Introduction
Liver cirrhosis is the manifestation of end-stage
chronic liver diseases caused by viral infection, immune disorder
and drug related factors and other factors (1). Histological manifestations of liver
cirrhosis include the diffused hepatocellular degeneration and
necrosis, and the followed hepatocellular nodular regeneration and
fibrous tissue hyperplasia. During the development of liver
cirrhosis, liver cell degeneration and necrosis, nodular
regeneration and fibrous tissue hyperplasia occur, which in turn
lead to liver deformation and hardening, resulting in cirrhosis
(1). Clinical manifestations usually
cannot be observed in patients with early stage of liver cirrhosis,
while liver dysfunction and portal hypertension can usually be
observed in later stage (2). Liver
fibrosis is the key stage of a variety of chronic hepatitis to
progress to liver cirrhosis. Studies have shown that (3,4), early
detection and treatment of liver fibrosis can effectively control
or even reverse the progression of this disease, thereby preventing
the progression to cirrhosis. Carbon monoxide (CO) and nitric oxide
(NO) and other endogenous gas signal molecules, which can
effectively maintain the portal vein relaxation, are important
mediators in expanding vessels and have important functions in
maintaining the diastolic state of sinus hepaticus (5). Endogenous hydrogen sulfide
(H2S) is a newly discovered endogenous gas signal
molecule, and the synthesis of H2S is regulated by a
number of metabolic pathways in vivo (6). H2S is mainly synthesized by
cystathionine-β-synthetase and cystathionine-γ-lyase using
l-cysteine (7). H2S has
similar pathophysiological functions to those of NO and CO
(8). H2S can reduce liver
cell damage in a variety of ways in the development of liver
disease (9,10). In this study, cirrhosis with portal
hypertension rat model was established and the model rats were
treated by propargylglycine (PPG), which is an inhibitor of
cystathionine-γ-lyase. Changes of H2S content in portal
veins, portal vein pressure (PVP) and indexes of liver function and
fibrosis were observed to investigate the changes of H2S
content in development portal hypertension and its correlation with
indexes of liver function and fibrosis, so as to provide the basis
for clinical diagnosis and treatment.
Materials and methods
Experimental reagents
PPG (Sigma, San Francisco, CA, USA); carbon
tetrachloride, zinc acetate, trichloroacetic acid and
p-amino-dimethyianiline dihydrochloride (Sinapharm Chemical Reagent
Co., Ltd.); laminin (LN), hyaluronic acid (HA), type III
procollagen (PCIII) detection kits (Naval Medical Research
Institute, Shanghai, China); TRIzol (Takara, Shiga, Japan); reverse
transcription kit (Toyobo, Osaka, Japan); primers for type I, type
III collagen and glyceraldehyde-3- phosphate dehydrogenase (GAPDH)
(Sangon, Shanghai, China); SYBR-Green PCR Master Mix (Takara).
Experimental instruments
Automatic biochemical analyzer (Mindray, Shenzhen,
China); 12 channels physiological record instrument (Biopac
Systems, Inc., Goleta, CA, USA); ultramicroscale ultraviolet
spectrophotometer (Thermo Fisher Scientific, Inc., Waltham, MA,
USA); continuous wavelength multifunctional microplate reader
(Tecan Austria GmbH, Grödig, Austria); real-time PCR instrument
(Eppendorf, Hamburg, Germany).
Model establishment and grouping
Thirty SPF grade female Sprague-Dawley rats (4–5
weeks, 180–200 g) were purchased from Shanghai SLAC Laboratory
Animal Co., Ltd., (Shanghai, China) license no: SCXK (Shanghai)
2012-0002. Rats were randomly divided into 3 groups including
normal control group (NC), liver cirrhosis group (LC) and cirrhosis
+ PPG group (LC+PPG) with 10 rats in each group. All rats were
raised in SPF environment with free access to food and water.
