Introduction
Osteoarthritis (OA) is a chronic whole-organ
disease, which is characterized by hyaline articular cartilage
degeneration, bone sclerosis and osteophyte formation. It also
involves the periarticular muscle, ligaments, synovium, the
neurosensory system and eventually the bone. It is increasingly
accepted that the subchondral bone has an important role in the
pathogenesis of OA, while the significance of changes inside the
subchondral bone remain controversial (1).
Emerging evidence supported that bone remodeling is
responsible for the progression of OA (2–4). Early
OA is associated with increased bone reabsorption, indicating that
increases in bone reabsorption are partly responsible for
initiating cartilage destruction under stressful conditions
(3,5). This pathological progression increases
the possibility that early application of a bone reabsorption
inhibitor may retard the progressive loss of articular
cartilage.
Bisphosphonates (BIS), including alendronate,
ibandronate and risedronate, are potent inhibitors of osteoclastic
bone reabsorption and have been used in the clinic for the
treatment of osteoporosis and for the treatment/prevention of
skeletal-associated events in multiple myeloma as well as breast
and prostate cancer patients (6–8). While
majority of studies demonstrated that BIS are effective in
inhibiting the progression of OA via increasing the periarticular
bone volume and bone mineral concentration (3,5), BIs
remain under debate, as other emerging studies suggested that BIS
aggravates cartilage degeneration in spite of enhancing subchondral
bone volume and thickness (9). For
decades, first- and second-generation BIS have been the focus of
major basic or clinical research. Zoledronic acid (ZOL), the
representative of the third generation of BIS, has a comparatively
higher efficacy and is commonly used in the treatment of
osteoporosis, particularly in post-menopausal women, but its
mechanism of action remains elusive (10). As a novel promising and potent BIS,
the effect and underlying mechanism of ZOL deserve to be
elucidated. The present study established an OA model in the knees
of rabbits (KOA model) through anterior cruciate ligament
transaction (ACLT). Intravenous injection of a high, medium or low
dose of ZOL was applied as an intervention. It was assessed
whether, by preventing increased subchondral bone reabsorption and
remodeling, ZOL treatment suppressed OA progression. It was assumed
that ZOL may intervene with cartilage degeneration in the early
stage of OA. The purpose of the present study was to determine the
chondroprotective effect of ZOL.
Materials and methods
Animals
A total of 32 New Zealand White rabbits (male; age,
5–7 months; weight, 2–3 kg) were provided by the Laboratory Animal
Center of the Academy of Guangdong Province (Guangzhou, China).
They were housed under constant laboratory conditions with free
access to food and distilled water and a natural light-dark cycle.
The present study was approved by the Laboratory Animal Ethics
Committee of Jinan University (Guangzhou, China; no. 2008A001).
Drugs and reagents
The following drugs and reagents were used in the
present study: Injection of ZOL (Novartis International AG, Basel,
Switzerland), injection of sumianxin II (Veterinary Institute of
Medical Science Military Academy, Changchun, China), pentobarbital
sodium (Shanghai Chemical Reagent Factory, Shanghai, China),
toluidine blue (Amresco, Inc., Framingham, MA, USA), eosin
hematoxylin, formaldehyde, xylene, anhydrous ethanol (all from
Guangzhou Chemical Reagent Factory, Guangzhou, China) and chloral
hydrate (Dalian Meilun factory, Dalian, China).
The dose of ZOL administered to the animals was
calculated by the conversion formula of surface area based on that
for humans. It was defined by the following equation: Dose for
rabbits (2.0 kg)=[dose for humans (60
kg)x0.07]2/3=(5,000×0.07)2/3=50 µg.
Therefore, a high dose of ZOL (ZH), a medium dose of ZOL (ZM) and a
low dose of ZOL (ZL) group were established, which were
administered ZOL at doses of 250, 50 and 10 µg/kg,
respectively.
Laboratory apparatus
Dual-energy X-ray absorptiometry (DXA) was performed
using the Lunar Prodigy instrument (GE Healthcare, Little Chalfont,
UK). Furthermore, a Signa1.5T HD Superconducting magnetic resonance
imaging (MRI) scanner (GE Healthcare) and an IX 71 inverted phase
contrast microscope (Olympus Co., Ltd., Tokyo, Japan) were
used.
Study design
After the rabbits had acclimatized to the new
environment for 2 weeks, the KOA model was established by ACLT. The
animals underwent ACLT on the left hind knee (ACLT knee) and a sham
operation on the right hind knee (sham knee), where the knee
underwent mostly similar operation steps except that the anterior
cruciate ligament was left untouched. The procedure of the surgery
was followed strictly as described previously (11). In addition, 3 days prior to and after
surgery, 400,000 units penicillin were intramuscularly injected
twice a day to prevent infection. The rabbits were allowed full
weight-bearing post-operatively and then randomized into 4 groups:
ZH group (group A; n=8), ZM group (group B; n=8), ZL group (group
C; n=8) and untreated group (group D; n=8), which received
intravenous injection of ZOL at 250, 50 10 or 0 µg/kg in saline,
respectively, via the ear marginal vein once post-surgery. DXA
scanning was performed at 0, 4 and 8 weeks after modeling. At the
8th week, MRI was performed and subsequently, all rabbits were
sacrificed by air embolism, during which the rabbits were
anesthetized by intraperitoneal injection of 10% chloral hydrate at
the dose of 400 mg/kg and 10 min later, 30 ml air was injected into
the ear margin. Cartilage samples were obtained to prepare sections
for hematoxylin and eosin (H&E) and toluidine blue staining.
Histological samples were statistically analyzed using the Mankin
scoring system (12) to evaluate the
pathological changes of the cartilage.
DXA scanning
DXA analysis was performed at 0, 4 and 8 weeks after
modeling. Measurements were taken in vivo with the rabbits
placed in the prone position under general anesthesia with 0.2
ml/kg Sumianxin II. DXA analysis was performed with analysis
software enCORE 10.50.086 (Lunar Prodigy Scientific Research; GE
Healthcare) for animals. The area was scanned between lower the
half of the thigh and the upper half of the tibia. Rectangular
regions (20×15 mm) at the center of the knee as well as the medial
and lateral condyle of the tibia were assessed to represent the
mean bone mineral density (BMD) of the whole knee joint (WKJ).
Another 4 square compartments (2.5×2.5 mm) were selected to assess
the subchondral bone density on the most protruding point of the
medial and lateral condyle of the femur and lateral condyle of the
tibia separately, which were defines as femoral medial condyle
(FMC), femoral lateral condyle (FLC), tibial medial condyle (TMC)
and tibial lateral condyle (TLC) for explicit recording. The
location of compartments was selected as illustrated in Fig. 1. The BMD at 0 week was considered as
the baseline parameter. As significant individual differences in
BMD were identified, changes in BMD following surgery in the ACLT
and sham joints were assessed by comparison with each joint's
baseline measurements (internal control) (4).
