Introduction
Osteoporosis is a common chronic progressive disease
and has the characteristics of osteopenia, bone microstructural
damage and bone strength reduction (1). In recent years, the morbidity of
osteoporosis has been increasing persistently. Patients with
osteopenia or osteoporosis in the US are predicted to reach 61
million by 2020, while patients with osteoporosis that are >50
years of age in China had already reached 69.44 million in 2006
(2). Current research has been
focusing on the key aspects of osteoporosis, mainly the absorption
of bone tissue, and uniform reduction of bone mineral content
(3). In addition, bone
microstructural damage, increase of osteopsathyrosis, bone load,
bending stress and reduction of biomechanical properties also occur
in patients with osteoporosis (4).
Furthermore, micro fractures or complete fractures can easily occur
in osteoporosis patients (4).
The sirtuin 1 (SIRT1) protein was the first member
of the Sirtuin protein family to be discovered (5). It has been reported that enzymes
associated with SIRT1 are class III histone acetylation enzymes
dependent on nicotinamide adenine dinucleotide (NAD+),
which is highly conserved (6). SIRT1
is able to participate in deacetylation between histone and
non-histone lysine residue, and regulates p53, nuclear factor
(NF)-κB and other transcription factors (6). In addition, these genes regulate cell
proliferation, differentiation and metabolism, and serve an
important regulating effect on chromatin structure, cell metabolism
and tumor morbidity processes (7,8). A
recent study has identified that SIRT1 also serves an important
role in the morbidity and prevention of osteoporosis (6).
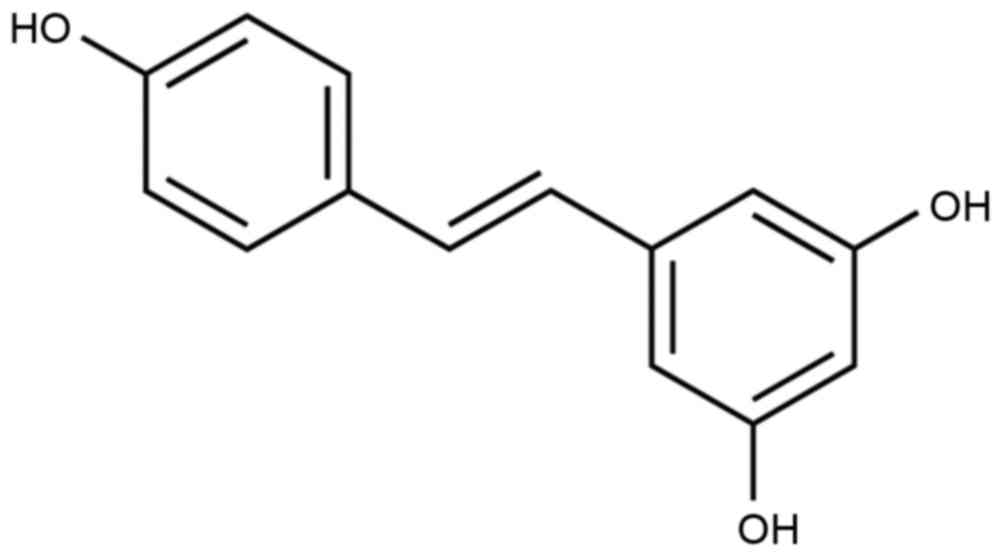
Resveratrol is also known as
3,5,4′-trihydroxystilbene and its molecular formula is
C14H12O3 (Fig. 1), and is a non-flavonoid polyphenolic
substance (9). In 1940, it was
obtained by separating it from the root of Veratrum
grandiflorum for the first time (10). Resveratrol is a natural plant
antitoxin that is extracted from numerous spermatophytes in severe
environments, including in grapes, berries and peanuts (11). Resveratrol is able to target
cytomembranes, intracellular receptors, signaling molecules,
enzymes, oxidative system, DNA repair system and transcription
factors (12). Cellular signal
transduction function is able to convert stimulation into a signal,
and this process frequently involves a series of biochemical
reactions (13). Resveratrol
regulates a series of signal transduction pathways, such as the
SIRT1 and NF-κB signaling pathways (14). Resveratrol is also able to increase
the quantity and function of mitochondria (15). Previous studies have demonstrated
that various organs, such as the liver, brain, kidney and lung, in
F2 hybrid mice appear to have oxidative DNA damage with aging
(12,16,17).
However, the oral absorption of resveratrol may reduce the high
levels of oxidative damage markers induced by aging (18). Therefore, the aim of the present
study was to investigate the protective effects of resveratrol in a
rat model of osteoporosis and examine the associated mechanisms of
its action.
Materials and methods
Animals and osteoporosis model
The procedures of the current study were conducted
in accordance with the Principles of Laboratory Animal Care
established by the National Institutes of Health (Bethesda, MD,
USA) and was approved by the Ethics Committee of Zhongnan Hospital
of Wuhan University (Wuhan, China). A total of 46 male Wistar rats
(180–200 g; 3-month-old) were obtained from the Animal Experiment
Center of Wuhan University and maintained under controlled
temperature at 23±1°C, humidity of 55±10% and a 12 h light/dark
cycle, and food and water were provided ad libitum. An
osteotomy was performed in the right femur of each rat according to
a previously described method (19).
All rats were anesthetized with 30 mg/kg pentobarbital
(intraperitoneally; 69020181; Sinopharm Chemical Reagent Co., Ltd.,
Shanghai, China), and then surgical procedures were performed,
including post-osteotomy fixation and tibia-tail fixation. A
lateral approach at the middle of femur was performed, as well as a
transverse fracture at the mid-diaphysis, with an oscillating power
saw under standard sterile conditions. The fracture site was fixed
and steadied by an intramedullary steel needle with a diameter of
1.2 mm under sterilized sutures.
Experimental groups
Rats were randomized into the following groups:
Control (n=6, sham operation), osteoporosis (n=10), osteoporosis +
low-dose resveratrol (5 mg/kg; n=10), osteoporosis + middle-dose
resveratrol (25 mg/kg; n=10) and osteoporosis + high-dose
resveratrol (45 mg/kg; n=10) groups. Resveratrol (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) treatment was provided at 7 days
after surgery for 8 weeks, and control and osteoporosis group were
was provided with normal saline.