Cirrhosis and portal hypertension model was established by carbon
tetrachloride induction. Rats in LC and LC+PPG groups were injected
subcutaneously with 40% carbon tetrachloride (diluted with cotton
seed oil) at a dose of 0.5 ml/0.1 kg for the first time and 0.3
ml/0.1 kg for the rest. Injection was performed every four days,
and a total of 13 injections were performed. At the same time, rats
in LC and LC+PPG groups were treated with 15% ethanol solution as
drinking water and fed with high fat and high cholesterol diet.
Rats in NC group were injected subcutaneously with the same dose of
cottonseed oil, and fed with normal food and water. After model
establishment, rats in LC+PPG rats were injected intraperitoneally
with PPG at a dose of 30 mg/kg, once per day, while rats in NC and
LC groups were injected intraperitoneally with normal saline, and
injection was performed for 7 days. The study was approved by the
Ethics Committee of Tongji Hospital.
Measurement of PVP
PVP was measured using portal vein catheterization.
Rats were injected intraperitoneally with 10% chloral hydrate for
anesthesia. An incision was made in the middle of the abdomen to
expose the visceral tissue. Small intestine was separated and
dragged to bottom left to expose the trunk of portal vein. A number
5.5 scalp needle was fixed in the trunk of portal vein, and the
other end was connected to 12 channels physiological record
instrument to measure PVP. PVP >12 mmHg was used as a standard
for formation of portal hypertension.
Measurement of H2S content
in plasma of portal vein
A 1 ml syringe was used to extract blood from portal
vein. Blood was transferred to an anticoagulant tube, followed by
centrifugation to separate plasma. Plasma H2S content
was determined by deproteinization (11). Plasma (0.1 ml) was mixed with 0.5 ml
of 10 g/l zinc acetate by vortex oscillation. Then 0.5 ml of 20
mmol/l p-aminodimethylaniline hydrochloride and 0.5 ml of 30 mmol/l
trichloroacetic acid were added. Finally, distilled water was added
to make a total volume of 5 ml. After vortex oscillation, the
mixture was centrifuged at (5,200 × g, 4°C) for 5 min to separate
the supernatant. OD values were measured at 670 nm using a
continuous wavelength multifunctional microplate reader. A standard
curve was drawn and H2S content was calculated.
Determination of liver function and
fibrosis indexes
Blood was extracted from abdominal aorta to prepare
serum samples. Levels of alanine aminotransferase (ALT) and
aspartate aminotransferase (AST) were determined by automatic
biochemical analyzer. Liver fibrosis indexes LN, HA and PC III were
determined using radioimmunoassay in strict accordance with the kit
instructions.
RNA extraction from liver tissue and
reverse transcription
Liver tissue was collected from the left lobe, and
total RNA was extracted using TRIzol according to the instructions.
Concentration and purity of RNA were determined by UV
spectrophotometer. Only RNA samples of satisfactory concentration
and purity were used for reverse transcription. Total RNA (2 µg)
was denatured at 65°C for 5 min, and was immediately placed on ice,
and 4 µl 5X RT Master Mix was then added, and DEPC treated water
was also added to make a total volume of 20 µl. Reaction conditions
were 37°C for 15 min, 52°C for 5 min and 98°C for 5 min.
qRT-PCR to determine the expression of
type I and type III collagen mRNAs in liver tissue
Primers for Type I collagen, type III collagen and
GAPDH were listed in Table I.
