Introduction
Inflammatory bowel disease (IBD) is characterized by
chronic intestinal inflammation and is predominantly comprised of
Crohn's disease (CD) and ulcerative colitis (UC) (1,2).
Approximately 3.6 million people are affected by IBD in the USA and
Europe (2), and the number of
patients with IBD in Asia has increased in recent years (3,4).
Although the exact etiology of IBD remains unknown, it has been
reported that dietary habits, environmental factors, genetic
susceptibility and infectious microbes may contribute to its
development (5–8). At the molecular level, the pathogenesis
of IBD involves an imbalance between proinflammatory cytokines,
including tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ),
interleukin (IL)-1β, IL-6 and IL-12, and anti-inflammatory
cytokines, including IL-4, IL-10 and IL-11 (9–11). As
one of the most important regulators of proinflammatory gene
expression, nuclear factor (NF)-κB serves several functions
(12). In the cytoplasm, it exists
in a stable complex form with inhibitory κB (IκB). The NF-κB-IκB
complex is disrupted by the phosphorylation of IκB, and NF-κB is
subsequently translocated to the nucleus and bound to DNA binding
sites, which inducesthe transcription of target genes associated
with inflammatory responses (13).
Attenuating the activity of NF-κB has been used to treat a variety
of immune disorders, including autoimmune and inflammatory diseases
(14).
Proinflammatory cytokines, including IL-1, IL-6, and
TNF-α, are overexpressed in patients with IBD (12). Furthermore, a large population of
infiltrated macrophages, which release inflammatory mediators
including histamine, prostaglandin E2, nitric oxide and reactive
oxygen species, may be detected in the patients' mucosa (3,4).
Macrophages also secrete proteases that damage tissue by degrading
the extracellular matrix (14).
Immunoregulation of active macrophages may therefore be an
efficient treatment for IBD (15).
It has been reported that limiting colonic
inflammation via anti-inflammatory agents may reduce the risk of
developing UC-associated cancer (2).
Various anti-inflammatory agents, including 5-aminosalic acid,
corticosteroids and immunosuppressive agents, are widely used to
treat IBD (14,16,17).
However, the serious side effects (including weight gain,
osteoporosis, nausea and poor immunity) and high recrudescence
rates of these agents limit their clinical applications (18). The conventional drug
salicylazosulfapyridine (SASP) has been reported to induce
chromosome aberrations and sister chromatid exchanges (19). Novel therapies and alternative
medicines are therefore urgently needed. Herbal medicines have
potential as treatments for IBD due to their low toxicity profiles
and high patient compliance (7).
Fagopyrum cymosum (Trev.) Meisn (Fag), which
is a herbal rhizome of the Polygonaceae family (20), and buckwheat species have been widely
used to treat bacterial dysentery, lung disease (21), irritable bowel syndrome (IBS) and
rheumatism (22–25). Previous chemical studies have
revealed that Fag rhizomes contain compounds that are effective for
the treatment of inflammatory disease, including phenolics,
flavonoids, β-sitosterol, hecogenin, p-coumaric acid,
ferulic acid, luteolin, dimericprocyanidin, glutinone,
protocatechuic acid, epicatechin and shakuchirin (20,21,26).
A previous study demonstrated that Fag is able to
ameliorate hyperalgesia in rats with IBS (25). The hypothesis is that Fag reduces
intestinal inflammation and enhances the function of mucosal
epithelium via regulating the structure and function of tight
junctions. To the best of our knowledge, the therapeutic effects of
Fag on IBD have not previously been explored. In the present study,
a 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced model of acute
murine colitis was used to assess the therapeutic effects of Fag
extract on IBD. To further investigate the anti-inflammatory
effects of Fag at the molecular level, the phosphorylation of IκB
and the nuclear concentration of NF-κB were measured in
lipopolysaccharide (LPS)-induced RAW264.7 macrophages in
vitro.
Materials and methods
Plant materials and reagents
Fagopyrum cymosum was purchased from Jiangsu
Traffic Hospital (Nanjing, China). All samples were identified by
Dr Shengjin Liu (Department of Medicinal Plants, Nanjing University
of Chinese Medicine, Nanjing, China). TNBS was obtained from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). SASP was supplied
by Aladdin Shanghai Biochemical Technology Co., Ltd. (Shanghai,
China). LPS was purchased from Sigma-Aldrich (Merck KGaA).
Extraction of Fag
Fag was extracted twice (100 g in 800 ml 50%
ethanol) by conventional refluxing for 2 h. The extracts were
combined, filtered and concentrated under reduced pressure in a
vacuum at 60°C to form a residue (9.48 g). The residue was resolved
in 0.5% sodium carboxyl methylcellulose (Sigma-Aldrich; Merck KGaA)
for animal experiments and in vitro testing.
High-performance liquid chromatography
(HPLC) analysis
The Agilent 1260 HPLC system (Agilent Technologies,
Inc., Santa Clara, CA, USA), consisting of a quaternary pump,
autosampler, column oven and diode array detector, was utilized to
analyze the samples. Chromatographic separation was performed using
a SepaxGP-C18 column (5.0 µm, 4.6×250 mm; Sepax Technologies, Inc.,
Newark, DE, USA) at 40°C. The flow rate was 1.0 ml/min and the
injection volume was 20 µl. The mobile phase was a mixture of
phosphoric acid solution (pH 3.0; A) and acetonitrile (B;
A:B=8:92). The chromatµograms were recorded at 220 nm. For
quantitative analysis, the Fag extract and the standard solution
mixtures of protocatechuic acid, catechin, epicatechin,
procyanidins B1 and procyanidins B2 were
analyzed under the conditions described above (40°C). Compounds
were run at six different concentrations (protocatechuic acid,
13.47–215.52 µg/ml; catechin, 10.21–163.36 µg/ml; epicatechin,
12.35–197.60 µg/ml; procyanidins B1, 6.16–98.56 µg/ml;
and procyanidins B2, 5.99 to 95.84 µg/ml) with a 20 µl
injection volume. Compound content in the extract was assessed
using linear regression analysis and was demonstrated to be linear
in the range with a correlation coefficient of 0.997–0.999.
Animal experiments
A total of 58 male BALB/c mice (8 weeks old, 18–22
g) were obtained from Nantong University (Nantong, China;
certificate no. SCXK-2008-0010). The mice were housed in an
air-conditioned room at 20–22°C and 80% humidity with a 12-h
light/dark cycle and were provided with ad libitum access to
standard laboratory chow and water. Animal experiments were
conducted in accordance with protocols approved by the Animal Ethic
Committee of the Nanjing University of Chinese Medicine. Mice were
randomly divided into the following six groups: Control group
(n=8), TNBS-induced colitis groups treated with different
concentrations of Fag (n=10 per group; 0, 0.57, 1.14 and 2.28 g/kg)
and the 200 mg/kg SASP group (n=10). Acute colitis was induced in
BALB/c mice as previously described with modifications (27). Briefly, mice were fasted for 24 h and
anesthetized with pentobarbital (50 mg/kg; Sigma-Aldrich; Merck
KGaA). TNBS solution (2.5% w/v; 100 µl) in 50% ethanol was
administered into the colon via a thin catheter 4 cm proximal to
the anus. The control group received vehicle (50% ethanol) alone.
Fag and SASP were orally administrated once a day for 3 days
following TNBS treatment. Mice were sacrificed 24 h following the
final administration of test agents.
Macroscopic scoring and histological
analysis of colitis
Mice were inspected and weighed daily. Following the
induction of colitis, animals were sacrificed and colons were
harvested and gently washed with ice-cold PBS. Colonic tissue was
then stored at −80°C for further experiments.
For histological analysis, colon tissues were fixed
in 4% paraformaldehyde at room temperature for 24 h, dehydrated in
a graded series of ethanol, embedded in paraffin and finally cut
into 4-µm thick sections. The samples were stained with hematoxylin
and eosin (room temperature, hematoxylin for 5 min and eosin for 2
min) in accordance with the standard procedures for histological
evaluation (28). Histological
scores were calculated by a blinded investigator on a scale from
0–9 based on the following criteria for inflammation: i) Erythema,
ii) hemorrhage, iii) edema, iv) stricture formation, v) ulceration,
vi) fecal blood, vii) presence of mucus, viii) diarrhea and ix)
adhesions, with 1 point awarded for each parameter observed using a
light microscope at magnification, ×20. A maximum score of 9
indicated severe colitis with an overall diffuse pattern of chronic
changes.
