Introduction
Carbon monoxide (CO) is one of the most common types
of asphyxiant poisoning gas in industrial manufacture and daily
life, and both the incidence and mortality rates (2.24/100,000 in
Europe in 2005) (1) of acute CO
poisoning are the highest among cases o f acute gas poisoning
(2). Clinically, hyperbaric oxygen
chamber therapy is the suggested treatment for acute CO poisoning
(3), as high pressure may accelerate
the dissociation of carboxyhaemoglobin (HbCO) to increase the
discharge of CO (4). However, the
therapeutic mechanism and effect of hyperbaric oxygen chamber
therapy for treating post-CO poisoning encephalopathy remain
unproven (5,6), and the therapy is not always readily
available. In addition, dexamethasone and hypertonic glucose
dehydration therapy are often used to prevent or treat delayed
encephalopathy (7); however these
treatments act only as symptomatic therapies or are used to prevent
CO poisoning complications. A recent hypothesis indicated that an
antioxidant such as caffeic acid phenethyl ester and anti-nitric
oxide therapy may be valuable for neuroprotection against CO
poisoning (8). Thus far, the
majority of research studies (9–11) have
focused on the treatment for delayed encephalopathy caused by CO
poisoning, whereas few (12) have
reported the emergency treatment for CO poisoning.
Hemin is an artificially synthesized chloride of
heme, with similar chemical characteristics (13). Furthermore, hemin may increase the
oxygen carrying capacity and may compete with HbCO to bind CO to
increase the body tolerance to hypoxia. In addition, hemin is an
activator of neuroglobin (14).
Neuroglobin participates in oxygen transport and storage in
neurons, helps increase the intracellular partial pressure of
oxygen in neurons, and is important in protecting neurons from
hypoxic injury. A previous animal study indicated that hemin is
able to induce heme oxygenase-1 activity to show neuroprotection
(15). Although there is no current
evidence in clinical trials, it was hypothesized that hemin may be
able to prevent and treat acute CO poisoning. Due to the lack of
effective drug therapies in clinically treating acute CO poisoning
and the high mortality rate among CO-poisoned patients, the aim of
the present study was to identify novel therapeutic options to
reduce the mortality rate of patients with CO poisoning. To test
this hypothesis, the potential protective effect of hemin on acute
CO poisoning was examined in mice. In the present study, an animal
model was generated by single intraperitoneal injection of CO to
Kunming mice (16). Using preventive
and therapeutic injection of hemin, its preventive function and
therapeutic effect was studied on acute CO poisoning in the mice
model; in particular, the present study attempted to establish
whether hemin administration was able to reduce the mortality
rate.
Materials and methods
Animals
A total of 280 Kunming mice (male:female ratio,
1:1), aged 5 weeks, weighing 18–22 g, were obtained from Guangdong
Medical Laboratory Animal Centre (Foshan, China). All mice were
raised in the Jinan University Medical Pharmacology Laboratory
(Guangzhou, China) with controlled temperature (25°C), humidity
(50–60%) and 12 h light:dark cycle. Animals were acclimatised with
ad libitum access to standard laboratory food and tap water
for one week and fasted for 24 h prior to all experiments. The
experimental protocol of the present study was approved by the
Jinan Medical University Animal Care Committee.
Chemicals and reagents
CO (purity >99.90%) was purchased from Guangzhou
Wanqiqiti, Ltd. (Guangzhou, China), hemin was purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) and a commercial
Malondialdehyde (MDA) Assay kit (TBA method) was purchased from the
Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Formaldehyde (3.5%) and sodium phosphate were purchased from
Guangzhou Chemical Reagent Factory (Guangzhou, China). All other
chemicals were of the highest quality commercially available.
Determination of CO model and optimal
dose of hemin
A total of 60 Kunming mice, weighing 18–22 g, were
divided into six groups (n=10 each) used for acute CO exposure in
order to determine the median lethal dose (LD50). A dose of
60 ml/kg was used as the initial exposure dose via intraperitoneal
injection. Results from the initial exposure dose (60 ml/kg) were
used to select the subsequent doses (90, 135, 202.5, 303.8 and
455.7 ml/kg), and by these up-and-down procedures (17), the mortality and 1-h mortality rate
were recorded. The modified Spearman-Karber method (18) was used to calculate LD50 and to
identify the lower limit of the calculated LD50 as the
administration dose (150 ml/kg).
The optimal dose of hemin used in the present study
was based on the results of preliminary experiments. In the
preliminary experiment, 100 Kunming mice were divided into five
groups (n=20 each), including the air control, CO-poisoning, low
(10 mg/kg), moderate (20 mg/kg) and high dose (40 mg/kg) of hemin
groups. CO was administered at the LD50 (150 ml/kg) via
intraperitoneal injection. Mice exposed to CO were administered
intraperitoneally with a dose of hemin (10, 20 or 40 mg/kg) when
they started to show symptoms of CO toxicity, such as anxiety and
hyperactivity. The air control group was administered
intraperitoneally with a dose of air (150 ml/kg) and an equivalent
volume of phosphate-buffered saline [PBS; 0.1 M sodium phosphate
(pH 7.2), 0.9% saline]. The 1-h mortality rate of mice in the
different groups was then recorded.
Animal grouping and drug
treatment
A total of 80 Kunming mice (18–22 g) were randomly
divided into four groups (n=20 each) as follows: i) Air control,
mice were injected intraperitoneally with air using the same dosage
as LD50 (150 ml/kg) and PBS solvent (40 mg/kg); ii) CO-poisoning
group, CO poisoning was induced in mice using the dosage of LD50 by
a single intraperitoneal injection (15) and treated with the same dose of PBS
solvent; iii) hemin-treatment + CO-poisoning group, mice were
injected intraperitoneally with hemin (40 mg/kg) 2 min after CO
exposure, when mice began to exhibit symptoms of toxicity; and iv)
hemin-pretreatment + CO-poisoning group, hemin (40 mg/kg) was
administered to mice 15 min prior to CO exposure.
