Introduction
Pancreatic β-cells secrete insulin via
insulin-containing granules that translocate from the intracellular
insulin reservoir to the cell surface membrane, fuse with the
membrane and secrete insulin through exocytosis (1,2).
Cytosolic Ca2+ ions serve a role in the process of
insulin secretion and can be classified into the following two
types: Endogenous and exogenous Ca2+. Endogenous
Ca2+ is primarily derived from intracellular organelles,
such as the endoplasmic reticulum (ER), which stores and releases
Ca2+ into the cytoplasm, resulting in an increased
cytosolic Ca2+ concentration. By contrast, exogenous
Ca2+ increases intracellular Ca2+
concentration [(Ca2+)i] via an influx of
Ca2+ through ion channels located on the cell surface
membrane. Thus far, the Ca2+ channels known to be on the
cell surface membranes of β-cells include voltage-dependent
Ca2+ channels (VDCCs), including Cav1.2 and Cav1.3, the
Ca2+ release-activated channel (CRAC) and transient
receptor potential (TRP) family channels. A previous study revealed
that Cav1.3 is associated with the regulation of insulin secretion
in β-cells (3). The CRAC channel on
the cell surface membrane of β-cells is activated by the release of
Ca2+ from the ER; this channel replenishes the ER with
Ca2+, and mediates ER-regulated membrane potential and
insulin secretion (4). TRP family
channels are non-selective cation channels in general, however some
members are selective with with differential permeability to
Ca2+ (5). There are ~30
types of TRP channels that have been reported to be expressed on
β-cells, including TRP vanilloid, TRP ankyrin, TRP canonical and
TRP melastatin (TRPM) (6). Among
these channels, TRPM2, a Ca2+-permeable channel, was
demonstrated to be associated with glucose metabolism and insulin
secretion, yet little is known about the underlying mechanisms of
these associations (7).
In recent years, glucagon like peptide 1 (GLP-1) and
its analogues, also known as incretins, have attracted much
attention for their anti-diabetic properties. A previous study
demonstrated that incretins are secreted following a meal and
promote insulin secretion, thereby minimizing the fluctuation of
postprandial blood glucose concentrations (8). However, the ‘incretin effect’ in
patients with type 2 diabetes is compromised. This is reflected in
the attenuated increase in GLP-1 concentration following dining,
accompanied by relatively normal incretin secretion and
hypoglycemic function (9).
Therefore, GLP-1 and its analogues have been proposed as promising
therapeutic agents for treating type 2 diabetes (10,11). A
previous study demonstrated that the binding of GLP-1 with the
GLP-1 receptor (GLP-1R) causes little Ca2+ influx or
insulin secretion when the concentration of glucose is low
(12). By contrast, when the glucose
concentration is high, the ATP/ADP ratio is increased, thus
ATP-dependent K+ (KATP) channels close and
the opening of voltage-gated Ca2+ channels is prolonged
(13). Therefore, GLP-1 causes
extensive Ca2+ influx and insulin secretion in the
presence of a high concentration of glucose.
Upon the binding of GLP-1 with GLP-1R on the cell
surface membrane, adenylyl cyclase activates and generates cAMP.
Subsequently, cAMP activates protein kinase A (PKA), which in turn
activates cAMP-response element binding protein (CREB). CREB binds
to the cAMP response element on the insulin gene promoter. This
signaling pathway results in the transcriptional activation of the
insulin gene and promotes insulin biosynthesis (14,15).
Additionally, glucose induces an increase in the intracellular
ATP/ADP ratio, leading to K+ channel closures and
membrane depolarization. Thus, extracellular Ca2+ enters
into the cell through activated VDCCs and increases the
[Ca2+]i (16).
Evidence suggests that the activation of TRPM2 in β-cells also
depends upon ATP (17). Providing
that GLP-1 prolongs the opening of Ca2+ channels in an
ATP-dependent manner and that the opening of TRPM2 is also
ATP-dependent (12), the present
study hypothesized that GLP-1 stimulates insulin secretion through
activating TRPM2.
Although little is known about TRPM2, it has been
hypothesized that TRPM2 is a type of non-selective cation channel
that is permeable to Ca2+ (18–20).
Multiple molecules can activate TRPM2, including ADP-ribose and
hydrogen peroxide. In addition, the activity of TRPM2 is associated
with a number of factors, including [Ca2+]i,
pH, temperature and intracellular Cl− concentration
(18,21–26). A
previous study by our group demonstrated that TRPM2 is sensitive to
temperature and exhibits Ca2+ permeability (27). Another previous study reported that
TRPM2-knockout mice exhibited impaired insulin secretion and
suffered from hyperglycemia (28).
Additionally, TRPM2-deficient β-cells have been identified to
exhibit suppressed intracellular Ca2+ signaling, in
addition to a reduction in insulin secretion in response to glucose
or incretins, such as GLP-1 (28).
These findings support the theory that TRPM2 serves an important
role in insulin secretion in β-cells.
As the driver of growth and differentiation of
pancreatic β-cells, GLP-1 can activate adenylyl cyclase and promote
cAMP production (29,30). In β-cells, cAMP activates the classic
PKA signaling pathway and the exchange protein directly activated
by cAMP (Epac) signaling pathway, which triggers the downstream
signaling of mitogen activated protein kinase and results in the
rapid phosphorylation of extracellular signal-regulated kinase 1/2
and phosphatidylinositol 4,5-bisphosphate 3-kinase-mediated
phosphorylation of protein kinase B (31–34). In
addition, the PKA and Epac signaling pathways are associated with
Ca2+-dependent insulin granule exocytosis from β-cells
(35,36). Furthermore, by interacting with Rim2,
a target of the small G-protein Rab3, Epac mediates cAMP-dependent
and PKA-independent insulin exocytosis (36–38).
Therefore, the activation of PKA or Epac may cause TRPM2
activation, which modulates Ca2+ influx and insulin
secretion. The aim of the present study was to investigate the role
of TRPM2 in GLP-1-stimulated insulin secretion and explore the
possible underlying mechanisms.
Materials and methods
Reagents
GLP-1 was purchased from EMD Millipore (Billerica,
MA, USA). ADP-ribose, 2-APB and ESI-09 were purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). H89 was purchased
from Calbiochem® (EMD Millipore). The 8-pCPT and 6-Benz
cAMP were purchased from BIOLOG Life Science Institute Laboratory
and Biochemivcal Sales GmbH (Bremen, Germany).
Isolation and culture of primary
pancreatic islets
The present study, involving primary animal cells,
was approved by the Ethics Committee of Tianjin Metabolic Diseases
Hospital affiliated to Tianjin Medical University (Tianjin, China).
Rat pancreatic islets were isolated from healthy Sprague Dawley
rats. A total of 80 6–8 week old SD rats, weighing 180–220 g, were
purchased from Huafukang Bioscience Co., Inc. (Beijing, China). The
animals were adaptively housed for one week in the Aminal Center of
Tianjin Medical University under standard conditions (23±2°C; 65±5%
humidity; 12 h light/dark cycle) with access to food and water
ad libitum. Approximately 105 pancreatic β-cells
were isolated from each rat. Briefly, the rats were sacrificed and
the common bile duct was ligated near the hepatic portal.