Colonic myeloperoxidase (MPO)
activity
MPO activity is an indicator of neutrophil
infiltration into the inflamed colon (n=6) (26,27). MPO
activity was assessed using an MPO activity kit (cat. no. A044;
Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Briefly, colons were homogenized in 10 mM PBS containing 0.5%
hexadecyltrimethylammonium bromide (pH 7.0), and centrifuged at 4°C
and 12,000 × g for 10 min. The supernatant (50 µl) was added to a
reaction mixture containing 0.1 mM H2O2 and
1.6 mM tetramethylbenzidine and incubated at 37°C. Absorbance was
measured at 650 nm. Myeloperoxidase activity was defined as the
quantity degrading 1 µmol/ml of peroxide at 37°C and expressed as
U/mg.
Immunohistochemical evaluation
F4/80 positive inflammatory cell infiltration
analysis was performed on paraffin-embedded colon tissue sections.
The sections were deparaffinized, rehydrated with xylene and graded
ethanol solutions, and washed with PBS. After blocking with 5%
bovine serum albumin (Sigma-Aldrich; Merck KGaA) for 1 h at 37°C,
the sections were incubated with primary antibodies against F4/80
(cat. no. 14-4801-81; eBioscience; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at a dilution of 1:50 for 2 h at 37°C, washed
with PBS and subsequently co-incubated with biotinylated secondary
antibody (cat. no. KS010; Nanjing Jiancheng Bioengineering
Institute) for 30 min at room temperature at a dilution of 1:2,000.
Following washing with PBS (pH 7.4), tissue sections were incubated
at 37°C with a horseradish peroxidase (HRP)-streptavidin complex
(cat. no. KS001; Nanjing Jiancheng Bioengineering Institute) to
detect secondary antibody for 30 min. Sections were stained with
DAB at room temperature for 5 min and mounted according to standard
protocols and observed under a light microscope at magnification,
×20.
Determination of cytokine and LPS in
plasma
The mouse plasma concentrations (n=6) of IL-1β (cat.
no. 432601), IL-6 (cat. no. 431304), and TNF-α (cat. no. 430904)
were determined using commercially available ELISA kits (BioLegend,
Inc., San Diego, CA, USA), and the lower limit of quantification
was 7.8 pg/ml for each cytokine. LPS (n=6) was measured using a
Limulus Amebocyte Lysate assay kit (Xiamen Bioendo Technology, Co.,
Ltd., Xiamen, China) according to the manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from colonic tissue (n=5)
using TRIzol (Thermo Fisher Scientific, Inc.). A Takara PrimeScript
First Strand cDNA Synthesis kit (Takara Bio, Inc., Otsu, Japan) was
employed for the RT reactions with a temperature protocol as
follows: 37°C for 15 min, 85°C for 15 sec and 4°C for 10 min. qPCR
analysis was performed in an ABI StepOnePlus Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) with a
temperature protocol as follows: 95°C for 3 min; 95°C for 30 sec,
58°C for 30 sec and 72°C for 30 sec (35 cycles). The sequences of
primers used in this experiment were as follows: IL-1β forward,
5′-CTGTGTCTTTCCCGTGGACC-3′ and reverse, 5′-CAGCTCATATGGGTCCGACA-3′;
IL-6 forward, 5′-CCAGAAACCGCTATGAAGTTCCT-3′ and reverse,
5′-CACCAGCATCAGTCCCAAGA-3′; TNF-α forward, 5′-ATCCGCGACGTGGAACTG-3′
and reverse, 5′-CAGCTCATATGGGTCCGACA-3′; and β-actin forward,
5′-TCTGGCACCACACCTTCTA-3′ and reverse, 5′-AGGCATACAGGGACAGCAC-3′.
The SYBR-Green PCR mix kit (Takara Bio, Inc.) was used to quantify
gene expression. Reactions were performed in a total volume of 20
µl following the manufacturer's protocol. Three replicates were
performed for each qPCR run. The mRNA concentrations of all target
genes were normalized to that of the β-actin in each sample using
the 2−∆∆Cq method (29).
Cell culture and treatment
Raw264.7 cells (Nanjing KeyGen Biotech Co., Ltd.,
Nanjing, China) were treated with 0, 5, 10, 20 or 50 µg/ml Fag for
30 min followed by incubation with 0.5 µg/ml LPS for 24 h. Cells
with no treatment were set as the control group, and the cells only
treated with LPS were set as model group. Dexamethasone (Dex; 1 µM)
was selected as the positive drug and the treatment time was the
same as the Fag treatment. The inhibitory effects of Fag on TNF-α
and IL-6 production were measured using the aforementioned ELISA
kits in supernatants collected from cells of 3 independent
experiments run in triplicate.
Western blotting
Raw264.7 cells were pretreated with 0, 10, 20, or 50
µg/ml Fag for 30 min. LPS was added to a final concentration of
0.5/ml and incubated at 37°C for 4 h. Cells were harvested and
nuclear and cytoplasmic extracts were prepared using Nuclear and
Cytoplasmic Extraction Reagents (Beyotime Institute of
Biotechnology, Haimen, China) with protease inhibitor cocktail
(Sangon Biotech Co., Ltd., Shanghai, China). All protein samples
were quantified using a BCA assay kit (Beyotime Institute of
Biotechnology). The protein samples (50 µg) were separated by 10%
SDS-PAGE and transferred onto a polyvinylidene difluoride membrane
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membrane was
blocked with 5% bovine serum albumin for 1 h at 37°C, followed by
incubation with antibodies against phosphorylated-IκBα (BS1190,
1:500), NF-κB (BS70527, 1:500), cyclooxygenase 2 (Cox-2) (BS1076,
1:500), inducible nitric oxide synthase (iNOS) (BS1267, 1:1,000),
β-actin (AP0060, 1:2,000) or Lamin A (BS1446, 1:2,000) (all from
Bioworld Technology, Inc., St. Louis Park, MN, USA) at 4°C
overnight. Membranes were subsequently incubated at 37°C for 1 h
with an HRP-conjugated secondary antibody (BS13278, 1:10,000;
Bioworld Technology, Inc.). Antibody signals were detected using an
enhanced chemiluminescence kit (Thermo Fisher Scientific, Inc.) and
images were captured using a ChemiDoc XRS+ system (Bio-Rad
Laboratories, Inc.).
Data analysis
Data are expressed as the mean ± standard deviation.
Statistical analysis was performed using a two-tailed Student's
t-test and one-way analysis of variance (with Tukey's post hoc
test). P<0.05 was considered to indicate a statistically
significant difference.
Clinical analysis
A total of 60 patients were recruited from Haian
Hospital of Traidtional Chinese Medicine (Haian, China) from May
2013 to July 2015 [37.8±7.8 years old; 32 (53.3%) male; 28 (46.7%)
female; 48 (80.0%) with stomachache; 58 (96.7%) with diarrhea; 46
(76.6%) with mucous bloody stool] were randomly divided into the
experimental and control groups (n=30 in each). Patients in the
experimental group were administered with 5 jinqiaomaipian tablets
(0.33 g Fag/tablet; Nantong Jinghua Pharmaceutical Co., Ltd.,
Nantong, China) 3 times daily for 2 months. Patients in the control
group were administered with 1 g SASP 4 times per day for the first
month and twice daily for the second month. Glucocorticoid and
5-aminosalicylic acid analogues were avoided throughout the study
period. The clinical study was approved by the Ethics Committee of
Haian Hospital of Traditional Chinese Medicine, and all patients
provided informed consent.
According to the Chinese Consensus on Diagnosis and
Treatment Standard of Inflammatory Bowel Disease (2007), curative
effects were grouped into three levels: Complete remission,
effective and invalid. The total efficiency was calculated by the
following formula: Total efficiency (%) = (total number of complete
remissions/total number of cases) × 100.
