Introduction
Syphilis is a sexually transmitted disease caused by
Treponema pallidum (Tp), which mainly attacks genital and
mucous membranes in the early stage and affects all systems in the
advanced stage (1). Based on the
disease course and symptoms, syphilis may be classified as primary,
secondary, tertiary and latent syphilis (2). The prognosis of syphilis patients is
good if the disease is detected early and subjected to standard
treatment (3). The incidence of
syphilis has been increasing worldwide (4,5); for
instance, it ranks first among all sexually transmitted diseases in
China (6,7). Atypical symptoms or untreated latent
syphilis may develop into serious cardiovascular syphilis and
neurosyphilis and may finally be life-threatening (8).
At present, the clinical diagnosis of syphilis is
based on a combination of the patient's personal history, clinical
symptoms and laboratory tests, the latter of which are particularly
important for patients with latent syphilis without any clinical
symptoms. At present, the laboratory diagnosis of syphilis is
mainly based on serological tests. The new syphilis algorithm is of
high specificity and sensitivity (9), using Tp-specific antibody tests such as
enzyme or chemiluminescent immunoassays as screening tests and the
rapid plasma regain (RPR) test or the Tolulized Red Unheated Serum
test (TRUST) for diagnosis as well as evaluation of treatment
efficacy and relapse (10). When a
patient with suspected Tp infection is false positive, specific
antibody detection methods such as Tp Particle Agglutination (TPPA)
or fluorescent treponemal antibody absorption are performed for
confirmation (10). However, the
sensitivity of Tp-specific antibody detection kits requires to be
improved for early diagnosis of syphilis (11). TRUST and RPR are non-specific
antibody detection assays with a high false-positive rate (12) and may not be suitable for evaluation
of treatment efficacy due to prolonged observation time and
serofast phenomenon (13),
particularly in patients with low titers.
The syphilis-specific recombinant diagnostic
antigens in Tp antibody detection kits are predominantly membrane
lipoproteins such as chimera of Tp17, Tp47, Tp15 and treponemal
membrane protein A (14,15). Detection of syphilis using these
antigens has certain disadvantages. For instance, Baughn et
al (16) found that human
fibronectin was in high homology with the 411PGTEYT426 sequence of
the Tp47 antigen, as well as with antigens of Treponema
endemicum and Treponema pertenue, leading to false
positive reactions. Furthermore, the number and types of Tp outer
membrane proteins are rare (17);
therefore, it is necessary to identify novel syphilis-specific
antigens.
Tp0693 is a hypothetical protein with unknown
function (18). The preliminary
results demonstrated that Tp0693 has strong immunogenicity and is
specifically recognized in sera from patients with syphilis
infection during the screening of antigens for syphilis. A
bioinformatics analysis, including transmembrane, signaling
peptide, subcellular localization and epitope analyses, indicated
that it is a secretion protein with strong immunogenicity in the
present study. Secretion proteins of other pathogens, such as
leishmaniasis (19), tuberculosis
(20), Fasciola (21) and gasterophilosis (22) have been found to serve as diagnostic
antigens. However, whether Tp0693 is a diagnostic antigen for
syphilis has remained elusive. The present study evaluated the
serodiagnostic value of Tp0693 in syphilis at various stages,
including early and latent syphilis.
Materials and methods
Strains and plasmids
Tp Nichols standard strains were preserved by
inoculation in a total of 8 male New Zealand rabbits (3.0–3.5 kg;
240 days old; Department of Laboratory Animal, University of South
China, Changde, China) and rabbits were housed at 18–20°C, 60–65%
humidity with a 12 h light/dark cycle and free access to food and
water. All animal experiments were approved by the Animal Welfare
Committee of University of South China. The Escherichia
(E.) coli strain JM109 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was used for DNA cloning and
BL21 (DE3; Merck KGaA, Darmstadt, Germany) was used for protein
expression were provided by the Institute of Pathogenic Biology,
University of South China (Chengyang, China).
Patients and samples
A total of 168 syphilis clinical serum samples were
obtained from patients with clinically diagnosed syphilis at the
First People's Hospital of Changde (Changde, China) from September
2014 to September 2015, including 36 cases of primary syphilis, 41
cases of secondary syphilis, 12 cases of tertiary syphilis, 75
cases of latent syphilis and 4 cases of congenital syphilis. A
total of 153 cases without syphilis infection were used as
controls, including 2 cases of epidemic hemorrhagic fever, 22 cases
of Epstein-Barr (EB) virus infection, 10 cases salmonella
infection, 29 cases of rheumatic disease, 1 case of multiple
myeloma, 1 case of cytomegalovirus infection, 51 healthy patients
who visited the hospital for physical examination and 27 pregnant
women. All clinical information was obtained from the medical
records of the subjects. Prior written informed consent was
obtained from each subject. The study was approved by the ethics
review board of the University of South China (Changde, China).
All specimens were stored at −20°C prior to
examination with a TPPA kit (VN40810; Fujirebio Diagnostics, Inc.,
Japan), a TRUST kit (2014082504; Shanghai Rongsheng Bio-Tech Co.,
Ltd., Shanghai, China) and a LiZhu™ Tp-ELISA kit (2014082601; Lvzhu
Pharmaceutical Group Co., Ltd., Beijing, China).
Construction of pET30a-Tp0693
plasmid
The primers for the Tp0693 gene were
5′-CGCGGATCCATGGACCGTTTTTTTTGTACGG-3′ and
5′-CCGCTCGAGTTACAGGAAGCACTGGAGC-3′. The Tp Nichols strain was used
as a template for polymerase chain reaction PCR amplification of
the Tp0693 gene. The Tp0693 gene was then cloned into a pET30a(+)
vector (Novagen; Merck KGaA) to construct the pET30a-Tp0693
plasmid. The pET30a-Tp0693 plasmid was identified by colony
screening and sequencing.
