Introduction
Angiogenesis is a biological process defined as the
formation of new blood vessels from pre-existing ones (1). Angiogenesis is involved in various
physiological processes, including tumor metastasis, tissue repair,
wound healing and embryonic development (2,3).
Endothelial cells constitute the inner wall of blood vessels
(4) Upon pro-angiogenic stimulation,
endothelial cells are activated and secrete proteolytic enzymes to
degrade the vascular basement membrane (5). Subsequently, the endothelial cells
proliferate and migrate, thus contributing to the formation and
growth of blood vessels (6,7). Increasing evidence has demonstrated
that angiogenesis is associated with various diseases including
cancer, inflammatory disease, cardiovascular disease and diabetes
(8,9). Previous studies have demonstrated that
tumor angiogenesis is regulated by multiple cytokines, including
prostaglandin E2, transforming growth factor-β (TGF-β), fibroblast
growth factor and vascular endothelial growth factor (10,11).
Magnesium, one of the most important minerals, is
essential for physiological processes and cellular metabolism
(12). Magnesium ions stabilize the
structures of cell membranes, nucleic acids and proteins, and
enhance the activities of ribozymes and enzymes (13). Zinc is an essential trace element for
humans, as it is a constituent of various enzymes, regulating their
catalyzing activity (14). Zinc ions
are easily absorbed and do not harm vital organs (15). Zinc is also a cofactor for many
transcription factors, proteins and enzymes that are involved in
DNA repair, cell apoptosis, cell cycle regulation and oxidative
stress (16). It has been reported
that calcium and magnesium ions serve vital roles in the growth,
mineralization and angiogenesis of bone tissues (17).
It has been demonstrated that cell migration and
cytoskeletal reorganization in endothelial cells is closely
associated with angiogenesis (18).
The epithelial-mesenchymal transition (EMT) is a process during
which cells lose their epithelial features and acquire mesenchymal
properties, including reduced adhesive and enhanced invasion
abilities (19,20). During EMT, E-cadherin expression is
downregulated, while expression of N-cadherin, vimentin,
fibronectin and transcription factors are upregulated (21,22). EMT
is associated with multiple biological processes, including organ
fibrosis, tissue regeneration, wound repair and tumor progression
(23,24). Angiogenesis is a multi-step process
that includes proliferation, migration and tube formation of
endothelial cells (25). HUVECs have
been widely used to investigate the effect of drugs on angiogenesis
(26). However, the effects of
MgCl2 and ZnCl2 on the biological
characteristics of human umbilical vein endothelial cells (HUVECs)
are not fully understood. In the present study, human HUVECs were
cultured in vitro and the possible roles and molecular
mechanisms of MgCl2 and ZnCl2 in the
metastasis and EMT of HUVECs were investigated.
Materials and methods
Cell culture
HUVECs were obtained from the China Center for Type
Culture Collection (Wuhan, China) and cultured in Dulbecco's
modified Eagle medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at 37°C in an incubator (HF-90; Shanghai Lishen
Scientific Equipment Co., Ltd., Shanghai, China) containing 5%
CO2. The culture medium was supplemented with 10% fetal
bovine serum (FBS; HyClone; GE Healthcare Life Sciences, Logan, UT,
USA).
MTT assay
HUVECs were plated at a density of 5×103
cells/well onto 96-well plates and cultured for 24 h at 37°C.
Following this, cells were incubated at 37°C with various doses of
MgCl2 (1.25, 2.5, 5, 6.25, 12.5, 25, 50 and 100 mM) or
ZnCl2 (1, 25, 50, 100, 300 and 500 µM; both
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Untreated HUVECs
served as a control. Following 24 h treatment, MTT solution (5
mg/ml; Sigma-Aldrich; Merck KGaA) was added to the cells and they
were incubated at 37°C for 4 h. Subsequently, the supernatant was
discarded and 200 µl dimethylsulfoxide (Sigma-Aldrich; Merck KGaA)
was added to dissolve the formazan crystals. The absorbance was
recorded at 490 nm using an ELX-800 microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA).
Migration and invasion assays
The upper chamber of a Transwell chamber (Corning
Life Sciences, Tewksbury, MA, USA) was coated with 45 µl diluted
Matrigel (BD Biosciences, San Jose, CA, USA) and placed in a
24-well plate for the invasion assay. HUVECs were cultured in DMEM
supplemented with 10% FBS. At 90% confluence, the medium was
replaced with serum-free DMEM and cells were incubated with 10
µg/ml mitomycin C (Sigma-Aldrich; Merck KGaA) for 2 h at 37°C.
Following this, cells were digested with 0.25% trypsin and
resuspended in serum-free DMEM to prepare a single cell suspension.
Cells were seeded into the upper chamber uncoated (8,000 cells in
200 µl cell suspension for migration assay) or coated with Matrigel
(2×104 cells in 200 µl cell suspension for invasion
assay) and DMEM supplemented with 20% FBS was added into the lower
chamber. Subsequently, the cells were incubated with 12.5 mM
MgCl2 or 100 µM ZnCl2. After 24 h cell
culture at 37°C, the cells on the upper side of the filters were
wiped with cotton swabs. The migrated or invaded cells were fixed
with 4% paraformaldehyde (Sinopharm Chemical Reagent Co., Ltd.,
Shanghai, China) for 20 min at room temperature and stained with
crystal violet (Amresco, LLC, Cleveland, OH, USA) for 5 min. The
migrated or invaded cell number was counted in five random fields
under an AE31 inverted microscope (Motic, Xiamen, China) at
magnification, ×200, and the average was calculated.