Results
Effective components in Fag
extracts
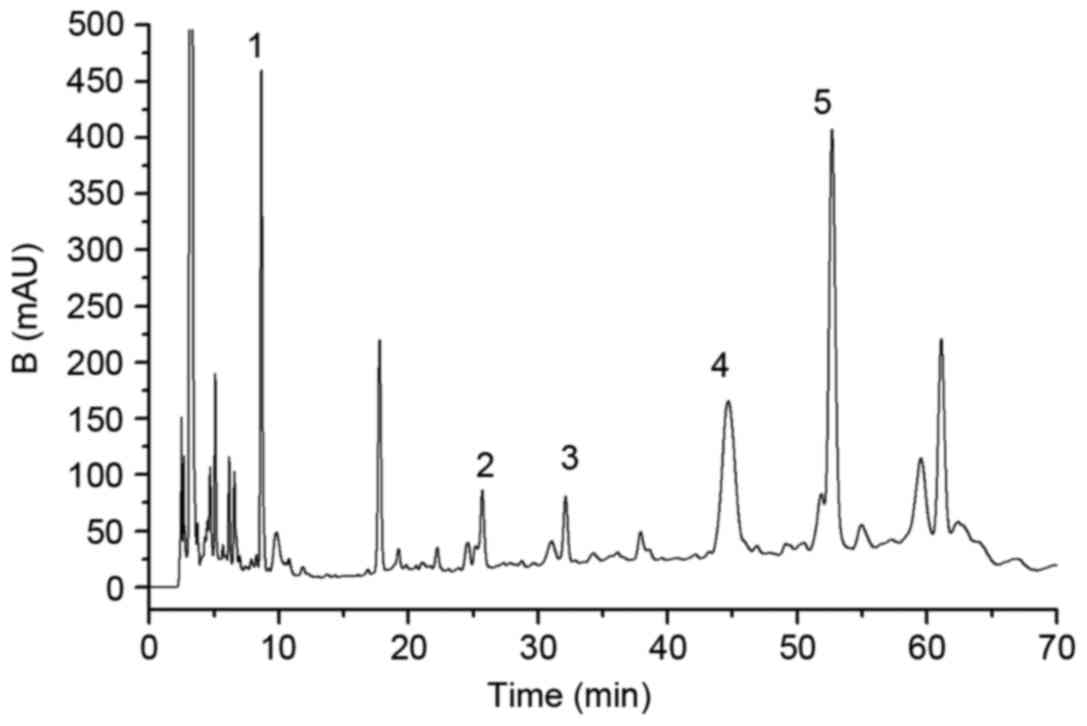
Fag extracts were analyzed using an established
sensitive HPLC method within 70 min and the components were well
separated (Fig. 1). The effective
compounds, including protocatechuic acid, procyanidin
B1, catechin, procyanidin B2 and epicatechin,
had retention times of 9.46, 25.84, 32.19, 44.87 and 53.17 min,
respectively (Fig. 1). Furthermore,
quantification was also performed using HPLC. The results revealed
that there was 6.98 mg protocatechuic acid, 4.07 mg procyanidin
B1, 2.64 mg catechin, 8.43 mg procyanidin B2
and 17.84 mg epicatechin per g of the crude 50% ethanol
extracts.
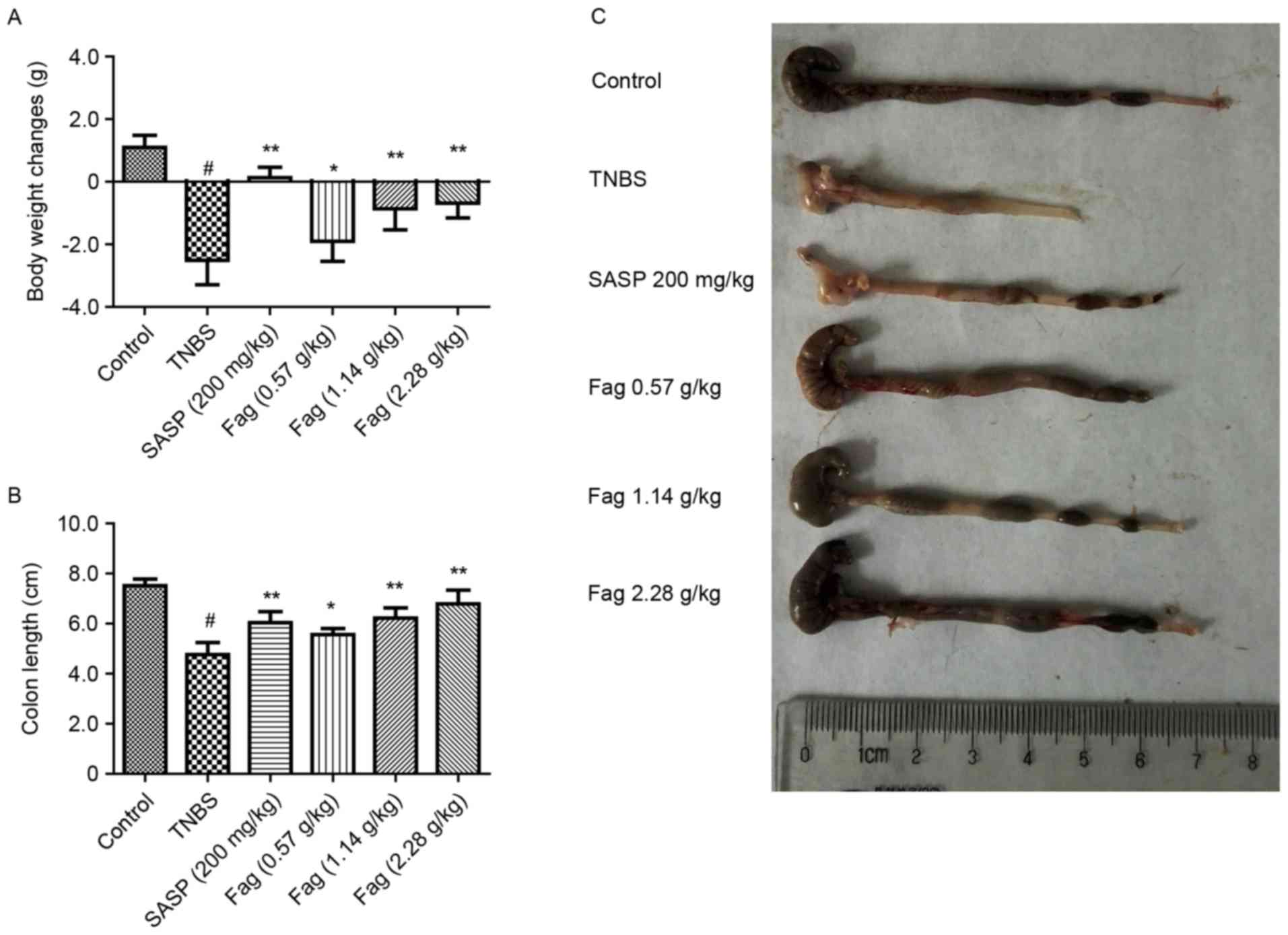
Fag ameliorates the symptoms of
TNBS-induced colitis in mice
It has previously been demonstrated that TNBS
induces colitis in mice (30).
Following treatment with TNBS in 50% ethanol, the mice were
inappetent, exhausted and emaciated, and had diarrhea with bloody
and purulent stool, indicating that severe inflammation in the
colon, or colitis. Weight loss and shortening of the colon were
detected in the TNBS-treated mice (Fig.
2). Treatment with SASP ameliorated TNBS-induced weight loss
and colon shortening when comparing with the TNBS treated mice
(P<0.01). Fag was demonstrated to significantly ameliorate
weight loss and colon shortening compared with the TNBS group in a
dose-dependent manner (0.57 g/kg, P<0.05; 1.14 and 2.28 g/kg,
P<0.01; Fig. 2). Although it was
not as effective as SASP, treatment with Fag extracts improved
colon health in mice with TNBS-induced colitis.
