Introduction
Colorectal cancer (CRC) is the third most common
cancer worldwide (1). Of patients
with CRC, 50–70% are diagnosed at advanced stages (2), and adjuvant chemotherapies are
recommended in addition to radical surgery to decrease the
possibility of recurrence and increase the success rate. However,
adjuvant chemotherapies, which are administrated systemically, are
unable to selectively target cancerous cells and, in turn cause
substantial toxicity (3), resulting
in an impaired quality of life for patients. Therefore, novel
therapeutic strategies are required.
Gene-direct enzyme/prodrug therapy (GEPT), also
named suicide gene therapy, has received considerable attention due
to its powerful anti-tumor efficacy without side effects (4,5). GEPT is
based on the intracellular delivery of genes encoding enzymes that
convert nontoxic prodrugs into highly cytotoxic metabolites
(6). Well-characterized GEPTs
include the herpes simplex virus thymidine kinase/ganciclovir
(HSV-TK/GCV) and cytosine deaminase/5-fluorocytosine (CD/5-FC)
(7). TK activates GCV to its
cytotoxic triphosphate derivative, which inhibits cellular DNA
synthesis, whereas CD deaminates 5-FC into the highly toxic
5-fluorouracil (5-FU), which may interfere with nucleoside
metabolism and lead to targeted cell death (8). However, GEPT is thought to be
insufficient to cure cancer alone (9). Previously, a number of studies have
aimed to enhance the therapeutic effect of GEPT through combination
with other gene therapies, including immuno-gene (10), anti-oncogene (11) and inhibition of multiple drug
resistance gene based on RNAi (12).
Survivin, which is known to be a member of the
inhibitor of apoptosis protein family (13), is overexpressed in a number of human
cancer types, including CRC (14–16).
Recent studies have indicated that Survivin serves an essential
role in tumor growth, infiltration and metastasis, and that it is
closely associated with the chemo-resistance of cancer cells
(17,18). Survivin has become a focus in cancer
therapy. RNA interference (RNAi) technology, based on
sequence-specific interactions between small interfering RNA
(siRNA) and mRNA (19), is
post-transcriptional gene silencing. Inhibition of Survivin by RNAi
has been demonstrated to restrain tumor growth and metastasis, and
increase sensitivity to anti-tumor agents (20). The anti-tumor effect of GEPT is
mediated by cytotoxic metabolites of prodrugs, such as 5-FU. The
downregulation of Survivin may help maintain the sensitivity of
colorectal cancer cells to the cytotoxic drugs. Therefore, a
combination of Survivin-targeted RNAi and the suicide gene may
exhibit synergistic effects for cancer treatment.
In the present study, a triple-gene vector
expressing Survivin-shRNA and fusion suicide gene yCDglyTK was
constructed to assess the feasibility of a novel therapeutic vector
system involving a combination of GEPT with Survivin-targeted RNAi
therapy. This novel vector was delivered into HCT116 cells (a colon
cancer cell line) by calcium phosphate nanoparticles (CPNPs), and
the anti-tumor effect was studied in vitro.
Materials and methods
Reagents
Restriction enzymes BsaI, MluI,
XhoI and NheI were purchased from MBI Fermentas
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). T4-DNA ligase
(New England Biolabs, Inc., Ipswich, MA, USA), rTaq DNA polymerase
(Takara Biotechnology Co., Ltd., Dalian, China), DNA Marker IV, DNA
Marker DL2000 (YRbio; Changsha, China), pYr1.1 vector (YRbio) and
pUC57 (YRbio) were applied. Lipofectamine 2000 (Invitrogen, Thermo
Fisher Scientific, Inc.), MinElute Gel Extration Kit (Qiagen GmbH;
Hilden, Germany), Geneticin (G418; Thermo Fisher Scientific, Inc.),
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
ReverTra Ace reverse transcription kit (Toyobo Co., Ltd., Osaka,
Japan), 2X Taq PCR MasterMix (Tiangen Biotech Co., Ltd., Beijing,
China), rabbit anti-Survivin antibody (ab76424; 1;5,000; Abcam,
Cambridge, UK), mouse anti-TK antibody (sc-53331; 1:200; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), mouse anti-β-actin antibody
(A5316; 1;5,000; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany),
goat anti-rabbit secondary antibody (SA00001-2; 1:2,000;
Proteintech Group, Inc., Chicago, IL, USA), goat anti-mouse
secondary antibody (SA00001-1; 1:2,000; Proteintech Group, Inc.),
rabbit anti-CD antibody (10348–924; 1:200; VWR International;
Randor, PA, USA), FITC-Goat Anti-Rabbit antibody (SA00003-2; 1:100;
Proteintech Group, Inc.), GCV (Sigma-Aldrich; Merck KGaA), 5-FC
(Sigma-Aldrich; Merck KGaA), MTT solution (Sigma-Aldrich; Merck
KGaA), dimethyl sulfoxide (Promega Corporation) and pyridine iodide
(PI; Sigma-Aldrich; Merck KGaA) were also used in the study.
Construction of Survivin-shRNA
expressing plasmid
The Survivin mRNA sequence in GenBank (https://www.ncbi.nlm.nih.gov/gene) was searched,
and three Survivin-specific target sequences were selected
according to the RNAi design tool (https://sg.idtdna.com/site/order/designtool/index/DSIRNA_CUSTOM).
The first siRNA sequence targeted the coding region 118–138
(5′-GAGGCTGGCTTCATCCACTGC-3′), the second sequence targeted the
coding region 323–342 (5′-GAGCCAAGAACAAAATTGC-3′) and the third
sequence targeted the coding region 387–405
(5′-GAAAGTGCGCCGTGCCAT-3′). Oligonucleotides that encoded the
corresponding small hairpin RNA (shRNA) were synthesized
commercially (Yrbio, Changsha, China), and the sequences are
presented in Table I.
 | Table I.Sequences of oligonucleotides
encoding Survivin-shRNA. |
Table I.
Sequences of oligonucleotides
encoding Survivin-shRNA.
| Survivin-shRNA | Sequences of
oligonucleotides |
|---|
| Survivin-sh1 | Forward:
5′-CACCGAGGCTGGCTTCATCCACTGCCTCGAGGCAGTGGATGAAGCCAGCCTCTTTTTTG-3′ |
|
| Reverse:
5′-AGCTCAAAAAAGAGGCTGGCTTCATCCACTGCCTCGAGGCAGTGGATGAAGCCAGCCTC-3′ |
| Survivin-sh2 | Forward:
5′-CACCGAGCCAAGAACAAAATTGCTTCAAGAGAGCAATTTTGTTCTTGGCTCTTTTTTG-3′ |
|
| Reverse:
5′-AGCTCAAAAAAGAGCCAAGAACAAAATTGCTCTCTTGAAGCAATTTTGTTCTTGGCTC-3′ |
| Survivin-sh3 | Forward:
5′-CACCGAAAGTGCGCCGTGCCATCTTCAAGAGAGATGGCACGGCGCACTTTCTTTTTTG-3′ |
|
| Reverse:
5′-AGCTCAAAAAAGAAAGTGCGCCGTGCCATCTCTCTTGAAGATGGCACGGCGCACTTTC-3′ |
The oligonucleotides were annealed in annealing
buffer (10 mM Tris-HCl pH 8.0, 50 mM NaCl and 1 mM EDTA), and
pYr1.1 vector was digested with BsaI at 37°C overnight.