Western blotting analysis
HUVECs were lysed with radioimmunoprecipitation
assay buffer (Beyotime Institute of Biotechnology, Haimen, China)
supplemented with 1% phenylmethanesulfonyl fluoride protease
inhibitor (ST506; Beyotime Institute of Biotechnology) on ice for 5
min and centrifuged at 10,005 × g for 10 min at 4°C. The
supernatant was collected and protein concentration was determined
using a bicinchoninic acid (BCA) protein assay kit (Beyotime
Institute of Biotechnology). Equal amounts of protein (40 µg/lane)
were separated by 8 or 10% SDS-PAGE and transferred to
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked with 5% non-fat milk at room
temperature for 1 h. Subsequently, membranes were incubated with
antibodies against matrix metalloproteinase (MMP)-2 (1:400; BA0569;
Wuhan Boster Biological Technology, Ltd., Wuhan, China), MMP-9
(1:400; BA0573; Wuhan Boster Biological Technology, Ltd.), vimentin
(1:500; bs-8533R; BIOSS, Beijing, China), Snail (1:500; bs-1371R;
BIOSS), N-cadherin (1:400; BA0673; Wuhan Boster Biological
Technology, Ltd.), Wnt1 (1:200; sc-5630; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) and β-catenin (1:400; BA0426; Wuhan Boster
Biological Technology, Ltd.) overnight at 4°C. Subsequent to
washing with Tris-buffered saline-Tween-20, membranes were
incubated with goat anti-rabbit horseradish peroxidase-labeled
immunoglobulin G (1:5,000; A0208; Beyotime Institute of
Biotechnology) for 45 min at 37°C and visualized using a high
sensitivity enhanced chemiluminescence reagent kit (WLA003;
Wanleibio Co., Ltd., Shenyang, China). The experiment was repeated
three times and results were quantified using Gel-Pro Analyzer v.4
(Media Cybernetics, Inc., Rockville, MD, USA).
ELISA
The supernatant of the cell culture was harvested by
centrifugation (1,000 × g for 10 min) following 24 h treatment with
MgCl2 or ZnCl2 at 37°C. The expression levels
of MMP-2 and MMP-9 in the supernatant were measured using ELISA
kits (DRE11368 and DRE10154; Shanghai WHB Biotech Co., Ltd.,
Shanghai, China), according to the manufacturer's instructions. The
plates were read on a microplate reader (BioTek Instruments, Inc.)
at 450 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from HUVECs using RL lysis
buffer containing DNase, according to the manufacturer's protocol
(BioTeke Corp., Beijing, China). RNA was then reverse transcribed
into cDNA using M-MLV reverse transcriptase (BioTeke Corp.).
Template RNA (1 µg) was mixed with 1 µl Oligo (dT)15, 1
µl random primer, and 2 µl dNTP and ddH2O (final volume
of 14.5 µl), incubated at 70°C for 5 min and then put on ice for 2
min. The mixture was subsequently incubated with 0.5 µl RNasin, 4
µl reaction buffer and 1 µl (200 U) M-MLV reverse transcriptase
(final volume of 20 µl) at 25°C for 10 min, followed by 42°C for 50
min and 95°C for 5 min. The primer sequences for qPCR were as
follows: E-cadherin, forward 5′-AGAACGCATTGCCACATACA-3′ and reverse
5′-TAAGCGATGGCGGCATTGTA-3′; N-cadherin, forward
5′-CAACACACTCGCAGACGCTCA-3′ and reverse 5′-AAGACGGCTCCAGGCAGTTT-3′;
and β-actin, forward 5′-CTTAGTTGCGTTACACCCTTTCTTG-3′ and reverse
5′-CTGTCACCTTCACCGTTCCAGTTT-3′. qPCR was performed using an
Exicycler™ 96 Real-Time Quantitative PCR system (Bioneer
Corporation, Daejeon, Korea). The cycling conditions were as
follows: 10 min at 95°C; followed by 40 cycles of 10 sec at 95°C,
20 sec at 60°C and 30 sec at 72°C (20 µl reaction volume comprising
1 µl cDNA template, 0.5 µl forward primer, 0.5 µl reverse primer,
10 µl SYBR Green PCR Mastermix and 8 µl ddH2O). The
experiment was repeated three times and β-actin was used as an
internal control. The relative mRNA expression levels were
normalized to β-actin, following the 2−ΔΔCq method
(27).
Gelatin zymography
HUVECs were treated with MgCl2 or
ZnCl2 for 24 h and subsequently lysed by repeated
freezing and thawing. Following centrifugation of the homogenate at
10,005 × g for 10 min at 4°C, the supernatant was harvested and the
protein concentration was determined by the BCA method (Beyotime
Institute of Biotechnology). Equal amounts of protein (30 µg/lane)
were separated by 10% SDS-PAGE containing 1 ml gelatin (10 mg/ml)
(Sigma-Aldrich; Merck KGaA). Following this, the gels were washed
twice with elution buffer (40 min; 2.5% Triton X-100, 50 mM
Tris-HCl, 5 mM CaCl2 and 1 µM ZnCl2; pH 7.6)
at room temperature and then twice with washing buffer (20 min; 50
mM Tris-HCl, 5 mM CaCl2 and 1 µM ZnCl2; pH
7.6). Subsequently, the gels were stained with Coomassie Brilliant
Blue R-250 (Amresco, LLC) for 3 h. Gels were then destained with
methanol and acetic acid, and imaged using a gel documentation
system (WD-9413B; Beijing Liuyi Instrument Factory, Beijing,
China).
Cytoskeletal staining
HUVECs were seeded in coverslips and incubated with
12.5 mM MgCl2 or 100 µM ZnCl2 for 24 h at
37°C. Following this, the cells were washed with phosphate-buffered
saline (PBS), fixed with 4% paraformaldehyde (Sinopharm Chemical
Reagent Co., Ltd., Shanghai, China) for 15 min at room temperature
and washed three times with PBS. Subsequently, coverslips were
incubated with Acti-stain™ 488 Fluorescent Phalloidin
(F-actin staining; Cytoskeleton, Inc., Denver, CO, USA) for 1 h.
Then, 4′,6-diamidino-2-phenylindole (DAPI; Biosharp, Hefei, China)
was added to counterstain the nuclei of the cells. Images were
captured under a fluorescent microscope (magnification, ×400; BX53;
Olympus Corp., Tokyo, Japan) and analyzed using Image Pro Plus
software v.6 (Media Cybernetics, Inc.).
Immunofluorescence staining
HUVECs were plated on slides, fixed with 4%
paraformaldehyde and permeabilized with 0.1% Triton X-100 for 30
min at room temperature. Subsequent to blocking with goat serum
(Beijing Solarbio Science and Technology Co., Ltd., Beijing, China)
for 15 min at room temperature, the slides were incubated with
antibody against E-cadherin (1:200; BA0474; Wuhan Boster Biological
Technology, Ltd.) at 4°C overnight and then with fluorescein
isothiocyanate-conjugated goat anti-rabbit secondary antibody for 1
h at room temperature (1:200; A0562; Beyotime Institute of
Biotechnology). Following this, the slides were stained with DAPI
and imaged under a fluorescent microscope (magnification, ×400;
BX53; Olympus Corp.).