Reaction system was: 2 µl of cDNA, 0.5 µl of each primer, 12.5 µl
of 2X SYBR Green PCR Master Mix and 9.5 µl of ultra-pure water. PCR
reaction was performed on a real-time PCR instrument. Reaction
conditions were 95°C for 30 sec, followed by 40 cycles of 95°C for
5 sec and 60°C for 30 sec. Ct values were processed using 2-∆∆Ct to
calculate the relative expression level of each gene with GAPDH as
endogenous control.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Sequences |
|---|
| Type I collagen | F:
5′-GGCTTCTTCAAACCACTGCTTT-3′ |
|
| R:
5′-AAAGTCATAGCCACCTCCGCTG-3′ |
| Type III
collagen | F:
5′-CTCCCAGAACATTACATAC-3′ |
|
| R:
5′-AATGTCATAGGGTGCGATA-3′ |
| GAPDH | F:
5′-AGGTCGGTGTGAACGGATTTG-3′ |
|
| R:
5′-GGGGTCGTTGATGGCAACA-3′ |
Statistical analysis
Experimental results were expressed as mean ±
standard deviation and SPSS 20.0 statistical software (IBM, Armonk,
NY, USA) was used for statistical analysis. Comparisons between two
groups were performed by independent sample t-test, and comparisons
among multiple groups were performed by single factor analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
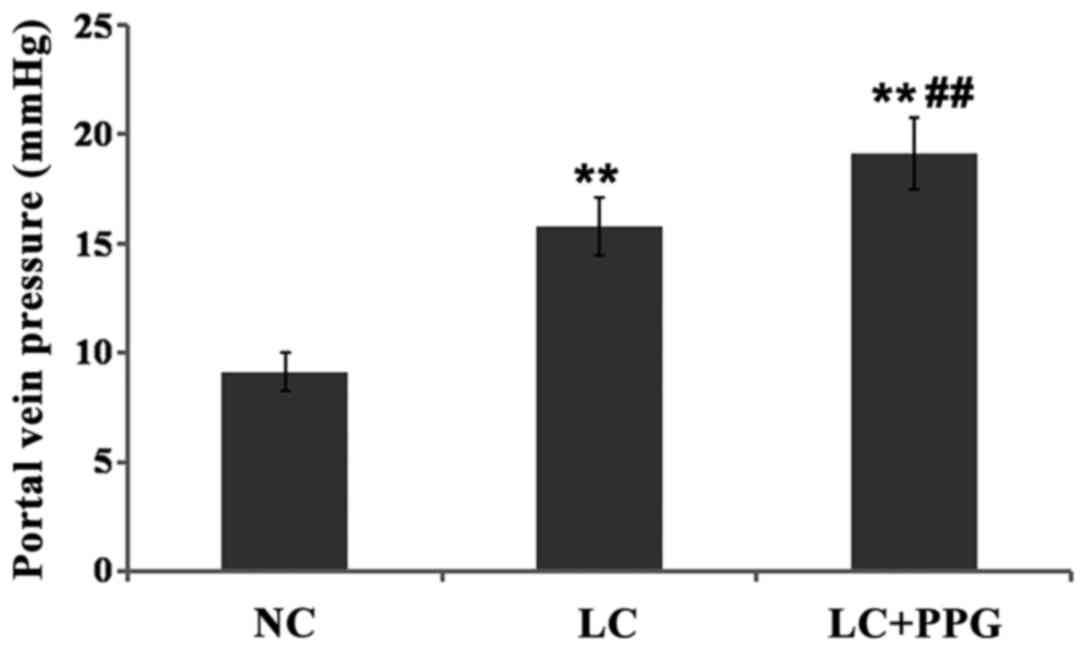
Changes in PVP
PVP of rats in LC and LC+PPG were all >12 mmHg,
indicating the successfully established cirrhosis and portal
hypertension model. Compared with NC group, PVP was significantly
increased in LC and LC+PPG groups (P<0.01). The PVP of LC+PPG
group was significantly higher than that of LC group (P<0.01)
(Fig. 1).