Expression and purification of
recombinant proteins
The recombinant plasmid pET30a-Tp0693 was
transfected into E. coli BL21 (DE3), which was then cultured
in lysogeny broth (Bioleaf Science, Inc., Shanghai, China) and
centrifuged at 37°C with kanamycin at 80 × g. Isopropyl
β-D-1-thiogalactopyranoside (IPTG; Bioleaf Science, Inc.) at a
final concentration of 0.5 mM was added when the optical density
(OD) reached ~0.6. The bacteria were then centrifuged at 30°C for 4
h with kanamycin at 50 × g. Finally, the bacteria were lysed in a
buffer containing 50 mM Trs-HCl (pH 7.8), 300 mM NaCl, 10 mM
imidazole, 20% glycerol and 1.5% Triton X-100, centrifuged at
16,000 × g for 10 min and the supernatant was collected. The
recombinant protein Tp0693 was purified by
Ni2+-nitroloacetic acid affinity chromatography (Qiagen
AB, Sollentuna, Sweden).
Western blot analysis
The purified recombinant protein Tp0693 (15 µg,
Lowory assay) was separated by 12% SDS-PAGE and then transferred
onto a polyvinylidene difluoride membrane. After blocking with 5%
skimmed milk, the membrane was incubated overnight at 4°C with
primary anti-polyhistidine tag (His)-labeled monoclonal antibody
(1:1,000; 20150201; Auragene Bioscience Co., Ltd., Changsha, China)
or with serum from syphilis-positive or -negative patients. After
washing for 3 times, secondary horseradish peroxidase (HRP)-labeled
goat anti-human immunoglobulin G (IgG) antibody (1:100; 20150312;
Shanghai Yanhui Biology Technology Co., Ltd., Shanghai, China) was
added, followed by incubation for 1 h at 37°C. Finally, the
membrane was developed with enhanced chemiluminescence plus reagent
(Pierce; Thermo Fisher Scientific, Inc.). The developed film was
scanned using the AlphaImager gel imaging system (G:BOX Chemi XXX9;
Syngene, Cambridge, UK). The western blot images were analyzed
using Quantity One software 3.0 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Establishment of indirect Tp0693-ELISA
method and TPPA, RPR and LiZhu™ Tp-ELISA testing
The recombinant protein Tp0693 was diluted with
carbonate buffer (pH 9.6) to a concentration of 15 µg/ml and was
used as the antigen. The Tp0693 solution was then added to 96-well
microtiter plates at 100 µl per well and incubated overnight at
4°C. The plate was washed 5 times with PBS containing Tween-20
(PBST), and was blocked by incubation with 5% skimmed milk
overnight at 4°C. Sample serum (100 µl; 1:100 dilution) was added
to each well and incubated for 60 min at 37°C. After washing with
PBST for 5 times, secondary antibody (1:1,000 dilution; HRP-labeled
goat anti-human IgG) was added, followed by incubation at 37°C for
1 h. The chromogenic agent was added and the absorbance was
detected at 450 nm wavelength for obtaining sample-to-cutoff ratio
(S/CO) values of sample serum. The experiment was repeated 3 times
in parallel. All samples were then analyzed by TPPA, RPR and LiZhu™
Tp-ELISA according to the kit instructions.
Statistical analysis
Statistical analysis was performed using SPSS 18.0
software (SPSS, Inc., Chicago, IL, USA). A receiver operating
characteristic (ROC) curve was plotted. The area under the curve
(AUC) was identified, and the sensitivity and specificity were
calculated. The S/CO values of Tp0693-ELISA and LiZhu™ Tp-ELISA
were continuous variables, and titers of TPPA and TRUST were
categorical variables. A Spearman correlation analysis was used.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Identification of engineered
strain
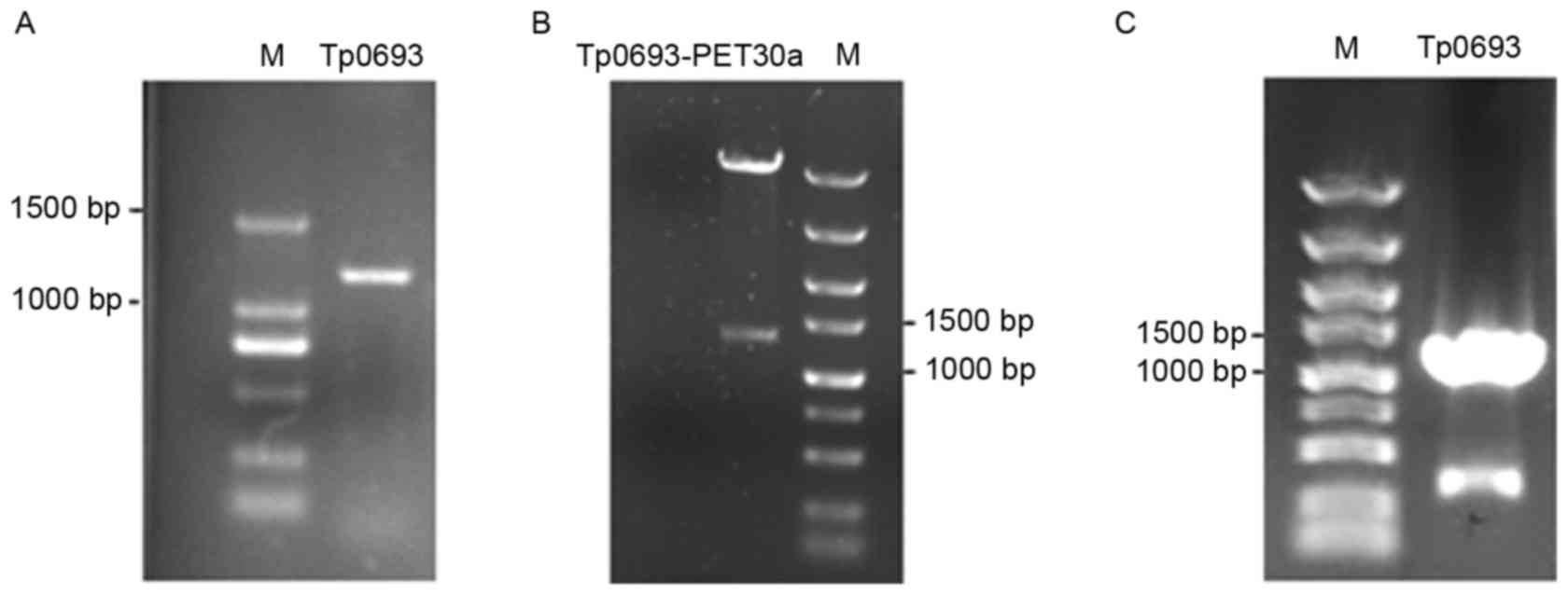
To identify the engineered Tp0693-expressing strain,
the recombinant plasmids were identified by PCR, restriction
digestion and sequencing. The expected specific target band at
1,323 bp was visible, as presented in Fig. 1. The gene sequences were compared
with the published gene sequences in GenBank (https://www.ncbi.nlm.nih.gov/genbank/)
through the Basic Local Alignment Search Tool with 100%
compatibility. This indicated that the recombinant
Tp0693-expressing plasmid was successfully constructed.