Subsequently, the linear fragment of pYr1.1 and the annealing
products were connected at 4°C overnight to construct
pYr1.1-Survivin-sh1, pYr1.1-Survivin-sh2 and pYr1.1-Survivin-sh3,
respectively. The expression of shRNA was regulated by the U6
promoter. Then the three interfering plasmids were sequenced. The
three interfering plasmids were then transfected into HCT116 cells
using Lipofectamine 2000 according to the manufacturer's
instructions, and the protein expression of Survivin was evaluated
by western blot analysis, as described below. pYr1.1-Survivin-sh2
was confirmed to be the most effective interfering plasmid.
Construction of the triple-gene
plasmid
The suicide gene should be expressed only in cancer
cells, and the human telomerase reverse transcriptase promoter
(hTERTp) was used to observe target expression. The hTERTp was
synthesized by Yrbio, according to a previous study (21), and the sequence was: 5′-ACGCGTGCTCCCAGTGGATTCGCGGGCACAGACGCCCAGGACCGCGCTCCCCACGTGGCGGAGGGACTGGGGACCCGGGCACCCGTCCTGCCCCTTCACCTTCCAGCTCCGCCTCCTCCGCGCGGACCCCGCCCCGTCCCGACCCCTCCCGGGTCCCCGGCCCAGCCCCCTCCGGGCCCTCCCAGCCCCTCCCCTTCCTTTCCGCGGCCCCGCCCTCTCCTCGCGGCGCGAGTTTCAGGCAGCGCTGCGTCCTGCTGCGCACGTGGGAAGCCCTGGCCCCGGCCACCCCCGCGGCTAGC-3′
(the underlined sections were MluI and NheI
restriction sites, respectively), and was subcloned into pUC57
vector, which was named pUC57-hTERTp. pUC57-hTERTp and pYr1.1 were
digested by MluI and NheI at 37°C overnight, respectively,
and the linear fragments were connected by T4 DNA ligase at 4°C
overnight to construct pYr1.1-hTERTp. A plasmid carrying fusion
suicide gene yCDglyTK was constructed as described in our previous
study (22), which was stored in the
department of Gastroenterology, Xiangya Hospital of Central South
University (Changsha, China). The fusion suicide gene yCDglyTK was
amplified through polymerase chain reaction (PCR). Primer sequences
used were as follows: P1,
5′-CTAGCTAGCGCCACCATGGTGACAGGGGGAATGGCAA-3′ (NheI restriction site
was introduced), and P2, 5′-CCGCTCGAGTCAGTTAGCCTCCCCCATCT-3′ (XhoI
restriction site was introduced). The reaction mixture for PCR
contained the following: 0.25 µl P1 (10 µM), 0.25 µl P2 (10 µM),
19.75 µl dH2O, 2.5 µl 10X LA PCR buffer (Mg2+
Plus), 1 µl dNTPs (2.5 mM), 0.25 µl LA Taq polymerase and 1 µl
template. The thermal cycle profile for PCR was 94°C for 5 min,
followed by 30 cycles of 20 sec at 94°C, 25 sec at an annealing
temperature of 58°C, 105 sec at 72°C, and an additional 3 min
incubation at 72°C following completion of the last cycle for
extension. Following electrophoresis on 1% agarose gel, PCR
products were extracted and stored at 4°C.
PCR products of yCDglyTK and pYr1.1-hTERTp were
subsequently digested by NheI and XhoI respectively
at 37°C overnight, and the two linear fragments were connected at
4°C overnight to develop the plasmid pYr1.1-hTERTp-yCDglyTK. In
this process, the enhanced green fluorescent protein (EGFP) of
pYr1.1-hTERTp was replaced by yCDglyTK.
pUC57-hTERTp and pYr1.1-Survivin-sh2 were digested
by MluI and NheI respectively at 37°C overnight, and
the linear fragments were connected at 4°C overnight to construct
pYr-1.1-hTERTp-Survivin-sh2. Subsequently, PCR products of yCDglyTK
and pYr-1.1-hTERTp-Survivin-sh2 were digested by NheI and
XhoI respectively at 37°C overnight, and the two linear
fragments were connected (at 4°C overnight) to construct a novel
triple-gene vector pYr1.1-hTERTp-yCDglyTK-shSurvivin2. Plasmids
used in the current study are presented in Table II.
 | Table II.Plasmids used in the present
study. |
Table II.
Plasmids used in the present
study.
| Plasmids | Abbreviations | Promoters | Inserts |
|---|
| pYr1.1 | pYr1.1 | hU6 | EGFP |
|
pYr1.1-Survivin-sh1/2/3 | shSur1/2/3 | hU6 |
Survivin-shRNA1/2/3 |
| pYr1.1-hTERTp | pYr1.1-hTERTp | hTERTp and hU6 | EGFP |
|
pYr1.1-hTERTp-yCDglyTK | hTERTp-CDTK | hTERTp and hU6 | yCDglyTK |
|
pYr1.1-hTERTp-yCDglyTK-sh Survivin2 | CDTK-shSur | hTERTp and hU6 | yCDglyTK and
Survivin-shRNA2 |
Cell line and cell culture
HCT116 (a human colon cancer cell line) and human
fibroblasts obtained from the Central Laboratory of the Second
Xiangya Hospital, Central South University (Changsha, China), were
used in the present study. The present study was approved by the
ethics committee of the Second Xiangya Hospital, Central South
University (Changsha, China) and informed consent was obtained from
patients prior to the use of human tissue. Cells were cultured in
RPMI 1640 medium (Hyclone; GE Healthcare Life Sciences, Logan, UT,
USA), supplemented with 10% fetal bovine serum (FBS, Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA) and maintained at 37°C in
a humidified atmosphere of 5% CO2 and 95% air.
Analysis of EGFP expression
HCT116 and human fibroblasts were seeded in 6-well
plates at a density of 2×105 cells/well. As described in
a previous study (22), calcium
phosphate nanoparticles (CPNPs) were produced, and 2 µg DNA
(pYr1.1-hTERTp) was mixed with 20 µg CPNPs to form the CPNP-DNA
complex, which was then added to each well. The expression of EGFP
was analyzed 48 h later using a fluorescence microscope (DMI 4000B;
Leica Microsystems GmbH, Wetzlar, Germany).
Stable transfection in vitro
HCT116 cells were seeded in 6-well plates at a
density of 2×105 cells per well. When the cell monolayer
reached 70–80% confluence, hTERTp-CDTK and CDTK-shSur were mixed
with CPNPs respectively. Each of the CPNP-DNA complexes was added
to different 6-well plates as described previously (22). The next day, a 1:10 passage of the
transfected HCT116 cells was performed, followed by the addition of
400 µg/ml G418 for selection. G418-resistant clones were isolated
and expanded in RPMI-1640 culture medium containing 200 µg/ml G418.
Surviving colonies transfected with hTERTp-CDTK or CDTK-shSur were
renamed HCT/CDTK, or HCT/CDTK-shSur, respectively, and subjected to
further studies.