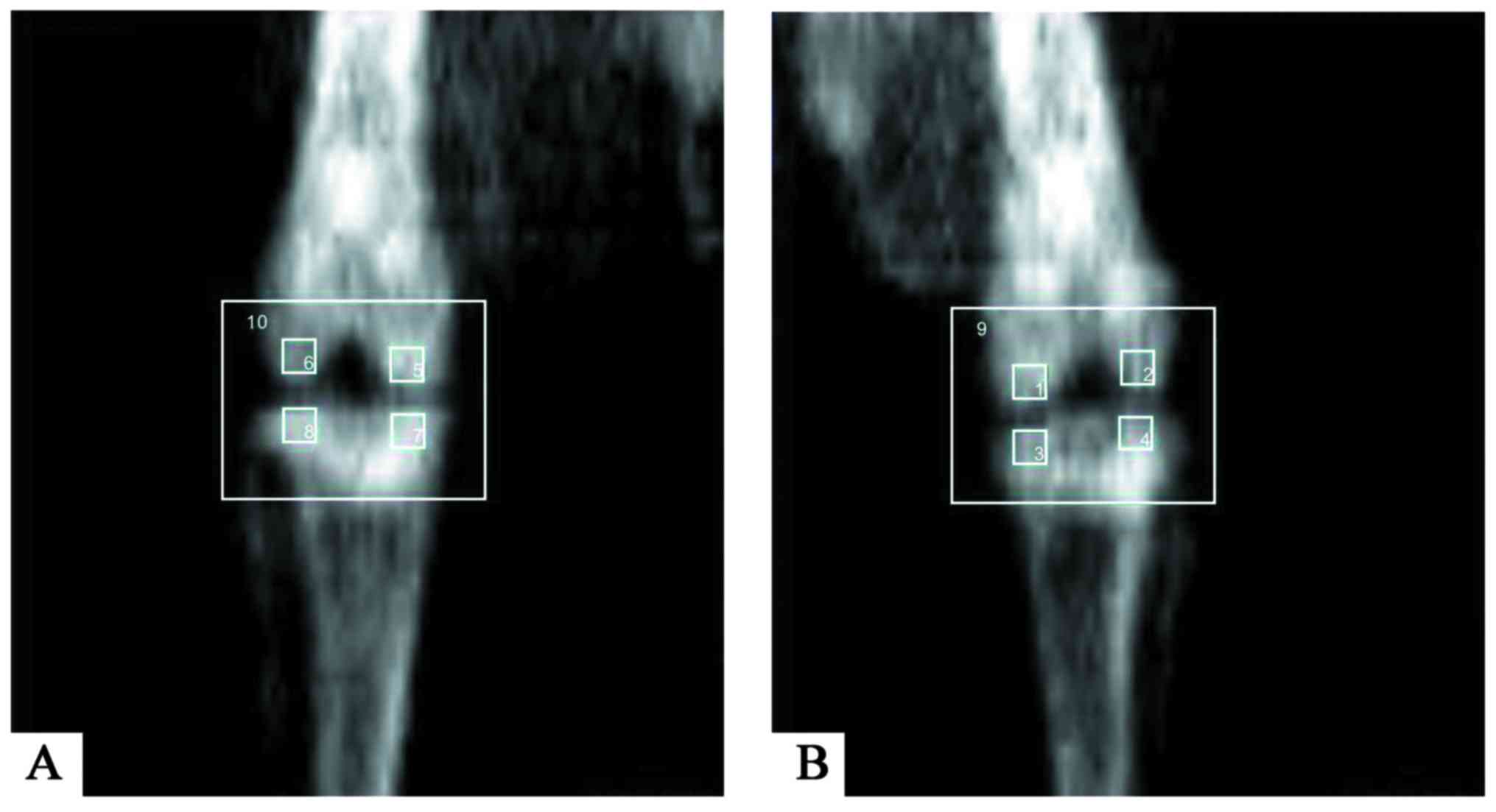 | Figure 1.DXA scanning in five compartments of
(A) the right knee and (B) the left knee. In total, the bone
mineral density of five compartments [femoral medial condyle (left
1, right 5), femoral lateral condyle (left 2, right 6), tibial
medial condyle (left 3, right 7), tibial lateral condyle (left 4,
right 8), and whole knee joint of the right (10) and left (9) control was evaluated by DXA]. DXA,
dual-energy X-ray absorptiometry. |
MRI scanning
At 8 weeks after modeling, rabbits were
anaesthetized with 3% pentobarbital sodium (30 mg/kg) administered
via the ear marginal vein and the two knees were examined
simultaneously. All examinations were standardized by using a
dedicated device allowing the rabbits to be placed in a supine
position with the leg placed in the scanner at slight flexion. The
knee underwent MRI scanning through the saggital position with
T2-weighted imaging, three-dimensional fat-suppressed spoil
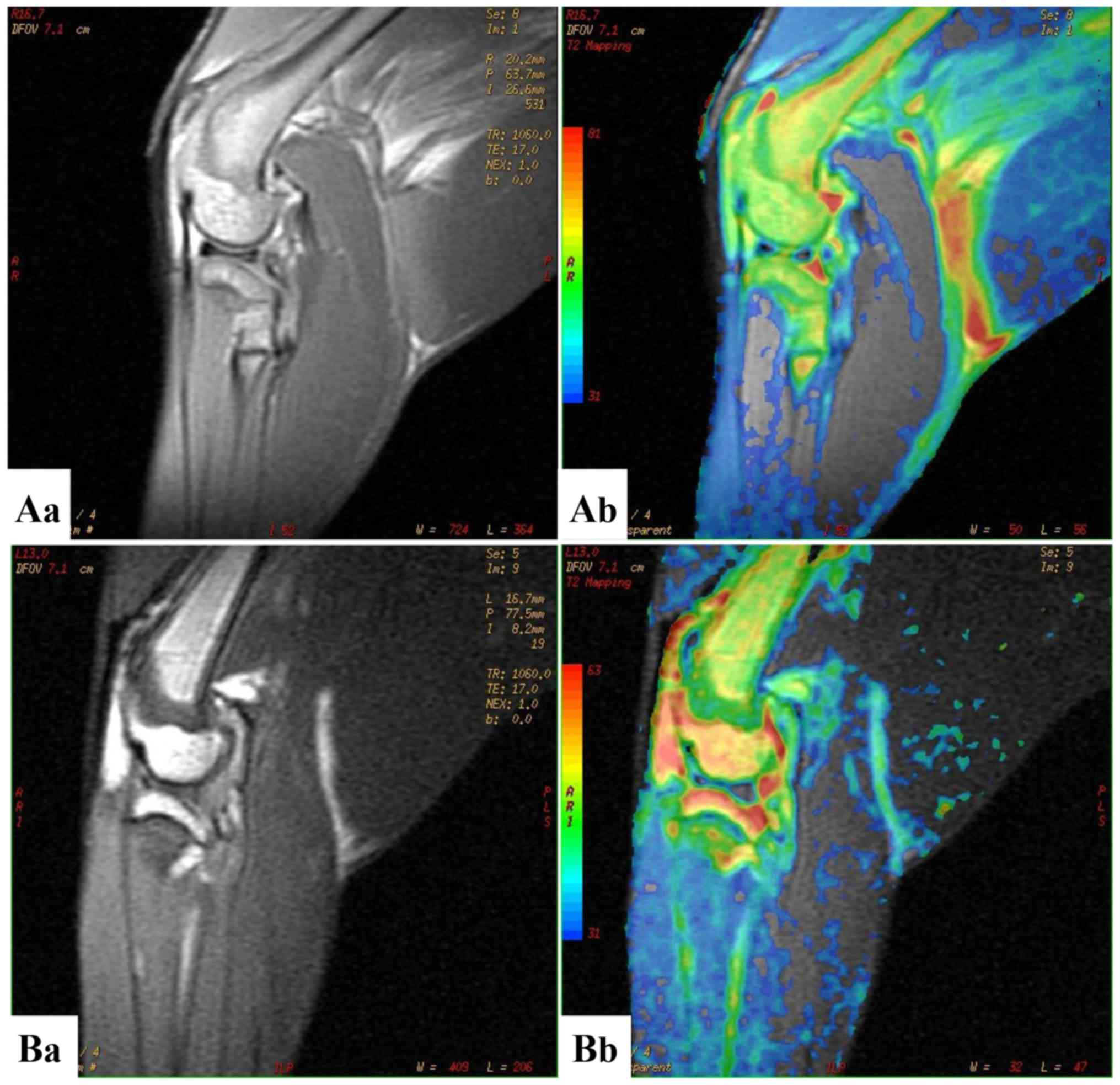
gradient-recalled sequence (3D-FS-SPGR) and T2 mapping (Fig. 2). The images were prospectively
analyzed by two independent musculoskeletal MRI radiologists
blinded to the grouping using the Sun Advantage Workstation 4.2
post-processing software (GE Healthcare). The radiologists had been
trained on the grading methods and were blinded to the macroscopic
analysis results. On the 3D-FS-SPDR, the cartilage thickness of 3
parts (anterior, middle and posterior) of the medial and lateral
femoral condyle was evaluated and the mean value was then used to
eradicate any discrepancies.