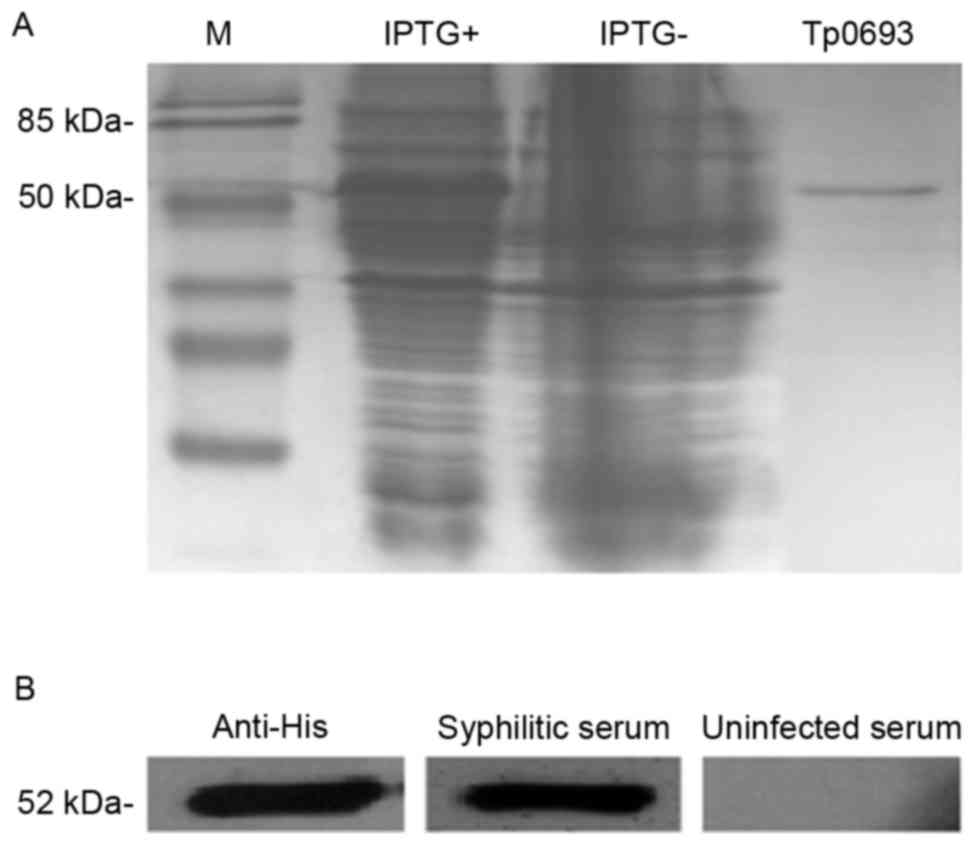
Expression, purification and
identification of recombinant proteins
To determine the expression, purification and
identification of recombinant proteins, the diluted anti-His
antibody, as well as serum from patients with or without syphilis
were used as the primary antibodies or negative control for western
blot analysis. Tp0693 expression was induced by IPTG. The
identification of the Ni2+ column-purified product was
examined by SDS-PAGE, as presented in Fig. 2A. The His antibody-reactive target
band with a molecular weight of ~52 kDa was slightly larger than
the standard value (47.66 kDa) (18), possibly due to the His tag at the N
terminus. As presented in Fig. 2B,
no immunoreactivity was observed with the serum from subjects
without syphilis, while immunoreactive bands were obtained with
anti-His antibody and serum from patients with syphilis. This
indicated that after the expression, purification and western blot
identification, the target protein was suitable for use in the
subsequent ELISA experiments.
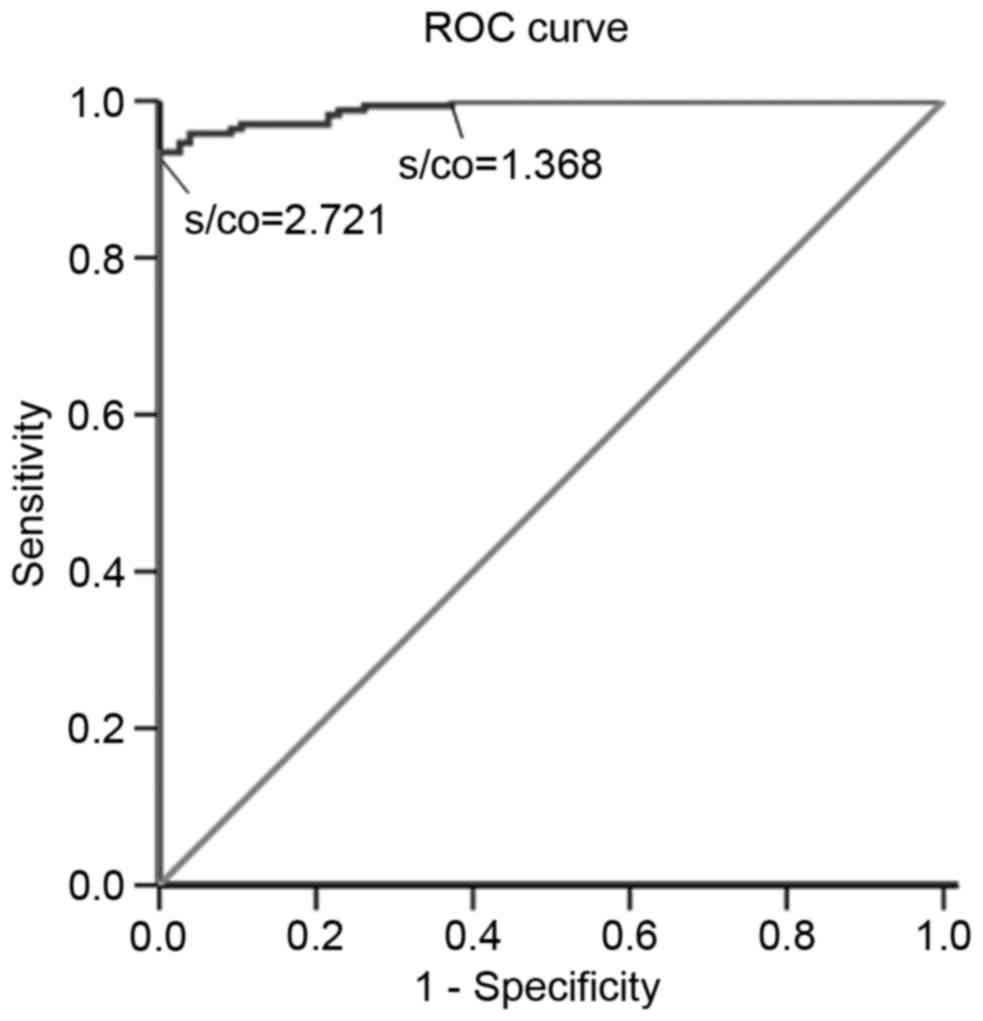
ROC curve analysis
The S/CO of the Tp0693-specific ELISA and clinical
diagnosis of syphilis were used to draw a ROC curve, which is
presented in Fig. 3. The AUC was
0.990. When the S/CO was <1.368, the sensitivity was 100% and
when the S/CO was >2.721, the specificity was 100%. This
indicated that Tp0693 had a good diagnostic value in syphilis
screening, and is expected to become a novel antigen candidate for
the diagnosis of syphilis.
