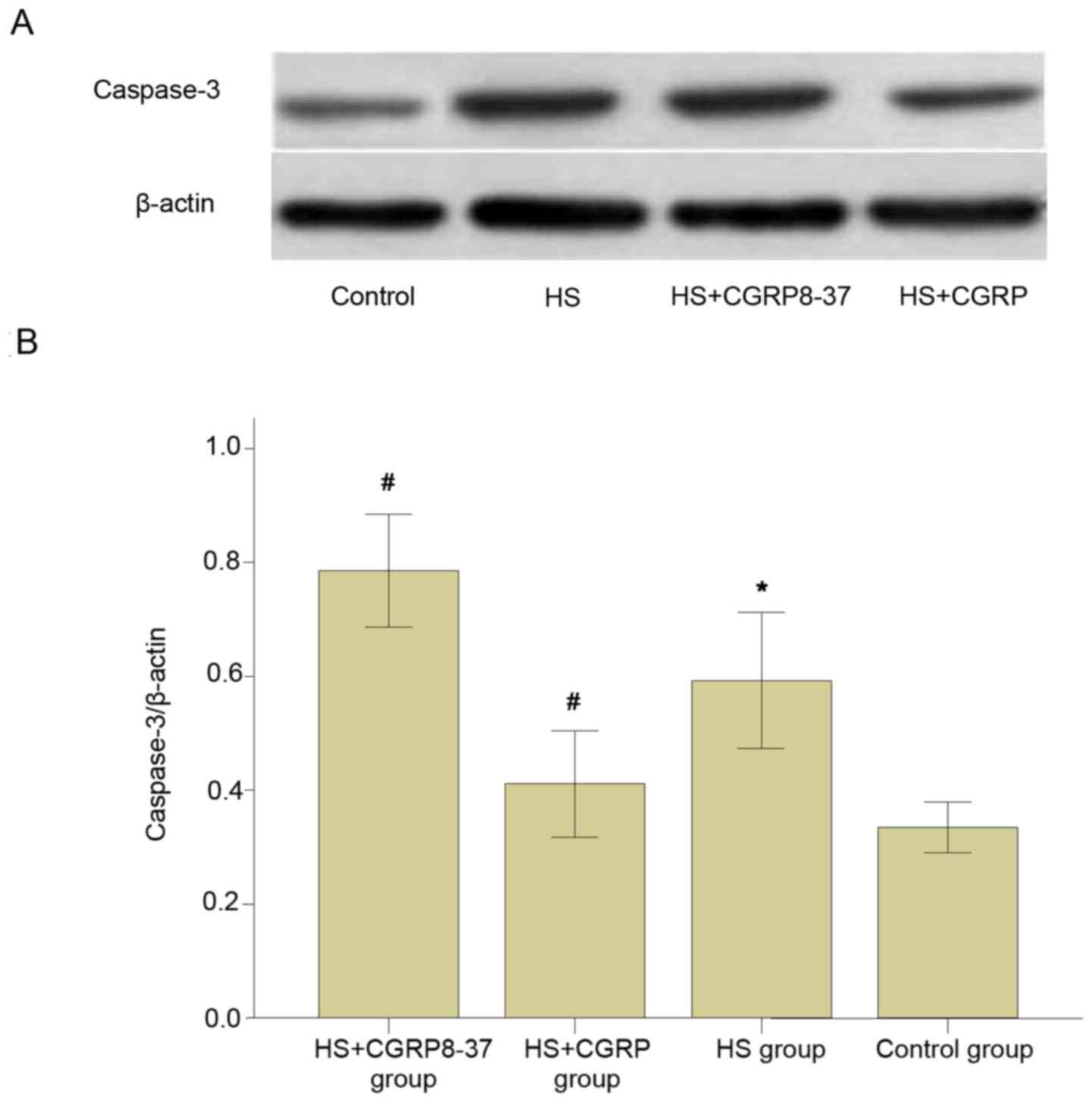
CGRP inhibits heat stroke-induced expression of
caspase-3 protein in the brains of rats. Western blot analysis
demonstrated that all rats subjected to heat stroke demonstrated a
significant increase in the protein levels of caspase-3 when
compared with those in the control group (P<0.05).
Administration of CGRP significantly prevented the increase of
caspase-3 protein expression. The protein levels of caspase-3 in
the HS+CGRP group significantly decreased compared with those in
the HS group (P<0.05). By contrast, there was an apparent
increase in the expression of caspase-3 protein in the brains of
rats in the CGRP8-37 group compared with those in the HS group
(P<0.05; Fig. 5). It was revealed
that CGRP8-37 significantly enhanced the protein levels of
caspase-3 in brain tissues. These results indicated that the
expression of caspase-3 protein induced by heat stroke was
considerably downregulated by CGRP protein in the brains of
rats.