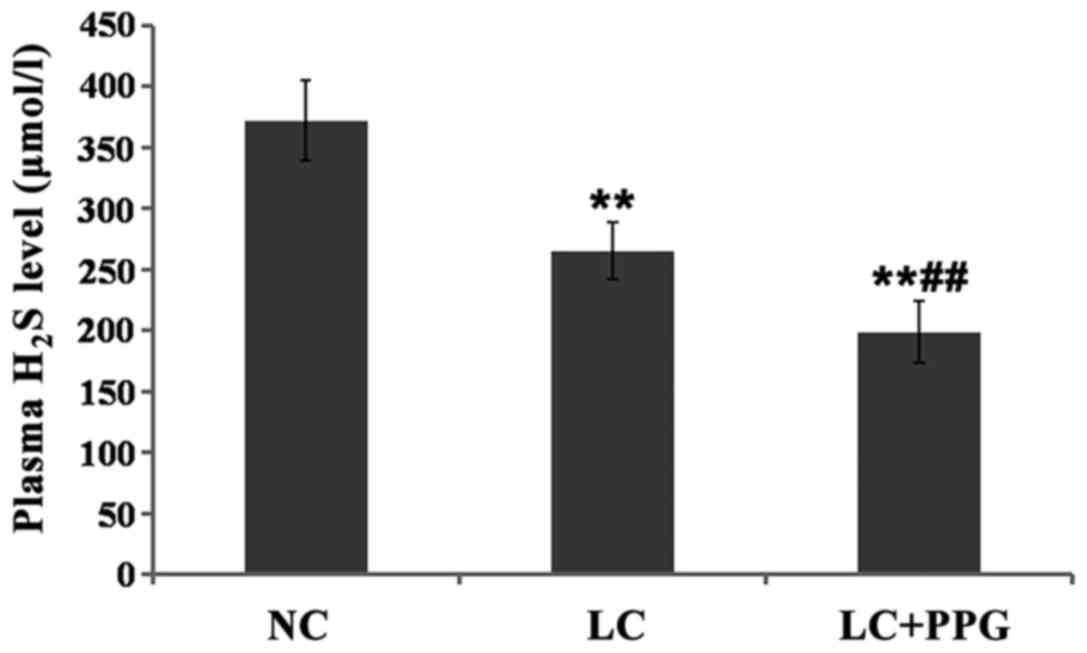
Changes in plasma H2S
levels
Compared with NC group, plasma H2S level
was significantly decreased in LC and LC+PPG groups (P<0.01).
Plasma H2S level in LC+PPG group was significantly lower
than that in LC group (P<0.01) (Fig.
2).
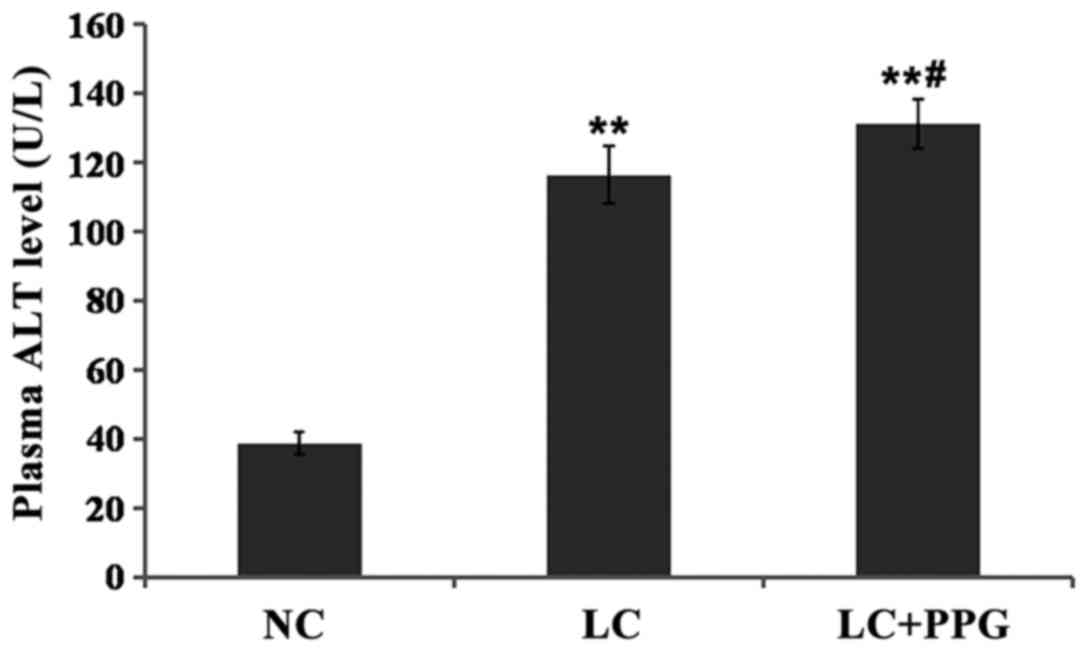
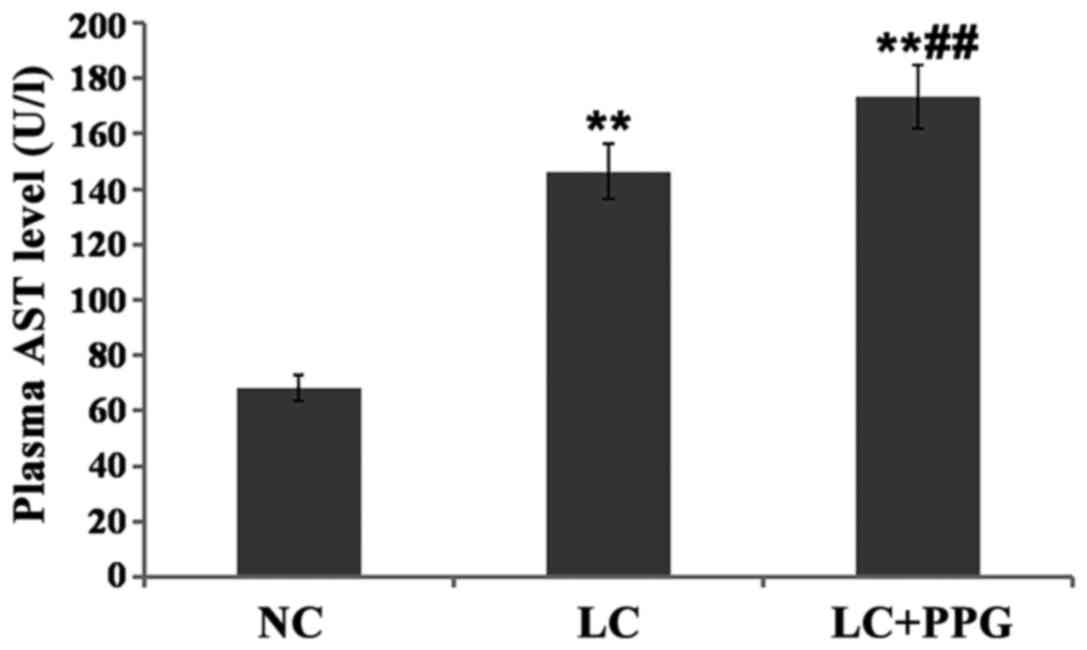
Comparison of liver function between
groups
Compared with NC group, levels of ALT and AST in LC
and LC+PPG groups were significantly increased (P<0.01). Levels
of ALT and AST in LC+PPG group were significantly higher than those
in LC group (P<0.05 or P<0.01) (Figs. 3 and 4).
Comparison of fibrosis indexes among
groups
Compared with NC group, levels of serum LN, HA and
PC III in LC and LC+PPG groups were significantly increased
(P<0.01). Levels of serum LN, HA and PC III in LC+PPG group were
significantly higher than those in LC group (P<0.01 or
P<0.05) (Table II).
 | Table II.Comparison of fibrosis indexes among
groups. |
Table II.