Comparison of TPPA, TRUST, LiZhu™
Tp-ELISA and Tp0693-ELISA
To determine the diagnostic efficacy of TPPA, TRUST,
LiZhu™ Tp-ELISA and Tp0693-ELISA, the ROC curve was
analyzed. The S/CO value of 2.721 in Tp0693-ELISAwas used as a
cut-off value. As presented in Tables
I and II, the detection
sensitivity of the Tp0693-ELISA method was 93.5%, which was higher
than that of TRUST (79.2%). The detection sensitivity of
Tp0693-ELISA was 97.2% for primary syphilis, 100% for secondary
syphilis, 91.7% for tertiary syphilis, 88% for latent syphilis and
100% for congenital syphilis. The distribution of the S/CO values
for Tp0693-ELISA is presented in Fig.
4, demonstrating that the mean S/CO value for serum samples
from patients with Tp infection was higher than that of serum from
patients without syphilis. This result indicated that analysis of
Tp0693 may be used to specifically identify syphilis-infected
sera.
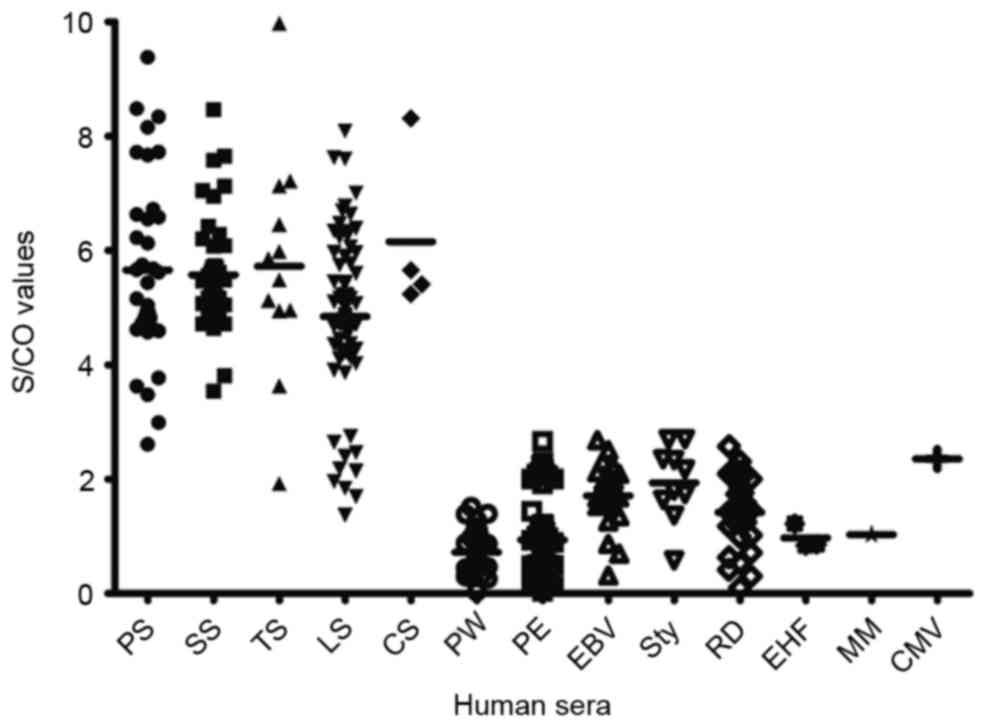 | Figure 4.S/CO values for Tp0693-specific ELISA
regarding the detection of different stages of syphilis and
cross-reactivity in serum of patients without syphilis infection.
The different stages of syphilis and other infections/diseases were
grouped on the x-axis and mean S/CO values of each group were
displayed on the y-axis. The overall mean S/CO value of each group
is represented by horizontal lines. PS, primary syphilis; SS,
secondary syphilis; TS, tertiary syphilis; LS, latent syphilis; CS,
congenital syphilis; PW, pregnant women; PE, physical examination
(healthy patients); EBV, Epstein-Barr virus infection; Sty,
Salmonella typhimurium infection; RD, rheumatic disease; EHF,
epidemic hemorrhagic fever; MM, multiple myeloma; CMV,
cytomegalovirus infection; S/CO, sample-to-cutoff ratio. |
 | Table I.Sensitivity of TRUST, TPPA,
Tp0693-ELISA and LiZhu™ Tp-ELISA in the detection of different
stages of syphilis. |
Table I.