Collagenase V (1 mg/ml) was injected from the common bile duct into
the pancreas to digest the pancreatic tissues. When the pancreas
had fully swollen, it was removed and incubated at 37°C for 25 min.
Following this, 40 ml cold Hanks' balanced salt solution was added
into the common bile duct to terminate the digestion. The
pancreatic tissues were dispersed by repeated pipetting, followed
by filtration through a 150-µm sieve and centrifugation at 300 × g
for 15 min at room temperature. The digested products were
resuspended in 25% Ficoll solution and separated by Ficoll gradient
centrifugation (23, 20 and 11%, from bottom to top) at 1,400 × g
for 20 min at 4°C. The pancreatic islets in the 23–20 and 10–11%
layers were collected and washed twice with 40 ml Hanks' buffer.
The round pancreatic islets with a regular shape were collected
under a light microscope. The islets were recovered and incubated
in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum
(Hyclone; GE Healthcare Life Sciences, Logan, UT, USA), 100 U/ml
penicillin and 100 µg/ml streptomycin for 3 h at 37°C in a
humidified atmosphere with 5% CO2. Following the
isolation, insulin release experiments, electrophysiological
measurements or viral infections were performed.
Silencing and overexpression of
TRPM2
Adenoviruses containing green fluorescent protein
(GFP) alone (pHBAd-MCMV-GFP), the TRPM2 gene
(pHBAd-MCMV-GFP-TRPM2), the negative control gene
(pHBAd-U6-GFP-scramble) or short hairpin RNA directed against TRPM2
(sense:
5′-AATTCGGACTAAGCTGGAGAAGTTCATTCAAGAGATGAACTTCTCCAGCTTAGTCCTTTTTTG-3′;
pHBAd-U6-GFP-TRPM2) were designed and prepared by Hanbio
Biotechnology Co., Ltd. (Shanghai, China). The isolated β-cells
were seeded in 24-well plates at a density of 30,000 cells per
well. Following adhesion to the plate, the cells were infected with
the viruses (50:1) and successful transduction was detected by the
presence of a GFP signal under a fluoresence microscope. Silencing
and overexpression of TRPM2 was also confirmed by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analyses 24 h post-infection, and non-infected cells
served as the control.
RT-qPCR
Total RNA was extracted from β-cells using a RNA
extraction kit (BioTeke Corporation, Beijing, China) according to
the manufacturer's protocol. The RNA was reverse transcribed to
cDNA by incubating RNA samples with super M-MLV reverse
transcriptase (BioTeke) and random primers at 42°C for 50 min. The
level of TRPM2 mRNA in each sample was measured by using
cDNA as the template and the following primers: TRPM2 forward,
5′-AAGTATGTCCGGGTCTCCC-3′ and reverse,
5′-TAACGGCCCAAATGAGAAGGTCACG-3′; β-actin forward,
5′-CTTAGTTGCGTTACACCCTTTCTTG-3′ and reverse,
5′-CTGTCACCTTCACCGTTCCAGTTT-3′. β-actin served as the internal
reference. qPCR was performed on an Exicycler 96 Quantitative
Thermal Block (Bioneer, Daejeon, Korea) using SYBR Green Master Mix
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China), and the amplification conditions were set as follows: 10
min at 95°C; 40 cycles of 10 sec at 95°C, 20 sec at 60°C and 30 sec
at 72°C; and finally 5 min at 4°C. The level of TRPM2 mRNA
was normalized to the non-infected (Control) cells using the
2−ΔΔCq method (39).
Western blot analysis
Proteins were extracted from β-cells using a protein
extraction kit (WanLeibio, Inc., Shenyang, China) according to the
manufacturer's protocol. The protein concentration was determined
with a BCA assay kit (WanLeibio, Inc.), and 40 µg proteins from
each sample were subjected to 7% SDS-PAGE. Subsequently, the
separated proteins were transferred onto a polyvinylidene
difluoride membrane (EMD Millipore, Billerica, MA, USA). Following
blocking with 5% skimmed milk for 1 hat room temperature, the
membrane was incubated with rabbit polyclonal anti-TRPM2 antibody
(1:400 dilution; cat. no. BA3459; Wuhan Boster Biological
Technology, Ltd., Wuhan, China) at 4°C overnight. Following washing
with TBS-Tween-20 (0.15% v/v), the membranes were incubated with
horseradish peroxidase-conjugated goat anti-rabbit IgG antibody
(1:5,000; cat. no. WLA023, WanLeibio, Inc.) for 45 min at 37°C.
Finally, the blots were developed with ECL reagent (WanLeibio,
Inc.), and the signals were exposed to films. Thereafter, the
membrane was stripped with stripping buffer (WanLeibio, Inc.) and
re-blotted with an anti-β-actin antibody (1:1,000; cat. no.
WL01845; WanLeibio, Inc.) to verify equal loading and transfer of
the proteins. The films were scanned, and the relative levels of
TRPM2 were calculated using Gel-Pro-Analyzer (Media Cybernetics,
Inc., Rockville, MD, USA) with β-actin as the internal
reference.
Insulin secretion assay
Selected pancreatic islets were transferred into
glass tubes (10 pancreatic islets/tube). A total of 500 µl of
modified Krebs-Ringer buffer (K-R buffer; 115 mM NaCl, 4.7 mM KCl,
2.5 mM CaCl2, 1.2 mM MgSO4. 7H2O,
1.2 mM KH2PO4, 25 mM NaHCO3 and 10
mM HEPES) containing 3.3 mM glucose, 1 mg/ml bovine serum albumin
(Sigma-Aldrich; Merck KGaA) was added into each tube, which was
then incubated at 37°C with 5% CO2 and continuous
agitation for 30 min. The islets either continued to be incubated
with the low-glucose K-R buffer (control), or were exposed to K-R
buffer containing one or more of the following reagents: 5.6 or
16.6 mM glucose, 30 mM KCl, 10 nM GLP-1, 100 µM ADP-ribose (TRPM2
activator), 10 µM 2-APB (TRPM2 inhibitor), 100 µM 6-Benz cAMP (PKA
activator), 10 µM H89 (PKA inhibitor), 10 µM 8-pCPT (Epac
activator), and 10 µM ESI-09 (Epac inhibitor). The islets were
incubated at 37°C, and the concentration of insulin in the
supernatant was measured at 0, 10 and 60 min post-exposure by ELISA
using a commercial kit (cat. no. 035-94, Phoenix Pharmaceuticals,
Inc., Burlingame, CA, USA). For the experiments involving PKA or
Epac activator/inhibitor, untreated cells were used as the
control.
Electrophysiological measurements
Following finding cells under an inverted light
microscope, a patch clamp amplifier (Axopatch-1D; Molecular
Devices, LLC, Sunnyvale, CA, USA) was used to measure the current
across the cell surface membrane. The bath solution was K-R buffer
(pH7.4, adjusted with NaOH), and the pipette solution contained 40
mM K2SO4, 50 mM KCl, 5 mM MgCl2,
0.5 mM EGTA and 10 mM HEPES, (pH 7.2, adjusted with KOH). The
pCLAMP™ software (version 10.2) and Digidata® 1440A
(both Molecular Devices, LLC) were adopted to give stimulatory
commands (voltage clamped at a holding potential of −70 mV) and
record data. The flow velocity of HEPES buffer (containing 2.2 mM
glucose) was 5 ml/min and the electrical resistance was ~3 MΩ. The
Ca2+ current and the changes in membrane capacitance
were recorded throughout continuous depolarization.