Mortality and survival curve
Physical and behavioral changes in the mice were
recorded. These included the time when the skin or mucosa of mice
turned to cherry red and the times when the mice exhibited
hyperactivity, opisthotonus or fatigue. Mortality at 1 h was
calculated as the number of dead mice at 1 h/number of mice in each
group. Furthermore, the time of death was recorded and the survival
curve plotted.
Determination of blood HbCO
concentration
HbCO was determined by double-wavelength
spectrophotometry (19), which was
measured as the resistance of HbCO to reduction by sodium
dithionite (Na2S2O4) compared with
reduced oxyhaemoglobin. HbCO has peak absorbance at a wavelength of
535 nm, whereas that of oxyhaemoglobin is at 578 nm. Therefore,
this quotient was used to determine the HbCO concentration
according to the experimental equation:
HbCO(%)=(2.44×A535A578–2.68)×100%
A535 is absorbance in λ at 535 nm; A578 is
absorbance in λ at 578 nm. A total of 10 mice were randomly
selected in each group and from each mouse 0.1 ml blood was
harvested from the tail 30 min after CO exposure. Blood was
collected in a dry Eppendorf tube that was rinsed with heparin in
advance. Subsequently, 0.1 ml blood was mixed thoroughly by
inversion with 20 ml of 0.4 mol/l ammonia solution and 20 mg
Na2S2O4 was then added. This mixed
reducing reagent was measured by a spectrophotometer (EnSpire 2300;
PerkinElmer, Inc., Waltham, MA, USA) at 535 and 578 nm wavelengths,
respectively, within 10 min due to the instability of the reducing
reagent. HbCO concentration could be calculated by the experimental
equation.
Determination of serum MDA
concentration
MDA, which is a parameter of oxidative stress, was
measured by the thiobarbituric acid (TBA) method (20). MDA reacts with TBA to form
red-coloured MDA-reactive products with a peak absorbance at a
wavelength of 535 nm. Therefore, spectrophotometry was used to
determine the serum MDA concentration.
In total, 10 mice in each group were randomly
selected, and from each mouse, 0.1 ml blood was collected from the
caudal veins at 5 and 30 min after CO exposure. Each blood sample
was thoroughly mixed with 0.5 ml normal saline (0.9%) and 0.5 ml
TBA (0.5%), and this mixture was heated at 95°C for 40 min.
Following cooling to room temperature, the sample was centrifuged
at 2,683 × g for 10 min. A 0.1-ml aliquot of protein-free
supernatant was separated from the mixture, and the intensity of
the end fraction product was examined at a wavelength of 532 nm
(21). Therefore, the serum MDA
concentration was calculated according to the specification
provided in the MDA kit.
Hematoxylin and eosin (H&E) and
Nissl staining
Mice were sacrificed 1 h after CO exposure, and
hippocampal tissues were removed immediately for histological
observation (22). The mice were
deeply anaesthetised with sodium pentobarbital (50 mg/kg,
intraperitoneally) and perfused through the heart with 200 ml of a
solution containing 3.5% formaldehyde and phosphate-buffered saline
[0.1 M sodium phosphate (pH 7.2), 0.9% saline]. The brain was
removed and preserved in the same fixative solution for 7 days at
room temperature (~22°C), then embedded in hard paraffin at 60°C
for 5 h. Then, 5-µm-thick coronal sections were prepared. The
hippocampal CAl area was selected from the serial sections, which
was 1.5 mm in length (6 mm posterior to the bulbous olfactorius,
according to the atlas) (23). The
selected sections were then treated with H&E and Nissl's
staining. Furthermore, the pathological changes in the hippocampal
CAl area of the specimens were observed under low-power
(magnification, ×40) and high-power field (magnification, ×400)
light microscopy, and images were captured using an image scanner
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
The data belonged to nonparametric data, which are
expressed as median and interquartile ranges. A significant
difference between the two groups was analyzed by the Mann-Whitney
U test, whereas categorical variables were analyzed by the
χ2 test. Furthermore, biochemical data are expressed as
the mean ± standard error of the mean, which were calculated by
one-way analysis of variance followed by the Bonferroni test for
multiple-group comparisons or evaluated by the Student's t-test for
two-group comparisons. Finally, the analyses were performed with
SSPS software (version 13.0; SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Establishment of an animal model of
acute CO poisoning
Initially, the LD50 of acute CO poisoning was
measured using the modified Spearman-Karber method. Six different
CO dosages were evaluated according to geometric progression, with
a group interval of 1.5 (60.0, 90.0, 135.0, 202.5, 303.8 and 455.7
ml/kg), and administered via a single intraperitoneal injection.
The 1-h mortality rate of mice was presented in Table I and the calculated LD50 was 150
ml/kg.
 | Table I.Mortality rates of mice after
intraperitoneal injection at different doses of carbon
monoxide. |
Table I.
Mortality rates of mice after
intraperitoneal injection at different doses of carbon
monoxide.
| Dose (ml/kg) | Mice (n) | Mortality (n) | 1-h mortality rate
(%) |
|---|
|
60.0 | 10 | 0 |
0 |
|
90.0 | 10 | 1 | 10 |
| 135.0 | 10 | 4 | 40 |
| 202.5 | 10 | 6 | 60 |
| 303.8 | 10 | 9 | 90 |
| 455.7 | 10 | 10 | 100 |
Determination of the optimal dose of
hemin
It was revealed that the mortality rate following
administration of a high dose of hemin (40 mg/kg) was markedly
lower than that of the low (10 mg/kg) and moderate (20 mg/kg)
dosage groups (Table II).
Therefore, in the following experiments, 40 mg/kg was used as the
optimum therapeutic dose of hemin.
 | Table II.Mortality rates of CO-poisoned mice
after treatment with different doses of hemin. |
Table II.