Tissue preparation
The rabbits were sacrificed by marginal venous air
embolism after anesthesia. Tissue samples were collected 2 cm
distal and proximal to the knee and fixed in 10% neutral buffered
formalin (4°C) for 24 h. After decalcification in 10% EDTA and
dehydration in ethanol/xylene, samples were embedded in paraffin.
Sections were cut using a microtome and stained using hematoxylin
for 5 min, eosin for 2 min (H&E) and toluidine blue for 10 min,
all at room temperature. The Mankin grading system was used to
characterize the pathological changes of the cartilage.
Statistical analysis
Values are expressed as the mean ± standard error.
One-way analysis of variance was performed for multi-group
comparison. The two-sample t test was utilized to analyze
differences between two groups. Levene's test and the
Student-Neuman-Keuls (SNK) test were utilized to analyze the Mankin
score. All statistical analyses were performed using SPSS 21.0
(International Business Machines, Corp., Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significantly
difference.
Results
General condition
All of the rabbits survived until the end of the
experiment. The wounds healed well without any complications.
Movements of the right knee were abnormal 2 weeks after the
modeling surgery, particularly in group D. Neither infection of the
knee joint nor patellar dislocation was revealed during tissue
sampling.
Imaging results
Bone mineral density of subchondral bone
Subchondral bone loss is directly reflected by the
BMD (4). The BMD in the FMC, FLC,
TMC, TLC and WJK compartments of the right and the left control
joints is illustrated in Fig. 1A and
B. The detailed BMD data are provided in Table I. The BMD was not significantly
different between the Sham and ALCT knees at week 0 after modeling
(P>0.05) in group D. The subchondral bone structure of the five
compartments in the ACLT knees exhibited dynamic alterations after
4 and 8 weeks of OA induction; specifically a progressive decrease
in BMD was observed (P<0.05). This indicated that the KOA
rabbits exhibited increasing bone reabsorption without
intervention. In comparison, the bone degeneration was
significantly suppressed in the ZH group (P<0.05). In addition,
the TMC compartments of ACLT knees and the Sham knees in the ZH
group had a higher BMD (P<0.05) at 4 and 8 weeks compared with
that at week 0, suggesting that a high dose of ZOL exerted a
protective effect and even fortified the bone. In the ZM group, the
BMD was stable and no significant changes were found between the
baseline (0 week) value and the value at 4 or 8 weeks (P>0.05),
which indicated the subchondral bone was preserved by a medium dose
of ZOL. However, in the ZL group, the FMC and TMC compartments of
ACLT knees exhibited a reduced BMD at 4 weeks (P<0.05 vs. week
0). After 8 weeks, all five compartments had a decreased BMD
(P<0.05 vs. week 0). All of the above implied that ZOL
efficiently inhibited bone reabsorption in a dose-dependent manner.
The doses of 250 and 50 µg/kg induced a favorable bone-preserving
effect.
 | Table I.Detailed data of bone mineral density
in each joint compartment of left and right knee
(mg/cm2). |
Table I.
Detailed data of bone mineral density
in each joint compartment of left and right knee
(mg/cm2).
|
| Left knee (Sham) | Right knee
(ACLT) |
|---|
|
|
|
|
|---|
| Group/knee
component | Week 0 | Week 4 | Week 8 | Week 0 | Week 4 | Week 8 |
|---|
| A, High-dose ZOL (250
µg/kg) |
|
| FMC |
336±13 |
345±11 |
350±16 |
346±12 |
354±15 |
340±14 |
| FLC |
352±17 |
357±13 |
364±12 |
338±14 |
343±17 |
329±23 |
| TMC |
340±10 |
343±08 |
358±14a |
329±23 |
351±28a |
344±17 |
| TLC |
363±21 |
352±25 |
366±31 |
351±20 |
360±13 |
355±18 |
| WKJ |
322±13 |
336±16 |
343±19a |
316±11 |
319±15 |
318±09 |
|
| B, Medium-dose ZOL
(50 µg/kg) |
|
| FMC |
373±26 |
360±21 |
368±18 |
361±22 |
370±26 |
357±19 |
| FLC |
359±18 |
367±16 |
370±27 |
367±25 |
358±19 |
361±20 |
| TMC |
348±13 |
355±17 |
362±21 |
349±18 |
356±22 |
360±16 |
| TLC |
356±20 |
370±33 |
361±15 |
370±30 |
382±17 |
368±23 |
| WKJ |
331±15 |
340±18 |
342±10 |
343±12 |
351±16 |
337±14 |
|
| C, Low-dose ZOL (10
µg/kg) |
|
| FMC |
350±16 |
347±13 |
343±17 |
362±13 |
340±16a |
332±15a |
| FLC |
381±18 |
395±22 |
377±25 |
403±30 |
388±12 |
376±18a |
| TMC |
359±11 |
366±17 |
345±14 |
371±22 |
353±20a |
335±30a |
| TLC |
367±23 |
358±14 |
353±30 |
380±18 |
367±15 |
348±22a |
| WKJ |
328±08 |
320±10 |
315±16 |
333±13 |
319±11 |
294±16a |
|
| D, Untreated
group |
|
| FMC |
349±15 |
360±19 |
353±14 |
358±13 |
335±18a |
314±21a |
| FLC |
383±27 |
377±16 |
390±23 |
391±33 |
369±26a |
355±24a |
| TMC |
364±18 |
359±12 |
355±17 |
374±25 |
356±23a |
339±19a |
| TLC |
358±10 |
364±09 |
368±13 |
360±11 |
341±19a |
325±16a |
| WKJ |
336±08 |
341±11 |
330±08 |
337±14 |
320±15a |
295±18a |
MRI scanning
MRI is able to accurately detect the progression of
cartilage lesions and subchondral bone edema over an eight-week
period (13). In the present study,
this quantitative MRI technique was used to measure the thickness
of cartilage. To reduce individual differences, cartilage thickness
of ACLT joints was compared with the relevant compartment of sham
joints in the same rabbit. As illustrated in Table II, in the untreated group without
any ZOL intervention, the thickness of the cartilage in the FMC of
the ACLT joint was identified to be significantly thinner than that
in the sham knee (P<0.01). In the ZH group, the FMC cartilage in
the ACLT joint was similar to that in the sham joint (P>0.05),
whereas the cartilage of the FMC compartment of the ACLT joint in
groups B and C was found to be thinner than that in the sham joint
(P<0.05). Furthermore, regarding the FLC compartment, the
cartilage in the ACLT joint in all ZOL-treated groups was no
different from that in the sham joint (P>0.05), indicating that
the high, medium and low dose of ZOL were effective to inhibit the
cartilage lesions in the FLC compartment. Overall, the results
implied that the high dose of ZOL exerted the highest
chondroprotective effect.