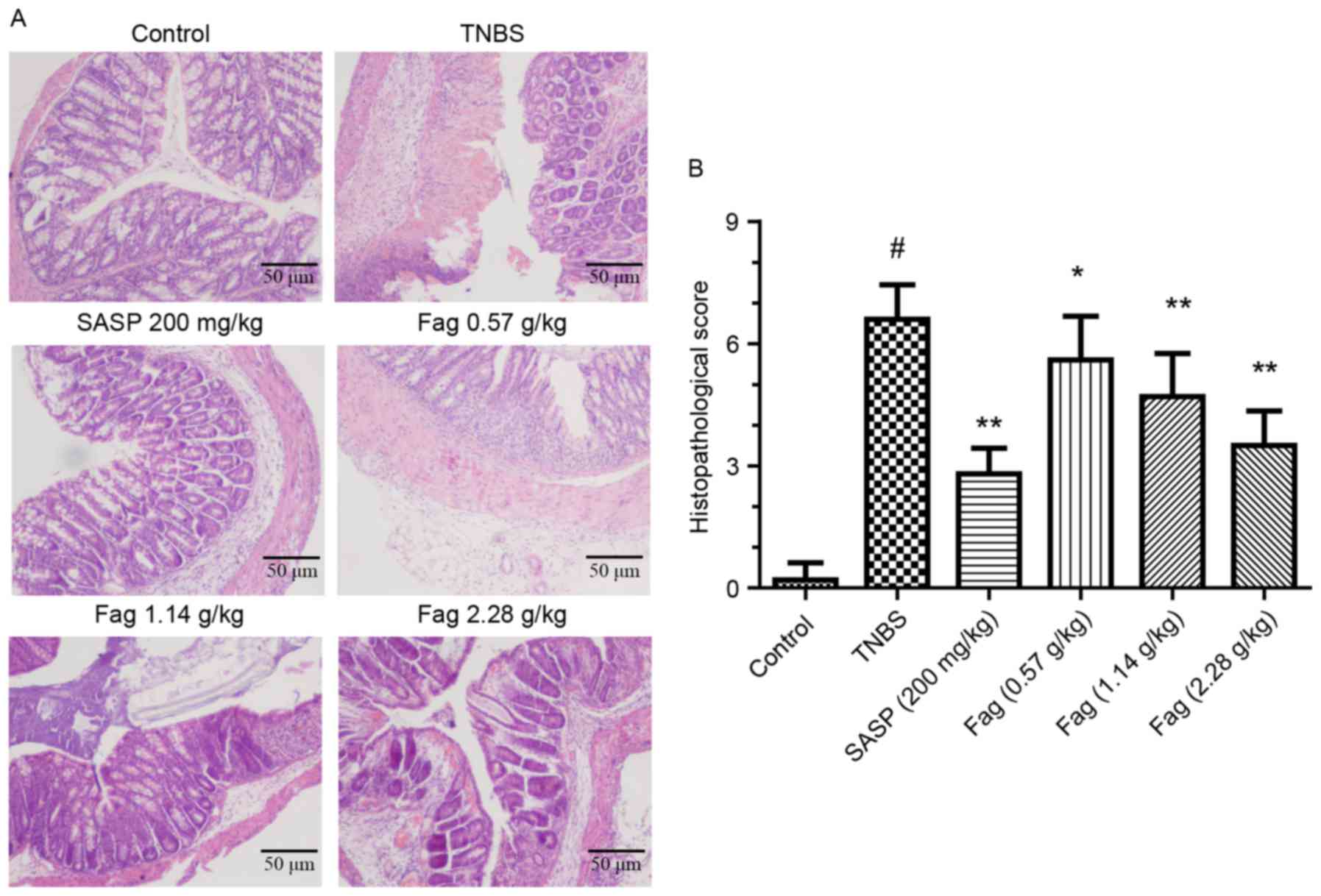
Histopathology
Histopathological analysis was utilized to evaluate
the severity of colonic inflammation and ulceration. There was
clear evidence of mucosal damage in the TNBS-treated mice,
characterized by crypt abscesses, neutrophils, mononuclear
infiltrating glandular epithelium and epithelial hyperplasia
(Fig. 3A). Following treatment with
Fag extract or SASP, mouse colons exhibited intact colonic
architecture with no apparent ulceration, indicating less
inflammatory cell infiltration compared with the TNBS group
(Fig. 3A). Histopathological scoring
was used to quantify the colon damage, and a marked increase was
observed in the TNBS group compared with the control group
(P<0.01; Fig. 3B). Treatment with
Fag extract induced a dose-dependent decrease in histopathological
scores (P<0.05 for the 0.57 g/kg group and P<0.01 for the
1.14 and 2.28 g/kg groups), which was also observed in the SASP
group (P<0.01; Fig. 3B).
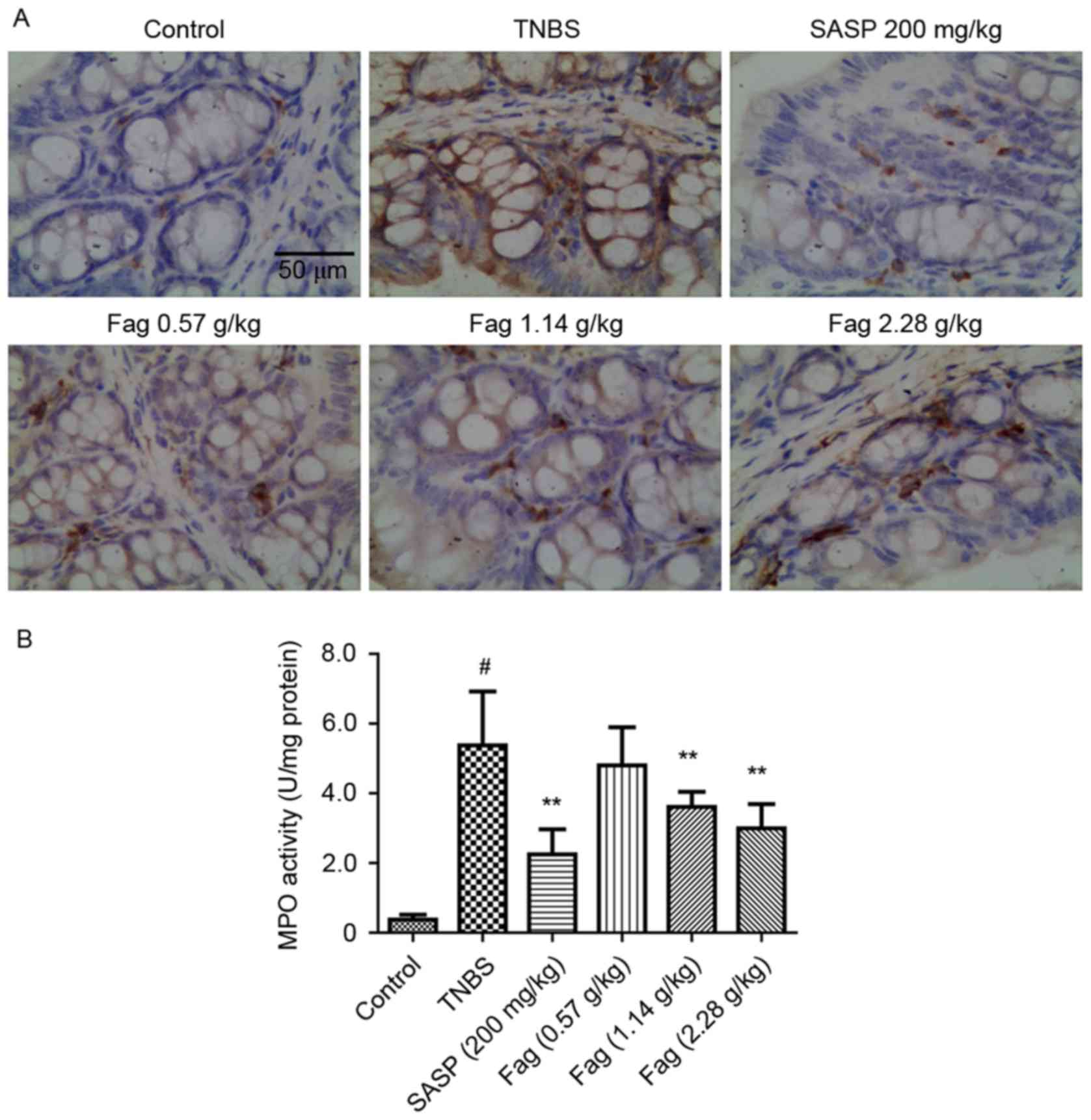
Immunohistochemistry
To further evaluate the protective effects of Fag on
TNBS-induced colitis, immunohistochemical analysis of F4/80 was
employed. An increased number of F4/80 positive inflammatory cells
was observed in TNBS-treated mice mucosa compared with the control
group, whereas treatment with Fag or SASP were observed to inhibit
this increase (Fig. 4A). MPO
activity was also evaluated, and was demonstrated to be
significantly increased in the TNBS group compared with the control
group (14.4 fold; P<0.01; Fig.
4B). The MPO activity increase was significantly ameliorated
following SASP treatment or treatment with 1.14 or 2.28 g/kg Fag
(P<0.01; Fig. 4B).