Comparison of fibrosis indexes among
groups.
| Groups | Cases | LN (µg/l) | HA (µg/l) | PC III (µg/l) |
|---|
| NC | 10 | 46.25±6.43 | 125.09±12.84 | 68.16±9.57 |
| LC | 10 |
80.68±7.70a |
276.36±14.63a |
183.48±15.92a |
| LC+PPG | 10 |
91.51±9.36a,b |
313.3±13.09a,c |
227.±17.03a,c |
Comparison of expression levels of
type I and type III collagen mRNAs among groups
Compared with NC group, expression levels of type I
and type III collagen mRNA in LC and LC+PPG groups were
significantly increased (P<0.01). In addition, expression levels
of type I and type III collagen mRNA LC+PPG group were
significantly higher than those in LC group (P<0.05 or
P<0.01) (Table III).
 | Table III.Comparison of expression levels of
type I and type III collagen mRNAs among groups. |
Table III.
Comparison of expression levels of
type I and type III collagen mRNAs among groups.
| Groups | Cases | Type I collagen | Type III
collagen |
|---|
| NC | 10 | 1.02±0.13 | 1.03±0.11 |
| LC | 10 |
2.14±0.32a |
1.88±0.41a |
| LC+PPG | 10 |
3.01±0.47a,c |
2.49±0.52a,b |
Discussion
Endogenous H2S, as a new type of gas
signaling molecule discovered after CO and NO, has been proved with
the functions of dilating blood vessels (12), protecting the myocardium (13) and anti-inflammatory (14,15).
Endogenous H2S is present in two forms including gases
or sodium hydrosulfide. Studies have shown that gas forms account
for about one-third, while sodium hydrosulfide forms account for
two-thirds. The dynamic balance between those forms maintains the
pH level of internal environment of the body (16). H2S is mainly synthesized
in the liver, and liver plays an important role in maintaining
blood concentration of H2S, while H2S can
regulate the microcirculation of the liver (17).
Cirrhosis is a liver disease caused by various
factors with portal hypertension and liver dysfunctions as the main
manifestations. The pathological features of cirrhosis include
increased extracellular matrix and hepatic stellate cell
activation, and cirrhosis is usually combined with liver cell
degeneration and necrosis, nodular regeneration and fibrous tissue
hyperplasia (1). The key step of the
occurrence of liver cirrhosis is in hepatic stellate cells.
Activated hepatic stellate cells have similar characteristics of
vascular smooth muscle cells, which can regulate blood flow of
sinus hepaticus and intrahepatic resistance (18). Studies have showed that the activity
of cystathionine-γ-lyase in hepatic stellate cells of cirrhosis
animal model was significantly reduced, resulting in a significant
reduction in H2S production, and then cause contraction
of hepatic stellate cells in sinus hepaticus, thereby increasing
the portal hypertension (17,19).
Another study found that H2S can regulate the portal
vein pressure by modulating hepatic artery buffer system and
dilating hepatic arteries (20). In
addition, H2S may affect the formation of portal
hypertension by participating in the opening of the
K+-ATP channel (21). In
vascular smooth muscle cells of portal vein, H2S can
activate K+-ATP channel to cause hyperpolarization of
cell membrane, thereby reducing the concentration of free calcium
ions in vascular smooth muscle cells, and ultimately lead to the
relaxation and expansion of vascular smooth muscle. This study
found that the plasma H2S content was significantly
reduced in rats with cirrhosis and portal hypertension, and
H2S content was further reduced ad PVP was further
increased after the treatment of PPG, indicating that
H2S can inhibit the formation of portal hypertension.
Higher portal hypertension is followed by the lower plasma
H2S content.
Hepatic fibrosis is the key step of a variety of
chronic hepatitis to progress to cirrhosis. The pathogenesis of
liver fibrosis is very complex. Liver damage caused by virus, drug
and other factor can stimulate macrophages to release inflammatory
cytokines, these cytokines can participate in the production and
degradation of extracellular collagen, and the activation and
apoptosis of hepatic stellate cell through a variety of ways
(22), which eventually leads to
fibrosis. It has been found that H2S can promote
apoptosis of vascular smooth muscle cells by downregulating the
level of apoptosis inhibitors such as Bcl-2 and NF-κB (23). Another study found that
H2S can inhibit the expression of type I and III
procollagen mRNA in the muscular arteries of the lungs, thereby
reducing the levels of collagen type I and type III proteins
(24). This study found that ALT and
AST were significantly increased, expression level of fibrosis
indexes LN, HA and PC III and type I and type III collagen were
also significantly increased in cirrhosis rats combined with portal
hypertension. The levels of those factors were further increased
after PPG treatment. Therefore, H2S content is
negatively correlated with the levels of liver function and
fibrosis indexes, suggesting that H2S can protect liver
function and inhibit fibrosis in portal hypertension.