Sensitivity of TRUST, TPPA,
Tp0693-ELISA and LiZhu™ Tp-ELISA in the detection of different
stages of syphilis.
|
| Tp0693-ELISA | LiZhu™ Tp-ELISA | TPPA | TRUST |
|---|
|
|
|
|
|
|
|---|
| Sample type | Positive | Negative | Sensitivity
(%) | Positive | Negative | Sensitivity
(%) | Positive | Negative | Sensitivity
(%) | Positive | Negative | Sensitivity
(%) |
|---|
| PS | 35 | 1 | 97.2 | 36 | 0 | 100 | 36 | 0 | 100 | 24 | 12 | 66.7 |
| SS | 41 | 0 | 100 | 41 | 0 | 100 | 41 | 0 | 100 | 37 | 4 | 90.2 |
| TS | 11 | 1 | 91.7 | 12 | 0 | 100 | 12 | 0 | 100 | 12 | 0 | 100 |
| LS | 66 | 9 | 88 | 75 | 0 | 100 | 75 | 0 | 100 | 56 | 19 | 74.7 |
| CS | 4 | 0 | 100 | 4 | 0 | 100 | 4 | 0 | 100 | 4 | 0 | 100 |
| AS | 157 | 11 | 93.5 | 168 | 0 | 100 | 168 | 0 | 100 | 152 | 16 | 79.2 |
 | Table II.Specificity of TRUST, TPPA,
Tp0693-ELISA and LiZhu™ Tp-ELISA in the detection of sera from
uninfected controls and potentially cross-reactive infections. |
Table II.
Specificity of TRUST, TPPA,
Tp0693-ELISA and LiZhu™ Tp-ELISA in the detection of sera from
uninfected controls and potentially cross-reactive infections.
|
| Tp0693-ELISA | LiZhu™
Tp-ELISA | TPPA | TRUST |
|---|
|
|
|
|
|
|
|---|
| Sample type | Positive | Negative | Specificity
(%) | Positive | Negative | Specificity
(%) | Positive | Negative | Specificity
(%) | Positive | Negative | Specificity
(%) |
|---|
| PM | 0 | 37 | 100 | 5 | 32 | 86.5 | 0 | 37 | 100 | 0 | 37 | 100 |
| PE | 0 | 51 | 100 | 12 | 39 | 76.5 | 0 | 51 | 100 | 0 | 51 | 100 |
| EBV | 0 | 22 | 100 | 0 | 22 | 100 | 0 | 22 | 100 | 0 | 22 | 100 |
| Sty | 0 | 10 | 100 | 0 | 10 | 100 | 0 | 10 | 100 | 0 | 10 | 100 |
| RD | 0 | 29 | 100 | 0 | 2 | 100 | 0 | 29 | 100 | 0 | 29 | 100 |
| EHF | 0 | 2 | 100 | 0 | 2 | 100 | 0 | 2 | 100 | 0 | 2 | 100 |
| AN | 0 | 151 | 100 | 15 | 136 | 88.9 | 0 | 151 | 100 | 0 | 151 | 100 |
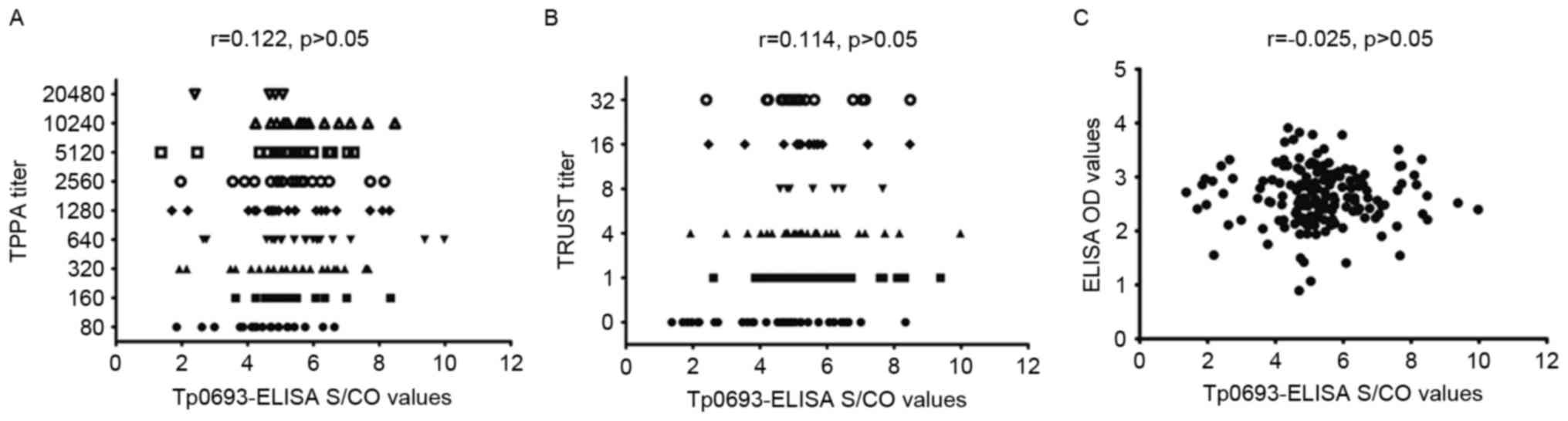
Correlation analysis of TPPA, TRUST,
LiZhu™ Tp-ELISA and Tp0693-ELISA
To determine the correlation of TPPA, TRUST, ELISA
and Tp0693-ELISA, a Spearman correlation analysis was performed.
The correlation coefficient was 0.122 (P>0.05) for the
Tp0693-ELISA S/CO value and the TPPA titer, 0.114 (P>0.05) for
the Tp0693-ELISA S/CO value and the TRUST titer, and 0.025
(P>0.05) for the Tp0693-ELISA S/CO value and the
LiZhu™ Tp ELISA OD value. As presented in Fig. 5, the S/CO value of the Tp0693-ELISA
was not increased with the TPPA titer, TRUST titer or
LiZhu™ Tp-ELISA OD value. These results indicated that
the S/CO value of Tp0693-ELISA was not correlated with TPPA or
TRUST titers, or the LiZhu™ Tp-ELISA OD value.
Grey area analysis
To analyze the grey area of the Tp0693-ELISA S/CO
value, its distribution was analyzed. The grey area of the
Tp0693-ELISA results was identified to be between 1.368 (highest
S/CO when the sensitivity was 100%) and 2.721 (the lowest S/CO when
the specificity was 100%). The grey area distribution is presented
in Table III, and it was highest
(14.8%) among patients with latent syphilis. In the cross-reaction
examination, typhoid fever (80%) and rheumatic diseases (55.2%)
ranked highest. As presented in Fig.