Reverse transcription-PCR
(RT-PCR)
Total RNA from parental and transfected HCT116 cells
was extracted using TRIzol reagent. The quantity and quality of RNA
were assessed by absorbance at 260 nm and 280 nm using an
ultraviolet spectrophotometer (DU800; Beckman Coulter, Inc., Brea,
CA, USA). The RT reaction was performed using the ReverTra Ace
reverse transcription kit according to the manufacturer's protocol.
Subsequently, PCR was performed on the cDNA product. For yCDglyTK,
a PCR product of 707 bp was produced by forward primer
5′-GGGAGATTAGAGGGCAAAGTGT-3′ and reverse primer
5′-ACGGCGTCGGTCACGGCATAA-3′. For Survivin, a PCR product of 107 bp
was produced by forward primer 5′-CATCCTGCGTCTGGACCTGG-3′ and
reverse primer 5′-TAATGTCACGCACGATTTCC-3′. β-actin was used as an
internal control, and the forward primer was
5′-AGCGAGCATCCCCCAAAGTT-3′ and the reverse primer was
5′-GGGCACGAAGGCTCATCATT-3′. The thermal cycle profile for PCR was
94°C for 3 min, followed by 28 cycles of 30 sec at 94°C, 30 sec at
an annealing temperature of 55°C and 60 sec at 72°C. PCR products
were electrophoresed on 2% agarose gels, and visualized using gel
image analysis system (BIO-PRO, SIM International group Co., Ltd.,
Los Angeles, CA, USA) and analyzed by Bandscan 5.0 (http://www.bbioo.com/download/58-140-1.html).
Western blot analysis
Parental and transfected HCT116 cells were lysed in
radioimmunoprecipitation assay buffer on ice containing
phenylmethylsulfonyl fluoride for 30 min with occasional agitation.
The lysates were transferred to E-tubes and clarified by
centrifugation at 14,000 × g for 15 min at 4°C. The supernatant was
collected and protein concentrations were evaluated using a BCA
protein assay. Identical amounts (40 µg protein) of cell lysates
were separated via 15% SDS-PAGE and transferred to polyvinylidene
fluoride membranes (GE Healthcare, Chicago, IL, USA). The membranes
were incubated in blocking solution, consisting of 5% skim milk in
Tris buffered saline with Tween-20 [10 mM Tris-HCl (pH 8.0), 150 mM
NaCl, and 0.1% Tween-20], for 1 h at room temperature, then probed
with rabbit anti-Survivin antibody, rabbit anti-TK antibody or
mouse anti-β-actin antibody at 4°C overnight. This was followed by
incubation with their respective peroxidase-conjugated secondary
antibodies for 1.5 h at room temperature. The blots were visualized
by the enhanced chemiluminescence detection system (GE Healthcare)
and analyzed by Bandscan 5.0.
Immunofluorescence assay
Parental and transfected HCT116 cells
(2×105 cells per well) were fixed in 4% formaldehyde for
20 min at room temperature. Cells were washed with cold PBS three
times, then permeabilized in 0.3% Triton X-100 and blocked with 1%
bovine serum albumin for 30 min at room temperature. The cells were
then treated with rabbit anti-CD antibody overnight at 4°C in a
humidified chamber. Then, cells were incubated with anti-rabbit
immunoglobulin G-fluorescein isothiocyanate antibody for 1 h at
37°C in the dark. After being washed three times with PBS,
coverslips were mounted with a drop of mounting medium (Beyotime
Institute of Biotechnology, Shanghai, China), sealed with clear
nail polish and visualized using a fluorescence microscope
(magnification, ×200).
MTT assay
HCT116 cells (transfected and untransfected) were
seeded in 96-well plates at a density of 6,000 cells per well. The
next day (at 37°C), cells were treated with prodrugs: 200 µg/ml
5-FC and 16 µg/ml GCV, which have been confirmed to have limited
toxicity on untransfected cells in a previous study (22), and cell viability was measured after
24, 48, 72 and 96 h of incubation at 37°C. A volume of 20 µl MTT
solution (5 mg/ml) was added and cells were further incubated at
37°C for 4 h. Then, the culture medium was removed and replaced
with 200 µl dimethyl sulfoxide to dissolve the blue crystals. The
optical density (OD) was determined using a multi-well plate reader
(Stat-Fax-2100; Awareness Technologies, Westport, CT, USA) by
measuring absorbance at 570 nm (OD570), with the absorbance at 690
nm as a reference. The background absorbance of medium was also
subtracted. Cell growth curves were produced with culture time on
the horizontal axis and OD570 on the vertical axis.
Cell apoptosis analyzed by flow
cytometry
A flow cytometry assay was performed to evaluate the
loss of cell viability in each experimental group. Parental and
transfected HCT116 cells were seeded into 75 cm2 cell
culture flasks at a density of 2×106 cells per flask.
RPMI 1640 medium (supplemented with 10% FBS) with 200 µg/ml 5-FC
and 16 µg/ml GCV was added when the cells reached 70% confluence.
48 h later, the cells were pelleted by centrifugation at 800 × g,
washed with cold PBS twice, fixed in 75% ethanol for 30 min at 4°C
and resuspended in a staining solution of PI (50 mg/ml) for 30 min
at 37°C. Finally, the cell apoptosis rate was analyzed using flow
cytometry (FACSCanto, BD Biosciences; San Jose, CA, USA).
Migration assay
A wound healing assay was applied to analyze cell
migration. HCT116 cells (transfected and untransfected) were seeded
in 6-well plates at a density of 5×105 cells/well in
RPMI 1640 medium with 10% FBS for 24 h at 37°C to reach 95%
confluence. The monolayers were then scratched with a 200 µl
pipette tip. The cells were washed three times with PBS, and
cultured in RPMI 1640 medium without FBS for 24 h at 37°C.
Migration of the cells was detected under a light microscope. The
wound margin distances between the two edges of the migrating cell
sheets were measured at 0 and 24 h following scratching. The
relative migrating distance of cells was measured as follows:
Distance of cell migration/the distance measured at 0 h.
Statistical analysis
All results were expressed as mean ± standard
deviation. Statistical analysis was performed using SPSS version
13.0 (SPSS, Inc., Chicago, IL, USA) The Student's t-test and
one-way analysis of variance assessments followed by the
Student-Newman-Keuls test was performed. P<0.05 was considered
to indicate statistically significant differences.