Fag decreases proinflammatory cytokine
and LPS levels
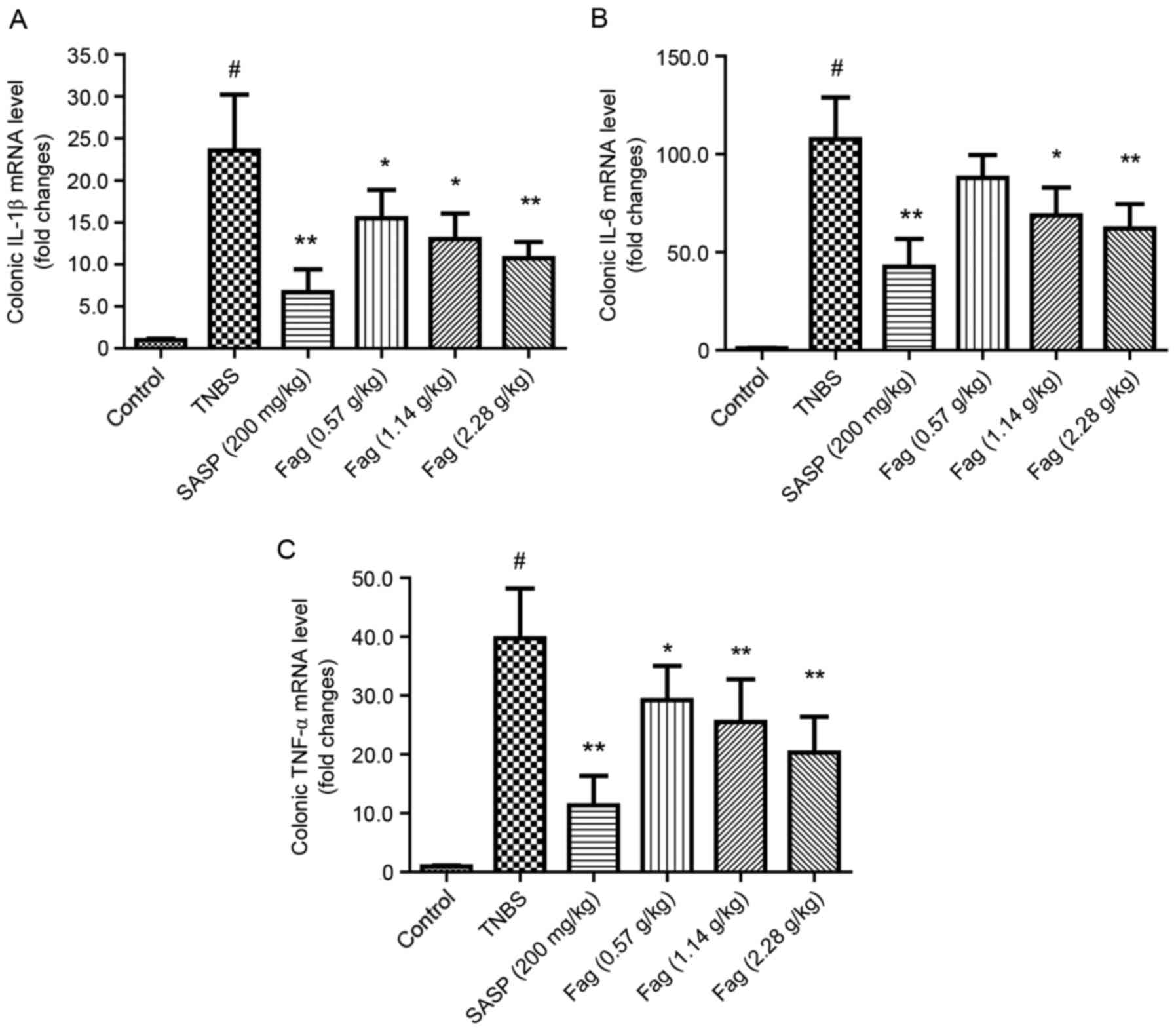
The levels of IL-1β, IL-6 and TNF-α mRNA were
significantly increased in the TNBS group compared with the control
group (P<0.01; Fig. 5). Following
treatment with SASP, the mRNA expressions of IL-1β, IL-6 and TNF-α
were significantly decreased compared with the TNBS-treated group
(all P<0.01; Fig. 5). Fag also
significantly attenuated the expression of IL-1β, IL-6 and TNF-α
compared with the TNBS group in a dose-dependent manner (P<0.05;
Fig. 5).
LPS is the major component of the outer membrane of
Gram-negative bacteria, and it has been reported that LPS is
upregulated in patients with IBD due to gut leakage and microbiota
dysbiosis (31). Following treatment
with TNBS for 3 days, the level of LPS in peripheral plasma was
significantly increased in the TNBS group compared with the control
group (P<0.01; Fig. 6A).
Treatment with Fag significantly decreased the LPS level in a
dose-dependent manner (1.14 g/kg, P<0.05; 2.28 g/kg, P<0.01;
Fig. 6A). Furthermore, SASP
treatment significantly ameliorated the TNBS-induced increase in
LPS (P<0.01; Fig. 6A). Plasma
levels of IL-1β, IL-6, and TNF-α were significantly increased in
the TNBS group compared with the control (P<0.01; Fig. 6B-D), and these increases were
significantly ameliorated by treatment with SASP (P<0.01) or Fag
in a dose-dependent manner (1.14 g/kg, P<0.05; 2.28 g/kg,
P<0.01; Fig. 6B-D).
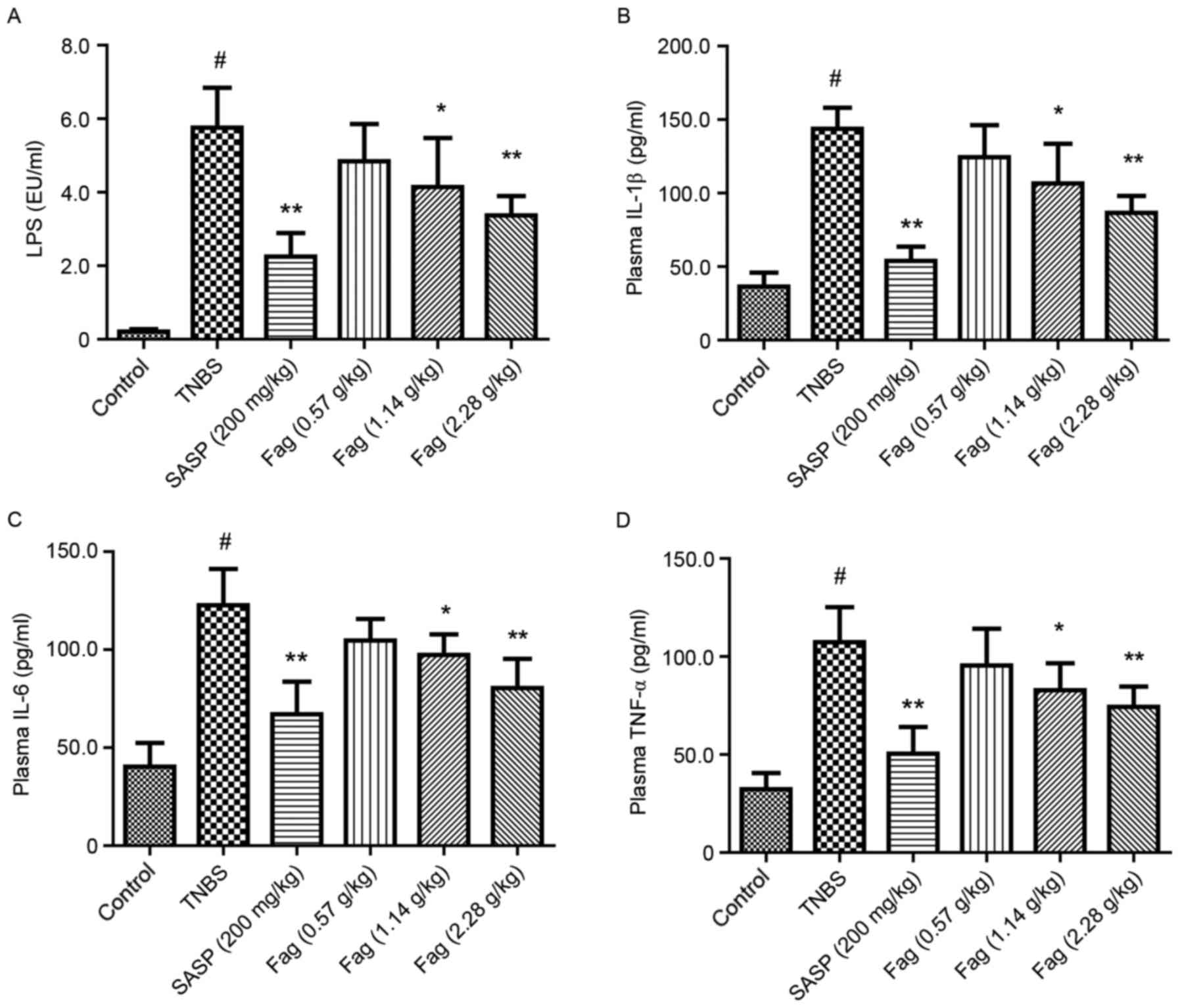 | Figure 6.Fag decreases the plasma levels of