Measurement of BMD
All rats were scanned with a dual-energy x-ray
absorptiometry system (Norland XR-46; Norland at Swissray, Fort
Atkinson, WI, USA) according to a previous method (20). Briefly, the bone mineral density
(BMD) value was measured for the proximal third of the right tibia
at a scan pitch of 1.5 mm and a scan speed of 60 mm/sec. The whole
femur was divided into three equal fields for X-ray analysis, which
included the distal femoral epiphysis and femoral shaft.
Histological analysis
All rats were sacrificed using decollation under 30
mg/kg pentobarbital and bone samples from the right proximal tibias
were harvested. Bone samples were subsequently fixed with 4%
formaldehyde for 24 h and dehydrated in a graded ethanol series,
cleared in xylene and embedded in molten paraffin at 62°C
overnight. Blocks were cut into 4-µm sections. Subsequently, the
sections were stained with hematoxylin and eosin and analyzed with
Image-Pro Plus software (version 6.0; Media Cybernetics, Inc.,
Rockville, MD, USA).
ELISA assay of alkaline phosphatase
(ALP) and osteocalcin (OC) protein levels
Blood samples (2 ml) were collected from the right
common carotid artery following resveratrol treatment and
centrifuged at a speed of 3,000 × g at 4°C for 20 min. The serum
levels of ALP (E-EL-R0113c) and OC (E-EL-R0243c) were measured by
colorimetric analysis at 450 nm, according to the manufacturer's
instructions (Elabscience Biotechnology Co., Ltd., Wuhan,
China).
Bone mechanical tests
All bone samples were assessed for femur strength by
the three-point bending test according to a previous method
(20). The femur of each rat was
removed from storage at −80°C, and lengths were measured using a
caliper. The hydrated weight of the bones was determined using a
four decimal place digital scale. Specimens were placed on two
supports (12 mm) and bent until fractured by lowering the crosshead
positioned at the mid-shaft. The peak load (N) and the ultimate
stiffness (N/mm) were obtained from the load placement curve.
Western blot assay
Bone samples from the right proximal tibias were
homogenized and lysed in ice-cold RIPA buffer containing 1%
protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA). The
supernatant was collected after centrifugation at 12,000 × g for 20
min at 4°C, and the protein concentration was measured by the
modified Bradford assay (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Protein samples (50 µg) were then separated by 8–12% SDS-PAGE
and transferred to nitrocellulose membranes (EMD Millipore,
Billerica, MA, USA). Next, membranes were blocked with 5% non-fat
milk in Tris-buffered saline with 0.1% Tween-20 (pH 7.4) for 2 h at
room temperature. Membranes were probed overnight at 4°C with the
following primary antibodies: Anti-SIRT1 (sc-15404; 1:4,000),
anti-NF-κB (sc-109, 1:200), anti-IkBα (sc-9130, 1:400) and
anti-β-actin (sc-7210, 1:2,000; all from Santa Cruz Biotechnology,
Inc., Dallas, TX, USA). This was followed by incubation with the
corresponding horseradish peroxidase-conjugated anti-rabbit
secondary antibodies (sc-2357; 1:2,000; Santa Cruz Biotechnology,
Inc.) at room temperature for 1 h. The western blot bands were
detected using an enhanced chemiluminescence reagent kit (EMD
Millipore) and visualized with UVP 210 Bio-Imaging Systems (UVP,
Upland, CA, USA).
Statistical analysis
All data are expressed as the mean ± standard
deviation. The control and treated groups were compared using
one-way analysis of variance. Statistically significant differences
between the results were analyzed using the SPSS software (version
15.0; SPSS, Inc., Chicago, IL, USA), and were indicated by
P<0.05.
Results
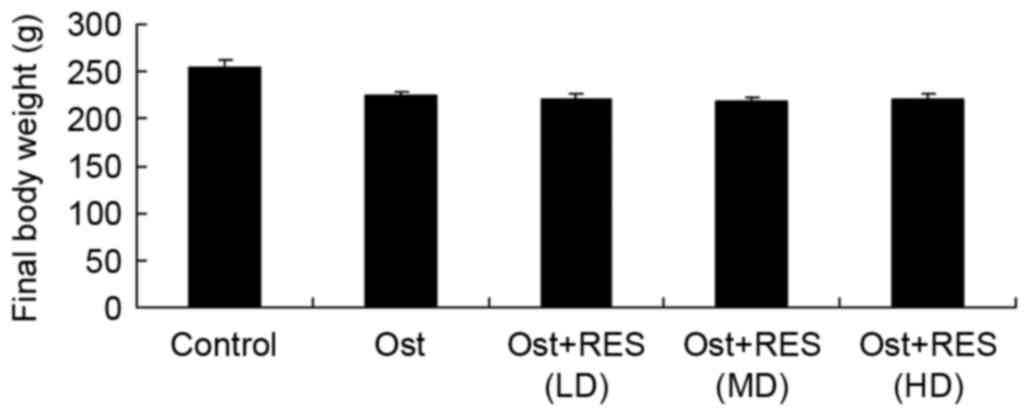
Final body weight
The final body weights of the rats in each group
were compared. As shown in Fig. 2,
the results indicated that the normal control group had a slightly
higher body weight compared with that of the other experimental
groups; however, there were no significant differences in body
weight between any groups (Fig.
2).