Mortality rates of CO-poisoned mice
after treatment with different doses of hemin.
| Group | Mice (n) | Mortality (n) | 1-h mortality rate
(%) |
|---|
| Control | 20 | 0 | 0.0 |
| CO poisoning | 20 | 10 | 50.0a |
| Low dose of hemin
(10 mg/kg) | 20 | 8 | 40.0b |
| Moderate dose of
hemin (20 mg/kg) | 20 | 7 | 35.0b |
| High dose of hemin
(40 mg/kg) | 20 | 4 | 20.0c |
Hemin reduced the mortality rate of
CO-poisoned mice
The mortality rate of mice at 1 h was 50.0% in the
acute CO-poisoning group; 20.0% in the hemin-treated and 5.0% in
the hemin-pretreated groups. Furthermore, the χ2 test
indicated that there was a significant difference in the mortality
rate of mice at 1 h between the CO-intoxication and hemin-treated
groups (P<0.05), and between the CO-intoxication and
hemin-pretreated groups (P<0.01) (Table III).
 | Table III.Mortality rate of acute CO-poisoned
mice. |
Table III.
Mortality rate of acute CO-poisoned
mice.
| Group | Mice (n) | Mortality (n) | 1-h mortality rate
(%) |
|---|
| Control | 20 | 0 |
0.0 |
| CO poisoning | 20 | 10 | 50.0a |
|
Hemin-treatment | 20 | 4 | 20.0b |
|
Hemin-pretreatment | 20 | 1 | 5.0c |
Hemin alleviated the symptoms of acute
CO poisoning
In the control group, no abnormal reaction
manifestation was observed. In the CO-poisoning group, following
intraperitoneal injection of CO, symptoms of poisoning were
evident. At 2 min, the skin and mucosa of mice began to turn cherry
red. At 5–7 min, mice became anxious and hyperactive and after 15
min mice exhibited fatigue and weakness of the limbs, and the skin
and mucosa turned cherry red as well. In addition, they showed
generalized convulsion, and 4 mice exhibited opisthotonus. After 30
min, CO-poisoned mice became less active and did not react to
tactile stimuli. In the hemin-treated CO-poisoned group, at 2 min
after CO injection, mice became anxious and the skin and mucosa
started to turn cherry red. However, following injection of hemin,
the time when the symptoms of poisoning appeared was postponed. In
total, 10 min after intoxication, mice became anxious again and
after 45 min the mice became quiet and stayed still together, while
2 mice appeared to be opisthotonus. In the hemin-pretreated
CO-poisoned group, at 3 min after CO injection, the mice started to
become hyperactive and the skin and mucosa started to turn cherry
red. At 14 min, mice exhibited anxiety, while none of the mice
exhibited opisthotonus. At 50 min, the mice appeared quiet
again.
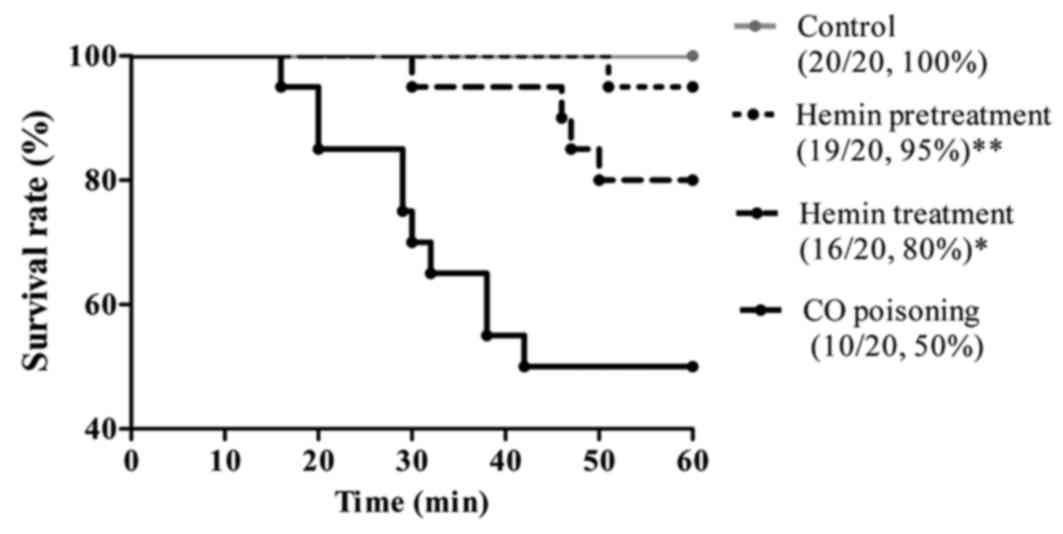
Hemin prolonged the survival time of
CO-poisoned mice
The mean mortality time in the CO-intoxication group
was 29.4 min (minimum, 16 min; maximum, 42 min), whereas it was
increased to a mean of 43.3 min in the hemin-treatment group
(minimum, 30 min; maximum, 50 min) and only 1 mouse died at 51.0
min in the hemin-pretreatment group. The mean mortality time of the
control group was >1 h; therefore, the survival rates of control
groups was 100% (Fig. 1).
Furthermore, there was a statistically significant difference
between the death time of both the hemin-treatment group and
hemin-pretreatment group and that of the CO-poisoning group
(P<0.05 and P<0.01, respectively).