Statistical analysis
Results were presented as the mean ± standard
deviation. The differences were analyzed by one-way analysis of
variance followed by Bonferroni post hoc tests using GraphPad Prism
v.5 software (GraphPad Software, Inc., La Jolla, CA, USA) or
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
MgCl2 and ZnCl2
do not significantly influence cell viability
HUVECs were incubated with various doses of
MgCl2 (0, 1.25, 2.5, 5, 6.25, 12.5, 25, 50 and 100 mM)
and ZnCl2 (0, 1, 25, 50, 100, 300 and 500 µM) for 24 h.
The cytotoxicity of MgCl2 or ZnCl2 was
measured by MTT assay. The results demonstrated that the cell
viability of HUVECs was not significantly affected following
exposure to all concentrations of MgCl2 and
ZnCl2 (Fig. 1).
Therefore, 12.5 mM MgCl2 and 100 µM ZnCl2
were selected for further experiments.
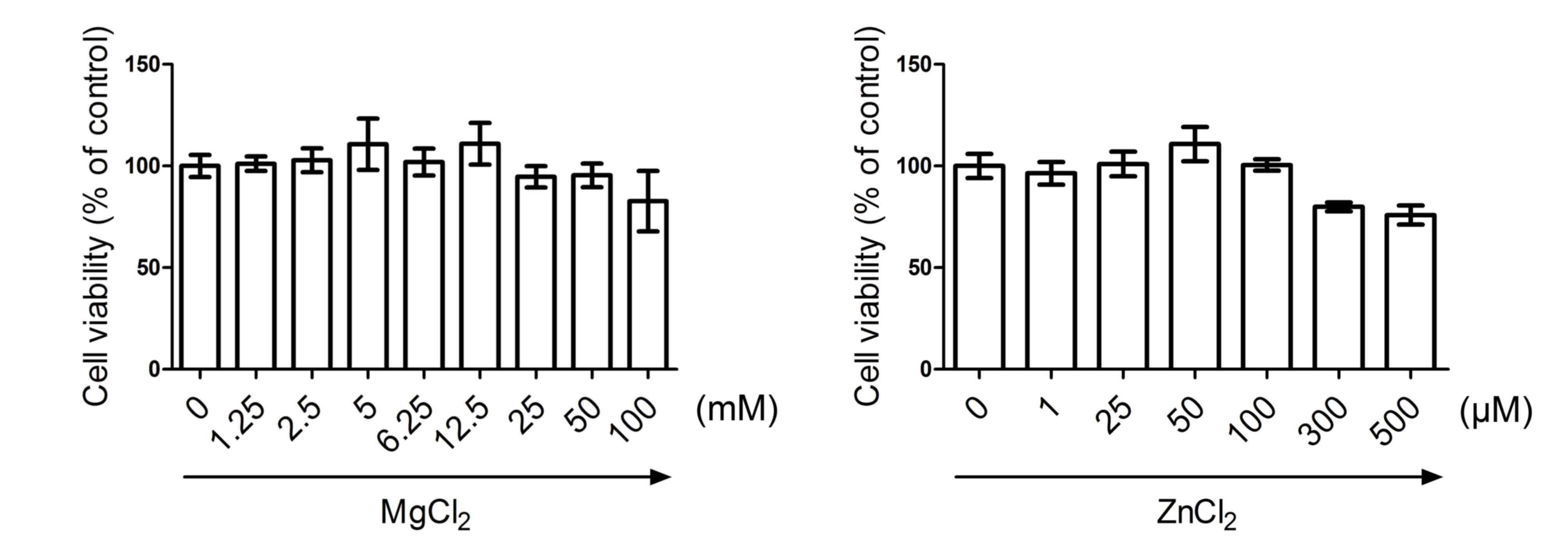 | Figure 1.Cytotoxic effect of MgCl2
and ZnCl2 on HUVECs. HUVECs were exposed to various
concentrations of MgCl2 (0, 1.25, 2.5, 5, 6.25, 12.5,
25, 50 and 100 mM) and ZnCl2 (0, 1, 25, 50, 100, 300 and
500 µM) for 24 h. Cells were then subjected to MTT assay and cell
viability was determined. Data are presented as mean ± standard
deviation. HUVECs, human umbilical vein endothelial cells. |
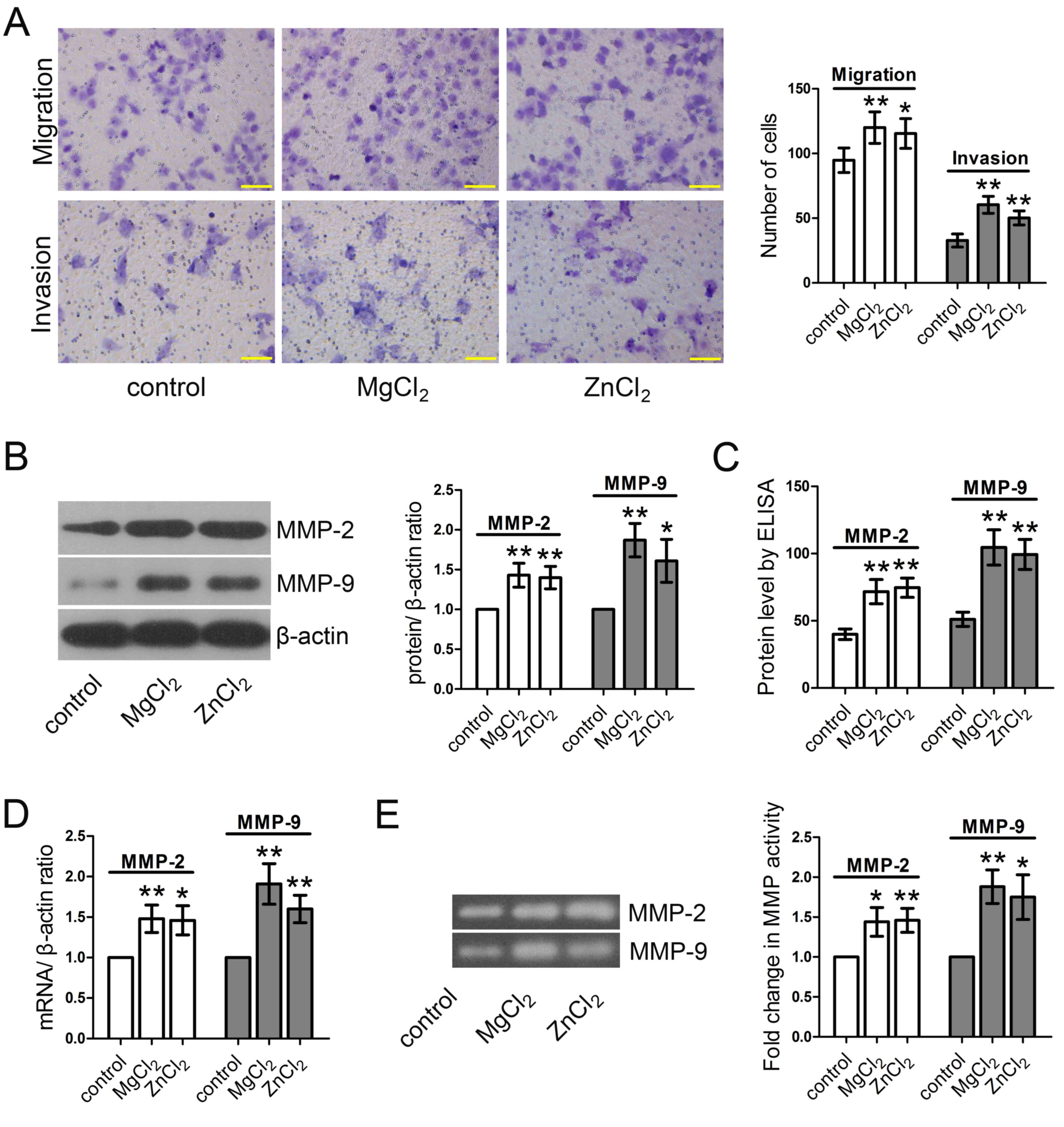
MgCl2 and ZnCl2
promote the migration and invasion of HUVECs in vitro