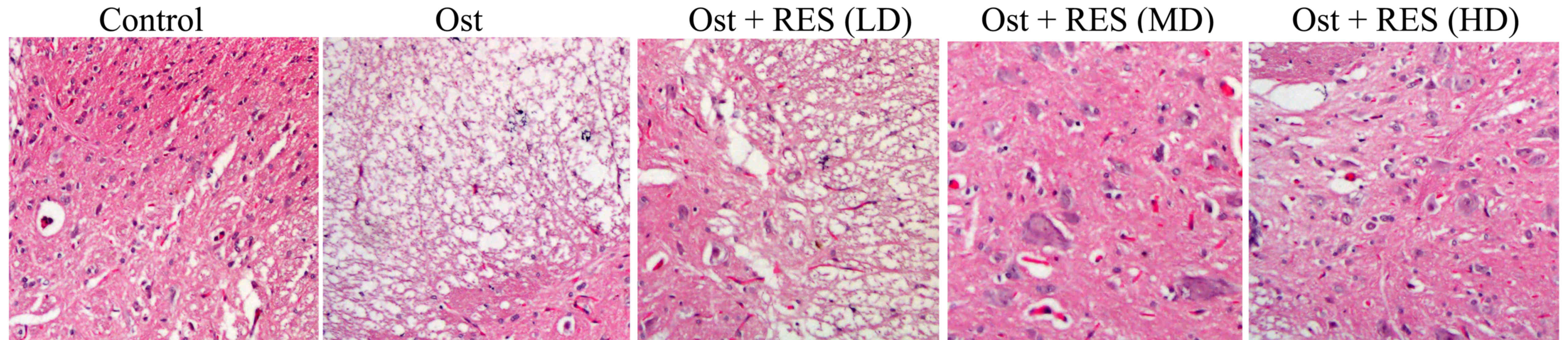
Histological analysis of BMD
Compared with the normal control group, the BMD was
evidently lower and a cavity was observed in the osteoporosis group
(Fig. 3). There were no considerable
differences between the osteoporosis and low-dose resveratrol
groups (Fig. 3). By contrast, the
BMD was markedly increased by treatment with middle-dose or
high-dose resveratrol in osteoporosis rats (Fig. 3).
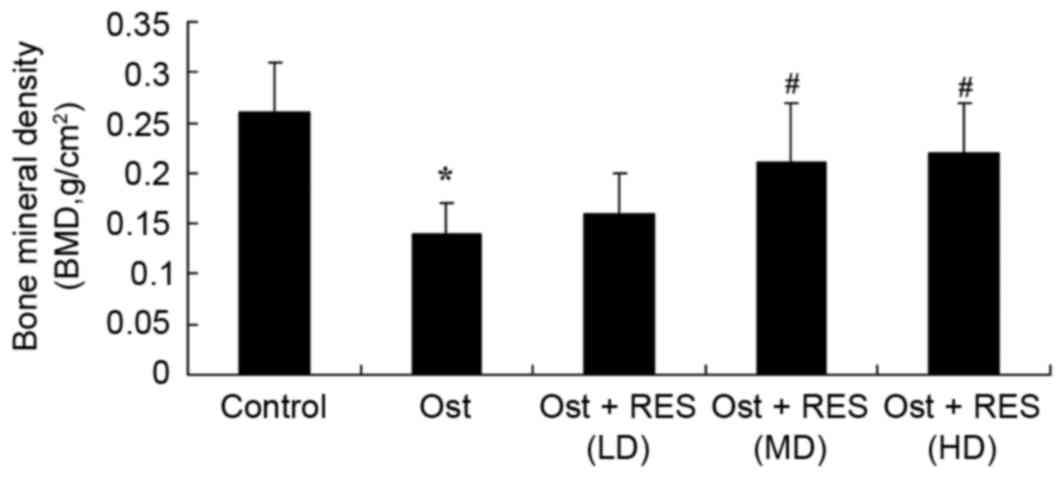
BMD value
In the osteoporosis group, the BMD value was
significantly decreased compared with that in the normal control
group (Fig. 4; P=0.0031). However,
there were no significantly differences between the osteoporosis
and low-dose resveratrol groups (Fig.
4). Treatment with middle-dose or high-dose resveratrol
significantly increased the BMD value in the osteoporosis rats
(Fig. 4; P=0.0065, P=0.0043,
respectively).
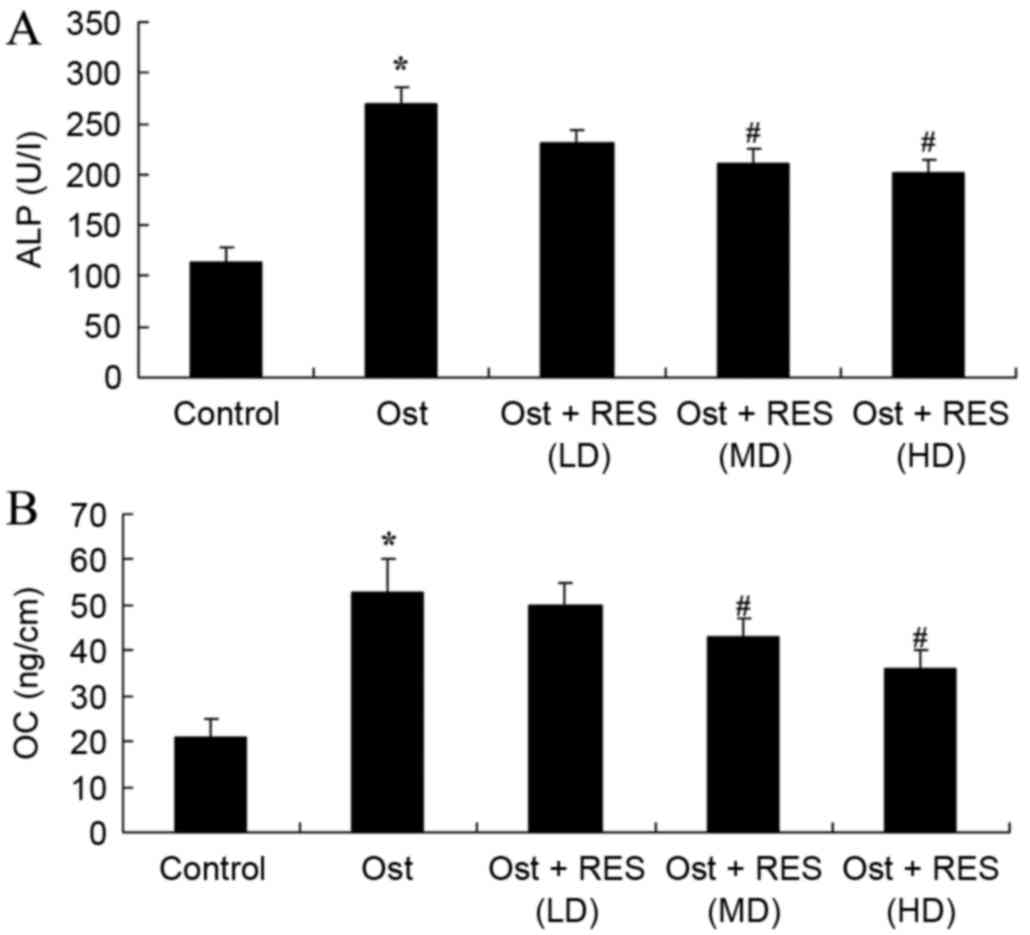
Serum levels of ALP and OC
Analysis of blood samples by ELISA identified that
the serum levels of ALP (P=0.0027) and OC (P=0.0022) in the
osteoporosis group were higher compared with those of the normal
control group (Fig. 5). However, no
significant changes were detected between the osteoporosis and
low-dose resveratrol group (Fig. 5).