(A) LPS, (B) IL-1β, (C) IL-6 and (D) TNF-α in TNBS-induced colitis
mice. n=6. #P<0.01 vs. control group; *P<0.05 and
**P<0.01, vs. TNBS group. Fag, Fagopyrum cymosum (Trev.)
Meisn; LPS, lipopolysaccharide; IL, interleukin; TNF, tumor
necrosis factor; TNBS, 2,4,6-trinitrobenzenesulfonic acid; SASP,
salicylazosulfapyridine. |
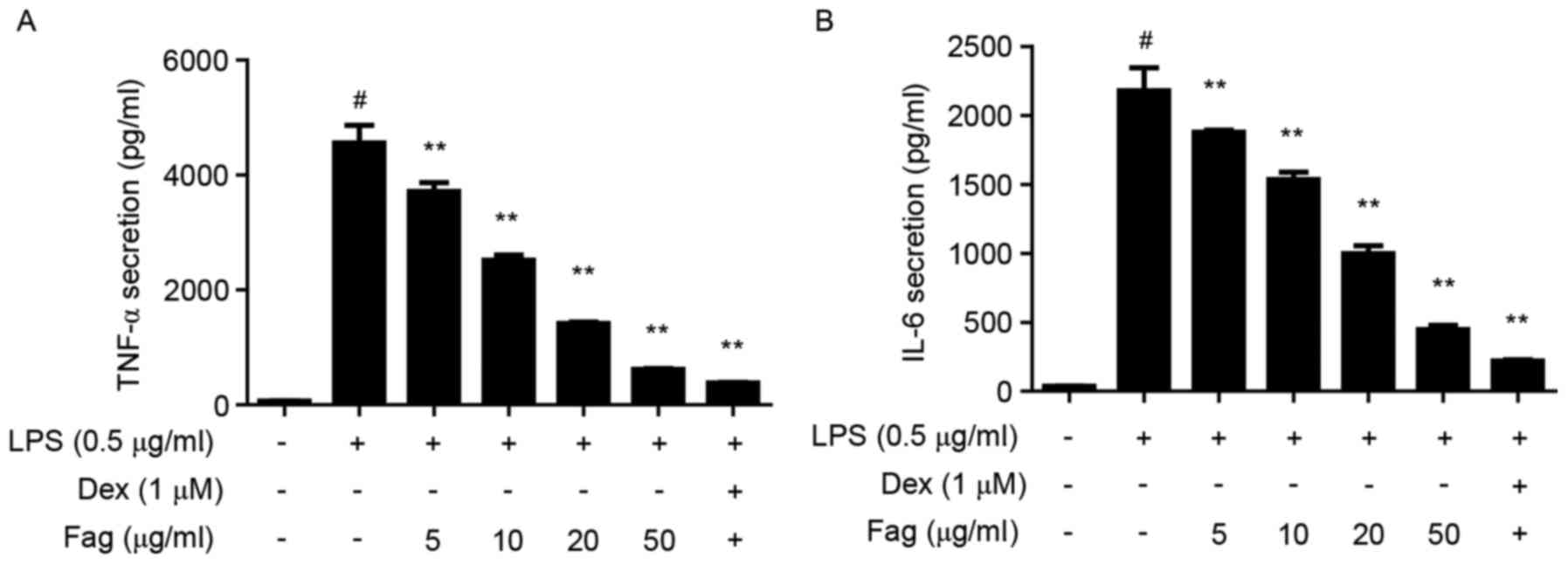
Fag reduces proinflammatory cytokines
in LPS-stimulated macrophages
LPS levels may be used as a marker of inflammation
in vivo, and LPS stimulates the inflammatory response of
macrophages in vitro (31).
Raw264.7 cells were used to verify the in vitro
anti-inflammatory effects of Fag. LPS stimulation significantly
increased the production of TNF-α and IL-6 in RAW264.7 cells
(P<0.01), and Fag treatment significantly ameliorated these
LPS-induced increases (P<0.01; Fig.
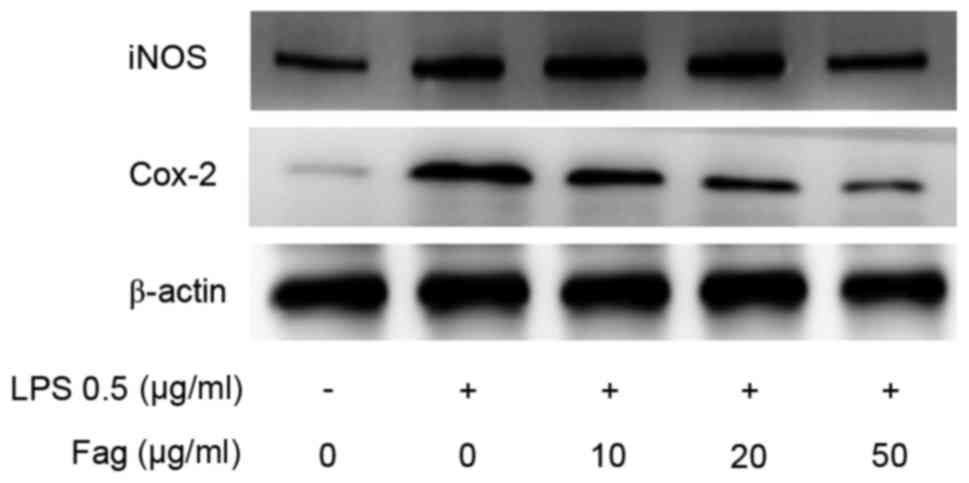
7) in a dose-dependent manner. iNOS and Cox-2 are two
injury-induced enzymes in macrophages. Western blotting results
demonstrated that LPS-induced overexpression of iNOS and Cox-2 was
ameliorated by Fag treatment in vitro (Fig. 8).v
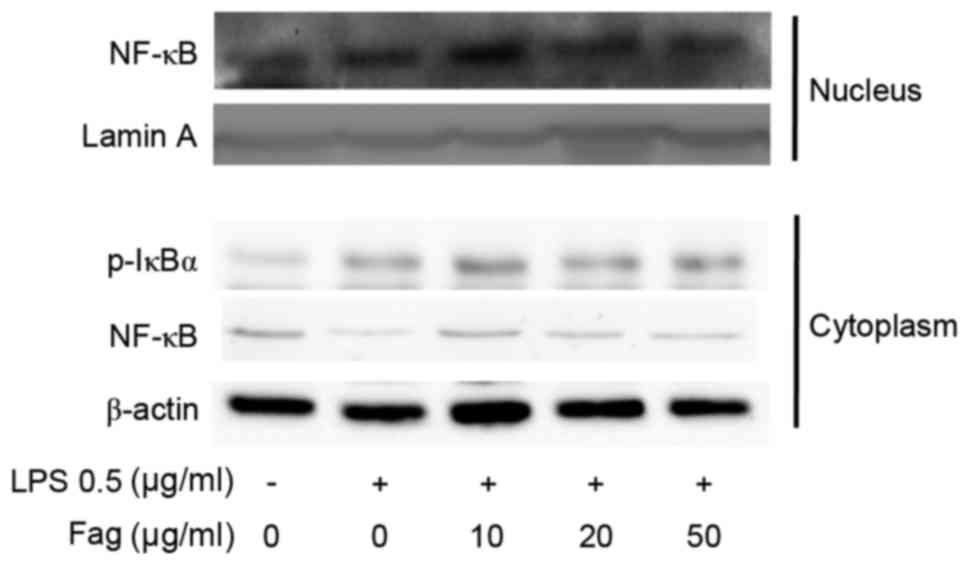
Western blotting was used to investigate the effect
of Fag on NF-κB activation signaling. The results revealed that Fag
attenuated the LPS-induced nuclear translocation of p65 NF-κB in a
dose-dependent manner (Fig. 9).
Although the exact mechanism by which this occurs is not fully
understood, the western blotting results indicate that Fag was able
to inhibit IκBα phosphorylation in LPS-induced cells in
vitro (Fig. 9).