 | Table II.Thickness of cartilage measured by
magnetic resonance imaging (mm). |
Table II.
Thickness of cartilage measured by
magnetic resonance imaging (mm).
|
| Left knee
(Sham) | Right knee
(ACLT) |
|---|
|
|
|
|
|---|
| Group | FLC | FMC | FLC | FMC |
|---|
| A |
1.00±0.04 |
0.96±0.04 |
0.96±0.08 |
0.93±0.03 |
| B |
1.08±0.05 |
1.11±0.03 |
0.98±0.03 |
1.02±0.02a |
| C |
0.98±0.05 |
1.08±0.05 |
0.86±0.09 |
0.84±0.04a |
| D |
1.06±0.05 |
1.02±0.02 |
0.78±0.03b |
0.80±0.03b |
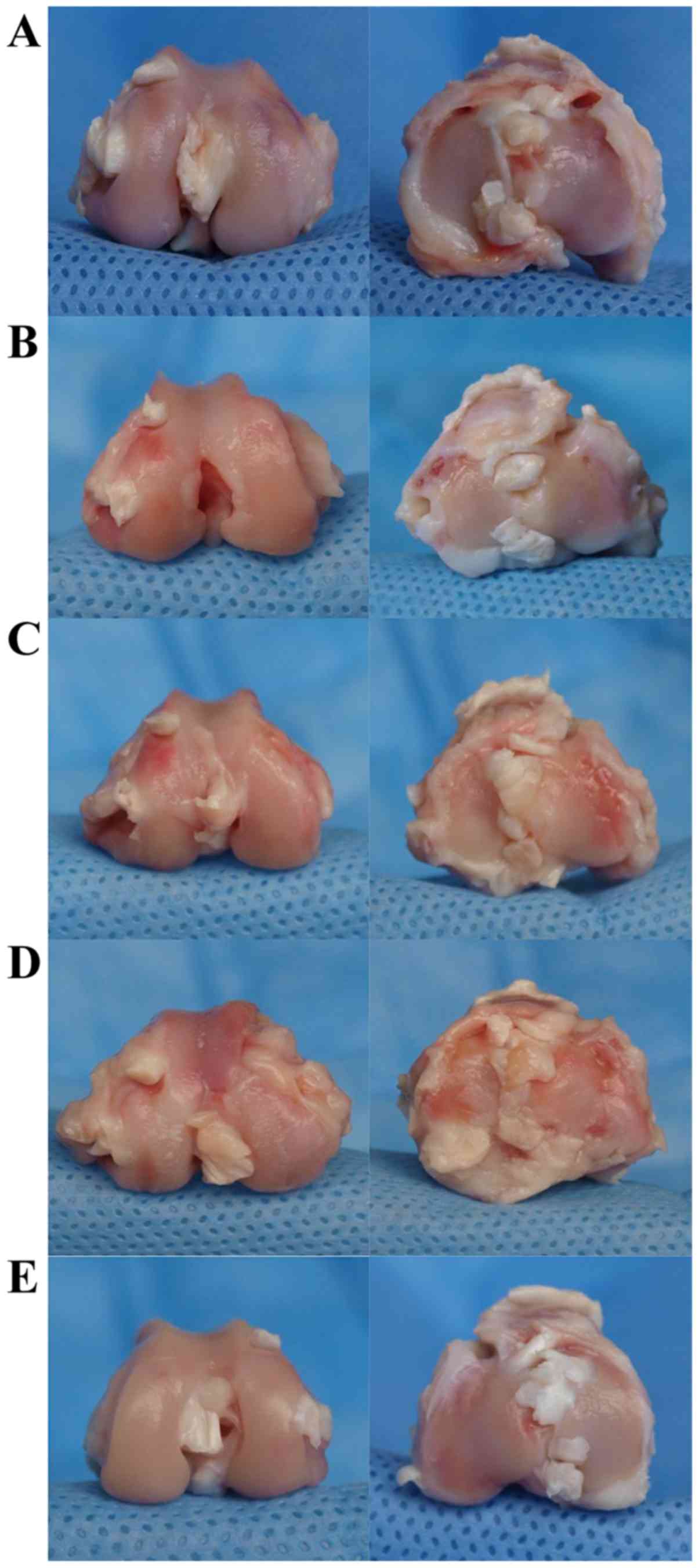
Macroscopic observation
The sham knees exhibited no detectable macroscopic
changes on the articular surface, which was smooth and intact with
no osteophyte formation (Fig. 3).
However, in the ACLT knees of the untreated group, the joint
surface collapsed and became obviously flattened. Extensive
cartilage erosion, and even strip and significant osteophyte
formation were detected as well. With ZOL intervention, an
improvement in the cartilage surface compared with that in the
untreated group was seen. The ACLT joints of the ZH group displayed
slight roughness and degeneration on the margin of the medial
condyle. In the ZM group, the cartilage partially stripped in the
medial and lateral condyle compartment. Hyperplastic fibrous
connective tissue was formed as well. In the ZL group, the surface
was somewhat collapsed with cartilage erosion, cartilage stripping,
exposure of subchondral bone and occasional small osteophytes.