As shown in Fig. 5, middle-dose or
high-dose resveratrol treatment significantly inhibited the serum
levels of ALP (P=0.0087 and P=0.0077, respectively) and OC
(P=0.0076 and P=0.0061, respectively) in the osteoporosis rats.
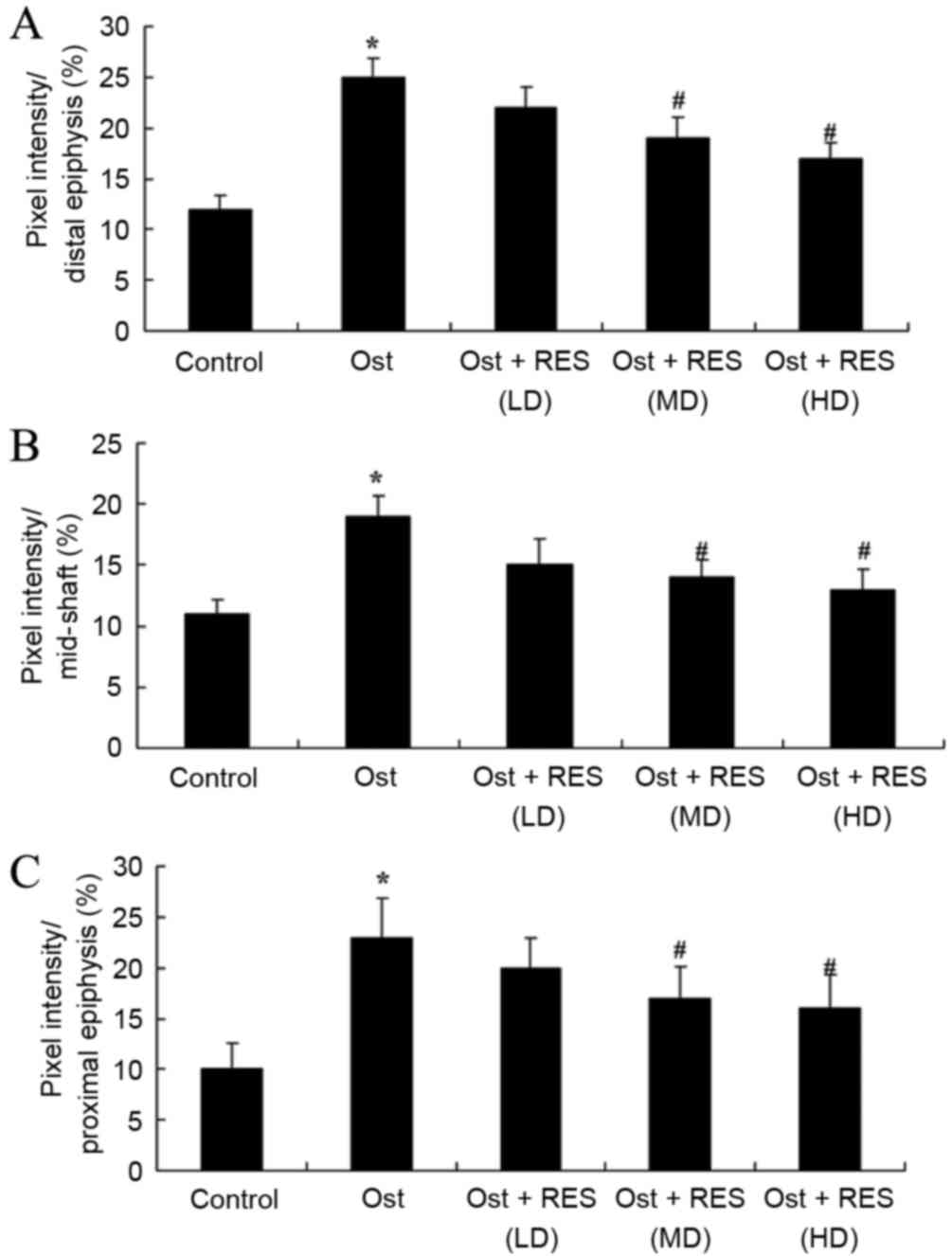
Femoral porosity
When compared with the normal control group, pixel
intensity (distal, mid-shaft and proximal epiphysis) was evidently
enhanced in the osteoporosis group (Fig.
6; P=0.0034, P=0.0069 and P=0.0041, respectively). No
significant intergroup difference was observed between the
osteoporosis and low-dose resveratrol groups for pixel intensity in
osteoporosis rats (Fig. 6).
Furthermore, treatment with middle- or high-dose resveratrol
significantly reduced the pixel intensity (distal, mid-shaft and
proximal epiphysis) in the osteoporosis rats (Fig. 6; P=0.0081 and P=0.0062; P=0.0093 and
P=0.0078; P=0.0087 and P=0.0067, respectively).