Statistical analysis
The data are expressed as the mean ± standard
deviation of repeated experiments. Comparisons of multiple groups
were performed using analysis of variance followed by Tukey's test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
TRPM2 channels are involved in
GSIS
To investigate the role of TRPM2 in insulin
secretion in pancreatic β-cells, primary β-cells were infected with
adenoviruses containing a TRPM2 expression construct or shTRPM2.
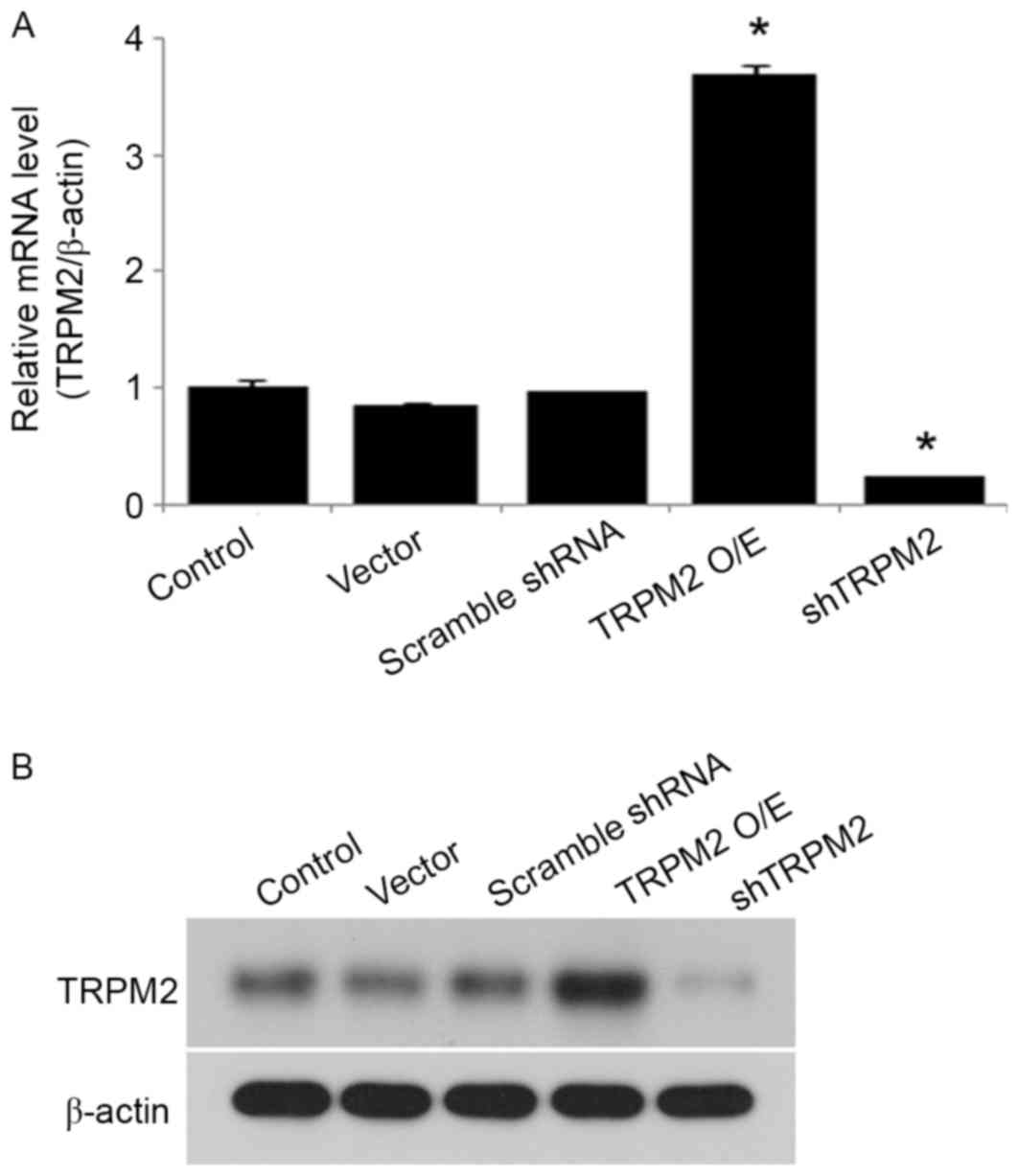
Overexpression or silencing of TRPM2 was achieved in the transduced
β-cells, and mRNA expression was significantly increased and
decreased compared with the control group in the overexpression and
shTRPM2 groups, respectively (Fig.
1A). A similar trend was observed in protein expression
(Fig. 1B).
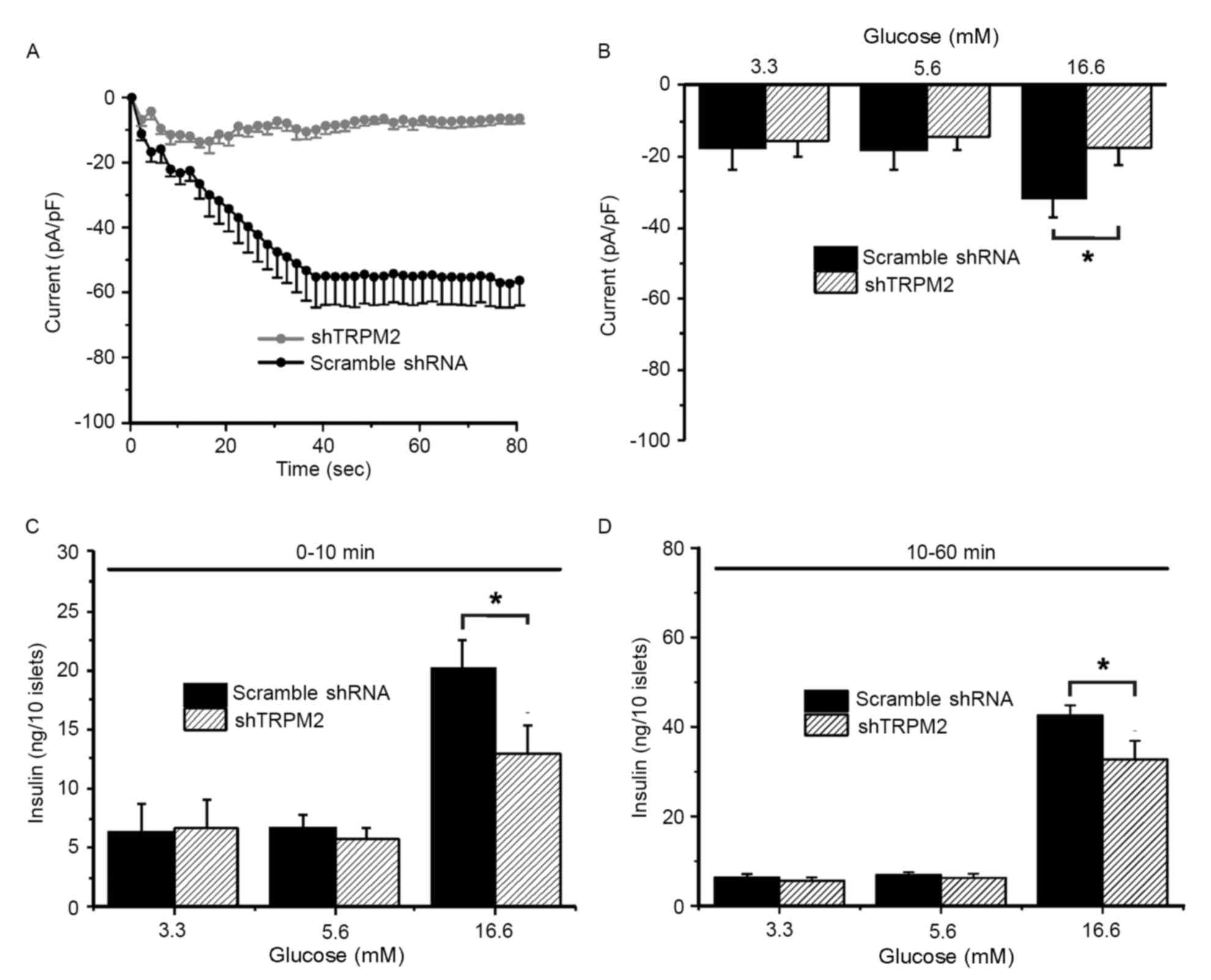
To examine whether TRPM2 is involved in GSIS in
primary β-cells, whole-cell patch clamp experiments and
measurements of insulin secretion were conducted. TRPM2 currents
were characterized in response to 100 µM ADP-ribose, a TRPM2
agonist, challenge in the cells transduced with scramble shRNA
(typical current: gradual decline to the lowest value of −60 mV
around 40 sec and remained at the lowest thereafter) or shTRPM2
(eliminated current) (Fig. 2A).