6, in the grey zone of syphilis-positive samples, the TPPA
titers were all ≥1:80, part of the TRUST results were negative, and
the OD values of the LiZhu™ Tp-ELISA were all >0.15.
In the syphilis-negative specimens, TPPA and TRUST results were all
negative; however, one sample was positive according to
LiZhu™ Tp-ELISA. This result indicated that Tp0693 is a
novel syphilis-specific antigen, whose diagnostic value may be
limited, and further study is required to explore its associated
functions.
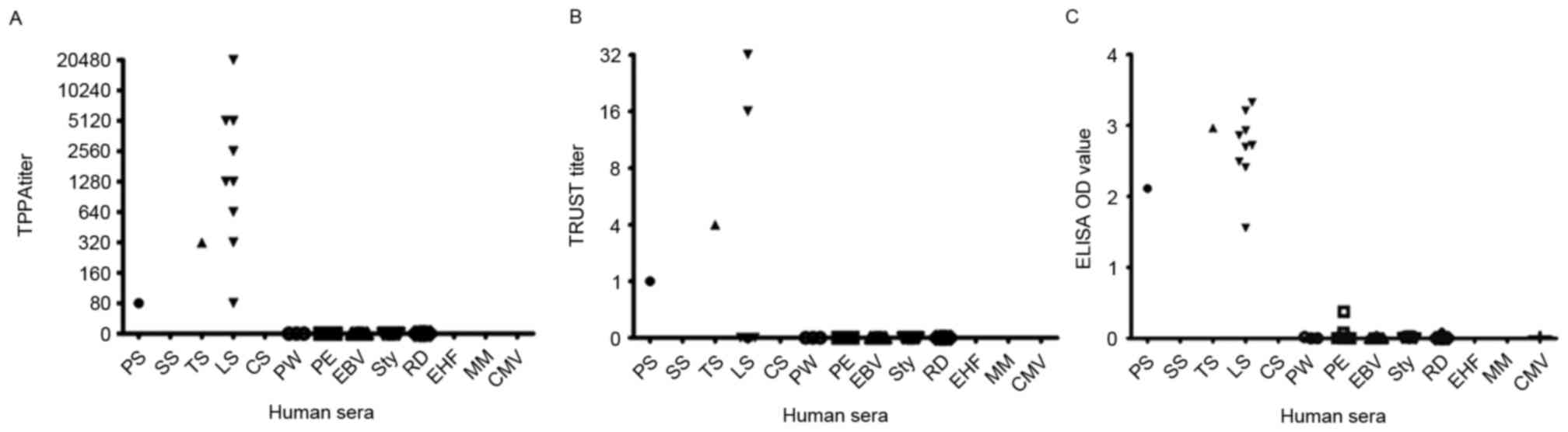 | Figure 6.Grey area of Tp0693-specific ELISA.
(A) TPPA titers, (B) TRUST titers and (C) ELISA S/CO values in
serum of patient groups with different stages of syphilis and those
without syphilis infection. PS, primary syphilis; SS, secondary
syphilis; TS, tertiary syphilis; LS, latent syphilis; CS,
congenital syphilis; PW, pregnant women; PE, physical examination
(healthy patients); EBV, Epstein-Barr virus infection; Sty,
Salmonella typhimurium infection; RD, rheumatic disease;
EHF, epidemic hemorrhagic fever; MM, multiple myeloma; CMV,
cytomegalovirus infection; S/CO, sample-to-cutoff ratio; TPPA, Tp
Particle Agglutination; TRUST, Tolulized Red Unheated Serum test;
OD, optical density; Tp, Treponema pallidum. |
 | Table III.Distribution of different specimens
in the detection of the grey zone of Tp0693-specific ELISA. |
Table III.
Distribution of different specimens
in the detection of the grey zone of Tp0693-specific ELISA.
| Sample type | Total (n) | Grey area samples,
n (%) |
|---|
| PS | 36 | 1 (2.78) |
| SS | 41 | 0 (0) |
| TS | 12 | 1 (8.3) |
| LS | 75 | 9 (12) |
| CS | 4 | 0 (0) |
| PW | 37 | 3 (8.1) |
| PE | 51 | 12 (23.5) |
| EBV | 22 | 17 (77.3) |
| Sty | 10 | 8 (80) |
| RD | 29 | 16 (55.2) |
| ECH | 3 | 0 (0) |
| MM | 1 | 0 (0) |
| CMV | 1 | 1 (100) |
| All | 321 | 68 (21.2) |
Discussion
At present, the antigen screening targets for
syphilis diagnosis are Tp membrane lipoprotein (14,15,23,24) or
flagellin (25); however, they have
certain limitations. The number and expression rate of
syphilis-specific plasma membrane lipoproteins is low. Membrane
protein TP47 and human as well as other treponema species (such as
treponema endemicum and treponema pertenue) share high antigenic
homology (16), leading to
false-positive reactions. Therefore, it is necessary to explore
novel types of recombinant antigens, and multi-epitope chimeric
antigens may improve the specificity and sensitivity of
detection.
The structure and function of Tp0693 in Tp have
remained elusive. Certain exocrine signaling peptides are present
on the outer membrane of pathogens, and Tp0693 protein is stably
expressed in the pathogenic strains of Tp according to a
bioinformatics analysis. It shares low homology with other
pathogens and the human genome. While McKevitt M (18) did not observe natural Tp0693 by
protein mass spectrometric analysis of Tp bacterial protein, this
may not necessarily indicate weak antigenicity and immunity; it may
have been due to Tp0693 being an exocrine protein that is secreted
by Tp during the early stages of infection. Other
pathogen-associated secreted proteins have been reported in
clinical studies (26).
The present study successfully constructed
pET30a/Tp0693 recombinant plasmid and purified Tp0693 recombinant
protein. This purified protein had no interaction with serum from
individuals without Tp infection, but strong immunoreactivity with
the serum of patients with syphilis, indicating the
antigen-specific reactions and potential diagnostic value of
Tp0693.