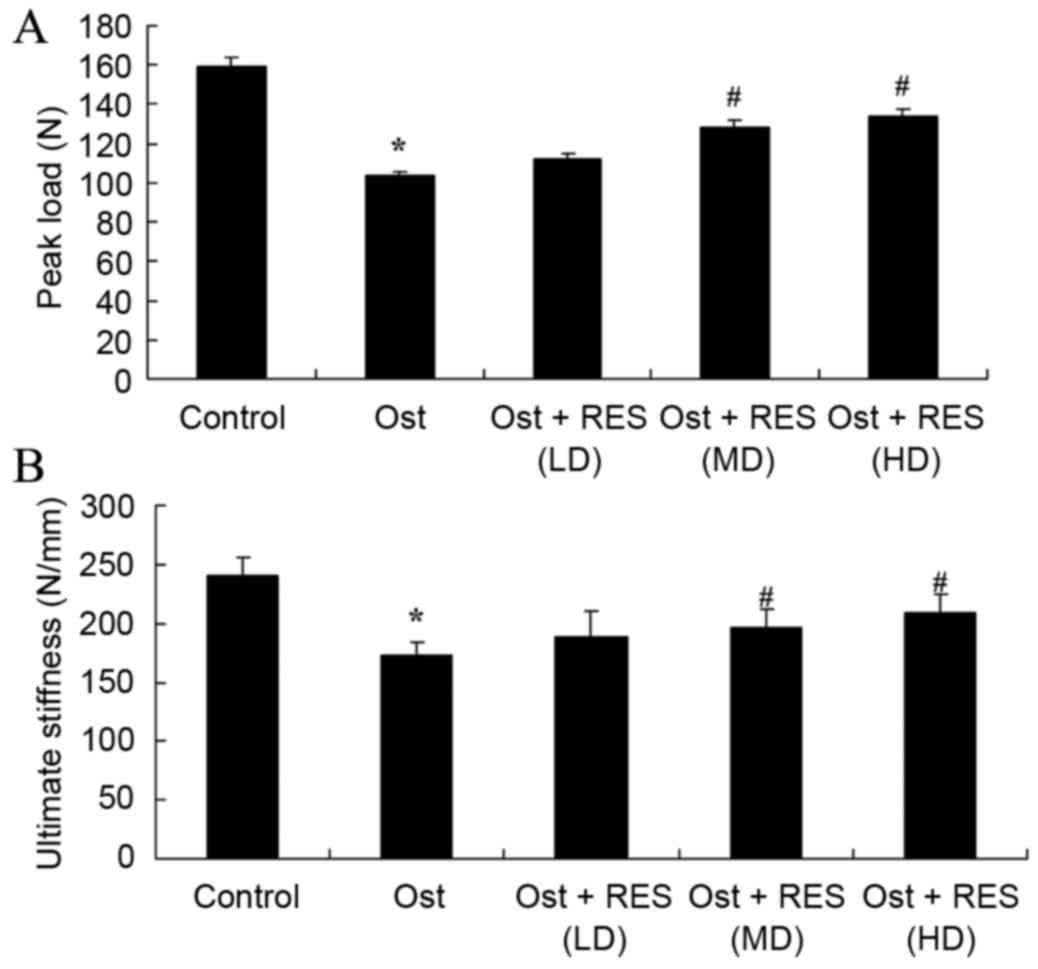
Bone mechanical tests
The present study found that there was a significant
inhibition in the percentages of peak load and ultimate stiffness
in the osteoporosis group compared with the normal control group
(Fig. 7; P=0.0044, P=0.0078).
However, there was no significant difference between the
osteoporosis and low-dose resveratrol groups (Fig. 7). By contrast, treatment with middle-
or high-dose resveratrol significantly inhibited the percentages of
peak load and ultimate stiffness in the osteoporosis rats, as shown
in Fig. 7 (P=0.0062, P=0.038;
P=0.0081, P=0.0073).
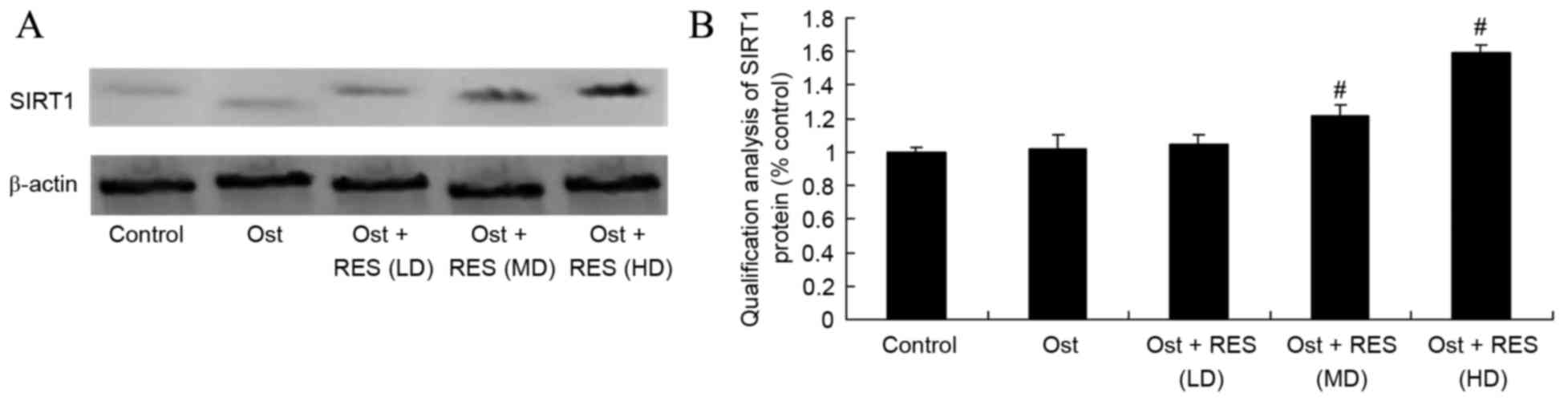
Treatment effect on SIRT1 signaling
pathway
Western blot assay was performed to measure the
protein expression of SIRT1 in the rat tissues. As shown in
Fig. 8, there was no significant
difference in the SIRT1 protein levels among the normal control,
osteoporosis and low-dose resveratrol groups. By contrast,
treatment with middle-dose or high-dose resveratrol significantly
activated the protein expression of SIRT1 in the osteoporosis rats
(Fig. 8, P=0.0076, P=0.0048).