During the exposure to a low (3.3 mM), normal (5.6 mM) and high
(16.6 mM) concentration of glucose, the TRPM2 peak current (60 sec
following ADP-ribose exposure) of the scramble shRNA-transduced
cells increased significantly upon high-glucose stimulation
compared with the shTRPM2-transduced cells (Fig. 2B). To determine whether high
glucose-induced activation of TRPM2 channels is associated with
GSIS and whether the modulatory effect is time-dependent, insulin
secretion during the first 10 min (first phase) and the following
50 min (second phase) was assessed. Insulin secretion stimulated by
a high glucose concentration was significantly reduced in shTRPM2
cells compared with scramble shRNA cells in the first and second
phases (Fig. 2C and D,
respectively), suggesting that the TRPM2 channels are involved in
GSIS in β-cells.
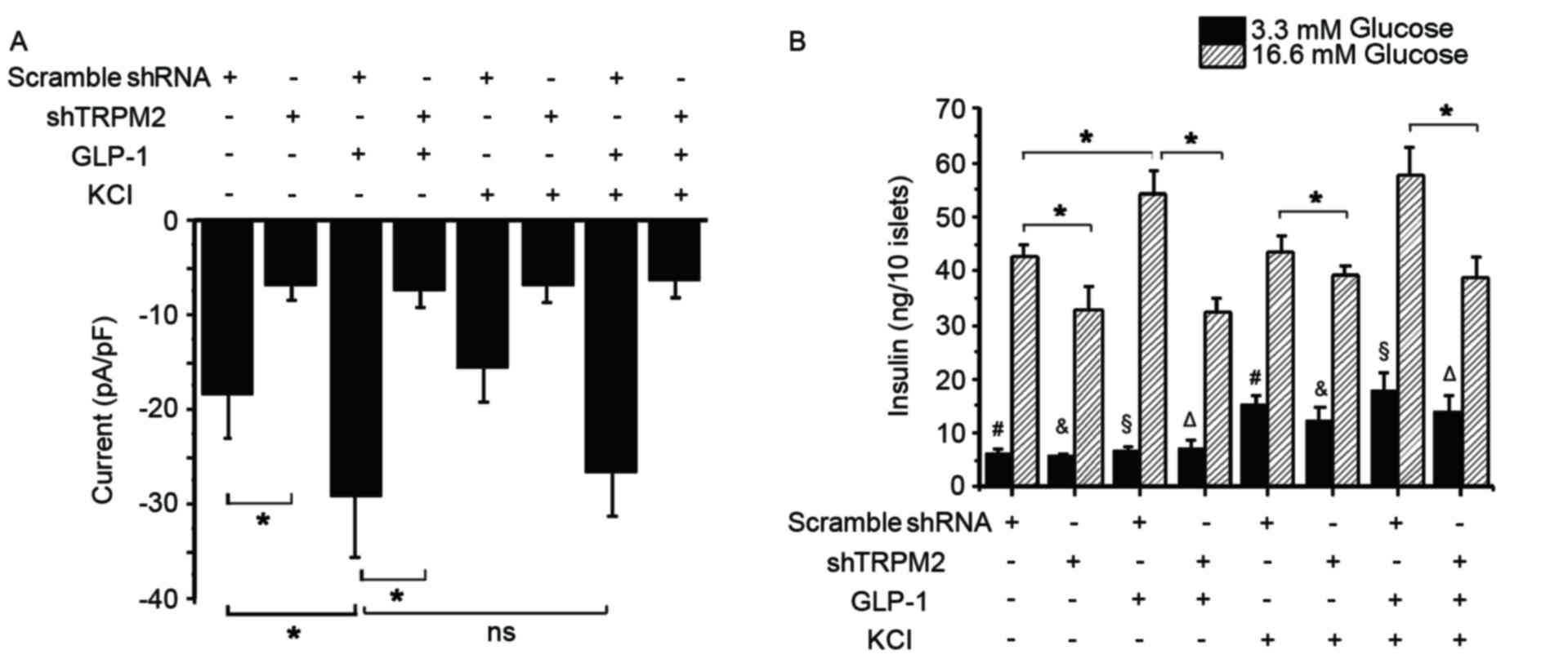
GLP-1 potentiates GSIS via TRPM2, but
TRPM2 activation is independent of K+-induced membrane
depolarization
Glucose is known to close KATP channels
and induce membrane depolarization, resulting in the opening of
VDCCs and subsequent Ca2+ influx, which ultimately
triggers the exocytosis of insulin-containing granules (40–42). To
investigate whether a high K+ concentration influences
the activation of TRPM2 during GSIS, the whole-cell TRPM2 current
and second phase insulin secretion in the control and
shTRPM2-transduced β-cells was examined. Exposure to 10 nM GLP-1
significantly elevated the peak current, whereas an additional 30
mM KCl did not produce a significant increase in the current
compared with the cells treated with GLP-1 alone (Fig. 3A). In addition, a high K+
concentration challenge alone failed to induce a significant change
in the current in the control cells (Fig. 3A). Silencing of TRPM2, however,
significantly reduced the basal and GLP-1-stimulated currents in
β-cells (Fig. 3A). These
electrophysiology results suggest that GLP-1 is an agonist of TRPM2
and that the activity of the TRPM2 channel is independent of
K+. In a low glucose concentration, KCl significantly
enhanced insulin secretion regardless of the presence or absence of
TRPM2 or GLP-1 (Fig. 3B). In a high
glucose concentration condition, GLP-1 potentiated insulin
secretion significantly, while shTRPM2 attenuated GSIS and
GLP-1-potentiated GSIS (Fig. 3B).