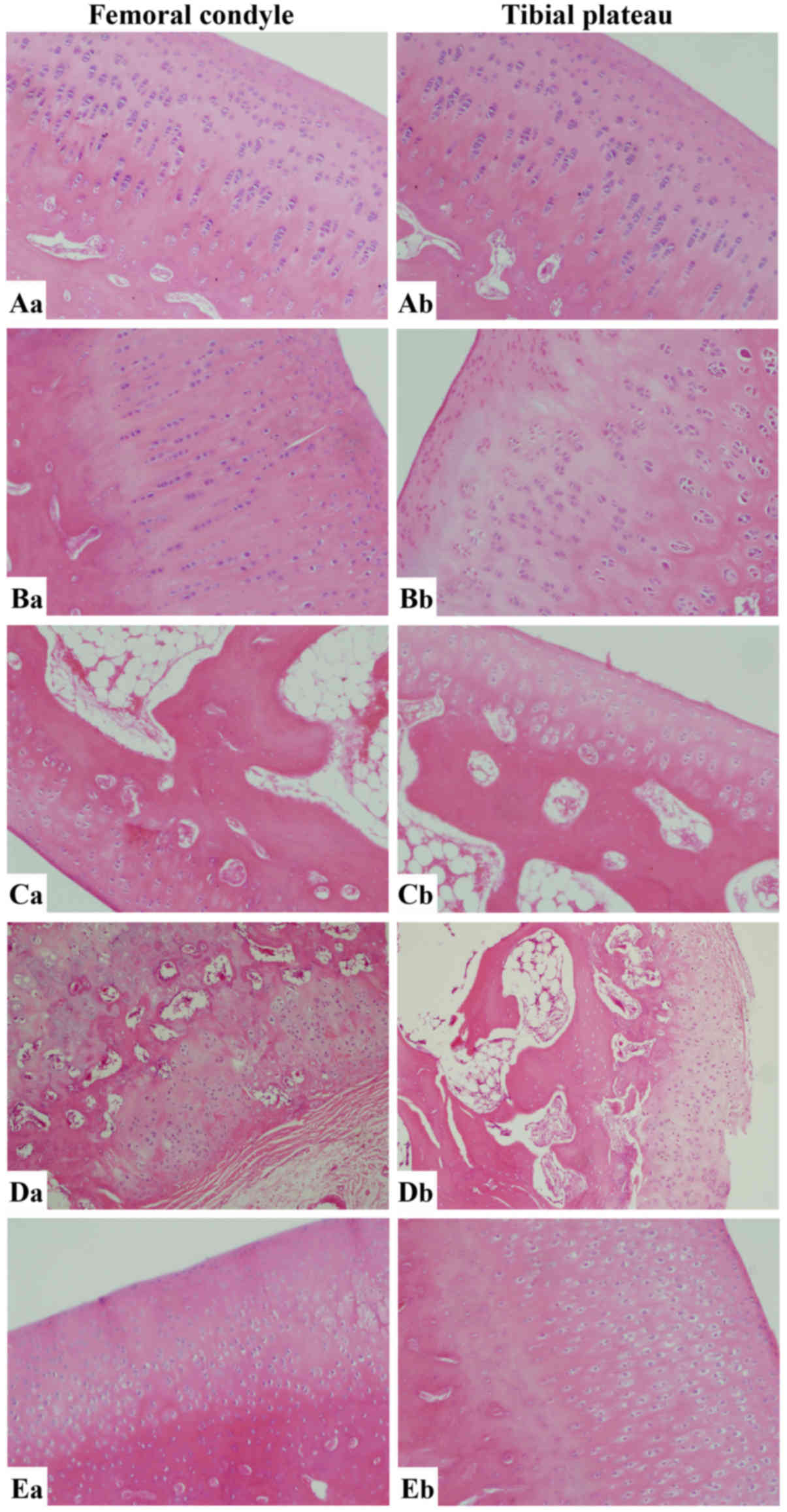
Histopathological analysis
Microscopic observation of cartilage cells with
hematoxylin and eosin staining
The cartilage cells of the ACLT knees in the ZH
group were uniformly distributed, and the tidemark was integral.
The matrix was dyed evenly without any loss of dye (Fig. 4). In the ZM group, the surface layer
was slightly uneven and cracks were identified in the transition
zone. Occasionally, confluent cells were seen. The matrix dye was
normal with uniform distribution. The degree of injury became
aggravated in the ACLT knees of the ZL group. Cartilage fibrosis,
cracks involving the interlayer, an irregular tidemark and
non-uniform staining of the matrix were observed. The number of
confluent cartilage cells was increased. In the untreated group,
the observations were similar to those in the ZL group, as the
cartilage displayed extension of the fibrosis range, loss of
cartilage cells, partial disappearance of the tidemark and unevenly
distributed matrix with severe loss of the dye.
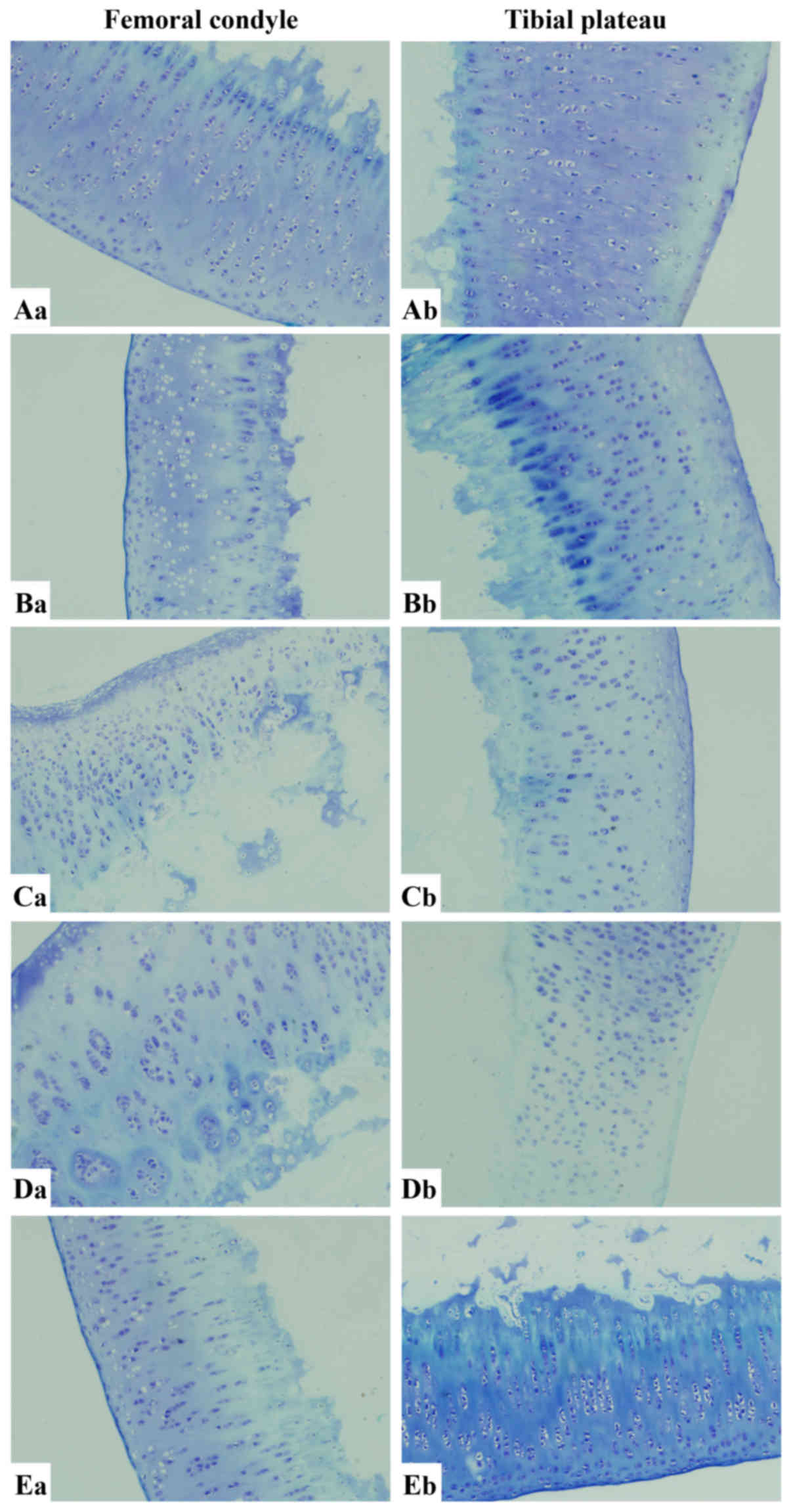
Microscopic observation of cartilage cells with
toluidine blue staining
As illustrated in Fig.