Treatment effect on NF-κB signaling
pathway
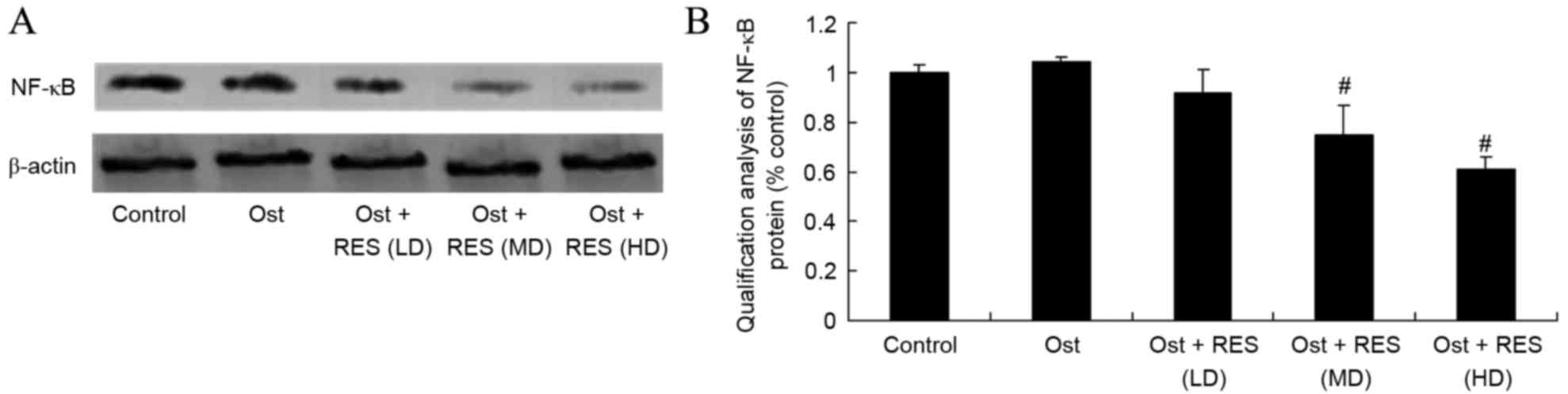
As shown in Fig. 9,
the protein expression of NF-κB/p65 in the normal control group was
very similar to that in the osteoporosis or low-dose resveratrol
groups (P=0.4368, P=0.0562). However, middle-dose or high-dose
resveratrol significantly suppressed the protein expression of
NF-κB/p65 in the osteoporosis rats (Fig.
9; P=0.0036, P=0.0013).
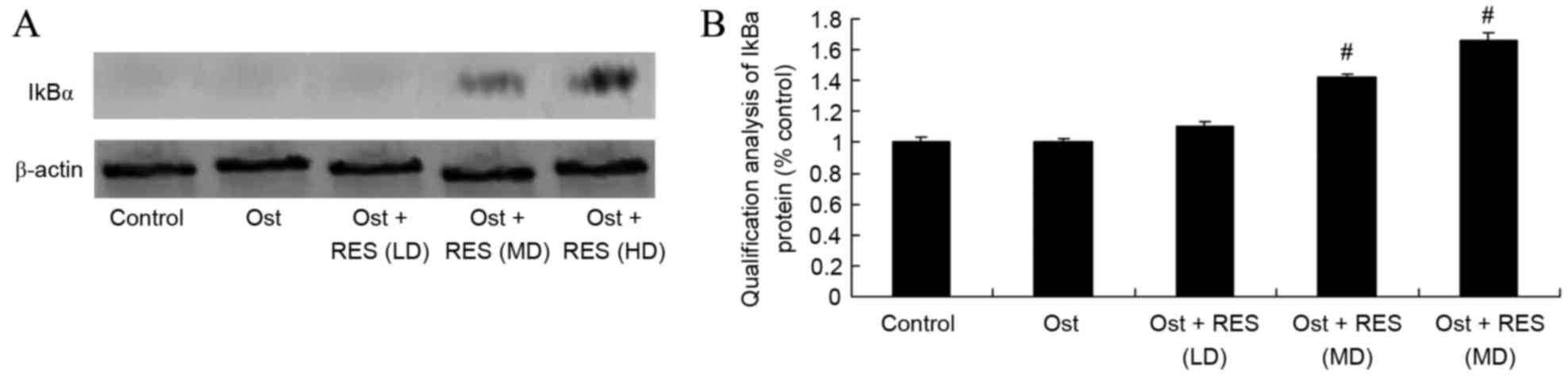
Treatment effect on IkBα signaling
pathway
As revealed by the western blot results, there was
no significant difference in the IkBa protein expression levels
among the normal control, osteoporosis and low-dose resveratrol
groups (Fig. 10). However,
middle-dose or high-dose resveratrol treatment significantly
promoted the protein expression of IkBα in the osteoporosis rats
(Fig. 10; P=0.0051, P=0.0037).
Discussion
As one of the diseases predominantly presenting in
the elderly, an increasing number of individuals suffer from
osteoporosis and osteoporotic fractures as a result of the increase
in the elderly population (21).
Osteoporosis has already become a problem for healthcare in the
aging population; however, it has yet to attract sufficient
research attention (22). The
morbidity of osteoporosis progresses rapidly and has become a
common disease in the elderly, due to the rapid development of
social civilization and economy, growth in the living standards,
and changes of life styles and habits, particularly in cities and
areas with increasing aging of population (23). In the present study, it was verified
that treatment with middle-dose or high-dose resveratrol markedly
increased the BMD in osteoporosis rats. The study by Zhao et
al (12) previously reported
that resveratrol suppresses the excess-iron-induced bone loss
through its antioxidative ability.
The bone matrix includes organic and inorganic
matter. Organic matter mainly contains collagenous fibers
(primarily types I and II collagenous fibers) and a few amorphous
matrixes (24). Inorganic matter is
referring to bone minerals, and includes the crystalline
hydroxyapatite and amorphous colloidal calcium phosphorus.