Results
Construction of the plasmid
pYr1.1-hTERTp-yCDglyTK-shSurvivin2
Three interfering plasmids targeting Survivin were
constructed and the most effective plasmid, pYr1.1-Survivin-sh2,
was selected. Subsequently, hTERTp was cloned into pYr1.1 to obtain
pYr1.1-hTERTp, and the specificity of hTERTp was confirmed by
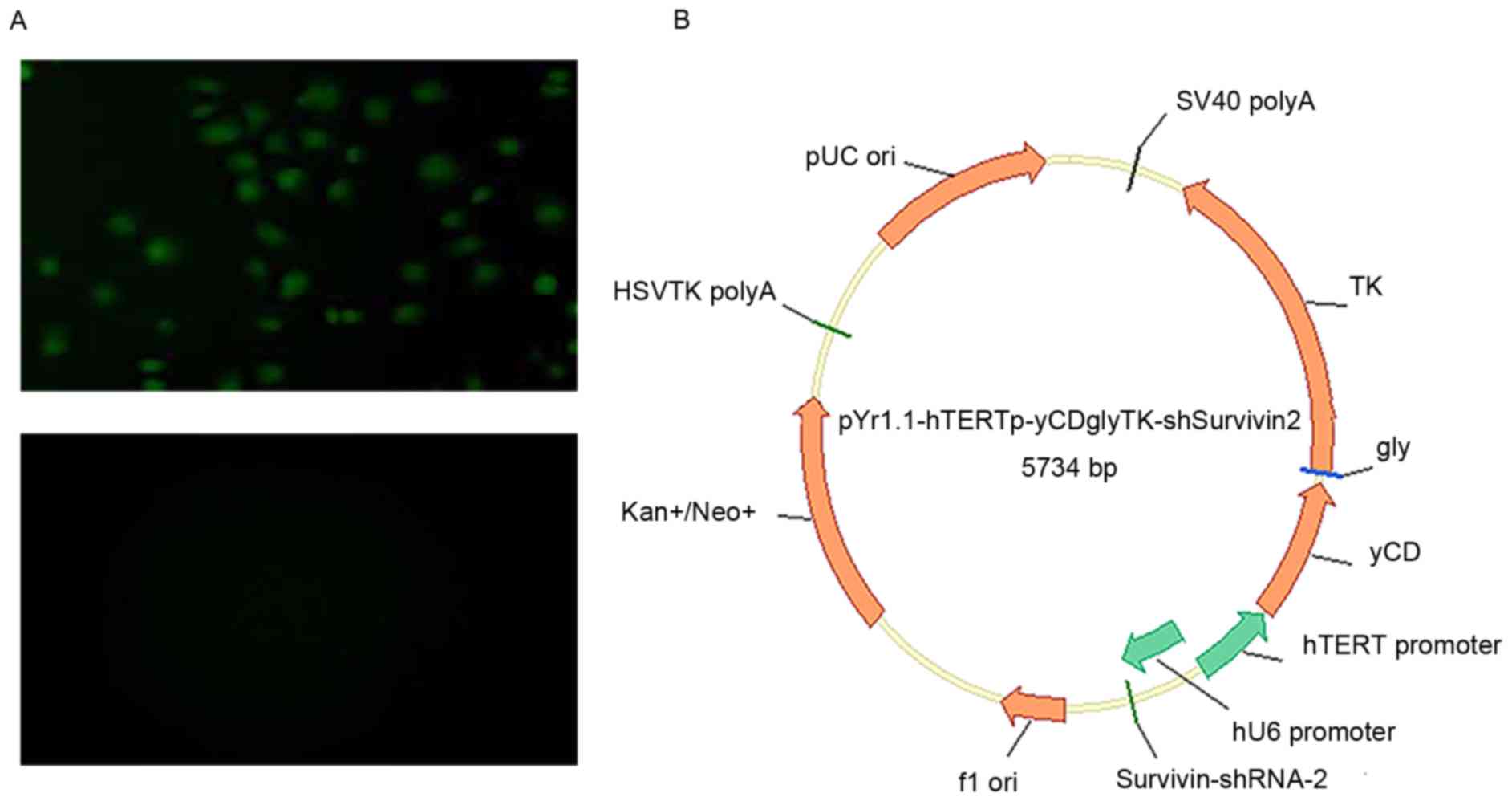
fluorescence microscopy, as presented in Fig. 1A. Then, yCDglyTK was cloned into
pYr1.1-hTERTp to generate pYr1.1-hTERTp-yCDglyTK. Finally,
Survivin-shRNA from pYr1.1-Survivin-sh2 was cloned into
pYr1.1-hTERTp-yCDglyTK to develop the triple-gene plasmid
pYr1.1-hTERTp-yCDglyTK-shSurvivin2. In this novel triple-expressing
plasmid, the Survivin-shRNA sequence was driven by a U6 promoter,
whereas fusion suicide gene yCDglyTK was regulated by hTERTp. The
construction scheme of the triple-gene plasmid