5, the femoral articular surface was smooth in ACLT knees of
the ZH group and the cartilage cells were regularly arranged
without any obvious loss of cells. The tibia had a flat surface
with a clear gradation. A slight disorder was observed in the
superficial layer of the tibial cartilage. A light loss of dye was
detected in the matrix. In ACLT knees of the ZM group, the
articular surface was less smooth compared with that in the ZM
group. The cartilage cells were irregularly arranged with numerous
vacuoles. The matrix was unevenly dyed. Vacuoles were sparse in the
superficial layer of the tibial cartilage. The matrix was slightly
hypochromic. In the ZL group, an irregular surface and stripped
cartilage in the femur were found. The cartilage cells were
unevenly dispersed with numerous vacuoles. The matrix exhibited
moderate loss of dye. The tibial cartilage had a similar
appearance. In ACLT knees of the untreated group, the arrangement
of chondrocytes was more irregular with obvious clusters. The
contour of the subchondral bone was destroyed. There was less
staining in the cartilage due to the lower chondrocyte numbers.
Mankin scoring results for cartilage
histopathology
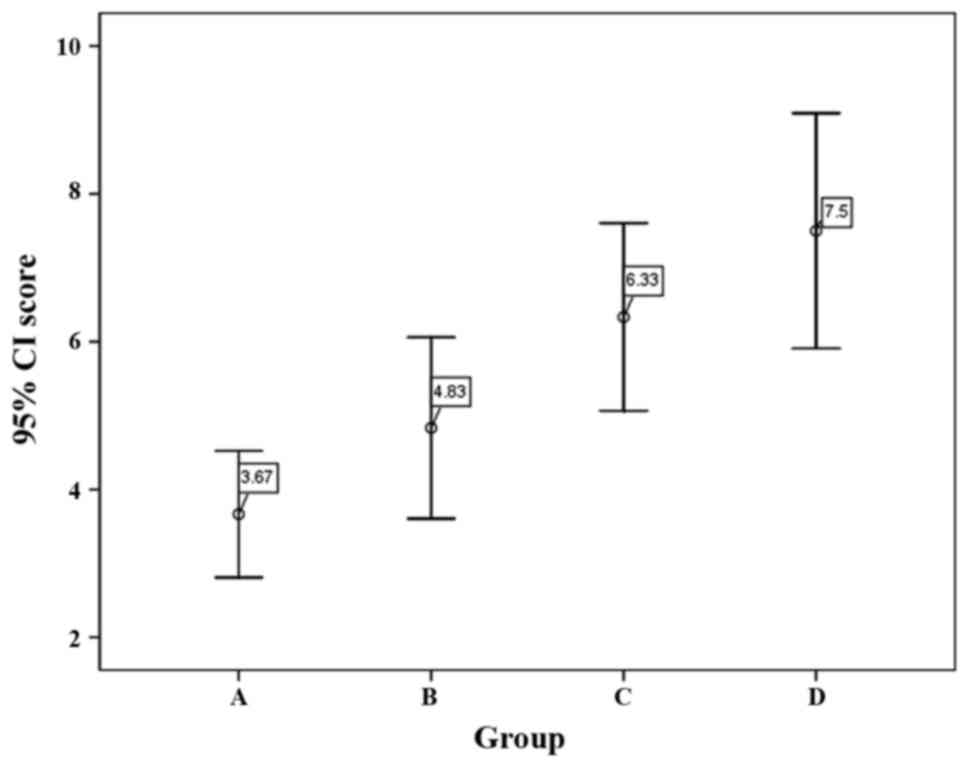
The mean Mankin score of groups A (ZH), B (ZM), C
(ZL) and D (untreated) were 3.67±0.82, 4.83±1.17, 6.33±1.21 and
7.50±1.52 (Fig. 6), respectively.
Based on Mankin scores, samples may be classified as 4 different
stages of OA: Nearly normal (0–2), early OA (2<, <6),
moderate OA (6<, <10) and late OA (10<, <14) (14–16).
According to this Groups A and B may be classified as early OA and
groups C and D may be classified as moderate OA. Significant
differences among the four groups were found by Levene's test
(F=11.686; P<0.001). Pairwise comparison by SNK test found that
the score of groups C and D were higher than those of groups A and
B (P<0.05).
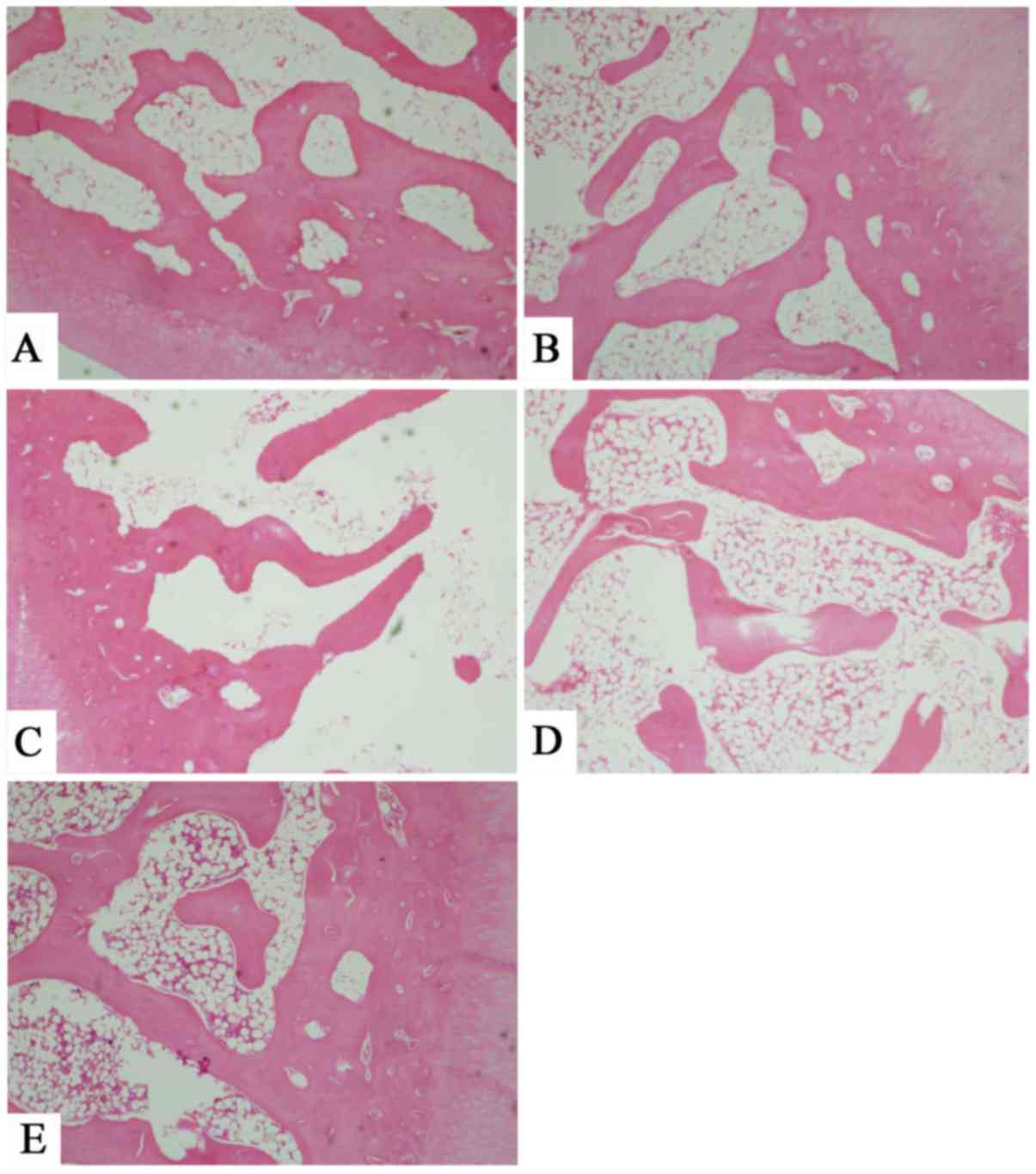
Microscopic observation of subchondral bone
The subchondral bone cells in ACLT knees of the ZH
group were regularly arranged. The bone trabecula was thick and
densely distributed. No cracks were observed and the matrix was
dyed evenly. In the medullary cavity, the number of blood cells
decreased and the size of fat cells was less homogenous as well
(Fig. 7). In the ZM group, the bone
trabecula was slightly thinned with intact structure. Tiny cracks
were detected locally and the dye was slightly unevenly
distributed. The medullar cavity was filled with hematopoietic
cells. The fat cells were homogenous in size and regularly
dispersed. The subchondral bone cells in the ZL group were
irregularly arranged. The trabeculae was thin with local breakdown.