The ROC curve indicated a correlation between
sensitivity and specificity, with the false-positive rate
(1-specificity) displayed on the y-axis and the true positive rate
(sensitivity) on the x-axis as. The AUC was used to evaluate the
diagnostic value. There is no diagnostic value when the AUC is
<0.5, a low diagnostic value when the AUC is <0.7, a moderate
diagnostic value when the AUC is 0.7–0.9, and a significant
diagnostic value when the AUC is >0.9. The AUC of the S/CO value
from the TP0693-ELISA assay was 0.99, indicating a high diagnostic
value.
In the present study, the diagnostic specificity of
Tp0693-ELISA reached 100% and the sensitivity was up to 93.5%,
which was higher than that of TRUST (79.2%). The sensitivity of
Tp0693-ELISA was 97.2% for primary syphilis. In the present study,
the specificity of TPPA and TRUST was 100% each, which was higher
than that determined by Liu et al (27) (98.38% for TPPA and 86.49% for TRUST).
This discrepancy may be due to the limited number of collected
samples and potential cross-reactions in the present study, for
which the specificity of Tp0693-ELISA (100%) was higher than that
of LiZhu™ Tp-ELISA (88.9%). However, in the clinical
setting, the sensitivity of screening tests is more important than
the specificity to avoid misdiagnosis (28), which may be avoided by another
specific syphilis test. Therefore, it is acceptable that in the
present study, the sensitivity of the ELISA screening test was
lower.
In the syphilis-positive samples, the S/CO value of
the Tp0693-ELISA was not correlated with the TPPA titer, RPR titer
or OD value of the LiZhu™ Tp-ELISA. The TPPA titer was
weakly correlated with the OD value of the LiZhu™
Tp-ELISA, which uses Tp membrane lipoprotein as the analyte antigen
(data not shown). Previously, Yoshioka et al (29) reported that chemiluminescent
microparticle immunoassay (CMIA) results were strongly correlated
with TPPA titers. Jiang et al (24) reported that membrane protein
TpF1-specific ELISA was correlated with TPPA titers. As TPPA
assesses Tp antigens as a whole, i.e. all membrane lipoproteins
combined, as antigens, its results are likely to be associated with
lipoprotein, as well as the results of membrane antigen Tp-ELISA,
CMIA and TpF1-ELISA. However, the Tp0693 protein is a completely
different type of secretion protein whose levels may not be
correlated with the results of other tests.
The gray area analysis missed a proportion of the
serum samples from patients with syphilis, including those with
high LiZhu™ Tp-ELISA OD values and high TPPA and RPR
titers, which may be due to the low or no production of Tp0693
during certain periods of Tp infection. Part of the S/CO values of
serum samples from syphilis-negative patients were in the grey
area, which may be due to the Tp0693 protein having weak
cross-reactivity with human proteins (30). In the future, epitope fitting of
Tp0693 and other strong and specific antigens will be assessed as
diagnostic antigens to improve the specificity and sensitivity of
detection. Those samples in the grey area of Tp0693-ELISA should
further perform TPPA for diagnosis instead of TRUST and ELISA. The
latter two methods were not able to distinguish between true
positives and true negatives.
In the present study, the Tp IgG antibody was
detected, which is not suitable for detecting early syphilis and
evaluating treatment efficacy, relapse and infectiousness. Bosshard
(31) found that patients in the
early infection stage may have negative TPPA results but may be
positive for Tp IgM detected by ELISA. Due to its high molecular
weight, the Tp IgM antibody is not able to get through the placenta
and blood brain barrier, and therefore, the identification of a
specific Tp IgM antibody in cerebrospinal fluid and amniotic fluid
may be of great importance for the diagnosis of congenital syphilis
and neurosyphilis (32). Tp0693 is
considered as a secretion protein that is only secreted during
active infection and therefore, it may be better for diagnosing
active infections. There are similar ongoing studies on the
diagnostic potential of secretion proteins of Mycobacterium
tuberculosis for active infection (26). Therefore, it is worthwhile to further
analyze Tp IgM antibody as an early sign of syphilis infection.
In conclusion, the present study evaluated the
diagnostic performance of a novel secretion protein, Tp0693, in
various stages of syphilis. Taken together, the results of the
present study indicate that Tp0693 may be a promising antigenic
marker of syphilis that can be developed into a diagnostic antigen
for the screening of syphilis.
Acknowledgements
The present study was supported by the National
Natural Science Foundation (grant nos. 81373230 and 81301470), the
Guangdong Provincial Science and Technology Department Foundation
(grant no. 2014A020212036), the Preclinical Medicine Hunan
Provincial Key Disciplines, Hunan Provincial Key Laboratory for
Special Pathogens Prevention and Control (grant no. 2014-5), the
Hunan Provincial Cooperative Innovation Center for Molecular Target
New Drug Study (grant no. 2015-08).
References
|
1
|
Chadwick JA, MacNab A, Sarma J, Ray S,
Kadir I and Muldoon EG: Secondary syphilis presenting with aortitis
and coronary ostial occlusion. Sex Transm Infect. 92:108–109. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sukthankar A: Syphilis. Medicine.
42:394–398. 2014. View Article : Google Scholar
|
|
3
|
Stamm LV: Syphilis: Re-emergence of an old
foe. Microb Cell. 3:363–370. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
World Health Organization, . Prevalence
and incidence of selected sexually transmitted infections.
Chlamydia trachomatis, Neisseria gonorrhoeae, syphilis and
Trichomonas vaginalis: Methods and results used by WHO to generate
2005 estimates. World Health Organization; Geneva: 2011
|
|
5
|
World Health Organization, . Global
prevalence and incidence of selected curable sexually transmitted
infections: Overview and estimates. World Health Organization;
Geneva: 2001
|
|
6
|
Tucker JD, Chen XS and Peeling RW:
Syphilis and social upheaval in China. N Engl J Med. 362:1658–1661.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tucker JD and Cohen MS: China's syphilis
epidemic: Epidemiology, proximate determinants of spread, and
control responses. Curr Opin Infect Dis. 24:50–55. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Klein J, McLaud M and Rogers D: Syphilis
on the Rise: Diagnosis, treatment and prevention. J Nurse Pract.
11:49–55. 2015. View Article : Google Scholar
|
|
9
|
Zanto SN: Changing algorithms in syphilis
laboratory diagnosis. Clin Microbiol Newsl. 32:59–64. 2010.