pYr1.1-hTERTp-yCDglyTK-shSurvivin2 is presented in Fig. 1B.
Establishment of stably transfected
cell lines
hTERTp-CDTK and CDTK-shSur were administered to
HCT116 cells using CPNPs. Following G418 selection, stably
transfected cell lines were established. HCT116 cells transfected
with hTERTp-CDTK were named HCT/CDTK, and those transfected with
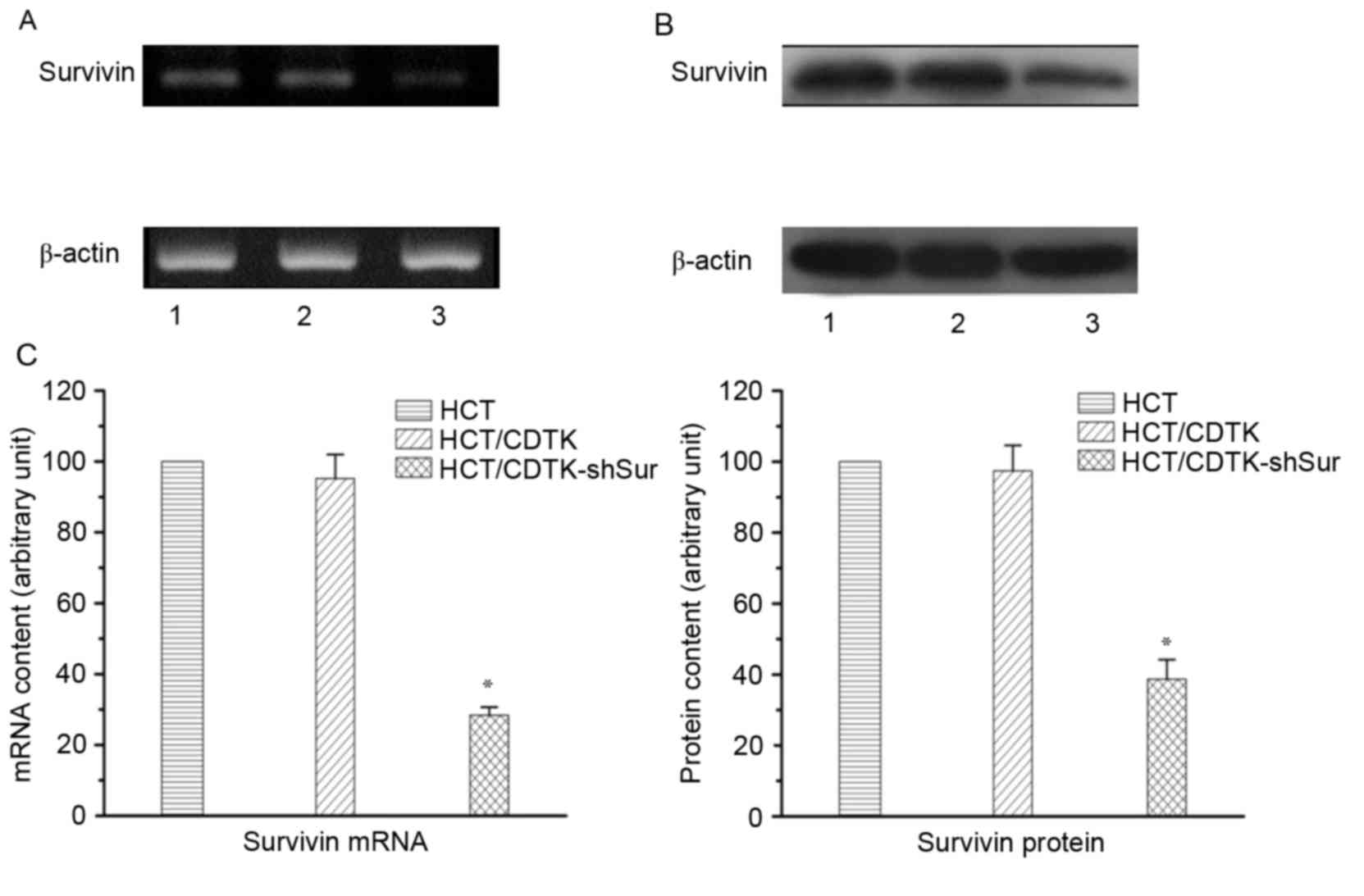
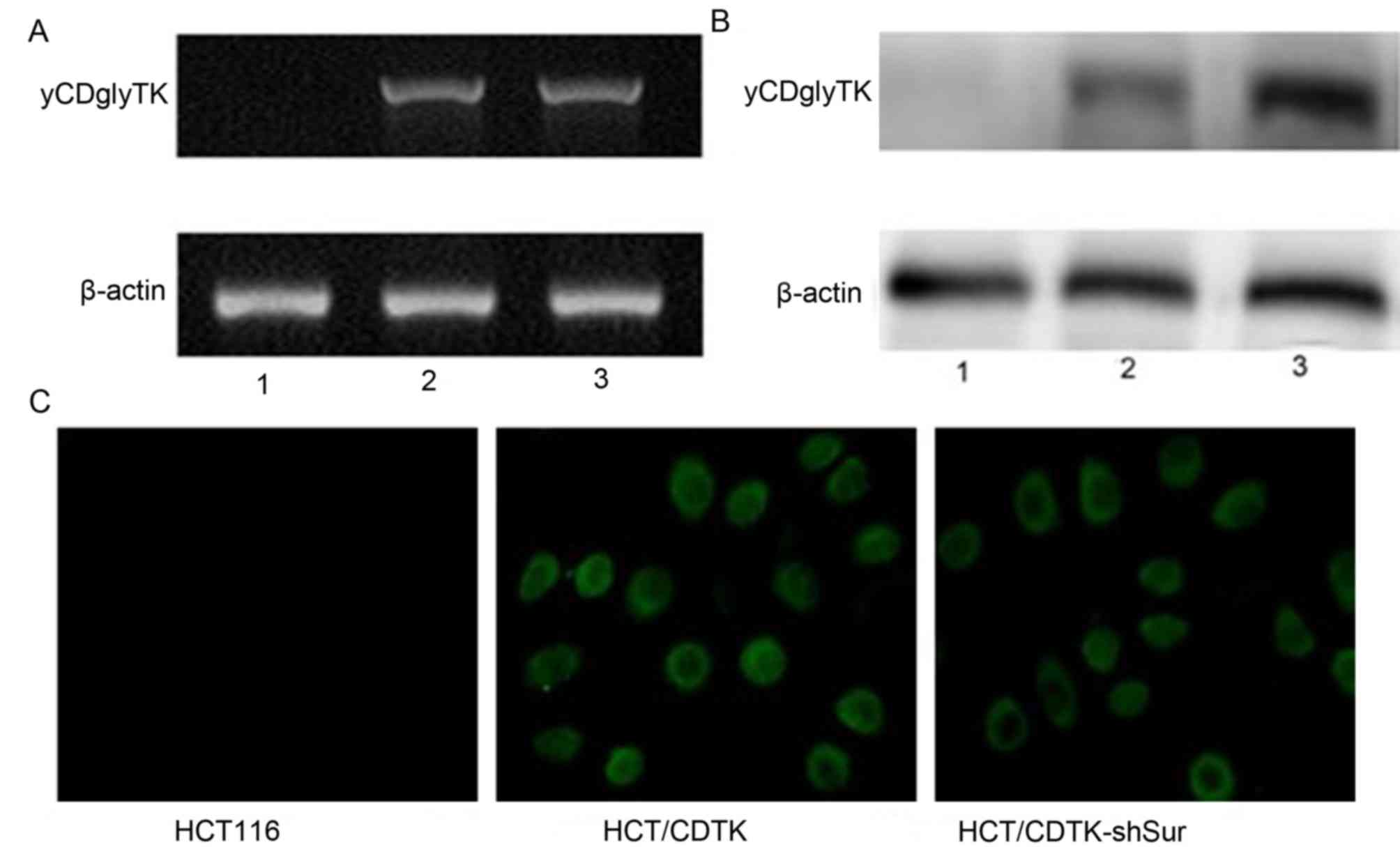
CDTK-shSur were named HCT/CDTK-shSur. RT-qPCR and western blot
analysis were performed to determine the expression of Survivin and
yCDglyTK, and immunofluorescence was conducted to determine the
expression of yCDglyTK (Figs. 2 and
3, respectively). Compared with
parent HCT116 cells and HCT/CDTK, mRNA and protein levels of
Survivin were significantly decreased in HCT/CDTK-shSur (P<0.01;
Fig. 2C). yCDglyTK was revealed to
only be expressed in HCT/CDTK and HCT/CDTK-shSur cells (Fig. 3).