Collagenous fibers are matrix proteins that constitute the bone
structure and maintain the bone mechanical strength (25). Thus, bone formation is equivalent to
bone mineralization, and refers to the process through which
amorphous calcium phosphate and its bone mineral are deposited in
bone organic matter intervals regularly (26). Ossein, referring to the collagen
content of bones, is the core of bone mineralization. In addition,
estrogen promotes the secretion of type I osteoblast collagen,
alkaline phosphatase and transforming growth factor, so as to
promote bone formation (27). The
current study found that resveratrol treatment significantly
inhibited the serum levels of ALP and OC in osteoporosis rats.
Similarly, Lee et al (14)
have previously suggested that resveratrol promoted bone growth
through the mediation of ALP levels in young rats.
SIRT1 is closely associated with bone metabolism and
bone mass. Histone is able to restrain bone formation, reduce the
expression of OC and ALP in osteoblasts, and reduce the
proliferation and differentiation of osteoblasts (28). The current osteoporosis medicines for
prevention, particularly traditional Chinese medicines, mainly
promote proliferation and differentiation of osteoblasts (29,30). The
inhibition of SIRT1 expression is important for osteoporosis and
for improving the BMD in old-age mice. It has been shown that
resveratrol, an activator of SIRT1 can improve the differentiation
of osteoblasts, as well as reduce the formation of marrow adipose
cells and the quantity of osteoclasts (29). In the present study experiments,
treatment with resveratrol significantly activated the protein
expression of SIRT1 in osteoporosis rats. Sin et al
supported that resveratrol has an effect on muscle injury induced
by compression through SIRT1 protein expression (31). Lee et al (14) also showed that resveratrol induces
human keratinocyte damage through the activation of SIRT1 (14).
NF-κB is closely associated with bone metabolism.
Reduction of NF-κB activity in osteoblasts can strengthen bone
cellular differentiation and mineralization, and reinforce bone
formation (32). It has been shown
that NF-κB activation depends on the phosphorylation of NF-κB/p65.
However, genes that regulate NF-κB in osteoblasts directly need to
be confirmed (33). Apoptosis of
osteoblasts can increase the generation of cancellous bone, and
previous research findings revealed that apoptosis in mice with
diabetes was evidently increased. The NF-κB pathway of osteoclasts
has already been widely studied (34). RANKL, TNF-α or IL-1 activated NF-κB
signal pathway can induce differentiation gene expression of
osteoclasts, lengthen the life of osteoclasts and increase bone
absorption (35). The present study
has clearly demonstrated that resveratrol significantly suppressed
the protein expression of NF-κB/p65 and activated IkBα protein
expression in osteoporosis rats. Similarly, Zhang et al
(32) reported that resveratrol
attenuates liver fibrosis through the suppression of NF-κB.
In conclusion, the present study confirmed that
resveratrol significantly increases BMD, and inhibits the
percentages of peak load and ultimate stiffness in osteoporosis
rats. An important finding is that the beneficial effects of
resveratrol also inhibited the serum levels of ALP and OC in
osteoporosis rats, in which mediated by SIRT1- NF-κB signaling
pathway. In future studies, the molecular mechanisms behind
resveratrol-associated protein regulation require further
investigation.
References
|
1
|
Mattei TA, Rehman AA, Issawi A and Fassett
DR: Surgical challenges in the management of cervical kyphotic
deformity in patients with severe osteoporosis: An illustrative
case of a patient with Hajdu-Cheney syndrome. Eur Spine J.
24:2746–2753. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ohtori S, Akazawa T, Murata Y, Kinoshita
T, Yamashita M, Nakagawa K, Inoue G, Nakamura J, Orita S, Ochiai N,
et al: Risedronate decreases bone resorption and improves low back
pain in postmenopausal osteoporosis patients without vertebral
fractures. J Clin Neurosci. 17:209–213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Otrock ZK, Azar ST, Shamseddeen WA, Habr
D, Inati A, Koussa S, Mahfouz RA and Taher AT: Intravenous
zoledronic acid treatment in thalassemia-induced osteoporosis:
Results of a phase II clinical trial. Ann Hematol. 85:605–609.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bastian L, Schils F, Tillman JB and
Fueredi G: SCORE Investigators: A randomized trial comparing 2
techniques of balloon kyphoplasty and curette use for obtaining
vertebral body height restoration and angular-deformity correction
in vertebral compression fractures due to osteoporosis. AJNR Am J
Neuroradiol. 34:666–675. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang Y, Zeng Y, Huang X, Qin Y, Luo W,
Xiang S, Sooranna SR and Pinhu L: Nur77 attenuates endothelin-1
expression via downregulation of NF-κB and p38 MAPK in A549 cells
and in an ARDS rat model. Am J Physiol Lung Cell Mol Physiol.
311:L1023–L1035. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dvir-Ginzberg M and Steinmeyer J: Towards
elucidating the role of SirT1 in osteoarthritis. Front Biosci
(Landmark Ed). 18:343–355. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bai B, Vanhoutte PM and Wang Y:
Loss-of-SIRT1 function during vascular ageing: Hyperphosphorylation
mediated by cyclin-dependent kinase 5. Trends Cardiovasc Med.
24:81–84. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jung-Hynes B and Ahmad N: SIRT1 controls
circadian clock circuitry and promotes cell survival: A connection
with age-related neoplasms. FASEB J. 23:2803–2809. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Javkhedkar AA and Banday AA: Antioxidant
resveratrol restores renal sodium transport regulation in SHR.
Physiol Rep. 3:pii: e12618. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McGill MR, Du K, Weemhoff JL and Jaeschke
H: Critical review of resveratrol in xenobiotic-induced
hepatotoxicity. Food Chem Toxicol. 86:309–318. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shankar S, Singh G and Srivastava RK:
Chemoprevention by resveratrol: Molecular mechanisms and
therapeutic potential. Front Biosci. 12:4839–4854. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao L, Wang Y, Wang Z, Xu Z, Zhang Q and
Yin M: Effects of dietary resveratrol on excess-iron-induced bone
loss via antioxidative character. J Nutr Biochem. 26:1174–1182.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Szkudelski T and Szkudelska K: Resveratrol
and diabetes: From animal to human studies. Biochim Biophys Acta.