In conclusion, in carbon tetrachloride-induced
cirrhosis and portal hypertension rat model, plasma H2S
level was inversely proportional to PVP, and H2S can
reduce portal hypertension, protect liver function and inhibit
fibrosis, thus delaying the progress of liver cirrhosis. Further
studies are still needed to investigate the possible synergistic or
antagonistic interactions of H2S with NO, CO and other
signal molecules in the development of cirrhosis.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China, no. 30901430.
References
|
1
|
Friedman SL: Molecular regulation of
hepatic fibrosis, an integrated cellular response to tissue injury.
J Biol Chem. 275:2247–2250. 2000. View Article : Google Scholar
|
|
2
|
Bonis PA, Friedman SL and Kaplan MM: Is
liver fibrosis reversible? N Engl J Med. 344:452–454. 2001.
View Article : Google Scholar
|
|
3
|
Loguercio C and Federico A: Oxidative
stress in viral and alcoholic hepatitis. Free Radic Biol Med.
34:1–10. 2003. View Article : Google Scholar
|
|
4
|
Kitade Y, Watanabe S, Masaki T, Nishioka M
and Nishino H: Inhibition of liver fibrosis in LEC rats by a
carotenoid, lycopene, or a herbal medicine, Sho-saiko-to. Hepatol
Res. 22:196–205. 2002. View Article : Google Scholar
|
|
5
|
La Villa G and Gentilini P: Hemodynamic
alterations in liver cirrhosis. Mol Aspects Med. 29:112–118. 2008.
View Article : Google Scholar
|
|
6
|
Geng B, Yang J, Qi Y, Zhao J, Pang Y, Du J
and Tang C: H2S generated by heart in rat and its
effects on cardiac function. Biochem Biophys Res Commun.
313:362–368. 2004. View Article : Google Scholar
|
|
7
|
Calvert JW, Coetzee WA and Lefer DJ: Novel
insights into hydrogen sulfide - mediated cytoprotection. Antioxid
Redox Signal. 12:1203–1217. 2010. View Article : Google Scholar
|
|
8
|
Hosoki R, Matsuki N and Kimura H: The
possible role of hydrogen sulfide as an endogenous smooth muscle
relaxant in synergy with nitric oxide. Biochem Biophys Res Commun.
237:527–531. 1997. View Article : Google Scholar
|
|
9
|
Kang K, Zhao M, Jiang H, Tan G, Pan S and
Sun X: Role of hydrogen sulfide in hepatic
ischemia-reperfusion-induced injury in rats. Liver Transpl.
15:1306–1314. 2009. View
Article : Google Scholar
|
|
10
|
Tan G, Pan S, Li J, Dong X, Kang K, Zhao
M, Jiang X, Kanwar JR, Qiao H, Jiang H, et al: Hydrogen sulfide
attenuates carbon tetrachloride-induced hepatotoxicity, liver
cirrhosis and portal hypertension in rats. PLoS One. 6:e259432011.