Cell migration and invasion assays using Transwell
chambers were performed to investigate the effect of
MgCl2 and ZnCl2 on the migration and invasion
capabilities of HUVECs. The results demonstrated that both
MgCl2 and ZnCl2 significantly enhanced the
migration and invasion abilities of HUVECs compared with the
control group (Fig. 2A). The
extracellular matrix and basement membrane provide the major
physical barriers to cell invasion. MMPs are important proteolytic
enzymes able to degrade the extracellular matrix and basement
membrane and serve critical roles in the invasion process (28). MgCl2 and ZnCl2
treatment significantly increased the expression of MMP-2 and MMP-9
proteins, as determined by western blotting (Fig. 2B) and ELISA (Fig. 2C). RT-qPCR determined that the
expression of MMP-2 and MMP-9 mRNA was significantly increased
(Fig. 2D). The gelatin zymography
assay demonstrated that MMP-2 and MMP-9 activity was significantly
enhanced by MgCl2 and ZnCl2 (Fig. 2E).
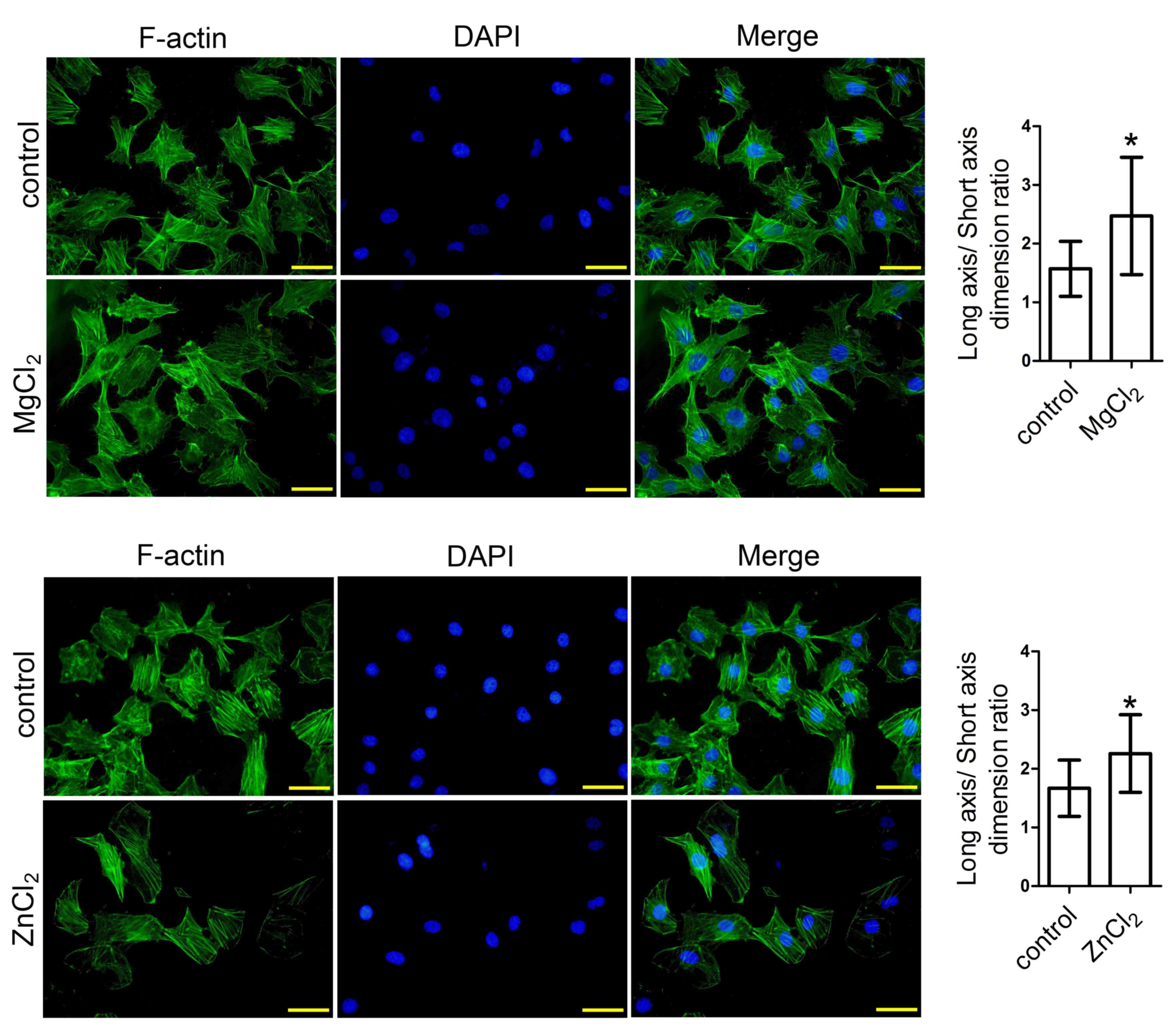
MgCl2 and ZnCl2
induce cytoskeletal reorganization in HUVECs
Subsequently, the effects of MgCl2 and
ZnCl2 on cytoskeletal reorganization in HUVECs were
investigated. The results demonstrated that MgCl2 and
ZnCl2 treatment promoted cytoskeletal reorganization,
with an increased long axis/short axis dimension ratio compared
with the control (Fig. 3).