The dye was moderately unevenly distributed. Sparse hemopoietic
cells and fat cells were present in the medullary cavity. In the
untreated group, the subchondral bone cells were disordered. The
severity of bone damage was aggravated with extensive breakdown of
the trabeculae. The matrix was dyed unevenly. In the medullary
cavity, the blood cells were hardly present and the shape of fat
cells was neither homogenous nor intact with different size and
uneven distribution.
Discussion
OA is a progressive rheumatic disease featuring the
degeneration of articular cartilage. It is the most common
rheumatic disorder and may become one of the most prevalent and
costly diseases (17). The bone
reduction in the OA model may be caused by post-operative synovitis
with an accompanying increase in blood flow (18), an increased turnover rate (19) or by disuse of the knee after ACLT
(9). In patients after ACL rupture,
the BMD in the injured knee was found to be lower than that in the
healthy contralateral knee (20).
Articular cartilage and synovial inflammation have been the primary
targets of pharmaceutical therapies for decades to attenuate joint
degeneration (21,22). However, the role of subchondral bone
has been largely ignored. An increasing number of studies supported
that the subchondral bone has an important role in the pathogenesis
of OA. Since ZOL is a potent inhibitor of osteoclastic bone
reabsorption, it may be a promising drug for ZOL reducing articular
cartilage damage in OA by preventing periarticular bone loss and
preserving subchondral architecture. In the present study, the ACLT
joint had more periarticular bone loss than the normal joint, while
ZOL treatment maintained bone density in the ACLT joint at a
similar level to that of the normal knee. The subchondral bone in
the ACLT joint was severely damaged and the bone trabeculae
featured extensive breakdown. However, in the ZH group, the normal
microstructure of the joint was preserved. These results supported
the hypothesis that ZOL is a potent and promising agent to inhibit
OA-associated bone reabsorption and remodeling. The use of MRI to
assess the cartilage thickness and DXA to invest the BMD
convincingly supported these conclusions.
Assessment of radiological joint space narrowing by
X-ray radiography is the ‘gold standard’ for assessing OA, while
there is currently no well-established imaging modality to
visualize and quantify changes in chondral and subchondral tissue
(13). MRI, with superior soft
tissue contrast, is the best technique available for the assessment
of normal articular cartilage and cartilage lesions (23). It makes non-invasive observation of
cartilage possible. The assessment of structural changes by MRI had
been previously investigated in a canine model of ACLT-induced OA
(24) and in a guinea pig model of
spontaneous OA (25). However, the
changes in chondral and subchondral structures in a rabbit model of
ACLT-induced OA have remained to be thoroughly investigated
(13). The present study used
3D-FS-SPGR and T2 mapping techniques. 3D-FS-SPGR mainly focused on
the morphology, particularly the detection of cartilage defects,
while T2 mapping was used to quantitatively assess the cartilage
(26,27). Regarding to the thickness of
cartilage tissue, the results for the FLC compartment were somewhat
inconsistent with those in the FMC compartment. In the FLC
compartment, the high, medium and low dose of ZOL were all
effective to inhibit the cartilage lesion. In the FMC compartment,
the cartilage was only preserved by the high dose of ZOL. The
cartilages in the ZM and ZL groups were found to be thinner than
those in the control group. The data of the FMC compartment were
taken into consideration, as cartilage lesions on MRI were more
severe and more commonly observed on the medial condyle (13).
The Mankin scoring system is a common
histopathological evaluation method to detect articular cartilage
injury. In this system, a higher the score indicates a higher the
level of OA (28,29). In the present study, according to
their Mankin scores, the sham knees were in the normal range, and
the ACLT knees in the ZH and ZM groups were categorized as early
OA. The articular surface in the ZL and the untreated group were in
the moderate stage of OA. This implied that reductions in the
Mankin score were present after ZOL intervention. The high and
medium dose of ZOL produced more favorable effects on the Mankin
scores compared with the low dosage.
The cartilage histopathology evaluated by the Mankin
score, cartilage thickness assessed by MRI and the macroscopic
changes of subchondral bone indicated that ZOL effectively
alleviated the OA-associated cartilage lesions and the dose of 250
µg/kg induced the most satisfactory intervention effect to protect
the cartilage. The data of the present study were sufficient to
confirm the hypothesis that ZOL intervened with cartilage
degeneration in OA.
Of note, the present study had certain shortcomings
and limitations. No blood was drawn from the rabbits due to
operating difficulties, and changes in blood parameters such as
bone calcium and bone turnover markers, which may have provided
information on the changes on a molecular level and would have been
helpful for evaluating the effect of ZOL more precisely, were not
determined. This remains to be assessed in future studies.
In conclusion, ZOL prevented the development of
abnormalities in subchondral bones to a large extent, and
suppressed bone reabsorption in early OA. Accordingly, cartilage
degeneration was reduced by ZOL. The cartilage histology,
subchondral bone morphology and MRI imaging all supported the
hypothesis that ZOL ameliorates OA-associated cartilage degradation
via intervening with subchondral bone loss. Thus, the present study
provided evidence supporting that ZOL presents an alternative
treatment option for OA patients and may serve as an innovative
therapeutic method to complement the treatment of OA.