View Article : Google Scholar
|
|
11
|
Ali MY, Ping CY, Mok YY, Ling L, Whiteman
M, Bhatia M and Moore PK: Regulation of vascular nitric oxide in
vitro and in vivo; a new role for endogenous hydrogen sulphide? Br
J Pharmacol. 149:625–634. 2006. View Article : Google Scholar
|
|
12
|
Nik Mel AV, Voloshchouk NI, Pentyuk NO and
Zaichko KO: Role of hydrogen sulfide and sulfur-containing amino
acids in regulation of tone of smooth muscles of the vascular wall
in rats. Neurophysiology. 42:126–131. 2010.
|
|
13
|
Chuah SC, Moore PK and Zhu YZ:
S-allylcysteine mediates cardioprotection in an acute myocardial
infarction rat model via a hydrogen sulfide-mediated pathway. Am J
Physiol Heart Circ Physiol. 293:H2693–H2701. 2007. View Article : Google Scholar
|
|
14
|
Sidhapuriwala JN, Ng SW and Bhatia M:
Effects of hydrogen sulfide on inflammation in caerulein-induced
acute pancreatitis. J Inflamm (Lond). 6:352009. View Article : Google Scholar
|
|
15
|
Hirata I, Naito Y, Takagi T, Mizushima K,
Suzuki T, Omatsu T, Handa O, Ichikawa H, Ueda H and Yoshikawa T:
Endogenous hydrogen sulfide is an anti-inflammatory molecule in
dextran sodium sulfate-induced colitis in mice. Dig Dis Sci.
56:1379–1386. 2011. View Article : Google Scholar
|
|
16
|
Chunyu Z, Junbao D, Dingfang B, Hui Y,
Xiuying T and Chaoshu T: The regulatory effect of hydrogen sulfide
on hypoxic pulmonary hypertension in rats. Biochem Biophys Res
Commun. 302:810–816. 2003. View Article : Google Scholar
|
|
17
|
Fiorucci S, Antonelli E, Mencarelli A,
Orlandi S, Renga B, Rizzo G, Distrutti E, Shah V and Morelli A: The
third gas: H2S regulates perfusion pressure in both the
isolated and perfused normal rat liver and in cirrhosis.
Hepatology. 42:539–548. 2005. View Article : Google Scholar
|
|
18
|
Bauer M, Bauer I, Sonin NV, Kresge N,
Baveja R, Yokoyama Y, Harding D, Zhang JX and Clemens MG:
Functional significance of endothelin B receptors in mediating
sinusoidal and extrasinusoidal effects of endothelins in the intact
rat liver. Hepatology. 31:937–947. 2000. View Article : Google Scholar
|
|
19
|
Bosy-Westphal A, Petersen S, Hinrichsen H,
Czech NJ and Müller M: Increased plasma homocysteine in liver
cirrhosis. Hepatol Res. 20:28–38. 2001. View Article : Google Scholar
|
|
20
|
Siebert N, Cantré D, Eipel C and Vollmar
B: H2S contributes to the hepatic arterial buffer
response and mediates vasorelaxation of the hepatic artery via
activation of K(ATP) channels. Am J Physiol Gastrointest Liver
Physiol. 295:G1266–G1273. 2008. View Article : Google Scholar
|
|
21
|
Dawe GS, Han SP, Bian JS and Moore PK:
Hydrogen sulphide in the hypothalamus causes an ATP-sensitive
K+ channel-dependent decrease in blood pressure in
freely moving rats. Neuroscience. 152:169–177. 2008. View Article : Google Scholar
|
|
22
|
Jeong EJ, Kim NH, Heo JD, Lee KY, Rho JR,
Kim YC and Sung SH: Antifibrotic compounds from Liriodendron
tulipifera attenuating HSC-T6 proliferation and TNF-α production in
RAW264.7 cells. Biol Pharm Bull. 38:228–234. 2015. View Article : Google Scholar
|
|
23
|
Liu YF, Chu YY, Zhang XZ, Zhang M, Xie FG,
Zhou M, Wen HH and Shu AH: TGFβ1 protects myocardium from apoptosis
and oxidative damage after ischemia reperfusion. Eur Rev Med
Pharmacol Sci. 21:1551–1558. 2017.
|
|
24
|
Wang YX, Liu ML, Zhang B, Fu EQ and Li ZC:
Fasudil alleviated hypoxia-induced pulmonary hypertension by
stabilizing the expression of angiotensin-(1–7) in rats. Eur Rev
Med Pharmacol Sci. 20:3304–3312. 2016.
|