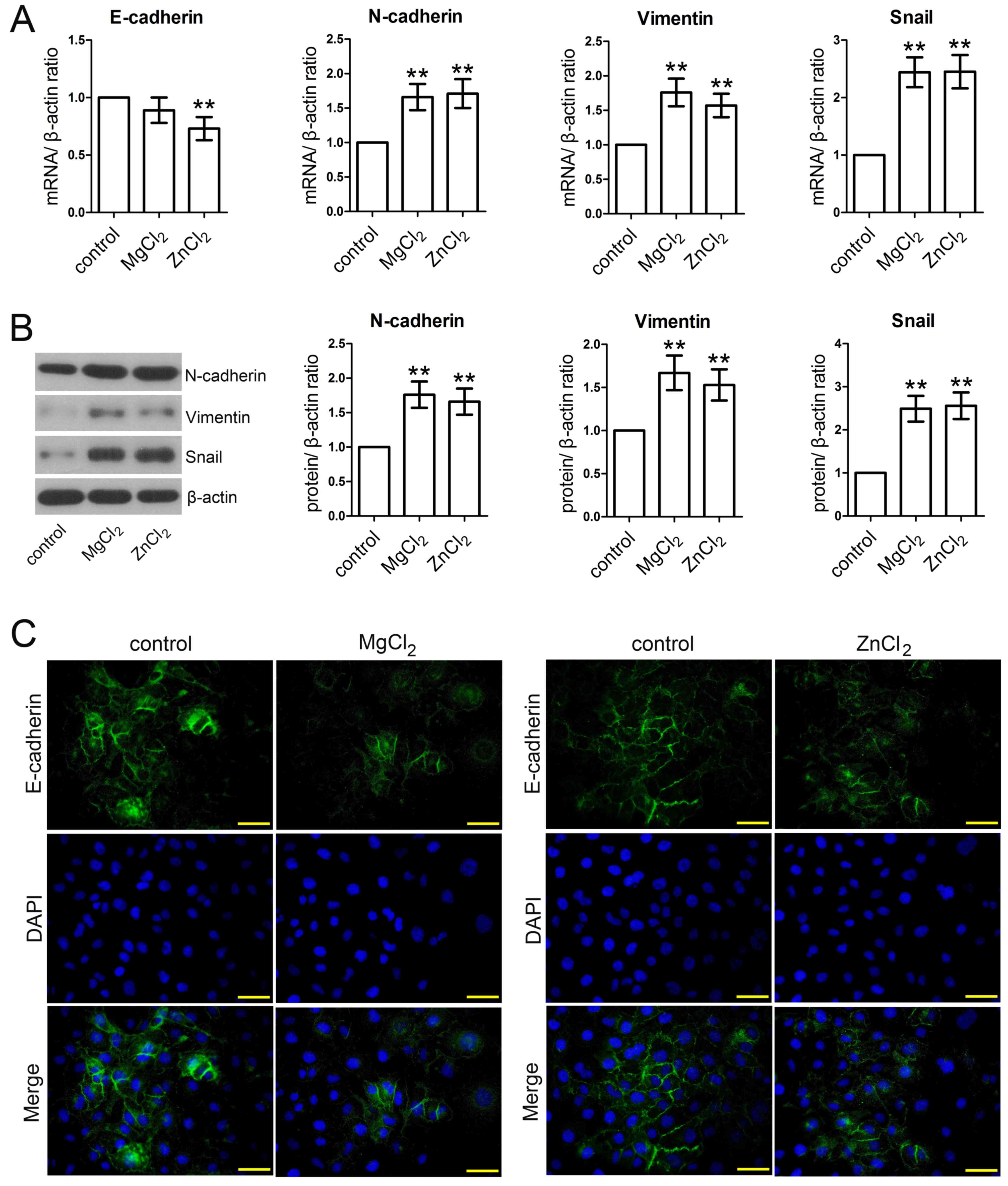
MgCl2 and ZnCl2
promote EMT
To further investigate whether MgCl2 and
ZnCl2 were able to modulate EMT, the expression of
several EMT-related genes were measured using RT-qPCR, western
blotting and immunofluorescence staining assays. The results
demonstrated that the expression of E-cadherin was downregulated
following MgCl2 and ZnCl2 stimulation;
however, this difference was only significant following
ZnCl2 stimulation (Fig.
4A). N-cadherin mRNA and protein expression was significantly
upregulated following MgCl2 and ZnCl2
stimulation compared with the control (Fig. 4A and B). Furthermore, the results
demonstrated that MgCl2 and ZnCl2 treatment
significantly increased the expression of vimentin and Snail at
both mRNA and protein levels (Fig. 4A
and B). The immunofluorescence staining assay further
demonstrated that E-cadherin was downregulated following
stimulation with MgCl2 and ZnCl2 (Fig. 4C).