Discussion
According to its pathophysiological responses, heat
stroke may be defined as a form of hyperthermia, associated with a
systemic inflammatory response, leading to a syndrome of
multi-organ dysfunction in which central nervous system injury
dominates (10,11). Heat-associated illnesses develop when
the pathological effects of heat load are not prevented. Syndromes
vary from less severe, such as heat syncope, to severe forms, such
as lethal heat stroke. Knowledge of the pathological changes of the
central nervous system is essential for understanding the
mechanisms of heat stroke. However, at present, little information
regarding the occurrence of brain lesions is available. Bouchama
et al (12) demonstrated that
tissue damage of varying degree was present in the brain after heat
stroke. In a rat model of heat stroke, injury to the brain appeared
even at 39°C, which was further aggravated with increases in
temperature, regardless of cooling treatment (13). However, the mechanism of brain injury
in heat stroke still remain elusive, and effective methods to
prevent the progression of brain injury in the clinic are currently
lacking. CGRP, a newly identified endogenous neural active peptide,
is widely distributed in the nervous system and has been considered
to have a positive role in central nervous system injury in this
decade (14–16). Increasing evidence has indicated that
CGRP has a protective effect on non-septic brain injury, such as
cerebral infarction, subarachnoid hemorrhage and traumatic brain
injury. In a rat model, CGRP reduced the degree of cerebral
ischemia/reperfusion injury and improved the tolerance of nerve
cells to hypoxia (6). Animal and
clinical experiments have indicated that CGRP also relieves
cerebral vasospasm after subarachnoid hemorrhage, improves the
blood supply of the brain and regulates the circulation of the
brain (17). Previous studies have
indicated a sharp release of CGRP from the brain tissue into the
serum after traumatic brain injury (5). Growing evidence has also suggested that
CGRP is an important mediator in septic and sepsis-like brain
injury. Studies have demonstrated that the expression of central
CGRP was significantly increased in a sepsis model and a scald
model (18,7). Considering that the mechanism of brain
injury after heat stroke is similar to that of sepsis, the present
study hypothesized that CGRP is also a potential protective factor
in brain injury after heat stroke. To the best of our knowledge, no
studies are available that explored the protective effects of CGRP
on brain injury induced by heat stroke.
To observe the potential protective effect of CGRP
against brain injury in heat stroke, CGRP and CGRP8-37 were
respectively injected into the rats after heat stroke through the
carotid artery trocar. The results indicated that brain injury was
present at an early stage after heat stroke and CGRP exerted a
protective effect on brain injury at the same time-point, as
directly evidenced by techniques such as EEG and histopathology.
EEG examination has the characteristics of high sensitivity and
fastness. In the present study, changes in the EEG were examined in
an attempt to observe the effect of CGRP on brain injury after heat
stroke. An extensive slow wave is the typical change in the EEG
after diffuse brain injury. The degree of the slow wave is linked
to the severity of brain damage (19). In the present study, heat stroke
evidently induced an abnormal EEG, which manifested as a large
quantity of representative slow waves, increased amplitude of
θ-wave and decreased amplitude of α-wave. Administration of CGRP
produced a rapid recovery of the electroencephalogram. The
appearance of a reverse tendency after bolus injection of CGRP8-37
further confirmed the brain-protective effect of CGRP. H&E
staining results further evidenced the protective effects of CGRP
on brain injury. Compared with those in the HS group, pathological
brain damage was significantly alleviated in the HS+CGRP group and
enhanced in the HS+CGRP8-37 group, which indicated that CGRP may
have a marked protective effect in heat stroke. In fact, CGRP is
the most potent microvascular neuropeptide vasodilator known to
date (20,21). Previous studies have demonstrated
that exogenous CGRP significantly increased the cerebral blood
flow, prevented blood-brain barrier injury and protected ischemic
neurons in cerebrovascular disease (22,6). The
results of the present study were consistent with those of studies
on ischemic brain injury, which suggested the presence of common
mechanisms. Associated studies have reported that heat stress
exerts cardioprotection, which is due to the synthesis and release
of CGRP via activation of capsaicin receptor (vanilloid receptor
subtype 1) on capsaicin-sensitive sensory neurons. The endothelial
cell-derived CGRP is considered as one of the key factors exerting
protective effects (23). It remains
elusive whether a similar mechanism may explain the protective
effect of CGRP on the central nervous system in sepsis-like heat
stroke.
Clinical and laboratory studies have confirmed that
neuronal apoptosis is one of the important causes of brain damage
in various types of brain injury. Apoptosis has a dual effect:
While it may clear aging cells and exert a protective effect on
tissues and organisms, excessive apoptosis may lead to massive
neuronal loss and then damage the nervous function, causing
secondary damage to the brain. With the continuous deepening of the
understanding of the damage heat stroke causes to the body, an
increasing number of studies have indicated that heat stroke also
induces apoptosis of brain neurons (13,24).