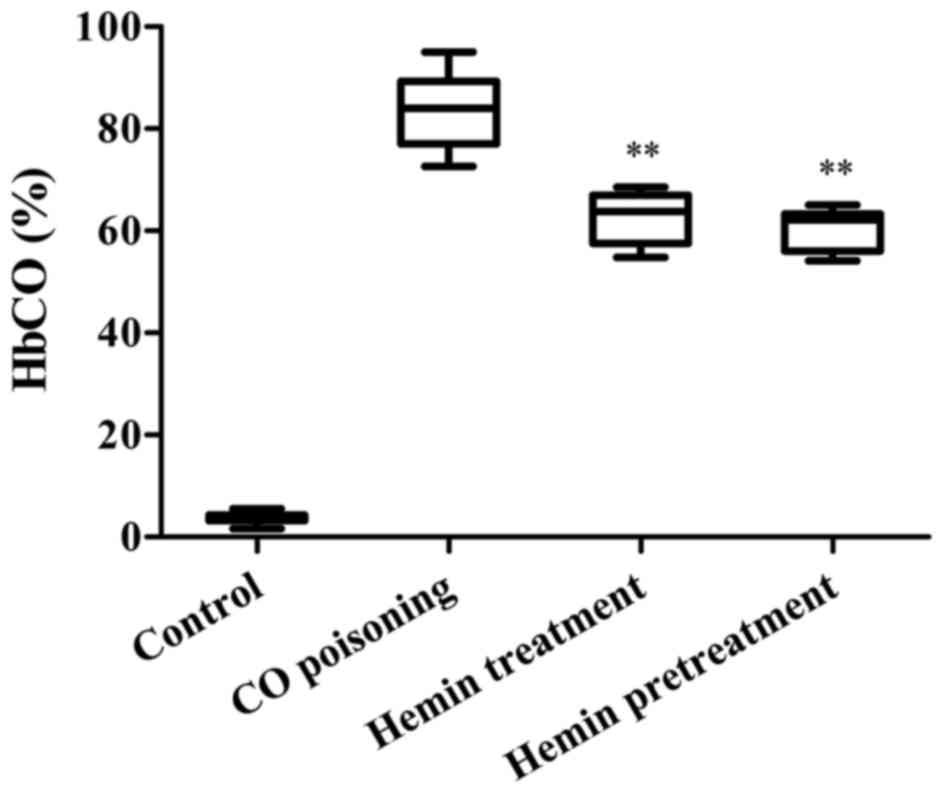
Hemin decreased the level of HbCO of
CO-poisoned mice
The HbCO level of mice 30 min following
administration of CO is presented in Fig. 2. The HbCO level of the CO-poisoning
group was significantly higher than that of the hemin-treatment
group (P<0.01). Additionally, there was a statistically
significant difference between the HbCO level of the
hemin-pretreatment and that of the CO-poisoned groups
(P<0.01).
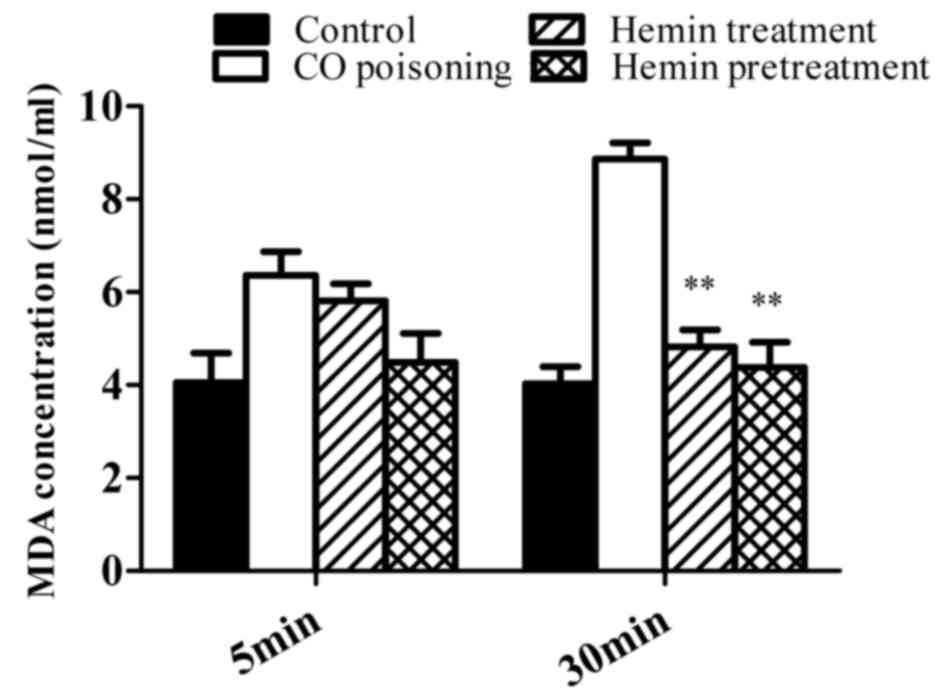
Hemin reduced the serum MDA
concentration in CO-poisoned mice
The serum MDA concentration in each group subjected
to CO poisoning increased following exposure, and there was no
statistically significant difference between the MDA concentration
of each group 5 min after intoxication. At 30 min following CO
intoxication, a statistically significant difference was observed
between the serum MDA concentration of the CO-poisoning group and
both the hemin treatment and hemin pretreatment groups (both
P<0.01). Furthermore, there was no statistically significant
difference between the MDA concentration of the control group at 5
and 30 min (Fig. 3).