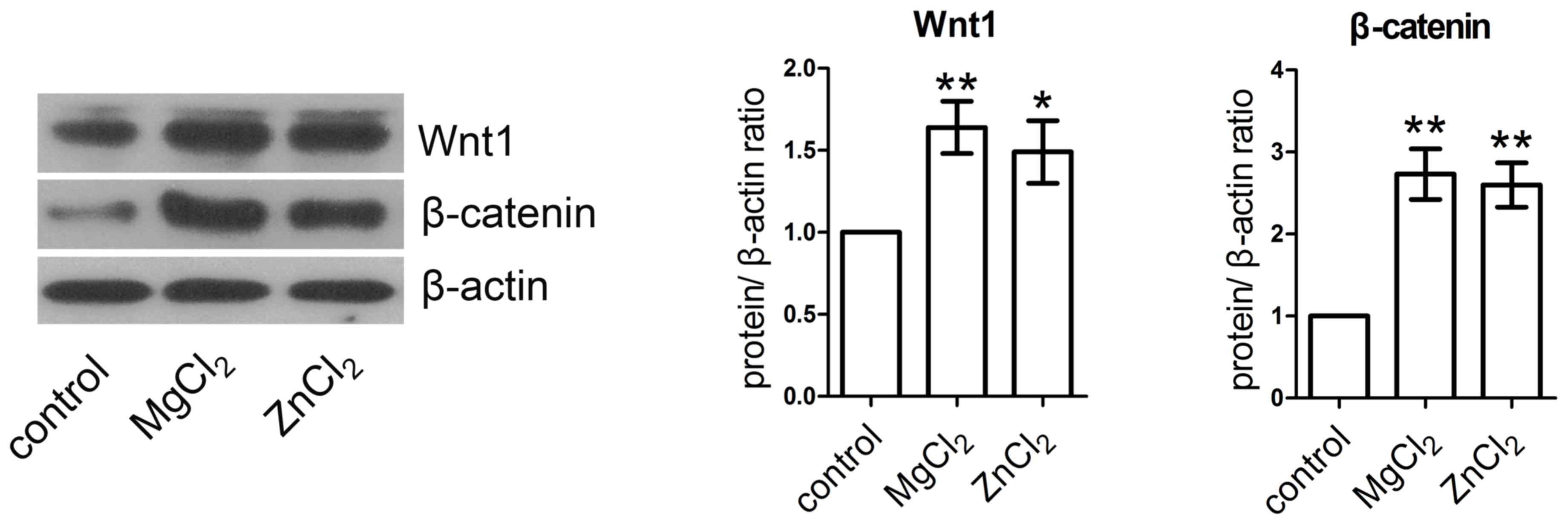
MgCl2 and ZnCl2
activate the Wnt/β-catenin signaling pathway
HUVECs were incubated with MgCl2 and
ZnCl2 for 24 h and the expression of Wnt1 and β-catenin
were measured by western blotting analysis. The expression of Wnt1
and β-catenin protein were significantly increased in
MgCl2- and ZnCl2-treated HUVECs compared with
the control (Fig. 5).
Discussion
In the present study, HUVECs were treated with
various concentrations of MgCl2 and ZnCl2.
Cell viability was measured by MTT assay and the optimum
concentrations of MgCl2 and ZnCl2 were
determined. The effect of MgCl2 and ZnCl2 on
the migration, invasion, cytoskeletal dynamics and EMT of HUVECs
was then investigated in vitro and the mechanisms involved
were also studied. To the best of our knowledge, the present study
was the first to demonstrate that MgCl2 and
ZnCl2 enhanced the migration and invasion abilities of
HUVECs, stimulated cytoskeletal reorganization and induced EMT via
the Wnt/β-catenin signaling pathway.
The impact of MgCl2 and ZnCl2
on the motility of HUVECs was examined. It was determined that
MgCl2 and ZnCl2 promoted the migration and
invasion of HUVECs. MMPs are key modulators of various biological
processes, including the EMT process, cancer, angiogenesis,
skeletal formation, inflammation and cell migration (29). Notably, both MMP-2 and MMP-9 are
crucial gelatinases that regulate angiogenesis in endothelial cells
(30). It has been observed that
ZnCl2 reverses the inhibitory effects of ellagic acid on
MMP-2 expression, MMP-2 activity and the migration of HUVECs
(31). Mg2+ is the most
abundant divalent cation in cells in the human body and has been
demonstrated to be associated with various cell functions (32). It has been determined that high
Mg2+ levels enhance microvascular endothelial cell (1G11
cell) migration and induce angiogenesis (33). A study by Takatani-Nakase et
al (34) demonstrated that
Zn2+ contributes to the promotion of cell migration in
breast cancer cells following exposure to high glucose. However,
the effects of MgCl2 and ZnCl2 treatment on
HUVEC motility, MMP-2 and MMP-9 levels and activities are not fully
understood. The present study indicated that MgCl2 and
ZnCl2 promoted HUVEC migration and invasion, as
determined by Transwell assays. Additionally, MgCl2 and
ZnCl2 treatment increased MMP-2 and MMP-9 expression at
the mRNA and protein levels. Meanwhile, MMP-2 and MMP-9 activities
were markedly enhanced. The results suggest that MgCl2
and ZnCl2 may promote HUVEC migration and invasion by
regulating the expression and activities of MMPs.
EMT is characterized by a loss of cell-cell adhesion
and increase in cell motility (35,36).
Cytoskeletal reorganization is involved in the process of EMT and
is a crucial hallmark of EMT (37,38). In
the present study, it was determined that MgCl2 and
ZnCl2 induced reorganization of the actin cytoskeleton
in HUVECs. Various genes are associated with the EMT process,
including epithelial markers, mesenchymal markers and transcription
factors (39,40). During EMT, E-cadherin and cytokeratin
(epithelial markers) are downregulated, whereas fibronectin,
N-cadherin and vimentin (mesenchymal markers) are upregulated
(41). E-cadherin is a transmembrane
glycoprotein that regulates cell-cell adhesion (42). It is a major epithelial marker and
its expression is reduced in cells that have undergone EMT
(39). N-cadherin is a
calcium-dependent adhesion molecule and in the presence of
Ca2+, N-cadherin resists hydrolysis by protease and
promotes tumor cell metastasis (43). Snail, a zinc finger transcription
factor, promotes EMT of microvascular endothelial cells (43). Vimentin is a type III intermediate
filament protein with a molecular weight of 57 kDa and is expressed
in non-epithelial cells, particularly in mesenchymal cells
(44,45). A study by Xiao et al (46) indicated that Zn2+ induces
EMT in human gastric adenocarcinoma cells via the gastrin gene. The
present study demonstrated that MgCl2 and
ZnCl2 incubation led to a significant decrease in
E-cadherin and increases in N-cadherin, vimentin and Snail
expression. These results suggest that MgCl2 and
ZnCl2 may promote the EMT of HUVECs by regulating the
expression of EMT markers.