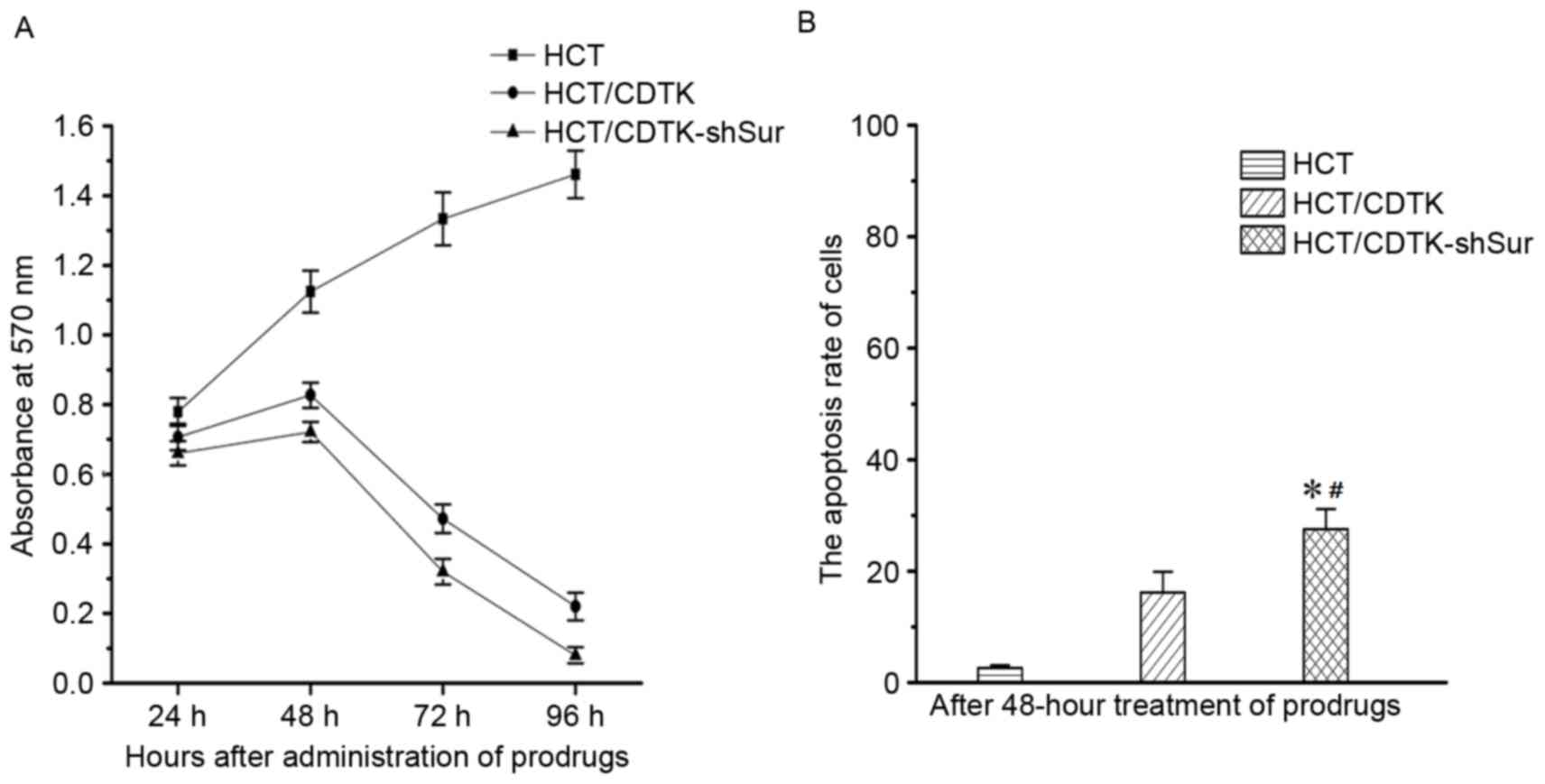
CDTK-shSur/prodrug system induced
cytotoxicity
Following 48 h treatment with 5-FC and GCV, the
OD570 of parental HCT116 cells was markedly increased compared with
HCT/CDTK and HCT/CDTK-shSur cells (Fig.
4A). Over time, untransfected HCT116 cells sustained a high
rate of proliferation, whereas the OD570 of HCT/CDTK and
HCT/CDTK-shSur cells decreased markedly, suggesting that the
majority of cells were killed. OD570 of HCT/CDTK-shSur remained the
lowest throughout.
CDTK-shSur/prodrug system induced cell
apoptosis
Each group was treated with prodrugs (5-FC and GCV)
for 48 h, and then subjected to flow cytometry to measure the
apoptosis rate (Fig. 4B). The
percentage of apoptotic cells in untransfected HCT116 cells was
2.63±0.48%, in HCT/CDTK cells was 16.17±3.71% and in HCT/CDTK-shSur
cells was 27.50±3.62%. The apoptosis rate of HCT/CDTK-shSur cells
was significantly higher in comparison with the untransfected
HCT116 and HCT/CDTK cells (P<0.05; Fig. 4B), indicating that the
CDTK-shSur/prodrug therapy system may induce cell apoptosis more
effectively.
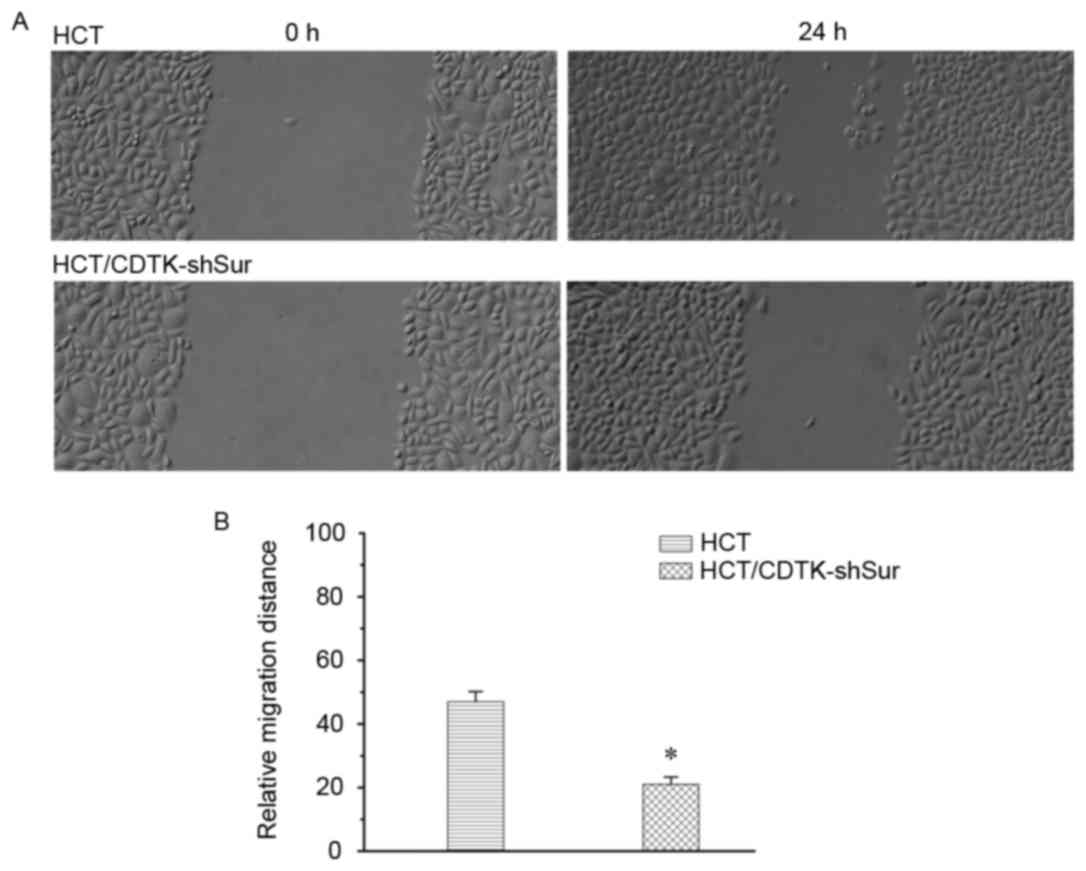
CDTK-shSur inhibits cancer cell
migration
The migration ability of HCT116 cells was measured
using a wound healing assay 24 h following scratching. As presented
in Fig. 5, compared with the
parental HCT116 cells, the migration of HCT/CDTK-shSur cells
decreased significantly (P<0.01; Fig.
5B).