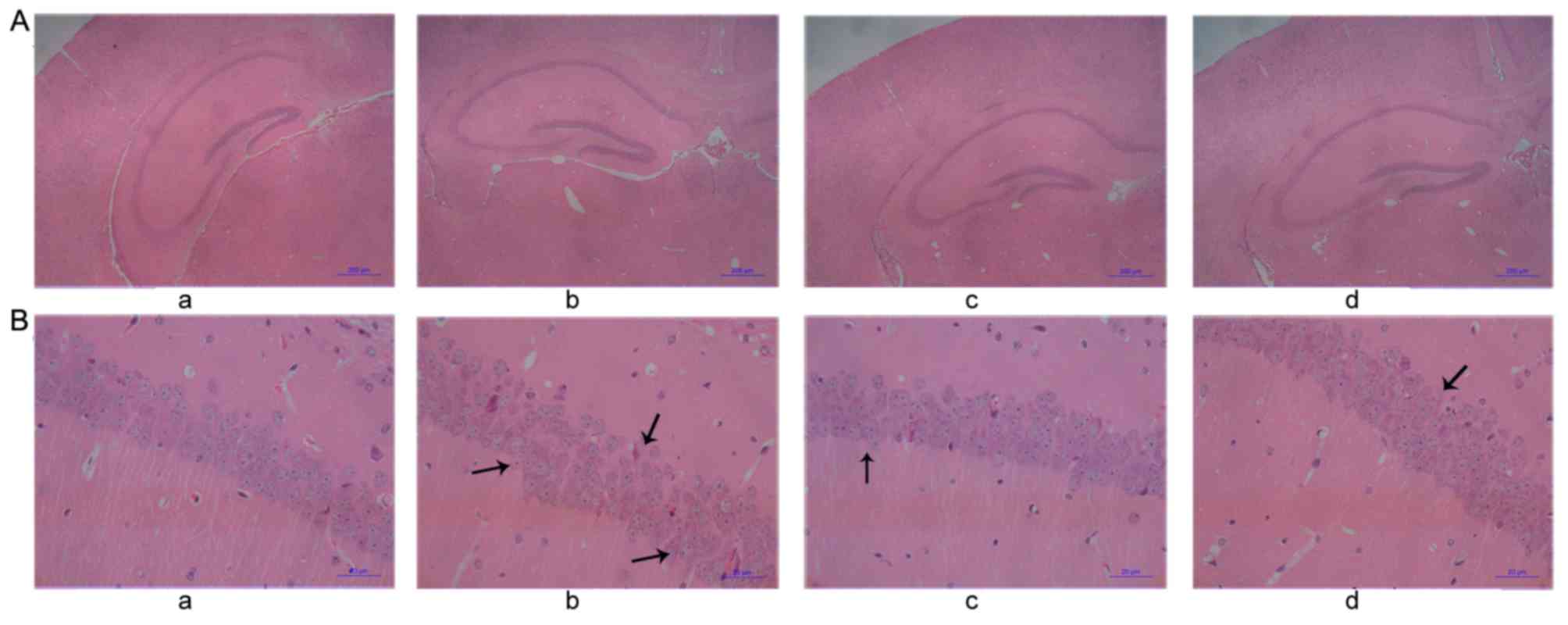
Pathological changes in the
hippocampus of mice
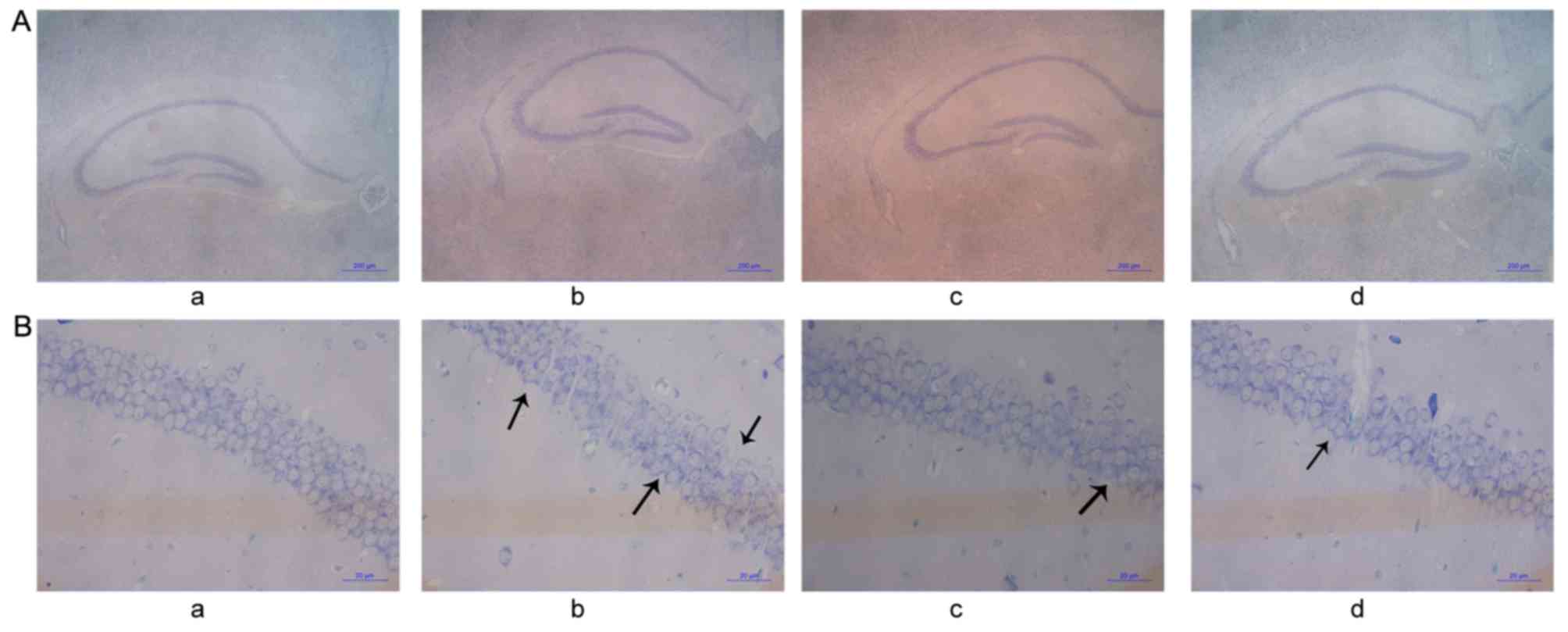
HE staining (Fig. 4)
indicated that there was swelling of cells at the CA1 region of the
hippocampus and dentate gyrus, and that the number of pyramidal
cells decreased. Some neurons underwent necrosis and exhibited
pyramidal cells that were triangular or polygonal in shape, reduced
in size and undergoing karyopyknosis with unclear nucleoli
(Fig. 4Ab). In the control group,
there were no marked changes to the neurons in any region of the
hippocampus in either side of the brain. Furthermore, there was no
swelling or necrosis, which indicated that the cells were healthy
(Fig. 4Aa). Compared with the
CO-poisoning group, the swelling of neurons in the hippocampus of
mice in the hemin-treated and hemin-pretreated groups was less
evident, the number of cells undergoing necrosis decreased and the
cell arrangements for both groups were more orderly (Fig. 4Ac and d). The morphology of cells
exhibited unclear cell boundaries with several necrotic cells
(Fig. 4Bb). In the control group,
there were no evident pathological changes in the nerve cells, and
they exhibited aligned nuclei (Fig.