It has been demonstrated that various signaling
pathways participate in the EMT process, including the Wnt
signaling pathway (47). A study by
Scheel et al (48) determined
that the Wnt signaling pathway and TGF-β induces EMT in mammary
epithelial cells. Furthermore, it has been determined that Wnt
signaling is the earliest event in the EMT process and cell
invasion (49). Thus, the present
experiments also investigated on the activation of the
Wnt/β-catenin signaling pathway. The results demonstrated that the
expression of Wnt1 and β-catenin protein were significantly
increased following MgCl2 and ZnCl2
treatment, indicating that MgCl2 and ZnCl2
may promote the migration and invasion of HUVECs via the
Wnt/β-catenin pathway. These results provide a potential
therapeutic strategy for the inhibition of HUVEC migration,
invasion, EMT and angiogenesis.
In conclusion, the results of the present study
suggest that MgCl2 and ZnCl2 may promote cell
migration and invasion and stimulate cytoskeletal reorganization
and EMT by activating the Wnt/β-catenin pathway. Further studies
are required to verify these observations.
References
|
1
|
Elshabrawy HA, Chen Z, Volin MV, Ravella
S, Virupannavar S and Shahrara S: The pathogenic role of
angiogenesis in rheumatoid arthritis. Angiogenesis. 18:433–448.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Srinivasan S, Chitalia V, Meyer RD,
Hartsough E, Mehta M, Harrold I, Anderson N, Feng H, Smith LE,
Jiang Y, et al: Hypoxia-induced expression of phosducin-like 3
regulates expression of VEGFR-2 and promotes angiogenesis.
Angiogenesis. 18:449–462. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rodriguez-Caso L, Reyes-Palomares A,
Sánchez-Jiménez F, Quesada AR and Medina MÁ: What is known on
angiogenesis-related rare diseases? A systematic review of
literature. J Cell Mol Med. 16:2872–2893. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kreuger J and Phillipson M: Targeting
vascular and leukocyte communication in angiogenesis, inflammation
and fibrosis. Nat Rev Drug Discov. 15:125–142. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brauer R, Beck IM, Roderfeld M, Roeb E and
Sedlacek R: Matrix metalloproteinase-19 inhibits growth of
endothelial cells by generating angiostatin-like fragments from
plasminogen. BMC Biochem. 12:382011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schuermann A, Helker CS and Herzog W:
Metallothionein 2 regulates endothelial cell migration through
transcriptional regulation of vegfc expression. Angiogenesis.
18:463–475. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kalluri R: Basement membranes: Structure,
assembly and role in tumour angiogenesis. Nat Rev Cancer.
3:422–433. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li P, Liu Y, Wang H, He Y, Wang X, He Y,
Lv F, Chen H, Pang X, Liu M, et al: PubAngioGen: A database and
knowledge for angiogenesis and related diseases. Nucleic Acids Res.
43:D963–D967. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu J, Yuan X, Liu Y, Zhang K, Wang J,
Zhang H and Liu F: Delayed administration of WP1066, an STAT3
inhibitor, ameliorates radiation-induced lung injury in mice. Lung.
194:67–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Inada M, Takita M, Yokoyama S, Watanabe K,
Tominari T, Matsumoto C, Hirata M, Maru Y, Maruyama T, Sugimoto Y,
et al: Direct melanoma cell contact induces stromal cell autocrine
prostaglandin E2-EP4 receptor signaling that drives tumor growth,
angiogenesis and metastasis. J Biol Chem. 290:29781–29793. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vormann J: Magnesium: Nutrition and
metabolism. Mol Aspects Med. 24:27–37. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang L, Arora K, Beard WA, Wilson SH and
Schlick T: Critical role of magnesium ions in DNA polymerase beta's
closing and active site assembly. J Am Chem Soc. 126:8441–8453.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peralta FA and Huidobro-Toro JP: Zinc as
allosteric ion channel modulator: Ionotropic receptors as
metalloproteins. Int J Mol Sci. 17:pii: E1059. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mostaed E, Vedani M, Hashempour M and
Bestetti M: Influence of ECAP process on mechanical and corrosion
properties of pure Mg and ZK60 magnesium alloy for biodegradable
stent applications. Biomatter. 4:e282832014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sheffer M, Simon AJ, Jacob-Hirsch J,
Rechavi G, Domany E, Givol D and D'Orazi G: Genome-wide analysis
discloses reversal of the hypoxia-induced changes of gene
expression in colon cancer cells by zinc supplementation.
Oncotarget. 2:1191–1202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ren N, Li J, Qiu J, Sang Y, Jiang H,
Boughton RI, Huang L, Huang W and Liu H: Nanostructured titanate
with different metal ions on the surface of metallic titanium: A
facile approach for regulation of rBMSCs fate on titanium implants.
Small. 10:3169–3180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Muñoz-Chápuli R, Quesada AR and Medina
Angel M: Angiogenesis and signal transduction in endothelial cells.
Cell Mol Life Sci. 61:2224–2243. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang L, Wang X, Wen C, Yang X, Song M,
Chen J, Wang C, Zhang B, Wang L, Iwamoto A, et al: Hsa-miR-19a is
associated with lymph metastasis and mediates the TNF-α induced
epithelial-to-mesenchymal transition in colorectal cancer. Sci Rep.
5:133502015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brito RB, Malta CS, Souza DM, Matheus LH,
Matos YS, Silva CS, Ferreira JM, Nunes VS, França CM and Dellê H:
1-Methyl-D-Tryptophan potentiates TGF-β-induced
epithelial-mesenchymal transition in T24 human bladder cancer
cells. PLoS One. 10:e01348582015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo Q, Ning F, Fang R, Wang HS, Zhang G,
Quan MY, Cai SH and Du J: Endogenous Nodal promotes melanoma
undergoing epithelial-mesenchymal transition via Snail and Slug in
vitro and in vivo. Am J Cancer Res. 5:2098–2112. 2015.PubMed/NCBI
|
|
22
|
Zhi Y, Mou Z, Chen J, He Y, Dong H, Fu X
and Wu Y: B7H1 expression and epithelial-To-Mesenchymal transition
phenotypes on colorectal cancer stem-like cells. PLoS One.