Additionally, cotreatment with GLP-1 and KCl did not significantly
elevate GSIS compared with GLP-1 treatment alone (Fig. 3B). These observations indicate that
TRPM2 is required for GSIS and GLP-1-potentiated GSIS, whereas a
high K+ concentration stimulates insulin release under a
low glucose concentration condition through another signaling
pathway.
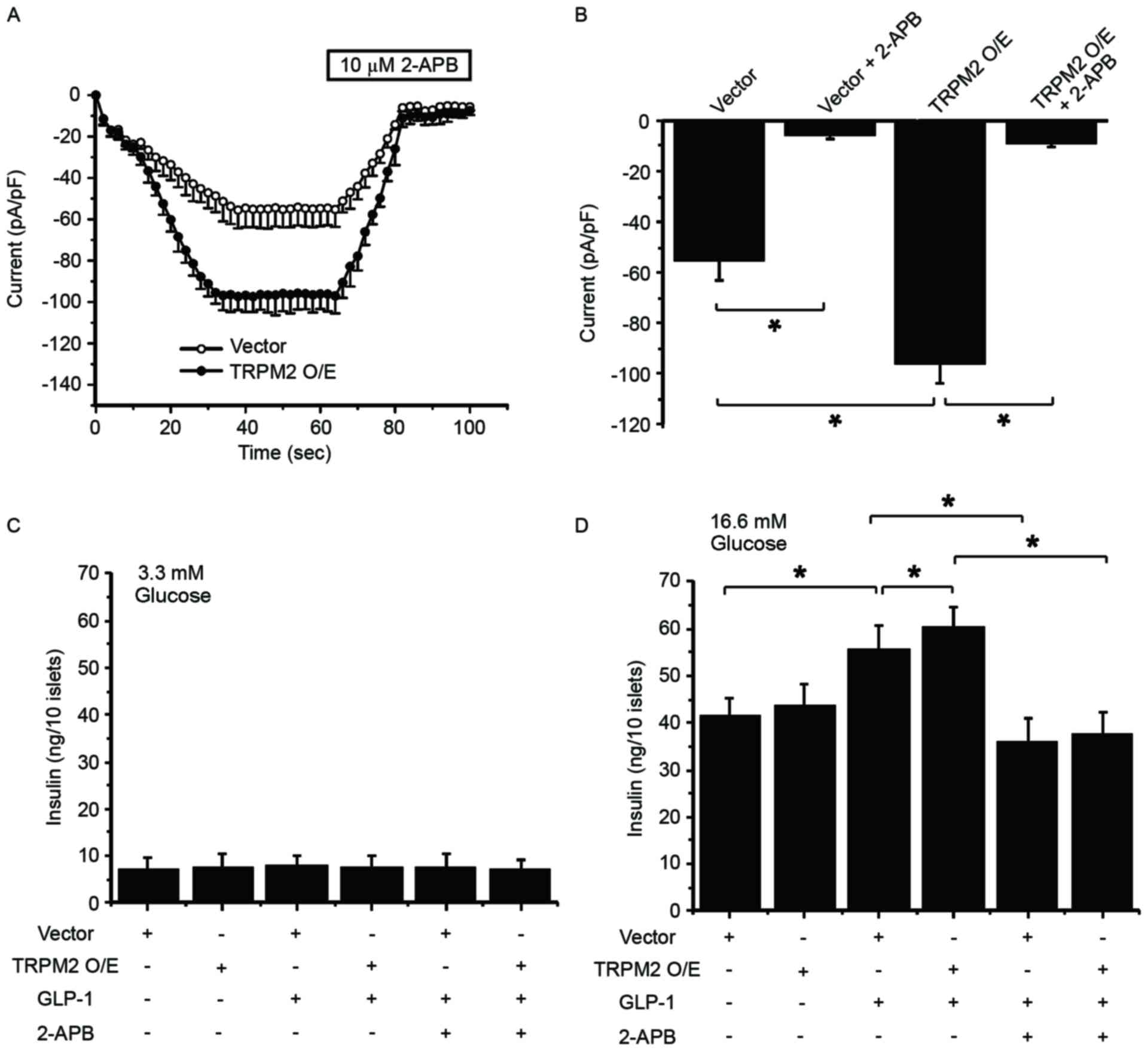
TRPM2 mediates GLP-1-potentiated
GSIS
To validate the role of TRPM2 in GLP-1-potentiated
GSIS, TRPM2 was overexpressed in β-cells, followed by whole-cell
patch clamp experiment and measurement of insulin secretion at 60
and 90 sec. In the presence of 100 µM ADP-ribose, TRPM2
overexpression enhanced the ADP-ribose-induced current in the cells
(Fig. 4A). Whereas 2-APB, a TRP
channel inhibitor added at 60 sec, significantly inhibited the
current in pHBAd-MCMV-GFP vector-transduced and
TRPM2-overexpressing cells (Fig.
4B). These patch clamp experiment results indicate that
exogenous TRPM2 is functional in β-cells. Next, the effect of TRPM2
overexpression on GSIS in the presence or absence of GLP-1
stimulation was examined. At a low glucose concentration, neither
GLP-1 treatment nor TRPM2 overexpression potentiated insulin
secretion (Fig. 4C). In addition,
2-APB exhibited no effect on insulin release under low glucose
conditions (Fig. 4C). At a high
glucose concentration, GLP-1 significantly increased insulin
release and TRPM2 overexpression significantly stimulated
GLP-1-potentiated GSIS (Fig. 4D). By
contrast, 2-APB significantly eliminated GLP-1-potentiated GSIS in
the control and TRPM2-overexpessing cells (Fig. 4D). These results highlight the
important role of TRPM2 in GLP-1-potentiated GSIS.
Epac serves a role in
GLP-1-potentiated GSIS, but GLP-1-potentiated GSIS does not act
through PKA
To explore the underlying mechanisms of TRPM2 in
GSIS, the roles of PKA and Epac in GLP-1-potentiated GSIS were
examined. To achieve this a PKA activator (6-Benz cAMP), a PKA
inhibitor (H89), an Epac activator (8-pCPT) and an Epac inhibitor
(ESI-09) were used. A GLP-1-induced current was not inhibited by
H89 in the presence of 5.6 mM glucose, and 6-Benz cAMP failed to
increase the current (Fig. 5A).
These results suggest that PKA is not be involved in the activation
of TRPM2. By contrast, 8-pCPT significantly increased the current
compared with the control, but there was no more increase following
GLP-1 stimulation (Fig. 5B).
Additionally, the GLP-1-stimulated current was significantly
inhibited by ESI-09 and GLP-1 compared with treatment with GLP-1
alone (Fig. 5B). These data suggest
that Epac serves an important role in the GLP-1-induced activation
of TRPM2.