Whether the effect of CGRP in brain damage induced by severe heat
stroke is associated with its regulation of the apoptosis of cells
of the central nervous system had remained to be determined. For
this reason, the present study assessed neuronal cell apoptosis in
rats. The results revealed that the heat stroke-induced
upregulation of cell apoptosis was significantly weakened after
administration of CGRP. Conversely, treatment with CGRP8-37
enhanced neuronal cell apoptosis. From these results, it may be
deduced that CGRP exerted an inhibitory effect on neuronal cell
apoptosis induced by heat stroke. CGRP is known to activate signal
transduction pathways through the G protein-coupled receptor.
Subsequently, the accumulation of intracellular cyclic adenosine
monophosphate leads to changes of cell function, including
apoptosis mediated through complex signaling pathways. Caspase-3
protein has a key role in the process of apoptosis and it is the
most common downstream effector of apoptotic pathways (25,26).
Associated studies have indicated that the cytoprotective role of
CGRP (i.e., its anti-apoptotic effect) after ischemia/reperfusion
injury of the heart, brain and gastrointestinal system is exerted
via downregulation of caspase-3 by modulating nitric oxide
production (27,28). The present study explored the
possible molecular mechanisms of the effect of CGRP. It was
examined whether CGRP regulated caspase-3 protein levels in rats
after heat stroke. The results indicated that the level of
caspase-3 in rats with heat stroke was significantly decreased
following a bolus injection of CGRP, while it was apparently
increased after administration of CGRP8-37. It was therefore
suggested that CGRP exerted its inhibitory effects on apoptosis in
rat brains interfering with caspase-3 signaling. The present
results are comparable with those of previous studies. However, it
remains elusive which upstream signaling proteins are directly
affected to promote this downregulation of caspase-3 and additional
research is required to clarify the exact mechanism.
Overall, the results of the present study
demonstrated that CGRP has a protective effect on early-stage brain
injury induced by heat stroke in rats. Furthermore, it was
demonstrated that the underlying mechanism of the protective effect
of CGRP includes inhibition of neuronal cell apoptosis via
downregulation of caspase-3. The present study provided novel
insight into the role of CGRP in heat stroke, but had certain
limitations. The damage in the brain was detected at a single
time-point after heat stroke, and no longer-term observation was
performed. Due to unpredictability, administration of CGRP is less
practical in the clinic; therefore, further study is required to
explore safer methods. In brief, the exact mechanism via which CGRP
reduces central nervous system injury after heat stroke remains to
be assessed to provide a theoretical basis for the treatment of
heat stroke by CGRP.
Acknowledgements
This study was supported in part by grants from the
National Natural Science Foundation of China (grant nos. 81071529
and 81272105).
References
|
1
|
Leon LR: Hypothermia in systemic
inflammation: Role of cytokines. Front Biosci. 9:1877–1888. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brownlow MA, Dart AJ and Jeffcott LB:
Exertional heat illness: A review of the syndrome affecting racing
Thoroughbreds in hot and humid climates. Aust Vet J. 94:240–247.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Székely M, Carletto L and Garami A: The
pathophysiology of heat exposure. Temperature (Austin). 2:4522015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang D, Zhang P, Wang Y, Han N, Tang C
and Jiang B: The influence of brain injury or peripheral nerve
injury on calcitonin gene-related peptide concentration variation
and fractures healing process. Artif Cells Blood Substit Immobil
Biotechnol. 37:85–91. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song Y, Bi L, Zhang Z, Huang Z, Hou W, Lu
X, Sun P and Han Y: Increased levels of calcitonin gene-related
peptide in serum accelerate fracture healing following traumatic
brain injury. Mol Med Rep. 5:432–438. 2012.PubMed/NCBI
|
|
6
|
Liu Z, Liu Q, Cai H, Xu C, Liu G and Li Z:
Calcitonin gene-related peptide prevents blood-brain barrier injury
and brain edema induced by focal cerebral ischemia reperfusion.
Regul Pept. 171:19–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu D, Chen B, Wang B and Ma D: The changes
in the morphology and distribution of substance P and
calcitonin-gene related peptide nerves in the anterior pituitary of
scalded rats. Zhonghua Shao Shang Za Zhi. 18:176–179. 2002.(In
Chinese). PubMed/NCBI
|
|
8
|
Satoh M, Kuraishi Y and Kawamura M:
Effects of intrathecal antibodies to substance P, calcitonin
gene-related peptide and galanin on repeated cold stress-induced
hyperalgesia: Comparison with carrageenan-induced hyperalgesia.
Pain. 49:273–278. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Legat FJ, Jaiani LT, Wolf P, Wang M, Lang
R, Abraham T, Solomon AR, Armstrong CA, Glass JD and Ansel JC: The
role of calcitonin gene-related peptide in cutaneous
immunosuppression induced by repeated subinflammatory ultraviolet
irradiation exposure. Exp Dermatol. 13:242–250. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bouchama A: Heatstroke: Facing the threat.