4Ba). Compared with the CO-poisoning group, the hemin-treated
and hemin-pretreated groups exhibited clearer boundaries with fewer
necrotic cells (Fig. 4Bc and d).
Nissl staining (Fig.
5) indicated that, in the CO-poisoning group, the neurons of
the hippocampus CA1 region were swollen and the number of Nissl
bodies decreased. Some neurons underwent necrosis with unclear
nucleoli and irregular boundaries (Fig.
5Ab). In the control group, there were no marked changes to the
neurons in any region of the hippocampus in either side of the
brain (Fig. 5Aa). Compared with the
CO-poisoning group, the swelling of neurons in the hippocampus of
mice in the hemin-treated and hemin-pretreated groups was less
evident; there were fewer necrotic cells and the cell arrangements
were more orderly (Fig. 5Ac and d).
In addition, the morphology of cells was irregular with unclear
boundaries accompanied by nuclei undergoing karyopyknosis (Fig. 5Bb). In the control group, there were
no evident pathological changes in the nerve cells, which indicated
that no swelling or necrosis had occurred (Fig. 5Ba). Compared with the CO-poisoning
group, the cell arrangements in the hemin-treated and
hemin-pretreated groups were more regular, the number of Nissl
bodies markedly decreased and fewer necrotic cells were observed
(Fig. 5Bc and d).
Discussion
In clinical settings, the initial management of
patients with CO poisoning consists of removing the patient from
exposure to the toxic atmosphere and supplying pure oxygen to
accelerate the elimination of CO and improve tissue oxygenation
(24). The exposure to acute CO
poisoning is primarily by inhalation. Therefore, CO poisoning
animal models were previously established by inhalation, which may
be classified as either dynamic or static exposure (25). Dynamic exposure, which is similar to
routine human inhalation, may negate the interference of other
non-toxic factors such as asphyxia. However, due to the high cost
of requirements and higher demand of hermeticity, the extensive use
of this approach is not convenient. Conversely, static exposure is
simple, but it is difficult to prevent hypoxia induced by
CO2 and other interferential factors. Therefore, the
results may not be accurate.
Previous research has demonstrated that the
establishment of an acute CO poisoning model by intraperitoneal
injection is a convenient and effective animal model (15). Compared with inhalation exposure,
intraperitoneal injection of CO results in a more precise CO
exposure dose which is more suitable for scientific research. It
has been demonstrated that if the level of HbCO in the blood is
>10%, toxic reactions can be observed; at levels of ≥30%,
moderate poisoning can be observed and at 50% severe poisoning
(26). Furthermore, a single
injection may result in HbCO levels rapidly reaching >60%, and
maintaining stability at 50% within 6 h. Additionally, a single
intraperitoneal injection of CO exhibits the same poisoning
mechanism and symptoms as those in the clinical settings (27), even though its exposure route is
different from clinical acute CO poisoning. In the present study, a
modified Spearman-Karber method was used to determine a single
intraperitoneal injection lethal dose that was the lower limit of
the median lethal dose (LD50) (28).
In the present study, following a single intraperitoneal injection
of CO, significant CO poisoning manifestations were observed in
mice. Furthermore, the binding ability of haemoglobin to oxygen was
significantly reduced resulting in tissue hypoxia and lipid
peroxidation, and blood MDA levels increased rapidly and
pathological changes in the hippocampal cells were observed. In
conclusion, the mouse model of the present study was established
via a single intraperitoneal injection of CO that successfully
simulated the pathogenic process of CO poisoning as observed in
clinical settings.
Hemin, as a synthetic heme chloride with a high
absorption rate, is recognized as a good source of iron to prevent
iron deficiency in anemia (14).
Hemin that has the property to combine with oxygen is able to
improve the oxygen carrying capacity of blood (13). Furthermore, hemin is also an
activator of neuroglobin (29), the
oxygen carrier that participates in transportation and storage of
oxygen in neurons and subsequently increases the oxygen
concentration within neurons. Therefore, hemin may be a potential
therapeutic agent to relieve hypoxia following CO exposure.
The present study demonstrated that both
pretreatment and treatment with hemin to mice with CO poisoning was
able to significantly reduce their mortality rate. Furthermore, it
is able to reduce toxic injury in mice, which was demonstrated by
the prolonged onset of symptoms in mice and a significant decrease
in the blood HbCO level. In CO poisoning, hypoxia has a
proportional association with the HbCO level (30), and therefore, the present results
indicated that the degree of poisoning in the pretreatment and
treatment groups has been reduced compared with that in the
CO-poisoning group. According to these results, the protective
mechanism of hemin against CO poisoning may be due to a reduction
in the blood HbCO level, which prevents oxygen transfer.
Furthermore, it may be because hemin replaces HbCO into
oxyhaemoglobin to expel CO and thus, oxygen may be transported to
the tissues by oxyhaemoglobin.
Hemin may also combine with free CO in the
bloodstream and thus relieve tissue injury. In addition, a previous
animal study provided evidence that hemin is able to induce heme
oxygenase-1 (HO-1) activity leading to neuroprotection against
acute CO poisoning (15). It is
believed that HO-1 exhibits protective effects against exogenous CO
toxicity (31) through degradation
of heme into biliverdin and free iron that show potential
biological effects (32,33). By combining the above evidence with
the results of the present study, it is suggested that it is
another possible mechanism that involves the production of HO-1
induced by hemin (34) following
acute CO exposure. However, further studies could be performed in
this field to investigate the underlying molecular mechanisms.