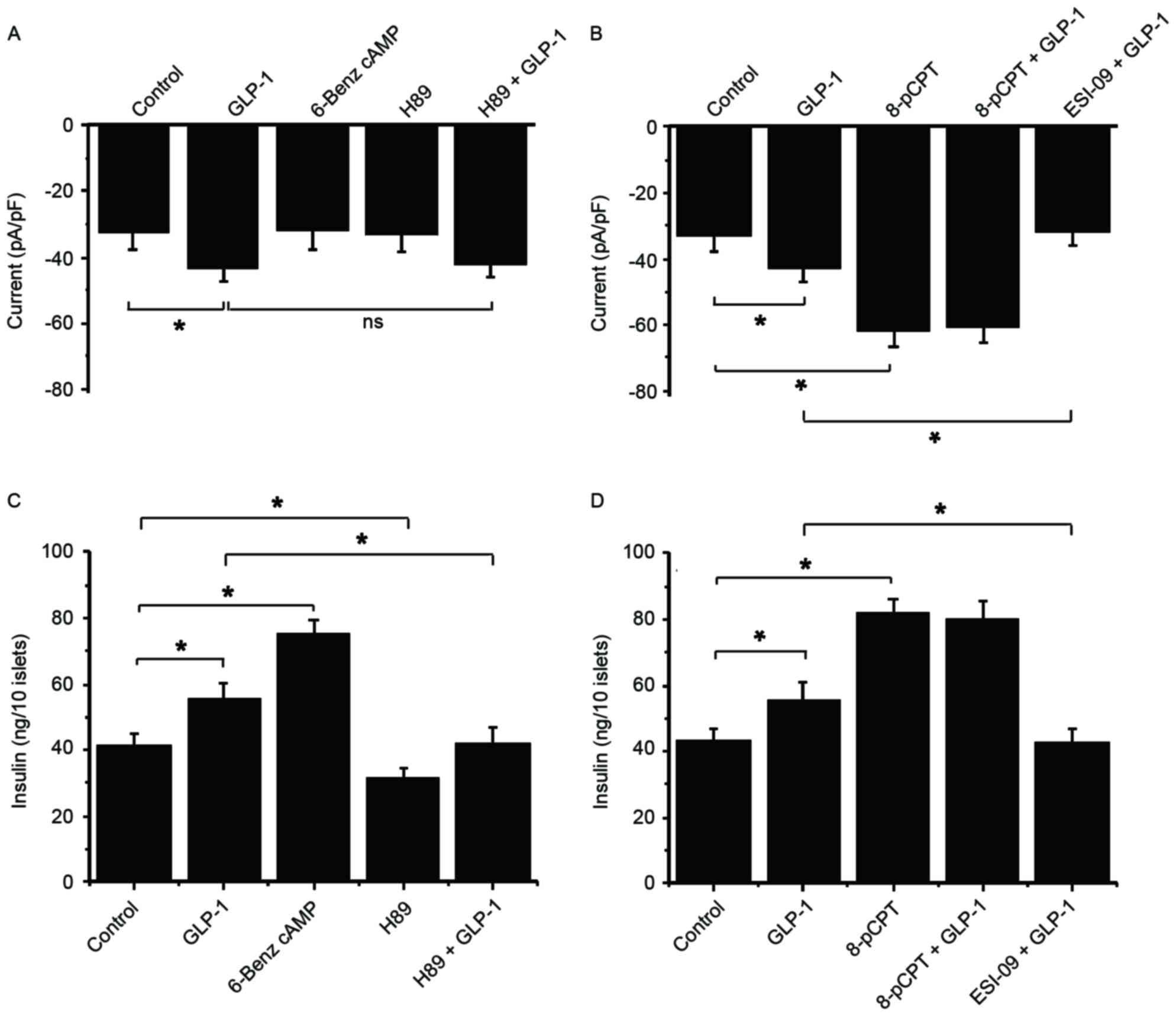 | Figure 5.Epac serves a role in
GLP-1-potentiated GSIS, but GLP-1-potentiated GSIS does not act
through PKA. The following drugs were used to examine the functions
of PKA and Epac in GSIS and GLP-1-potentiated GSIS in β-cells:
6-Benz cAMP (PKA activator), H89 (PKA inhibitor), 8-pCPT (Epac
activator) and ESI-09 (Epac inhibitor). Whole-cell currents in
response to 5.6 mM glucose and (A) GLP-1, 6-Benz cAMP and H89, and
(B) GLP-1, 8-pCPT and ESI-09 were recorded at 60 sec following
glucose administration. An insulin secretion experiment was
performed at a high glucose concentration (16.6 mM) in order to
observe the second phase of insulin secretion in response to (C)
GLP-1, 6-Benz cAMP and H89, and (D) GLP-1, 8-pCPT and ESI-09. n=9.
*P<0.05. ns, not significant; GLP-1, glucagon-like protein-1;
Epac, exchange protein directly activated by cAMP; GSIS,
glucose-stimulated insulin secretion; PKA, protein kinase A; TRPM2,
transient receptor potential melastatin 2. |
In a high glucose concentration (16.6 mM) condition,
6-Benz cAMP exhibited a stronger efficacy compared with GLP-1 on
potentiating insulin secretion, while H89 markedly reduced GSIS and
GLP-1-potentiated GSIS (Fig. 5C).
This result indicates that GLP-1 and PKA stimulate insulin
secretion via different signaling pathways. Conversely, 8-pCPT
stimulation significantly increased insulin secretion compared with
the control, which was not enhanced by additional GLP-1 (Fig. 5D). Additionally, ESI-09 significantly
inhibited GLP-1-induced insulin secretion compared with
GLP-1-potentiated insulin secretion under high glucose conditions
(Fig. 5D). These data are consistent
with current knowledge and suggest that GLP-1 potentiates GSIS via
the Epac rather than PKA signaling pathway.
Discussion
The present study demonstrated that TRPM2 channel
opening and KATP channel closure promoted GSIS in
pancreatic β-cells. Typically, the closure of the KATP
channels results in membrane depolarization, which initiates the
activation of VDCCs. Ca2+ influx through VDCCs leads to
an increase in [Ca2+]i and
Ca2+-induced Ca2+ release (CICR) from the ER.
This elevated [Ca2+]i is an effective
stimulus for insulin secretion. Therefore, insulin release in
response to a high K+ concentration challenge exhibits
independency from glucose metabolism. By contrast, in the presence
of glucose, glucose metabolism causes an increase in the cytosolic
ATP/ADP ratio, which induces the closure of KATP
channels, resulting in membrane depolarization and periodic influx
of Ca2+. In such circumstances, high K+
treatment cannot further enhance insulin secretion.
In response to an elevated ATP level upon glucose
induction, adenylyl cyclase produces the second messenger cAMP,
which serves a critical role in the activation of TRPM2. GLP-1R is
a ligand-specific G-protein-coupled receptor. When activated by
GLP-1, GLP-1R couples with a trimeric G-protein complex and
facilitates the release of the activated Gαs subunit
from the complex, and Gαs, in turn, activates
membrane-bound adenylyl cyclase to produce cAMP (43). During this process, ATP acts as the
substrate for the generation of cAMP. Thus, glucose is essential
for GLP-1/TRPM2-mediated insulin secretion. In addition to cAMP,
glucose stimulates the generation of cADP-ribose, another second
messenger important for Ca2+ mobilization from the ER
and subsequent insulin secretion from the pancreatic islets
(44). cADP-ribose activates TRPM2
and is involved in the GLP-1-mediated Ca2+ signal for
the regulation of insulin secretion (21,45).
Therefore, cAMP and cADP-ribose may be involved in GLP-1-regulated
GSIS via TRPM2.