Crit Care Med. 34:1272–1273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leon LR and Helwig BG: Heat stroke: Role
of the systemic inflammatory response. J Appl Physiol (1985).
109:1980–1988. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bouchama A, Roberts G, Al Mohanna F,
El-Sayed R, Lach B, Chollet-Martin S, Ollivier V, Al Baradei R,
Loualich A, Nakeeb S, et al: Inflammatory, hemostatic, and clinical
changes in a baboon experimental model for heatstroke. J Appl
Physiol (1985). 98:697–705. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu ZF, Li BL, Tong HS, Tang YQ, Xu QL,
Guo JQ and Su L: Pathological changes in the lung and brain of mice
during heat stress and cooling treatment. World J Emerg Med.
2:50–53. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Edvinsson L: The Journey to establish CGRP
as a migraine target: A retrospective view. Headache. 55:1249–1255.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schebesch KM, Herbst A, Bele S, Schödel P,
Brawanski A, Stoerr EM, Lohmeier A, Kagerbauer SM, Martin J and
Proescholdt M: Calcitonin-gene related peptide and cerebral
vasospasm. J Clin Neurosci. 20:584–586. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kageneck C, Nixdorf-Bergweiler BE,
Messlinger K and Fischer MJ: Release of CGRP from mouse brainstem
slices indicates central inhibitory effect of triptans and
kynurenate. J Headache Pain. 15:72014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tian XH, Wang ZG, Meng H, Wang YH, Feng W,
Wei F, Huang ZC, Lin XN and Ren L: Tat peptide-decorated
gelatin-siloxane nanoparticles for delivery of CGRP transgene in
treatment of cerebral vasospasm. Int J Nanomedicine. 8:865–876.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
De winter BY, Bredenoord AJ, Van Nassauw
L, De Man JG, De Schepper HU, Timmermans JP and Pelckmans PA:
Involvement of afferent neurons in the pathogenesis of
endotoxin-induced ileus in mice: Role of CGRP and TRPV1 receptors.
Eur J Pharmacol. 615:177–184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sinha RK: Electro-encephalogram
disturbances in different sleep-wake states following exposure to
high environmental heat. Med Biol Eng Comput. 42:282–287. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Smillie SJ and Brain SD: Calcitonin
gene-related peptide (CGRP) and its role in hypertension.
Neuropeptides. 45:93–104. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mai TH, Wu J, Diedrich A, Garland EM and
Robertson D: Calcitonin gene-related peptide (CGRP) in autonomic
cardiovascular regulation and vascular structure. J Am Soc
Hypertens. 8:286–296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang ZH, Fang XB, Xi GM, Li WC, Ling HY
and Qu P: Calcitonin gene-related peptide enhances CREB
phosphorylation and attenuates tau protein phosphorylation in rat
brain during focal cerebral ischemia/reperfusion. Biomed
Pharmacother. 64:430–436. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ye F, Deng PY, Li D, Luo D, Li NS, Deng S,
Deng HW and Li YJ: Involvement of endothelial cell-derived CGRP in
heat stress-induced protection of endothelial function. Vascul
Pharmacol. 46:238–246. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kibayashi K, Nakao K and Shojo H:
Hyperthermia combined with ethanol administration induces c-fos
expression in the central amygdaloid nucleus of the mouse brain. A
possible mechanism of heatstroke under the influence of ethanol
intake. Int J Legal Med. 123:371–379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ji J, Kline AE, Amoscato A, Samhan-Arias
AK, Sparvero LJ, Tyurin VA, Tyurina YY, Fink B, Manole MD, Puccio
AM, et al: Lipidomics identifies cardiolipin oxidation as a
mitochondrial target for redox therapy of brain injury. Nat
Neurosci. 15:1407–1413. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rao RV, Hermel E, Castro-Obregon S, del
Rio G, Ellerby LM, Ellerby HM and Bredesen DE: Coupling endoplasmic
reticulum stress to the cell death program. Mechanism of caspase
activation. J Biol Chem. 276:33869–33874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Duan L, Lei H, Zhang Y, Wan B, Chang J,
Feng Q and Huang W: Calcitonin gene-related peptide improves
hypoxia-induced inflammation and apoptosis via nitric oxide in H9c2
cardiomyoblast cells. Cardiology. 133:44–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luo CC, Huang CS, Ming YC, Chu SM and Chao
HC: Calcitonin gene-related peptide downregulates expression of
inducible nitride oxide synthase and caspase-3 after intestinal
ischemia-reperfusion injury in rats. Pediatr Neonatol. 57:474–479.
2016. View Article : Google Scholar : PubMed/NCBI
|