The present study also demonstrated that MDA, the
blood oxidative stress indicator, decreased significantly in both
the hemin-pretreated and hemin-treated groups compared with in the
CO-poisoning model group. It is believed that oxidative stress is
essential in CO-induced neuronal damage (35). Furthermore, MDA is a common product
of lipid peroxidation, which is able to reflect the systemic lipid
peroxidation level and therefore indirectly reflects the degree of
free radical attack and cell injury extent (36). Therefore, it is possible to measure
oxygen radicals and lipid peroxidation level in the brain tissue
via serum MDA content. The observations of the present study were
consistent with the hypothesis that reactive oxygen species (ROS)
may be associated with the acute toxic effects of CO on the central
nervous system (8). Additionally, a
sudden burst of ROS during ischemic-reperfusion injury in CO
toxicity leads to cellular lipid peroxidation, and thus MDA may
increase in acute CO poisoning. The decrease in MDA indicated that
the protective effect of hemin for acute CO poisoning injury may be
associated with the inhibition of lipid peroxidation, reducing ROS
in the brain. Furthermore, in clinical settings, oxygen therapy
following CO-induced tissue hypoxia may be followed by
ischemic-reperfusion injury in the CNS (37), leading to increased production of ROS
such as nitric oxide (38).
Therefore, it may be hypothesized that hemin may also be an
alternative therapy to prevent secondary injury and thus protect
the brain.
Histopathological examinations demonstrated that
both hemin pretreatment and the hemin treatment in early stages
could not only reduce hippocampus oedema and necrosis, but also the
number of abnormal cells and neuronal damage. Since the hippocampal
area is important in memory, learning and emotional activities, it
is highly sensitive to hypoxia, asphyxia and ischemia because of
its high metabolic rate (25).
Furthermore, white and gray matter in the brain are sensitive to
hypoxic damage due to the anatomic structure of poor vasculature
(39). Therefore, the hippocampal
area is able to show evident pathological changes in acute CO
poisoning. Furthermore, the improvement in pathological changes in
the hippocampal area revealed that CO-induced impairment was
significantly alleviated in mice pretreated or treated with
hemin.
In conclusion, the present study found that hemin
has protective effects of decreasing mortality and relieving
hippocampus oedema in mice with acute CO poisoning. Furthermore,
the potential protective mechanisms may include a decrease in the
level of HbCO, inhibition of lipid peroxidation and reduction of
oxygen free radicals in brain cells. Meanwhile, the present study
could provide reliable evidence of animal experiments for the
treatment of acute CO poisoning. Finally, applications of hemin for
acute CO poisoning may provide a reliable basis for future clinical
treatment to gain rescue time and further decrease the mortality
rate.
Acknowledgements
The present study was funded by the Natural Science
Foundation of China (grant no. 81202519) and Guangdong Province
(grant no. S2011040002140), and the Science and Technology Program
of Guangzhou (grant no. 201607010216) and Challenge Cup of Jinan
University (grant no. 15112024).
References
|
1
|
Braubach M, Algoet A, Beaton M, Lauriou S,
Héroux ME and Krzyzanowski M: Mortality associated with exposure to
carbon monoxide in WHO European Member States. Indoor Air.
23:115–125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prockop LD and Chichkova RI: Carbon
monoxide intoxication: An updated review. J Neurol Sci.
262:122–130. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weaver LK, Hopkins RO, Chan KJ, Churchill
S, Elliott CG, Clemmer TP, Orme JF Jr, Thomas FO and Morris AH:
Hyperbaric oxygen for acute carbon monoxide poisoning. N Engl J
Med. 347:1057–1067. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pace N, Strajman E and Walker EL:
Acceleration of carbon monoxide elimination in man by high pressure
oxygen. Science. 111:652–654. 1950. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hawkins M, Harrison J and Charters P:
Severe carbon monoxide poisoning: Outcome after hyperbaric oxygen
therapy. Br J Anaesth. 84:584–586. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hampson NB and Zmaeff JL: Outcome of
patients experiencing cardiac arrest with carbon monoxide poisoning
treated with hyperbaric oxygen. Ann Emerg Med. 38:36–41. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Q, Song JJ, Zhang HY, Fu K, Lan HB and
Deng Y: Dexamethasone therapy for preventing delayed encephalopathy
after carbon monoxide poisoning. Biotech Histochem. 90:561–567.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Akyol S, Yuksel S, Pehlivan S, Erdemli HK,
Gulec MA, Adam B and Akyol O: Possible role of antioxidants and
nitric oxide inhibitors against carbon monoxide poisoning: Having a
clear conscience because of their potential benefits. Med
Hypotheses. 92:3–6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang H, Li Y, Wu Q, Xu C and Liu Q:
Combination of butylphthalide with umbilical mesenchymal stem cells
for the treatment of delayed encephalopathy after carbon monoxide
poisoning. Medicine (Baltimore). 95:e54122016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ochi S, Abe M, Li C, Mori Y, Ishimaru T,
Yoshino Y, Yamazaki K, Mori T, Fukuhara R, Tanimukai S, et al: The
nicotinic cholinergic system is affected in rats with delayed
carbon monoxide encephalopathy. Neurosci Lett. 569:33–37. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu MC, Shiah IS, Yeh CB, Chen HK and Chen
CK: Ziprasidone in the treatment of delayed carbon monoxide
encephalopathy. Prog Neuropsychopharmacol Biol Psychiatry.
30:755–757. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
O'Bryan EC, Veser FH, Veser B and Casey R:
Therapeutic effects of glucagon on carbon monoxide poisoning.