Fag efficiently treatsv IBD with fewer
side effects than SASP
The results of the clinical study demonstrate that
jinqiaomaipian was able to ameliorate stomachache, diarrhea and
mucous bloody stool with a total efficiency of ~80% (Table I). No significant difference was
observed between the control and experimental groups, suggesting
that Fag may be as effective as SASP for the treatment of IBD.
 | Table I.Clinical study of jinqiaomaipian
treatment for ulcerative colitis. |
Table I.
Clinical study of jinqiaomaipian
treatment for ulcerative colitis.
| Group | Clinical
manifestation | Total cases
(n) | Complete remission
(n) | Effective (n) | Invalid (n) | Total efficiency
(%) |
|---|
| Experimental
group | Stomachache | 23 | 13 | 6 | 4 | 82.6 |
|
| Diarrhea | 28 | 12 | 10 | 6 | 78.6 |
|
| Mucous bloody
stool | 24 | 11 | 8 | 5 | 79.2 |
|
| Colonoscopy | 30 | 12 | 13 | 5 | 83.3 |
| Control group | Stomachache | 25 | 11 | 10 | 4 | 84.0 |
|
| Diarrhea | 30 | 9 | 13 | 8 | 73.3 |
|
| Mucous bloody
stool | 22 | 10 | 8 | 4 | 81.8 |
|
| Colonoscopy | 30 | 12 | 11 | 7 | 76.7 |
Of the 30 patients in the SASP group, 9 (30.0%)
experienced side effects (3 nausea, 3 abdominal discomfort, 1
headache, and 2 leukopenia). However, only 1 out of 30 patients
(3.3%) in the experimental group experienced nausea. The incidence
of side effects was significantly lower in the experimental group
(P<0.01; data not shown).
Discussion
Recent studies have demonstrated that IBD cannot be
cured using medication, and treatment mainly focuses on increasing
remission periods and improving patient quality of life (17,32).
Novel pharmacological agents and natural medicines for treating IBD
are urgently needed. TNBS-induced colitis in mice is a
well-established animal model with an enhanced Th1/Th17 response
(27). As the process is similar to
the cluster of differentiation-related immune response (28), the TNBS-induced murine model has been
widely used for screening IBD treatments and exploring the
therapeutic effects of potential agents.
Fag is a traditional Chinese medicine used to treat
bacterial dysentery, lung infection and rheumatism (22,24,26). It
has previously been demonstrated that there are effective treatment
components in the ethanol extract of Fag, including luteolin
(22) and epicatechin (24). Fag has been reported to exert
anti-inflammatory effects in Raw264.7 cells via inhibiting p38 and
c-Jun N-terminal kinase phosphorylation (33) or inducing 67 kD alaminin receptor
internalization (34). To the best
of our knowledge, the present study is the first time that the
anti-inflammatory effects of Fag extract on TNBS-induced colitis
have been reported.
In the present study, the TNBS-induced mouse model
was used to evaluate the protective effects of ethanol extract of
Fag on colitis. The anti-inflammatory effects of Fag in LPS-induced
RAW264.7 macrophages were also verified. The results demonstrated
that oral administration of ethanol extract of Fag ameliorated
TNBS-induced colitis in mice, reducing body weight loss and colon
shortening. The staining results confirmed that Fag effectively
decreased mucosal erosion, submucosal edema and disruption of
crypts and villi, which are crucial for maintaining normal colonic
function (25,35). These mucosal events significantly
affect the structure and function of tight junctions, leading to a
damaged intestinal barrier and immune activation (36). Subsequently, proinflammatory
cytokines are overexpressed, triggering the signaling cascade of
inflammation and leading to inflammatory cell infiltration in the
inflamed mucosa (37). The results
of the present study revealed that ethanol extract of Fag
effectively reduced macrophage infiltration in the colon of
TNBS-induced mice, with F4/80 and colonic MPO activity used as
macrophage markers (35).
On the molecular level, the colonic levels of
proinflammatory cytokines, including IL-1β, IL-6 and TNF-α,
correlate with the degree of inflammation (35). These cytokines serve an important
role in the pathogenesis of various inflammatory diseases (38,39).
Colonic levels of IL-1β, IL-6 and TNF-α mRNA in TNBS-induced
colitis mice were markedly reduced following treatment with Fag.
Previous studies have also reported that IL-6 and TNF-α are able to
significantly decrease barrier function by influencing tight
junction proteins, including claudin, occludin, and zonula
occludens-1 (40,41). The inflamed colon may result in gut
leakage and bacterial translocation (37). In the present study, an increased
plasma LPS level was identified in TNBS-induced colitis mice,
suggesting that bacteria were translocated to the circulation via
the leaky gut (42). The
administration of Fag significantly decreased the plasma LPS level
in TNBS-treated mice, as well as the levels of IL-1β, IL-6 and
TNF-α. To further verify the anti-inflammatory effects of Fag, an
LPS-stimulated murine macrophage cell line was used. The results
revealed that Fag significantly inhibited LPS-induced secretion of
TNF-α and IL-6. Further experiments demonstrated that Fag inhibited
the expression of proinflammatory cytokines via inhibiting the
phosphorylation of IκB.
Compared with the conventional treatment for IBD,
SASP, the incidence of side effects was significantly decreased
with Fag treatment, whereas the therapeutic efficiency was similar.
The results of the present study provide evidence that Fag may be a
suitable alternative herbal medicine for the treatment of IBD in
clinical practice, although identifying the optimal dosage requires
further investigation.
In conclusion, the present study demonstrates that
the ethanol extract of Fag exerts protective effects in
TNBS-induced colitis mice via its anti-inflammatory action. These
results indicate that Fag may be an effective therapeutic treatment
for IBD.
Acknowledgements
The present study was supported by the Specialized
Research Fund for the Doctoral Program of Higher Education (grant
no. 20133237110008), the National Natural Science Funds of China
(grant no. 81503536), the Natural Science Foundation of Jiangsu
Province (grant nos. BK20151567, BK20151008 and BK20140959), the
Jiangsu Provincial Bureau of Traditional Chinese Medicine (grant
no. YB2017066), the Natural Science Foundation of Universities in
Jiangsu Province (grant no. 15KJB360008) and the Key Subject of
Zhoupu Hospital, Pudong New Area, Shanghai (grant no.
zp-xk-2015b-5).
References
|
1
|
Lutgens M, Oijen M, van Mooiweer E, van
der Valk M, Vleggaar F, Siersema P and Oldenburg B: A
risk-profiling approach for surveillance of inflammatory bowel
disease-colorectal carcinoma is more cost-effective: A comparative
cost-effectiveness analysis between international guidelines.
Gastrointest Endosc. 80:842–848. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rubin DT: Why it's time for updated U.S.
colorectal cancer prevention guidelines in inflammatory bowel
disease. Gastrointest Endosc. 80:849–851. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huttenhower C, Kostic AD and Xavier RJ:
Inflammatory bowel disease as a model for translating the
microbiome. Immunity. 40:843–854. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsai SY, Yang TY, Lin CL, Tsai YH, Kuo CF
and Kao CH: Increased risk of varicella zoster virus infection in
inflammatory bowel disease in an Asian population: A nationwide
population-based cohort study. Int J Clin Pract. 69:228–234. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paul S, Wand M, Emerick GT and Richter JM:
The role of latanoprost in an inflammatory bowel disease flare.
Gastroenterol Rep (Oxf). 2:232–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deepak P and Stobaugh DJ: Maternal and
foetal adverse events with tumour necrosis factor-alpha inhibitors
in inflammatory bowel disease. Aliment Pharm Therap. 40:1035–1043.
2014. View Article : Google Scholar
|
|
7
|
Ghouri YA, Richards DM, Rahimi EF, Krill
JT, Jelinek KA and DuPont AW: Systematic review of randomized
controlled trials of probiotics, prebiotics and synbiotics in
inflammatory bowel disease. Clin Exp Gastroenterol. 7:473–487.
2014.PubMed/NCBI
|
|
8
|
Kang A, Zhang S, Shan J and Di L: Gut
microbiota-mediated deglycosylation of ginsenoside Rb-1 in rats: In
vitro and in vivo insights from quantitative ultra-performance
liquid chromatography-mass spectrometry analysis. Anal Methods.