1852:1145–1154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee JH, Kim JS, Park SY and Lee YJ:
Resveratrol induces human keratinocyte damage via the activation of
class III histone deacetylase, Sirt1. Oncol Rep. 35:524–529. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kumar S, Eroglu E, Stokes JA III,
Scissum-Gunn K, Saldanha SN, Singh UP, Manne U, Ponnazhagan S and
Mishra MK: Resveratrol induces mitochondria-mediated,
caspase-independent apoptosis in murine prostate cancer cells.
Oncotarget. 8:20895–20908. 2017.PubMed/NCBI
|
|
16
|
Lopez MS, Dempsey RJ and Vemuganti R:
Resveratrol neuroprotection in stroke and traumatic CNS injury.
Neurochem Int. 89:75–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Ligt M, Timmers S and Schrauwen P:
Resveratrol and obesity: Can resveratrol relieve metabolic
disturbances? Biochim Biophys Acta. 1852:1137–1144. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ozcicek A, Cetin N, Cimen Keskin F,
Tumkaya L, Malkoc I, Gulaboglu M, Yarali O and Suleyman B: The
impact of resveratrol on oxidative stress induced by methotrexate
in rat ileum tissue: Evaluation of biochemical and
histopathological features and analysis of gene expression. Med
Princ Pract. 25:181–186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mathavan N, Bosemark P, Isaksson H and
Tägil M: Investigating the synergistic efficacy of BMP-7 and
zoledronate on bone allografts using an open rat osteotomy model.
Bone. 56:440–448. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khajuria DK, Razdan R, Mahapatra DR and
Bhat MR: Osteoprotective effect of propranolol in ovariectomized
rats: A comparison with zoledronic acid and alfacalcidol. J Orthop
Sci. 18:832–842. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gamsjaeger S, Buchinger B, Zwettler E,
Recker R, Black D, Gasser JA, Eriksen EF, Klaushofer K and
Paschalis EP: Bone material properties in actively bone-forming
trabeculae in postmenopausal women with osteoporosis after three
years of treatment with once-yearly Zoledronic acid. J Bone Miner
Res. 26:12–18. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haines CJ, Chung TK, Leung PC, Hsu SY and
Leung DH: Calcium supplementation and bone mineral density in
postmenopausal women using estrogen replacement therapy. Bone.
16:529–531. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang T, Pang L, Huang H and Wang WY:
Observation on influence of bone metabolism biochemical indices of
senile osteoporosis treated with distant acupuncture and nearby
tuina. Zhongguo Zhen Jiu. 32:13–16. 2012.(In Chinese). PubMed/NCBI
|
|
24
|
Pedraza CE, Marelli B, Chicatun F, McKee
MD and Nazhat SN: An in vitro assessment of a cell-containing
collagenous extracellular matrix-like scaffold for bone tissue
engineering. Tissue Eng Part A. 16:781–793. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reid JW, Pietak A, Sayer M, Dunfield D and
Smith TJ: Phase formation and evolution in the silicon substituted
tricalcium phosphate/apatite system. Biomaterials. 26:2887–2897.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Levaot N, Simoncic PD, Dimitriou ID,
Scotter A, La Rose J, Ng AH, Willett TL, Wang CJ, Janmohamed S,
Grynpas M, et al: 3BP2-deficient mice are osteoporotic with
impaired osteoblast and osteoclast functions. J Clin Invest.
121:3244–3257. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nowwarote N, Osathanon T, Jitjaturunt P,
Manopattanasoontorn S and Pavasant P: Asiaticoside induces type I
collagen synthesis and osteogenic differentiation in human
periodontal ligament cells. Phytother Res. 27:457–462. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He N, Zhu X, He W, Zhao S, Zhao W and Zhu
C: Resveratrol inhibits the hydrogen dioxide-induced apoptosis via
Sirt 1 activation in osteoblast cells. Biosci Biotechnol Biochem.
79:1779–1786. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shakibaei M, Shayan P, Busch F, Aldinger
C, Buhrmann C, Lueders C and Mobasheri A: Resveratrol mediated
modulation of Sirt-1/Runx2 promotes osteogenic differentiation of
mesenchymal stem cells: Potential role of Runx2 deacetylation. PLoS
One. 7:e357122012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saravanan S, Vimalraj S, Vairamani M and
Selvamurugan N: Role of mesoporous wollastonite (Calcium Silicate)
in mesenchymal stem cell proliferation and osteoblast
differentiation: A cellular and molecular study. J Biomed
Nanotechnol. 11:1124–1138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sin TK, Yung BY, Yip SP, Chan LW, Wong CS,
Tam EW and Siu PM: SIRT1-dependent myoprotective effects of
resveratrol on muscle injury induced by compression. Front Physiol.
6:2932015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang F, Lu M, Wang H and Ren T: Aspirin
attenuates angiotensin II-induced inflammation in bone marrow
mesenchymal stem cells via the inhibition of ERK1/2 and NF-κB
activation. Biomed Rep. 1:930–934. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu CM, Chen PC, Li TM, Fong YC and Tang
CH: Si-Wu-tang extract stimulates bone formation through
PI3K/Akt/NF-κB signaling pathways in osteoblasts. BMC Complement
Altern Med. 13:2772013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yao Z, Li Y, Yin X, Dong Y, Xing L and
Boyce BF: NF-κB RelB negatively regulates osteoblast
differentiation and bone formation. J Bone Miner Res. 29:866–877.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tsubaki M, Takeda T, Kino T, Itoh T, Imano
M, Tanabe G, Muraoka O, Satou T and Nishida S: Mangiferin
suppresses CIA by suppressing the expression of TNF-α, IL-6, IL-1β
and RANKL through inhibiting the activation of NF-κB and ERK1/2. Am
J Transl Res. 7:1371–1381. 2015.PubMed/NCBI
|