The present study demonstrated that interfering with
the expression or activity of TRPM2 influenced GLP-1-stimulated
GSIS. These results provide support for the following hypothesis of
the present study: That GLP-1 potentiates GSIS via the TRPM2
channel. The two primary downstream effectors of GLP-1R are PKA and
Epac, and by using their agonists and antagonists the present study
revealed that GLP-1R activated the TRPM2 channel via the cAMP-Epac
signaling pathway rather than the PKA signaling pathway.
Nonetheless, the activation of the Epac or PKA signaling pathways
can result in the augmentation of GSIS, since their inhibitors were
demonstrated to suppress GSIS.
Taken together, these results indicate potential
mechanisms through which the Epac and PKA signaling pathways
regulate GSIS. The binding of GLP-1 with GLP-1R leads to the
phosphorylation of adenylyl cyclase and an elevated extracellular
glucose concentration, which increases ATP as a consequence of
intracellular glucose metabolism. The elevated ATP is converted to
cAMP by adenylyl cyclase, and cAMP then promotes gene expression
via PKA-mediated phosphorylation of CREB family activators for
insulin biosynthesis. Additionally, Epac-mediated activation of the
TRPM2 channel triggers Ca2+ influx, which serves an
important role in the exocytosis of insulin-containing granules
(46). In addition, the increase in
[Ca2+]i acts as a stimulus for CICR from the
ER through ryanodine receptors (RyRs). ER-released Ca2+
is an agonist of the inositol 1,4,5-triphosphate receptor (IP3R)
and stimulates additional Ca2+ release from
IP3R-regulated Ca2+ stores; RyR- and IP3R-mediated
Ca2+ release is facilitated by the PKA signaling pathway
(3,47). As a result, increases in insulin
biosynthesis and exocytosis cause enhanced insulin secretion, and
the PKA and Epac signaling pathways serve different roles in the
process of GSIS.
In conclusion, the results of the present study
demonstrated that the expression level and activity of TRPM2
affects GSIS. GLP-1 was demonstrated to enhance GSIS by activating
TRPM2 via the Epac signaling pathway, whereas KATP
closure-mediated activation of VDCCs facilitated insulin release in
a glucose-independent manner. Thus, the present study highlights a
novel area for the prevention and treatment of diabetes and its
complications.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81300664 and
81470187), the Natural Science Foundation of Tianjin (grant no.
14JCYBJC26200) and the Foundation of Tianjin Medical University
(grant no. 279). The authors thank all clinical and laboratory
staff in the Metabolic Diseases Hospital of Tianjin Medical
University and the Department of physiology at Seoul National
University College of Medicine for their dedication to this
study.
References
|
1
|
Rorsman P, Eliasson L, Renström E, Gromada
J, Barg S and Göpel S: The cell physiology of biphasic insulin
secretion. News Pysiol Sci. 15:72–77. 2000.
|
|
2
|
Efanov AM, Zaitsev SV and Berggren PO:
Inositol hexakisphosphate stimulates non-Ca2+-mediated and primes
Ca2+-mediated exocytosis of insulin by activation of protein kinase
C. Proc Natl Acad Sci USA. 94:4435–4439. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reinbothe TM, Alkayyali S, Ahlqvist E,
Tuomi T, Isomaa B, Lyssenko V and Renström E: The human L-type
calcium channel Cav1.3 regulates insulin release and polymorphisms
in CACNA1D associate with type 2 diabetes. Diabetologia.
56:340–349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dyachok O and Gylfe E: Store-operated
influx of Ca(2+) in pancreatic β-cells exhibits graded dependence
on the filling of the endoplasmic reticulum. J Cell Sci.
114:2179–2186. 2001.PubMed/NCBI
|
|
5
|
Owsianik G, Talavera K, Voets T and Nilius
B: Permeation and selectivity of TRP channels. Annu Rev Physiol.
68:685–717. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Colsoul B, Vennekens R and Nilius B:
Transient receptor potential cation channels pancreatic-β cells.
Rev Physiol Biochem Pharmacol. 161:87–110. 2011.PubMed/NCBI
|
|
7
|
Zhang Z, Zhang W, Jung DY, Ko HJ, Lee Y,
Friedline RH, Lee E, Jun J, Ma Z, Kim F, et al: TRPM2 Ca2+ channel
regulates energy balance and glucose metabolism. Am J Physiol
Endocrinol Metab. 302:E807–E816. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim W and Egan JM: The role of incretins
in glucose homeostasis and diabetes treatment. Pharmacol Rev.
60:470–512. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang XL, Ye F, Li J, Zhu LY, Feng G, Chang
XY and Sun K: Impaired secretion of glucagon-like peptide 1 during
oral glucose tolerance test in patients with newly diagnosed type 2
diabetes mellitus. Saudi Med J. 37:48–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Freeman JS: Improving glucagon-like
peptide-1 dynamics in patients with type 2 diabetes mellitus. J Am
Osteopath Assoc. 112 1 Suppl 1:S2–S6. 2012.PubMed/NCBI
|
|
11
|
Gavin JR III: Initiating a glucagon-like
peptide-1 receptor agonist in the management of type 2 diabetes
mellitus. J Am Osteopath Assoc. 112 1 Suppl 1:S16–S21.
2012.PubMed/NCBI
|
|
12
|
Meloni AR, DeYoung MB, Lowe C and Parkes
DG: GLP-1 receptor activated insulin secretion from pancreatic
β-cells: Mechanism and glucose dependence. Diabetes Obes Metab.
15:15–27. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bahlouli S, Mokaddem A, Hamdache F, Riane
H and Kamenche M: Fractal behavior of the pancreatic β-cell near
the percolation threshold: Effect of the KATP channel on the
electrical response. IEEE/ACM Trans Comput Biol Bioinform.
13:112–121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Toft-Nielsen MB, Madsbad S and Holst JJ:
Determinants of the effectiveness of glucagon-like peptide-1 in
type 2 diabetes. J Clin Endocrinol Metab. 86:3853–3860. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oetjen E, Diedrich T, Eggers A, Eckert B
and Knepel W: Distinct properties of the cAMP-responsive element of
the rat insulin I gene. J Biol Chem. 269:27036–27044.
1994.PubMed/NCBI
|
|
16
|
Henquin JC: Triggering and amplifying
pathways of regulation of insulin secretion by glucose. Diabetes.
49:1751–1760. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fonfria E, Marshall IC, Benham CD,
Boyfield I, Brown JD, Hill K, Hughes JP, Skaper SD and McNulty S:
TRPM2 channel opening in response to oxidative stress is dependent
on activation of poly(ADP-ribose) polymerase. Br J Pharmacol.
143:186–192. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamamoto S, Shimizu S, Kiyonaka S,
Takahashi N, Wajima T, Hara Y, Negoro T, Hiroi T, Kiuchi Y, Okada
T, et al: TRPM2-mediated Ca2+ influx induces chemokine production
in monocytes that aggravates inflammatory neutrophil infiltration.
Nat Med. 14:738–747. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hong CW, Kim TK, Ham HY, Nam JS, Kim YH,
Zheng H, Pang B, Min TK, Jung JS, Lee SN, et al:
Lysophosphatidylcholine increases neutrophil bactericidal activity
by enhancement of azurophil granule-phagosome fusion via glycine.