View Article : Google Scholar
|
|
10
|
Workowski KA and Bolan GA: Centers for
Disease Control and Prevention: Sexually transmitted diseases
treatment guidelines, 2015. MMWR Recomm Rep. 64:1–137.
2015.PubMed/NCBI
|
|
11
|
Morales-Múnera CE, Fuentes-Finkelstein PA
and Vall Mayans M: Update on the diagnosis and treatment of
RR-Sífilis: Actualización en el manejo diagnóstico y terapéutico.
106:1–69. 2015.
|
|
12
|
Liu F, Liu LL, Guo XJ, Xi Y, Lin LR, Zhang
HL, Huang SJ, Chen YY, Zhang YF, Zhang Q, et al: Characterization
of the classical biological false-positive reaction in the
serological test for syphilis in the modern era. Int
Immunopharmacol. 20:331–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eickhoff CA and Decker CF: Syphilis. Dis
Mon. 62:280–286. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun AH, Mao YF, Hu Y, Sun Q and Yan J:
Sensitive and specific ELISA coated by TpN15-TpN17-TpN47 fusion
protein for detection of antibodies to Treponema pallidum. Clin
Chem Lab Med. 47:321–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moreno AI Brito, Bas C Acosta, Rodríguez
M, Conde IB Baluja, Carballo S Feal and Martínez L: Monoclonal
antibodies to the recombinant protein TmpA of the Treponema
pallidum. Hybrid Hybridomics. 22:393–396. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baughn RE, Jiang A, Abraham R, Ottmers V
and Musher DM: Molecular mimicry between an immunodominant amino
acid motif on the 47-kDa lipoprotein of Treponema pallidum (Tpp47)
and multiple repeats of analogous sequences in fibronectin. J
Immunol. 157:720–731. 1996.PubMed/NCBI
|
|
17
|
Lafond RE and Lukeart SA: Biological basis
for syphilis. Clin Microbiol Rev. 19:29–49. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McKevitt M, Brinkman MB, McLoughlin M,
Perez C, Howell JK, Weinstock GM, Norris SJ and Palzkill T: Genome
scale identification of Treponema pallidum antigens. Infect Immun.
73:4445–4450. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pinedo-Cancino V, Laurenti MD, Kesper N
and Umezawa ES: Evaluation of Leishmania (Leishmania) infantum
excreted-secretion antigens for detection of canine leishmaniasis.
Acta Trop. 161:41–43. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rizvi N, Singh A, Yadav M, Hussain SR,
Siddiqui S, Kumar V, Ali S and Agarwal A: Role of alpha-crystallin,
early-secretion antigenic target 6-kDa protein and culture filtrate
protein 10 as novel diagnostic markers in osteoarticular
tuberculosis. J Ortho Trans. 6:18–26. 2016.
|
|
21
|
Sabry MA, Taher ES, Allah NF and Mahgoub
AM: Diagnosis of Fasciola infection by SDS-PAGE eluted excretory
secretory (ES) protein fractions using dot-ELISA. Int J Vet Sci
Med. 2:130–135. 2014. View Article : Google Scholar
|
|
22
|
Sánchez-Andrade R, Cortiñas FJ, Francisco
I, Sánchez JA, Mula P, Cazapal C, Vázquez L, Suárez JL, Francisco
R, Arias MS, et al: A novel second instar Gasterophilus
excretory/secretory antigen-based ELISA for the diagnosis of
gasterophilosis in grazing horses. Vet Parasitol. 171:314–320.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu M, Xie Y, Jiang C, Xiao Y, Kuang X,
Zhao F, Zeng T, Liu S, Liang M, Li L, et al: A novel ELISA using a
recombinant outer membrane protein, rTp0663, as the antigen for
serological diagnosis of syphilis. Int J Infect Dis. 43:51–57.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang C, Zhao F, Xiao J, Zeng T, Yu J, Ma
X, Wu H and Wu Y: Evaluation of the recombinant protein TpF1 of
Treponema pallidum for serodiagnosis of syphilis. Clin Vaccine
Immunol. 20:1563–1568. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang C, Xiao J, Xie Y, Xiao Y, Wang C,
Kuang X, Xu M, Li R, Zeng T, Liu S, et al: Evaluation of FlaB1,
FlaB2, FlaB3, and Tp0463 of Treponema pallidum for serodiagnosis of
syphilis. Diagn Microbiol Infect Dis. 84:105–111. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tiwari D, Haque S, Tiwari RP, Jawed A,
Govender T and Kruger HG: Fast and efficient detection of
tuberculosis antigens using liposome encapsulated secretory
proteins of Mycobacterium tuberculosis. J Microbiol Immunol Infect.
50:189–198. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu C, Ou Q, Chen H, Chen J, Lin S, Jiang
L and Yang B: The diagnostic value and performance evaluation of
five serological tests for the detection of Treponema pallidum. J
Clin Lab Anal. 28:204–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cantor AG, Pappas M, Daeges M and Nelson
HD: Screening for Syphilis: Updated evidence report and systematic
review for the US preventive services task force. JAMA.
315:2328–2337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoshioka N, Deguchi M, Kagita M, Kita M,
Watanabe M, Asari S and Iwatani Y: Evaluation of a chemiluminescent
microparticle immunoassay for determination of Treponema pallidum
antibodies. Clin Lab. 53:597–603. 2007.PubMed/NCBI
|
|
30
|
Wang L, Deng X, Liu H, Zhao L, You X, Dai
P, Wan K and Zeng Y: The mimic epitopes of Mycobacterium
tuberculosis screened by phage display peptide library have
serodiagnositic potential for tuberculosis. Pathog Dis. 74:pii:
ftw0912016. View Article : Google Scholar
|
|
31
|
Bosshard PP: Usefulness of IgM-specific
enzyme immunoassays for serodiagnosis of syphilis: Comparative
evaluation of three different assays. J Infect. 67:35–42. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Herremans M, Notermans DW, Mommers M and
Kortbeek LM: Comparison of a Treponema pallidum IgM immunoblot with
a 19S fluorescent treponemal antibody absorption test for the
diagnosis of congenital syphilis. Diagn Microbiol Infect Dis.
59:61–66. 2007. View Article : Google Scholar : PubMed/NCBI
|