7:6173–6181. 2015. View Article : Google Scholar
|
|
9
|
Heilmann RM, Otoni CC, Jergens AE,
Grutzner N, Suchodolski JS and Steiner JM: Systemic levels of the
anti-inflammatory decoy receptor soluble RAGE (receptor for
advanced glycation end products) are decreased in dogs with
inflammatory bowel disease. Vet Immunol Immunopathol. 161:184–192.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shouval DS, Ebens CL, Murchie R, McCann K,
Rabah R, Klein C, Muise A and Snapper SB: Large b-cell lymphoma in
an adolescent patient with IL-10 receptor deficiency and history of
infantile inflammatory bowel disease. J Pediatr Gastroenterol Nutr.
63:e15–e17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hyams JS: Standardized recording of
parameters related to the natural history of inflammatory bowel
disease: From Montreal to Paris. Dig Dis. 32:337–344. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Wang Y, Zhang F, Wang K, Liu G,
Yang M, Luan Y, Zhao Z, Zhang J, Cao X and Zhang D: Allyl methyl
disulfide inhibits IL-8 and IP-10 secretion in intestinal
epithelial cells via the NF-кB signaling pathway. Int
Immunopharmacol. 27:156–163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nishitani Y, Yamamoto K, Yoshida M, Azuma
T, Kanazawa K, Hashimoto T and Mizuno M: Intestinal
anti-inflammatory activity of luteolin: Role of the aglycone in
NF-κB inactivation in macrophages co-cultured with intestinal
epithelial cells. Biofactors. 39:522–533. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu J, Zhou F, Chen Q, Kang A, Lu M, Liu
W, Zang X, Wang G and Zhang J: Chronic inflammation up-regulates
P-gp in peripheral mononuclear blood cells via the STAT3/Nf-κb
pathway in 2,4,6-trinitrobenzene sulfonic acid-induced colitis
mice. Sci Rep. 5:135582015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Freeman JJ, Rabah R, Hirschl RB, Maspons
A, Meier D and Teitelbaum DH: Anti-TNF-α treatment for
post-anastomotic ulcers and inflammatory bowel disease with
Crohn's-like pathologic changes following intestinal surgery in
pediatric patients. Pediatr Surg Int. 31:77–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Loftus EJ: Biologic therapy in
inflammatory bowel disease. Gastroenterol Clin North Am.
43:xv–xvii. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dave M, Papadakis KA and Faubion WA Jr:
Immunology of inflammatory bowel disease and molecular targets for
biologics. Gastroenterol Clin North Am. 43:405–424. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maranville JC, Micic D, Hanauer SB, Rienzo
AD and Kupfer SS: In vitro sensitivity assays and clinical response
to glucocorticoids in patients with inflammatory bowel disease. J
Crohns Colitis. 8:1539–1547. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bishop JB, Witt KL, Gulati DK and
MacGregor JT: Evaluation of the mutagenicity of the
anti-inflammatory drug salicylazosulfapyridine (SASP). Mutagenesis.
5:549–554. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tian L, Xu L and Yang S: Chemical
composition of Fagopyrum cymosum (Trev.) Meisn. Zhongguo
Zhong Yao Za Zhi. 22:743–765. 1997.(In Chinese). PubMed/NCBI
|
|
21
|
Dong LY, Wang CY, Wu CQ, Jiang Q and Zhang
ZF: Protection and mechanism of Fagopyrum cymosum on lung
injury in rats with Klebsiella pneumonia. Zhong Yao Cai.
35:603–607. 2012.(In Chinese). PubMed/NCBI
|
|
22
|
Chan PK: Inhibition of tumor growth in
vitro by the extract of Fagopyrum cymosum (fago-c). Life
Sci. 72:1851–1858. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen L, Wang P, Guo J and Du G:
Anti-arthritic activity of ethanol extract of Fagopyrum
cymosum with adjuvant-induced arthritis in rats. Pharm Biol.
51:783–789. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu LN, Yan J and Sun ZG: Effect of
Fagopyrum cymosum (Trev.) Meisn alcohol extract on
defecation and isolated colon of diarrhea-IBS rats and its
mechanism. Zhongguo Zhong Xi Yi Jie He Za Zhi. 34:1469–1475.
2014.(In Chinese). PubMed/NCBI
|
|
25
|
Liu L, Cai X, Yan J, Luo Y, Shao M, Lu Y,
Sun Z and Cao P: In Vivo and In Vitro Antinociceptive effect of
Fagopyrum cymosum (Trev.) Meisn extracts: A possible action
by recovering intestinal barrier dysfunction. Evid Based Complement
Alternat Med. 2012:9838012012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shao M, Yang YH, Gao HY, Wu B, Wang LB and
Wu LJ: Phenolic acid derivatives from the rhizome of Fagopyrum
cymosum. Zhongguo Zhong Yao Za Zhi. 30:1591–1593. 2005.(In
Chinese). PubMed/NCBI
|
|
27
|
Witaicenis A, Luchini AC, Hiruma-Lima CA,
Felisbino SL, Garrido-Mesa N, Utrilla P, Gálvez J and Di Stasi LC:
Suppression of TNBS-induced colitis in rats by 4-methylesculetin, a
natural coumarin: Comparison with prednisolone and sulphasalazine.
Chem Biol Interact. 195:76–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cui L, Feng L, Zhang ZH and Jia XB: The
anti-inflammation effect of baicalin on experimental colitis
through inhibiting TLR4/NF-κB pathway activation. Int
Immunopharmacol. 23:294–303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
te Velde AA, Verstege MI and Hommes DW:
Critical appraisal of the current practice in murine TNBS-induced
colitis. Inflamm Bowel Dis. 12:995–999. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pastor RO, Lopez SR, Albéniz AE, Martínez
AH, Sevillano ER and Martínez AA: Serum lipopolysaccharide-binding
protein in endotoxemic patients with inflammatory bowel disease.
Inflamm Bowel Dis. 13:269–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guandalini S: Are probiotics or prebiotics
useful in pediatric irritable bowel syndrome or inflammatory bowel
disease? Front Med (Lausanne). 1:232014.PubMed/NCBI
|
|
33
|
Choi EY, Jin JY, Choi JI, Choi IS and Kim
SJ: Effects of luteolin on the release of nitric oxide and
interleukin-6 by macrophages stimulated with lipopolysaccharide
from Prevotella intermedia. J Periodontol. 82:1509–1517.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang CP, Huang GJ, Huang HC, Chen YC,
Chang CI, Wang SY, Chang HS, Tseng YH, Chien SC and Kuo YH: The
effect of the aerial part of Lindera akoensis on
lipopolysaccharides (LPS)-induced nitric oxide production in
RAW264.7 cells. Int J Mol Sci. 14:9168–9181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Funakoshi T, Yamashita K, Ichikawa N,
Fukai M, Suzuki T, Goto R, Oura T, Kobayashi N, Katsurada T,
Ichihara S, et al: A novel NF-κB inhibitor,
dehydroxymethylepoxyquinomicin, ameliorates inflammatory colonic
injury in mice. J Crohns Colitis. 6:215–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hardee S, Alper A, Pashankar DS and
Morotti RA: Histopathology of duodenal mucosal lesions in pediatric
patients with inflammatory bowel disease: Statistical analysis to
identify distinctive features. Pediatr Dev Pathol. 17:450–454.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Beniwal-Patel P and Saha S: The role of
integrin antagonists in the treatment of inflammatory bowel
disease. Expert Opin Biol Ther. 14:1815–1823. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Munakata S, Tashiro Y, Nishida C, Sato A,
Komiyama H, Shimazu H, Dhahri D, Salama Y, Eiamboonsert S, Takeda
K, et al: Inhibition of plasmin protects against colitis in mice by
suppressing matrix metalloproteinase 9-mediated cytokine release
from myeloid cells. Gastroenterology. 148:565–578. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Castro J, Ocampo Y and Franco L: Cape
gooseberry (Physalis peruviana L.) calyces ameliorate TNBS
acid-induced colitis in rats. J Crohns Colitis. 9:1004–1015. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fischer A, Gluth M, Weege F, Pape UF,
Wiedenmann B, Baumgart DC and Theuring F: Glucocorticoids regulate
barrier function and claudin expression in intestinal epithelial
cells via MKP-1. Am J Physiol Gastrointest Liver Physiol.
306:G218–G228. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li X, Wang Q, Xu H, Tao L, Lu J, Cai L and
Wang C: Somatostatin regulates tight junction proteins expression
in colitis mice. Int J Clin Exp Pathol. 7:2153–2162.
2014.PubMed/NCBI
|
|
42
|
Hering NA and Schulzke JD: Therapeutic
options to modulate barrier defects in inflammatory bowel disease.
Dig Dis. 27:450–454. 2009. View Article : Google Scholar : PubMed/NCBI
|