10:e01355282015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang YQ, Wei XL, Liang YK, Chen WL, Zhang
F, Bai JW, Qiu SQ, Du CW, Huang WH and Zhang GJ: Over-expressed
twist associates with markers of epithelial mesenchymal transition
and predicts poor prognosis in breast cancers via ERK and Akt
activation. PLoS One. 10:e01358512015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cohen EN, Gao H, Anfossi S, Mego M, Reddy
NG, Debeb B, Giordano A, Tin S, Wu Q, Garza RJ, et al: Inflammation
mediated metastasis: Immune induced epithelial-To-Mesenchymal
transition in inflammatory breast cancer cells. PLoS One.
10:e01327102015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng HW, Chen YF, Wong JM, Weng CW, Chen
HY, Yu SL, Chen HW, Yuan A and Chen JJ: Cancer cells increase
endothelial cell tube formation and survival by activating the
PI3K/Akt signalling pathway. J Exp Clin Cancer Res. 36:272017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang S, Li Y, Lin T, Yuan L, Li Y, Wu S,
Xia L, Shen H and Lu J: IL-35 inhibits angiogenesis through
VEGF/Ang2/Tie2 pathway in rheumatoid arthritis. Cell Physiol
Biochem. 40:1105–1116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li C, Zhou Y, Peng X, Du L, Tian H, Yang
G, Niu J and Wu W: Sulforaphane inhibits invasion via activating
ERK1/2 signaling in human glioblastoma U87MG and U373MG cells. PLoS
One. 9:e905202014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Awasthi N, Wang-Su ST and Wagner BJ:
Downregulation of MMP-2 and −9 by proteasome inhibition: A possible
mechanism to decrease LEC migration and prevent posterior capsular
opacification. Invest Ophthalmol Vis Sci. 49:1998–2003. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. FEBS J. 278:16–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang ST, Yang RC, Wu HT, Wang CN and Pang
JH: Zinc-chelation contributes to the anti-angiogenic effect of
ellagic acid on inhibiting MMP-2 activity, cell migration and tube
formation. PLoS One. 6:e189862011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hong BZ, Kang HS, So JN, Kim HN, Park SA,
Kim SJ, Kim KR and Kwak YG: Vascular endothelial growth factor
increases the intracellular magnesium. Biochem Biophys Res Commun.
347:496–501. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bernardini D, Nasulewic A, Mazur A and
Maier JA: Magnesium and microvascular endothelial cells: A role in
inflammation and angiogenesis. Front Biosci. 10:1177–1182. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takatani-Nakase T, Matsui C, Maeda S,
Kawahara S and Takahashi K: High glucose level promotes migration
behavior of breast cancer cells through zinc and its transporters.
PLoS One. 9:e901362014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guarino M: Epithelial-mesenchymal
transition and tumour invasion. Int J Biochem Cell Biol.
39:2153–2160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Q, Liu LN, Yong Q, Deng JC and Cao
WG: Intralesional injection of adipose-derived stem cells reduces
hypertrophic scarring in a rabbit ear model. Stem Cell Res Ther.
6:1452015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mori M, Nakagami H, Koibuchi N, Miura K,
Takami Y, Koriyama H, Hayashi H, Sabe H, Mochizuki N, Morishita R
and Kaneda Y: Zyxin mediates actin fiber reorganization in
epithelial-mesenchymal transition and contributes to endocardial
morphogenesis. Mol Biol Cell. 20:3115–3124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim JH, Hwang YJ, Han SH, Lee YE, Kim S,
Kim YJ, Cho JH, Kwon KA, Kim JH and Kim SH: Dexamethasone inhibits
hypoxia-induced epithelial-mesenchymal transition in colon cancer.
World J Gastroenterol. 21:9887–9899. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pang L, Li Q, Wei C, Zou H, Li S, Cao W,
He J, Zhou Y, Ju X, Lan J, et al: TGF-β1/Smad signaling pathway
regulates epithelial-to-mesenchymal transition in esophageal
squamous cell carcinoma: In vitro and clinical analyses of cell
lines and nomadic Kazakh patients from northwest Xinjiang, China.
PLoS One. 9:e1123002014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xishan Z, Ziying L, Jing D and Gang L:
MicroRNA-320a acts as a tumor suppressor by targeting BCR/ABL
oncogene in chronic myeloid leukemia. Sci Rep. 5:124602015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen L, Jian W, Lu L, Zheng L, Yu Z and
Zhou D: Elevated expression of E-cadherin in primary breast cancer
and its corresponding metastatic lymph node. Int J Clin Exp Med.
8:11752–11758. 2015.PubMed/NCBI
|
|
43
|
Wang YL, Zhao XM, Shuai ZF, Li CY, Bai QY,
Yu XW and Wen QT: Snail promotes epithelial-mesenchymal transition
and invasiveness in human ovarian cancer cells. Int J Clin Exp Med.
8:7388–7393. 2015.PubMed/NCBI
|
|
44
|
Liu CY, Lin HH, Tang MJ and Wang YK:
Vimentin contributes to epithelial-mesenchymal transition cancer
cell mechanics by mediating cytoskeletal organization and focal
adhesion maturation. Oncotarget. 6:15966–15983. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mendez MG, Kojima S and Goldman RD:
Vimentin induces changes in cell shape, motility, and adhesion
during the epithelial to mesenchymal transition. FASEB J.
24:1838–1851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xiao L, Kovac S, Chang M, Shulkes A,
Baldwin GS and Patel O: Zinc ions upregulate the hormone gastrin
via an E-box motif in the proximal gastrin promoter. J Mol
Endocrinol. 52:29–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu Y, Gai L, Liu J, Cui Y, Zhang Y and
Feng J: Expression of poly(C)-binding protein 1 (PCBP1) in NSCLC as
a negative regulator of EMT and its clinical value. Int J Clin Exp
Pathol. 8:7165–7172. 2015.PubMed/NCBI
|
|
48
|
Scheel C, Eaton EN, Li SH, Chaffer CL,
Reinhardt F, Kah KJ, Bell G, Guo W, Rubin J, Richardson AL and
Weinberg RA: Paracrine and autocrine signals induce and maintain
mesenchymal and stem cell states in the breast. Cell. 145:926–940.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Elsarraj HS, Hong Y, Valdez KE, Michaels
W, Hook M, Smith WP, Chien J, Herschkowitz JI, Troester MA, Beck M,
et al: Expression profiling of in vivo ductal carcinoma in situ
progression models identified B cell lymphoma-9 as a molecular
driver of breast cancer invasion. Breast Cancer Res. 17:1282015.
View Article : Google Scholar : PubMed/NCBI
|