Annals Emergency Med. 44:S922004. View Article : Google Scholar
|
|
13
|
Cuadrado A and Rojo AI: Heme oxygenase-1
as a therapeutic target in neurodegenerative diseases and brain
infections. Curr Pharm Des. 14:429–442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu Y, Sun Y, Jin K and Greenberg DA:
Hemin induces neuroglobin expression in neural cells. Blood.
100:2494–2498. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guan L, Wen T, Zhang Y, Wang X and Zhao J:
Induction of heme oxygenase-1 with hemin attenuates hippocampal
injury in rats after acute carbon monoxide poisoning. Toxicology.
262:146–152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fechter LD, Thorne PR and Nuttall AL:
Effects of carbon monoxide on cochlear electrophysiology and blood
flow. Hear Res. 27:37–45. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bruce RD: An up-and-down procedure for
acute toxicity testing. Fundam Appl Toxicol. 5:151–157. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hamilton MA, Russo RC and Thurston RV:
Trimmed Spearman-Karber method for estimating median lethal
concentrations in toxicity bioassays. Environmental Sci Technol.
11:714–719. 1977. View Article : Google Scholar
|
|
19
|
Ramieri A Jr, Jatlow P and Seligson D: New
method for rapid determination of carboxyhemoglobin by use of
double-wavelength spectrophotometry. Clin Chem. 20:278–281.
1974.PubMed/NCBI
|
|
20
|
Placer ZA, Cushman LL and Johnson BC:
Estimation of product of lipid peroxidation (malonyl dialdehyde) in
biochemical systems. Anal Biochem. 16:359–364. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Todorova I, Simeonova G, Kyuchukova D,
Dinev D and Gadjeva V: Reference values of oxidative stress
parameters (MDA, SOD, CAT) in dogs and cats. Comparative Clinical
Pathol. 13:190–194. 2005. View Article : Google Scholar
|
|
22
|
Nabeshima T, Katoh A, Ishimaru H, Yoneda
Y, Ogita K, Murase K, Ohtsuka H, Inari K, Fukuta T and Kameyama T:
Carbon monoxide-induced delayed amnesia, delayed neuronal death and
change in acetylcholine concentration in mice. J Pharmacol Exp
Ther. 256:378–384. 1991.PubMed/NCBI
|
|
23
|
Sidman RL, Angevine JB and Pierce ET:
Atlas of the mouse brain and spinal cord. Harvard University Press;
Cambridge, Mass: 1971
|
|
24
|
Raub JA, Mathieu-Nolf M, Hampson NB and
Thom SR: Carbon monoxide poisoning-a public health perspective.
Toxicology. 145:1–14. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Penney DG: Acute carbon monoxide
poisoning: Animal models: A review. Toxicology. 62:123–160. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lopez DM, Weingarten-Arams JS, Singer LP
and Conway EE Jr: Relationship between arterial, mixed venous, and
internal jugular carboxyhemoglobin concentrations at low, medium
and high concentrations in a piglet model of carbon monoxide
toxicity. Crit Care Med. 28:1998–2001. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fechter LD, Thorne PR and Nuttall AL:
Effects of carbon monoxide on cochlear electrophysiology and blood
flow. Hear Res. 27:37–45. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fan Y, Li J, Yin Q, Zhang Y, Xu H, Shi X,
Li C, Zhou Y and Zhou C: Effect of extractions from Ephedra sinica
Stapf on hyperlipidemia in mice. Exp Ther Med. 9:619–625. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Burmester T, Weich B, Reinhardt S and
Hankeln T: A vertebrate globin expressed in the brain. Nature.
407:520–523. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen NC, Huang CW, Lui CC, Lee CC, Chang
WN, Huang SH, Chen C and Chang CC: Diffusion-weighted imaging
improves prediction in cognitive outcome and clinical phases in
patients with carbon monoxide intoxication. Neuroradiology.
55:107–115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kurauchi Y, Hisatsune A, Isohama Y and
Katsuki H: Nitric oxide-cyclic GMP signaling pathway limits
inflammatory degeneration of midbrain dopaminergic neurons: Cell
type-specific regulation of heme oxygenase-1 expression.
Neuroscience. 158:856–866. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Morse D and Choi AM: Heme oxygenase-1:
From bench to bedside. Am J Respir Crit Care Med. 172:660–670.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu L and Wang R: Carbon monoxide:
Endogenous production, physiological functions, and pharmacological
applications. Pharmacol Rev. 57:585–630. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Elbirt KK and Bonkovsky HL: Heme
oxygenase: Recent advances in understanding its regulation and
role. Proc Assoc Am Physicians. 111:pp. 438–447. 1999; PubMed/NCBI
|
|
35
|
Akyol S, Erdogan S, Idiz N, Celik S, Kaya
M, Ucar F, Dane S and Akyol O: The role of reactive oxygen species
and oxidative stress in carbon monoxide toxicity: An in-depth
analysis. Redox Rep. 19:180–189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Amemiya S, Kamiya T, Nito C, Inaba T, Kato
K, Ueda M, Shimazaki K and Katayama Y: Anti-apoptotic and
neuroprotective effects of edaravone following transient focal
ischemia in rats. Eur J Pharmacol. 516:125–130. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hampson NB, Simonson SG, Kramer CC and
Piantadosi CA: Central nervous system oxygen toxicity during
hyperbaric treatment of patients with carbon monoxide poisoning.
Undersea Hyperb Med. 23:215–219. 1996.PubMed/NCBI
|
|
38
|
Ulbrich F and Goebel U: Argon: A novel
therapeutic option to treat neuronal ischemia and reperfusion
injuries? Neural Regen Res. 10:1043–1044. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
O'Donnell P, Buxton PJ, Pitkin A and
Jarvis LJ: The magnetic resonance imaging appearances of the brain
in acute carbon monoxide poisoning. Clin Radiol. 55:273–280. 2000.
View Article : Google Scholar : PubMed/NCBI
|