Discussion
Gene therapy has emerged as a promising strategy for
treating malignant tumors (23). As
the genesis, development and metastasis of cancer is a complicated
process involving multiple factors (24), single gene therapy alone is not
effective enough to eradicate cancer cells. Combination gene
therapy may be an efficient approach to obtaining greater
anti-tumor efficacy. Combination gene therapy may be achieved by
co-transferring vectors carrying different genes; however, it is
impossible to ensure that all of the different vectors are
delivered into the cell simultaneously. The approach of one vector
expressing multiple therapeutic genes has been suggested to enhance
the therapeutic efficacy (25–27). In
the current study, a triple-gene vector expressing Survivin-shRNA
and fusion suicide gene yCDglyTK was constructed, in which
Survivin-shRNA was regulated by U6 promoter whereas fusion suicide
gene yCDglyTK was driven by hTERTp.
Different GEPTs exhibit different characteristics
(7). For example, the HSV-TK/GCV
system has a more powerful killing efficacy, whereas the CD/5-FC
system exerts a superior bystander effect. Furthermore, cell type
dependency may exist with GEPT, as HSV-TK/GCV is typically employed
in treating gliomas (28), and the
CD/5-FC system is often adopted in treating gastrointestinal tumors
(29). Double suicide gene combined
with HSV-TK/GCV and CD/5-FC may break the dependence of tumor cell
types and exhibit a synergistic effect (30). The suicide gene should be expressed
only in cancer cells, so GEPT may be regarded as intratumoral
chemotherapy and cause little systematic toxicity. In a previous
study, a vector expressing the fusion suicide gene yCDglyTK was
constructed, and a CEA promoter was used to drive the expression of
yCDglyTK, a treatment that specifically killed CEA-positive cancer
cells (22). However, not all
colorectal cancer cells are CEA-positive (31), and yCDglyTK driven by a CEA promoter
has little effect on the CEA-negative cancer cells. Therefore, in
order to expand the applicability of fusion suicide gene therapy, a
more prevalent promoter is required. Telomerase is activated in
>85% of all malignant tumor cells, including colorectal cancer
cells, but is repressed in normal somatic cells (32–34), the
transcriptional activity that is regulated by hTERTp. hTERTp was
confirmed to drive specific target gene expression in various tumor
cells (9,35–37).
Therefore, hTERTp was used in the current study to cause
tumor-specific gene expression of yCDglyTK. When pYr1.1-hTERTp was
delivered into both HCT116 cells and human fibroblasts, EGFP was
only expressed in HCT116 cells and not in human fibroblasts,
suggesting that hTERTp was specific enough to drive target gene in
cancer cells.
The function of Survivin in tumor progression,
metastasis and chemo-resistance has been well documented (38). In the present study, RNAi technology
was used to inhibit its expression. Three Survivin-specific target
sequences were selected and corresponding Survivin-shRNA expression
plasmids were developed, from which the more effective one was
selected. Introduction of a Survivin-targeted shRNA increased the
cytotoxicity of yCDglyTK. The reasons for this synergistic effect
may be as follows: Inhibition of Survivin may promote cell
apoptosis and decrease cell mitosis (39); or downregulation of Survivin may
maintain and enhance the sensitivity of colorectal cancer cells to
cytotoxic metabolites of prodrugs. Furthermore, HCT116 cells
transfected with CDTK-shSur exhibited a decreased migration
ability, which determines invasiveness and metastasis of cancer
cells, even without the presence of prodrugs. These data
demonstrated that a combination of Survivin-siRNA and yCDglyTK may
be a promising approach to treating cancer in the future.
The novel triple-gene plasmid produced in the
current study may eradicate colon cancer cells and decrease their
migration effectively in vitro. However, there are potential
limitations of this novel system. Survivin was revealed to be
expressed in normal cells, such as T-cells, hematopoietic
progenitor cells, vascular endothelial cells, liver cells,
gastrointestinal tract mucosa and polymorphonuclear cells (40), and participates in numerous cell
processes including apoptosis, cell proliferation, cell cycle,
chromosome movement, mitosis and regulation of response to cellular
stress (41). The U6 promoter is not
tissue-specific, and CPNPs do not target specific tissues.
Strategies aiming to improve the safety of RNAi-based gene therapy
are therefore required.
In conclusion, the current study has demonstrated
that a combination of Survivin-targeted RNAi and suicide gene
therapies exhibits a synergistic effect. Introduction of
Survivin-shRNA into the CDTK/prodrug system may be an effective and
feasible strategy to eradicate colon cancer cells and inhibit their
migration in vitro. Although there are a number of
limitations to be resolved for further application, the current
study provides a novel gene therapy strategy for treating
colorectal cancer.
Acknowledgements
The present study was supported by the Hunan
Provincial Science and Technology Program of China (grant no.
2011SK3239) and the Technology Program of Hunan Provincial
Development and Reform Commission (grant no. 2011-1318).
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marin JJ, de Medina Sanchez F, Castaño B,
Bujanda L, Romero MR, Martinez-Augustin O, Moral-Avila RD and Briz
O: Chemoprevention, chemotherapy and chemoresistance in colorectal
cancer. Drug Metab Rev. 44:148–172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wiela-Hojeńska A, Kowalska T,
Filipczyk-Cisarz E, Łapiński Ł and Nartowski K: Evaluation of the
toxicity of anticancer chemotherapy in patients with colon cancer.
Adv Clin Exp Med. 24:103–111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nawa A, Tanino T, Luo C, Iwaki M, Kajiyama
H, Shibata K, Yamamoto E, Ino K, Nishiyama Y and Kikkawa F: Gene
directed enzyme prodrug therapy for ovarian cancer: Could GDEPT
become a promising treatment against ovarian cancer? Anticancer
Agents Med Chem. 8:232–239. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hedley D, Ogilvie L and Springer C:
Carboxypeptidase-G2-based gene-directed enzyme-prodrug therapy: A
new weapon in the GDEPT armoury. Nat Rev Cancer. 7:870–879. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karjoo Z, Chen X and Hatefi A: Progress
and problems with the use of suicide genes for targeted cancer
therapy. Adv Drug Deliv Rev. 99:113–128. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nouri FS, Wang X and Hatefi A: Genetically
engineered theranostic mesenchymal stem cells for the evaluation of
the anticancer efficacy of enzyme/prodrug systems. J Control
Release. 200:179–187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zu B, Shi Y, Xu M, You G, Huang Z, Gao M
and Feng W: ARE/SUZ12 dual specifically-regulated adenoviral TK/GCV
system for CML blast crisis cells. J Exp Clin Cancer Res.
34:562015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rainov NG: A phase III clinical evaluation
of herpes simplex virus type 1 thymidine kinase and ganciclovir
gene therapy as an adjuvant to surgical resection and radiation in
adults with previously untreated glioblastoma multiforme. Hum Gene
Ther. 11:2389–2401. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chai LP, Wang ZF, Liang WY, Chen L, Chen
D, Wang AX and Zhang ZQ: In vitro and in vivo effect of 5-FC
combined gene therapy with TNF-alpha and CD suicide gene on human
laryngeal carcinoma cell line Hep-2. PLoS One. 8:e611362013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang Q, Xia Z, You Y and Pu P: Wild Type
p53 gene sensitizes rat C6 glioma cells to HSV-TK/ACV treatment in
vitro and in vivo. Pathol Oncol Res. 16:509–514. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park SY, Lee W, Lee J and Kim IS:
Combination gene therapy using multidrug resistance (MDR1) gene
shRNA and herpes simplex virus-thymidine kinase. Cancer Lett.