GlyR alpha 2/TRPM2/p38 MAPK signaling. J Immunol. 184:4401–4413.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wehrhahn J, Kraft R, Harteneck C and
Hauschildt S: Transient receptor potential melastatin 2 is required
for lipopolysaccharide-induced cytokine production in human
monocytes. J Immunol. 184:2386–2393. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Togashi K, Hara Y, Tominaga T, Higashi T,
Konishi Y, Mori Y and Tominaga M: TRPM2 activation by cyclic
ADP-ribose at body temperature is involved in insulin secretion.
EMBO J. 25:1804–1815. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Perraud AL, Fleig A, Dunn CA, Bagley LA,
Launay P, Schmitz C, Stokes AJ, Zhu Q, Bessman MJ, Penner R, et al:
ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed
by Nudix motif homology. Nature. 411:595–599. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Beck A, Kolisek M, Bagley LA, Fleig A and
Penner R: Nicotinic acid adenine dinucleotide phosphate and cyclic
ADP-ribose regulate TRPM2 channels in T lymphocytes. FASEB J.
20:962–964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Starkus JG, Fleig A and Penner R: The
Calcium-permeable non-selective cation channel TRPM2 is modulated
by cellular acidification. J Physiol. 588:1227–1240. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kühn FJ, Heiner I and Lückhoff A: TRPM2: A
calcium influx pathway regulated by oxidative stress and the novel
second messenger ADP-ribose. Pflugers Arch. 451:212–219. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Naziroğlu M: New molecular mechanisms on
the activation of TRPM2 channels by oxidative stress and
ADP-ribose. Neurochem Res. 32:1990–2001. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pang B, Shin DH, Park KS, Huh YJ, Woo J,
Zhang YH, Kang TM, Lee KY and Kim SJ: Differential pathways for
calcium influx activated by concanavalin A and CD3 stimulation in
Jurkat T cells. Pflugers Arch. 463:309–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Uchida K, Dezaki K, Damdindor B, Inada H,
Shiuchi T, Mori Y, Yada T, Minokoshi Y and Tominaga M: Lack of
TRPM2 impaired insulin secretion and glucose metabolisms in mice.
Diabetes. 60:119–126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Egan JM, Bulotta A, Hui H and Perfetti R:
GLP-1 receptor agonists are growth and differentiation factors for
pancreatic islet beta cells. Diabetes Metab Res Rev. 19:115–123.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yusta B, Baggio LL, Estall JL, Koehler JA,
Holland DP, Li H, Pipeleers D, Ling Z and Drucker DJ: GLP-1
receptor activation improves beta cell function and survival
following induction of endoplasmic reticulum stress. Cell Metab.
4:391–406. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gomez E, Pritchard C and Herbert TP:
cAMP-dependent protein kinase and Ca2+ influx through L-type
voltage-gated calcium channels mediate Raf-independent activation
of extracellular regulated kinase in response to glucogon-like
peptide-1 in pancreatic beta-cells. J Biol Chem. 277:48146–48151.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arnette D, Gibson TB, Lawrence MC, January
B, Khoo S, McGlynn K, Vanderbilt CA and Cobb MH: Regulation of ERK1
and ERK2 by glucose and peptide hormones in pancreatic beta cells.
J Biol Chem. 278:32517–32525. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Buteau J, Roduit R, Susini S and Prentki
M: Glucagon-like peptide-1 promotes DNA synthesis, activates
phosphatidylinositol 3-kinase and increase transcription factor
pancreatic and duodenal homeobox gene 1 (PDX-1) DNA binding
activity in beta (INS-1)-cells. Diabetologia. 42:856–864. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ozaki N, Shibasaki T, Kashima Y, Miki T,
Takahashi K, Ueno H, Sunaga Y, Yano H, Matsuura Y, Iwanaga T, et
al: cAMP-GEFII is a direct target of cAMP in regulated exocytosis.
Nat Cell Biol. 2:805–811. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Renstrom E, Eliasson L and Rorsman P:
Protein kinase A-dependent and -independent stimulation of
exocytosis by cAMP in mouse pancreatic β-cells. J Physiol.
502:105–118. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kang G, Joseph JW, Chepurny OG, Monaco M,
Wheeler MB, Bos JL, Schwede F, Genieser HG and Holz GG:
Epac-selective cAMP analog 8-pCPT-2′-O-Me-cAMP as a stimulus for
Ca2+-induced Ca2+ release and exocytosis in pancreatic beta-cells.
J Biol Chem. 278:8279–8285. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fujimoto K, Shibasaki T, Yokoi N, Nashima
Y, Matsumoto M, Sasaki T, Tajima N, Iwanaga T and Seino S: Piccolo,
a Ca2+ sensor in pancreatic beta-cells. Involvement of cAMP-GEFII.
Rim2. Piccolo complex in cAMP-dependent exocytosis. J Biol Chem.
277:50497–50502. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kashima Y, Miki T, Shibasaki T, Ozaki N,
Miyazaki M, Yano H and Seino S: Critical role of cAMP-GEFFII-Rim2
complex in incretin-protentiated insulin secretion. J Biol Chem.
276:46046–46053. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Overbergh L, Vig S, Coun F and Mathieu C:
Chapter 4: Quantitative polymerase chain reactionMolecular
Diagnostics. 3rd. Patrinos GP: Academic Press; pp. 41–58. 2017,
View Article : Google Scholar
|
|
40
|
Damdindorj B, Dezaki K, Kurashina T, Sone
H, Rita R, Kakei M and Yada T: Exogenous and endogenous ghrelin
counteracts GLP-1 action to stimulate cAMP signaling and insulin
secretion in islet β-cells. FEBS Lett. 586:2555–2562. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Holz GG, Heart E and Leech CA:
Synchronizing Ca2+ and cAMP oscillations in pancreatic beta-cells:
A role for glucose metabolism and GLP-1 receptors? Focus on
‘regulation of cAMP dynamics by Ca2+ and G protein-coupled
receptors in the pancreatic beta-cell: A computational approach’.
Am J Physiol Cell Physiol. 294:C4–C6. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shigeto M, Katsura M, Matsuda M, Ohkuma S
and Kaku K: Low, but physiological, concentration of GLP-1
stimulates insulin secretion independent of the cAMP-dependent
protein kinase pathway. J Pharmacol Sci. 108:274–279. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Doyle ME and Egan JM: Mechanisms of action
of glucagon-like peptide 1 in the pancreas. Pharmacol Ther.
113:546–593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Takasawa S, Nata K, Yonekura H and Okamoto
H: Cyclic ADP-ribose in insulin secretion from pancreatic beta
cells. Science. 259:370–373. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim BJ, Park KH, Yim CY, Takasawa S,
Okamoto H, Im MJ and Kim UH: Generation of nicotinic acid adenine
dinucleotide phosphate and cyclic ADP-ribose by glucagon-like
peptide-1 evokes Ca2+ signal that is essential for insulin
secretion in mouse pancreatic islets. Diabetes. 57:868–878. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fridlyand LE and Philipson LH: Coupling of
metabolic, second messenger pathways and insulin granule dynamics
in pancreatic beta-cells: A computational analysis. Prog Biophys
Mol Biol. 107:293–303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Park S, Dong X, Fisher TL, Dunn S, Omer
AK, Weir G and White MF: Exendin-4 uses Irs2 signaling to mediate
pancreatic beta cell growth and function. J Biol Chem.
281:1159–1168. 2006. View Article : Google Scholar : PubMed/NCBI
|