References
|
1
|
Felson DT and Neogi T: Osteoarthritis: Is
it a disease of cartilage or of bone? Arthritis Rheum. 50:341–344.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hayami T, Pickarski M, Zhuo Y, Wesolowski
GA, Rodan GA and Duong LT: Characterization of articular cartilage
and subchondral bone changes in the rat anterior cruciate ligament
transection and meniscectomized models of osteoarthritis. Bone.
38:234–243. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hayami T, Pickarski M, Wesolowski GA,
McLane J, Bone A, Destefano J, Rodan GA and Duong LT: The role of
subchondral bone remodeling in osteoarthritis: Reduction of
cartilage degeneration and prevention of osteophyte formation by
alendronate in the rat anterior cruciate ligament transection
model. Arthritis Rheum. 50:1193–1206. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bouchgua M, Alexander K, Carmel EN,
d'Anjou MA, Beauchamp G, Richard H and Laverty S: Use of routine
clinical multimodality imaging in a rabbit model of
osteoarthritis-part II: Bone mineral density assessment.
Osteoarthritis Cartilage. 17:197–204. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shirai T, Kobayashi M, Nishitani K, Satake
T, Kuroki H, Nakagawa Y and Nakamura T: Chondroprotective effect of
alendronate in a rabbit model of osteoarthritis. J Orthop Res.
29:1572–1577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kimmel DB: Mechanism of action,
pharmacokinetic and pharmacodynamic profile, and clinical
applications of nitrogen-containing bisphosphonates. J Dent Res.
86:1022–1033. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bauer DC: Osteoporotic fractures:
Ignorance is bliss? Am J Med. 109:338–339. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Black DM, Thompson DE, Bauer DC, Ensrud K,
Musliner T, Hochberg MC, Nevitt MC, Suryawanshi S and Cummings SR:
Fracture Intervention Trial: Fracture risk reduction with
alendronate in women with osteoporosis: The fracture intervention
trial. FIT research group. J Clin Endocrinol Metab. 85:4118–4124.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ding M, Danielsen CC and Hvid I: The
effects of bone remodeling inhibition by alendronate on
three-dimensional microarchitecture of subchondral bone tissues in
guinea pig primary osteoarthrosis. Calcif Tissue Int. 82:77–86.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shuai B, Shen L, Yang Y, Ma C, Zhu R and
Xu X: Assessment of the impact of zoledronic acid on ovariectomized
osteoporosis model using micro-CT scanning. PLoS One.
10:e01321042015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoshioka M, Coutts RD, Amiel D and Hacker
SA: Characterization of a model of osteoarthritis in the rabbit
knee. Osteoarthritis Cartilage. 4:87–98. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mankin HJ, Dorfmon H, Lipiello L and
Zarins A: Biochemical and metabolic abnormalities in articular
cartilage from osteo-arthritic human hips. II. Correlation of
morphology with biochemical and metabolic data. J Bone Joint Surg
Am. 53:523–537. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jia L, Chen J, Wang Y, Liu Y, Zhang Y and
Chen W: Magnetic resonance imaging of osteophytic, chondral, and
subchondral structures in a surgically-induced osteoarthritis
rabbit model. PLoS One. 9:e1137072014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zuo H, Jiang L, Qu N, Wang J, Cui X and
Yao W: The biomarkers changes in serum and the correlation with
quantitative MRI markers by histopathologic evaluation of the
cartilage in surgically-induced osteoarthritis rabbit model. PLoS
One. 10:e01247172015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ehrlich MG, Houle PA, Vigliani G and
Mankin HJ: Correlation between articular cartilage collagenase
activity and osteoarthritis. Arthritis Rheum. 21:761–766. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murata M, Trahan C, Hirahashi J, Mankin HJ
and Towle CA: Intracellular interleukin-1 receptor antagonist in
osteoarthritis chondrocytes. Clin Orthop Relat Res. 285–295. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brooks PM and March LM: New insights into
osteoarthritis. Med J Aust. 163:367–369. 1995.PubMed/NCBI
|
|
18
|
Dedrick DK, Goldstein SA, Brandt KD,
O'Connor BL, Goulet RW and Albrecht M: A longitudinal study of
subchondral plate and trabecular bone in cruciate-deficient dogs
with osteoarthritis followed up for 54 months. Arthritis Rheum.
36:1460–1467. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Myers SL, Brandt KD, Burr DB, O'Connor BL
and Albrecht M: Effects of a bisphosphonate on bone
histomorphometry and dynamics in the canine cruciate deficiency
model of osteoarthritis. J Rheumatol. 26:2645–2653. 1999.PubMed/NCBI
|
|
20
|
van Meer BL, Waarsing JH, van Eijsden WA,
Meuffels DE, van Arkel ER, Verhaar JA, Bierma-Zeinstra SM and
Reijman M: Bone mineral density changes in the knee following
anterior cruciate ligament rupture. Osteoarthritis Cartilage.
22:154–161. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kapoor M, Martel-Pelletier J, Lajeunesse
D, Pelletier JP and Fahmi H: Role of proinflammatory cytokines in
the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 7:33–42.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Martel-Pelletier J, Boileau C, Pelletier
JP and Roughley PJ: Cartilage in normal and osteoarthritis
conditions. Best Pract Res Clin Rheumatol. 22:351–384. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bruyere O, Genant H, Kothari M, Zaim S,
White D, Peterfy C, Burlet N, Richy F, Ethgen D, Montague T, et al:
Longitudinal study of magnetic resonance imaging and standard
X-rays to assess disease progression in osteoarthritis.
Osteoarthritis Cartilage. 15:98–103. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boileau C, Martel-Pelletier J, Abram F,
Raynauld JP, Troncy E, D'Anjou MA, Moreau M and Pelletier JP:
Magnetic resonance imaging can accurately assess the long-term
progression of knee structural changes in experimental dog
osteoarthritis. Ann Rheum Dis. 67:926–932. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tessier JJ, Bowyer J, Brownrigg NJ, Peers
IS, Westwood FR, Waterton JC and Maciewicz RA: Characterisation of
the guinea pig model of osteoarthritis by in vivo three-dimensional
magnetic resonance imaging. Osteoarthritis Cartilage. 11:845–853.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Disler DG, McCauley TR, Kelman CG, Fuchs
MD, Ratner LM, Wirth CR and Hospodar PP: Fat-suppressed
three-dimensional spoiled gradient-echo MR imaging of hyaline
cartilage defects in the knee: Comparison with standard MR imaging
and arthroscopy. AJR Am J Roentgenol. 167:127–132. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee SY, Jee WH, Kim SK, Koh IJ and Kim JM:
Differentiation between grade 3 and grade 4 articular cartilage
defects of the knee: Fat-suppressed proton density-weighted versus
fat-suppressed three-dimensional gradient-echo MRI. Acta Radiol.
51:455–461. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rezende MU, Gurgel HM, Junior Vilaça PR,
Kuroba RK, Lopes AS, Phillipi RZ and Hernandez AJ: Diacerhein
versus glucosamine in a rat model of osteoarthritis. Clinics (Sao
Paulo). 61:461–466. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Armstrong S, Read R and Ghosh P: The
effects of intraarticular hyaluronan on cartilage and subchondral
bone changes in an ovine model of early osteoarthritis. J
Rheumatol. 21:680–688. 1994.PubMed/NCBI
|