261:205–214. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Altieri DC: Survivin-The inconvenient IAP.
Semin Cell Dev Biol. 39:91–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu JL, Gao W, Kang QM, Zhang XJ and Yang
SG: Prognostic value of survivin in patients with gastric cancer: A
systematic review with meta-analysis. PLoS One. 8:e719302013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xia H, Chen S, Huang H and Ma H: Survivin
over-expression is correlated with a poor prognosis in esophageal
cancer patients. Clin Chim Acta. 446:82–85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Krieg A, Werner TA, Verde PE, Stoecklein
NH and Knoefel WT: Prognostic and clinicopathological significance
of survivin in colorectal cancer: A meta-analysis. PLoS One.
8:e653382013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang J, Lyu H, Wang J and Liu B:
Influence of survivin-targeted therapy on chemosensitivity in the
treatment of acute myeloid leukemia. Cancer Lett. 366:160–172.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jaiswal PK, Goel A and Mittal RD:
Survivin: A molecular biomarker in cancer. Indian J Med Res.
141:389–397. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liao Y and Tang L: Inducible RNAi system
and its application in novel therapeutics. Crit Rev Biotechnol.
36:630–638. 2016.PubMed/NCBI
|
|
20
|
Liu W, Zhu F, Jiang Y, Sun D, Yang B and
Yan H: siRNA targeting survivin inhibits the growth and enhances
the chemosensitivity of hepatocellular carcinoma cells. Oncol Rep.
29:1183–1188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takakura M, Kyo S, Kanaya T, Hirano H,
Takeda J, Yutsudo M and Inoue M: Cloning of human telomerase
catalytic subunit (hTERT) gene promoter and identification of
proximal core promoter sequences essential for transcriptional
activation in immortalized and cancer cells. Cancer Res.
59:551–557. 1999.PubMed/NCBI
|
|
22
|
Liu T, Tang A, Zhang G, Chen Y, Zhang J,
Peng S and Cai Z: Calcium phosphate nanoparticles as a novel
nonviral vector for efficient transfection of DNA in cancer gene
therapy. Cancer Biother Radiopharm. 20:141–149. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Libutti SK: New horizons for cancer gene
therapy. Cancer Gene Ther. 21:12014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Backman V and Roy HK: Advances in
biophotonics detection of field carcinogenesis for colon cancer
risk stratification. J Cancer. 4:251–261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu T, Ye L, He Y, Chen X, Peng J, Zhang
X, Yi H, Peng F and Leng A: Combination gene therapy using
VEGF-shRNA and fusion suicide gene yCDglyTK inhibits gastric
carcinoma growth. Exp Mol Pathol. 91:745–752. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Long H, Li Q, Wang Y, Li Q, Liu T and Peng
J: Effective combination gene therapy using CEACAM6-shRNA and the
fusion suicide gene yCDglyTK for pancreatic carcinoma in vitro. Exp
Ther Med. 5:155–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li J, Zhang G, Liu T, Gu H, Yan L and Chen
B: Construction of a novel vector expressing the fusion suicide
gene yCDglyTK and hTERT-shRNA and its antitumor effects. Exp Ther
Med. 4:442–448. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Paíno T, Gangoso E, Medina JM and
Tabernero A: Inhibition of ATP-sensitive potassium channels
increases HSV-tk/GCV bystander effect in U373 human glioma cells by
enhancing gap junctional intercellular communication.
Neuropharmacology. 59:480–491. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang G, Liu T, Chen YH, Chen Y, Xu M,
Peng J, Yu S, Yuan J and Zhang X: Tissue specific cytotoxicity of
colon cancer cells mediated by nanoparticle-delivered suicide gene
in vitro and in vivo. Clin Cancer Res. 15:201–207. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Niu J, Xing C, Yan C, Liu H, Cui Y, Peng
H, Chen Y, Li D, Jiang C, Li N and Yang H: Lentivirus-mediated
CD/TK fusion gene transfection neural stem cell therapy for C6
glioblastoma. Tumour Biol. 34:3731–3741. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stiksma J, Grootendorst DC and van der
Linden PW: CA 19-9 as a marker in addition to CEA to monitor
colorectal cancer. Clin Colorectal Cancer. 13:239–244. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Glybochko PV, Zezerov EG, Glukhov AI,
Alyaev YG, Severin SE, Polyakovsky KA, Varshavsky VA, Severin ES
and Vinarov AZ: Telomerase as a tumor marker in diagnosis of
prostatic intraepithelial neoplasia and prostate cancer. Prostate.
74:1043–1051. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Crees Z, Girard J, Rios Z, Botting GM,
Harrington K, Shearrow C, Wojdyla L, Stone AL, Uppada SB, Devito JT
and Puri N: Oligonucleotides and G-quadruplex stabilizers:
Targeting telomeres and telomerase in cancer therapy. Curr Pharm
Des. 20:6422–6437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ayiomamitis GD, Notas G, Zaravinos A,
Zizi-Sermpetzoglou A, Georgiadou M, Sfakianaki O and Kouroumallis
E: Differences in telomerase activity between colon and rectal
cancer. Can J Surg. 57:199–208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu L, Wu W, Zhu G, Liu L, Guan G, Li X,
Jin N and Chi B: Therapeutic efficacy of an hTERT promoter-driven
oncolytic adenovirus that expresses apoptin in gastric carcinoma.
Int J Mol Med. 30:747–754. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song Y, Xin X, Zhai X, Xia Z and Shen K:
Sequential combination therapy with flavopiridol and autocatalytic
caspase-3 driven by amplified hTERT promoter synergistically
suppresses human ovarian carcinoma growth in vitro and in mice. J
Ovarian Res. 7:1212014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tian D, Sun Y, Yang Y, Lei M, Ding N and
Han R: Human telomerase reverse-transcriptase promoter-controlled
and herpes simplex virus thymidine kinase-armed adenoviruses for
renal cell carcinoma treatment. Onco Targets Ther. 6:419–426.
2013.PubMed/NCBI
|
|
38
|
Cheung CH, Huang CC, Tsai FY, Lee JY,
Cheng SM, Chang YC, Huang YC, Chen SH and Chang JY:
Survivin-biology and potential as a therapeutic target in oncology.
Onco Targets Ther. 6:1453–1462. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li Y, Zhou Y, Zheng J, Niu C, Liu B, Wang
M, Fang H and Hou C: Downregulation of survivin inhibits
proliferation and migration of human gastric carcinoma cells. Int J
Clin Exp Pathol. 8:1731–1736. 2015.PubMed/NCBI
|
|
40
|
Mobahat M, Narendran A and Riabowol K:
Survivin as a preferential target for cancer therapy. Int J Mol
Sci. 15:2494–2516. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Altieri DC: Targeting survivin in cancer.
Cancer Lett. 332:225–228. 2013. View Article : Google Scholar : PubMed/NCBI
|