Introduction
Oxidative stress results from an imbalance between
in vivo oxidative and anti-oxidative effects (1). Oxidative stress is involved in the
pathophysiological processes of various diseases, including
arteriosclerosis, diabetes, ischemia/reperfusion injury, ethanol
intoxication and liver steatosis (2). When the body encounters harmful
stimuli, reactive oxygen species (ROS) are produced that may not be
effectively removed by antioxidants (3). ROS may be produced by non-alcoholic
fatty liver disease (NAFLD) oxidase, xanthine oxidase and the
mitochondrial electron transport system (4). Increased levels of ROS have been
indicated to enhance the expression of apoptotic genes (5). Furthermore, apoptosis has been
indicated as a prominent pathology of liver diseases and is a
well-acknowledged type of programmed cell death (6). For protection against oxidative
stress-related injury, the body possesses several scavenger
systems, including enzymatic and non-enzymatic antioxidants.
Enzymatic antioxidants include superoxide dismutase (SOD), catalase
(CAT) and the selenium dependent enzyme, glutathione peroxidase
(GSH-PX) (7). As antioxidant enzymes
may affect lipid peroxidation, an increase in the activities of
these enzymes may delay liver steatosis. Furthermore, oxidative
stress has been indicated to induce structural and functional
cellular effects through damage to DNA, proteins or membrane lipids
(8). With respect to hepatocytes,
lipid metabolism function may be impaired.
Clinically, liver steatosis without excessive
alcohol consumption is known as NAFLD, which is the most common
liver condition in the world (9).
Abnormal lipid metabolism, which is a high risk for
atherosclerosis, is a condition that may result in various types of
cardiovascular diseases (10).
Previous studies have demonstrated that steatotic livers have
increased susceptibility to oxidative stress and that increased ROS
may trigger an apoptotic cascade (2,11).
Although the scavenging mechanisms of the human body that act
against excess ROS-induced cellular injury are typically
sufficient, these may not always be enough to combat external
stresses. Therefore, there is a pressing requirement to discover
natural antioxidants (12). The
dried roots of Salvia miltiorrhiza Bge, a perennial herb in
the mint family, are used as a traditional Chinese medicine. In
China, the herb is used as an adjunct therapy to treat particular
circulatory diseases by promoting blood circulation and removing
blood stasis (13). Tanshinone IIA
(TSIIA) is one of the major lipophilic components isolated from
Salvia miltiorrhiza Bge (14). Previous clinical trials and
experimental studies have reported the antioxidant and
anti-apoptotic properties of TSIIA (15,16). As
a natural antioxidant, TSIIA is able to protect cardiac myocytes
through anti-oxidative pathways, inhibit oxidation of LDL in
vitro and reduce the serum levels of oxidized low-density
lipoprotein in mice (17–19). In our previous study, we showed that
TSIIA was able to ameliorate atherosclerosis by suppressing
toll-like receptor 4 (TLR4) and tumor necrosis factor (TNF)-α
expression, in vitro (20).
Large-scale prospective studies have also shown that TSIIA can be
beneficial for cardiovascular diseases (14–16).
However, there are few studies on the specific mechanism of TSIIA
activity on the liver. We have previous indicated that TSIIA is
able to protect cells from apoptosis in vitro (21). Thus, the purpose of the present study
was to evaluate the effects of TSIIA on oxidative stress and
hyperlipidemia in rats with fatty livers and to further explore the
mechanism of action of TSIIA.
Materials and methods
Pharmacological agents, chemicals and
reagents
Sodium TIIA sulfonate was purchased from Shanghai
No. 1 Biochemical Pharmaceutical Co., Ltd. (Shanghai, China).
Lactate dehydrogenase (LDH), MDA, peroxidase (POD), T-SOD and
creatine kinase (CK) test kits were obtained from Nanjing Jiancheng
Bioengineering Institute (Nanjing, China). Total cholesterol (TC),
thyroglobulin (TG), low density lipoprotein cholesterol (LDL-C),
high density lipoprotein cholesterol (HDL-C) test kits were
purchased from Sichuan Maker Biotechnology Co., Ltd. (Chengdu,
China). TRIzol reagent and the reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) kit were purchased from Takara
Biotechnology Co., Ltd. (Dalian, China). The BCA Protein Assay kit,
SDS-PAGE Gel Preparation kit and all horseradish
peroxidase-conjugated secondary antibodies were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). RIPA lysis buffer
was purchased from Beyotime Institute of Biotechnology (Shanghai,
China).
Animals
A total of 90 male Sprague-Dawley rats (300±10 g)
aged 6 weeks were purchased from Vital River Laboratories (Beijing,
China). Rats were maintained under standard conditions of 12-h
light-dark cycle (temperature, 22±1°C; humidity, 50±5%) with free
access to water. All animal studies were approved by the Local
Ethics Committee for Animal Experimentation (approval number
2012-113), according to the guidelines of the Animal Committee of
Liaoning University of Traditional Chinese Medicine (Shenyang,
China). All efforts were made to minimize the number of animals
used and their suffering throughout the study. Rats were randomly
divided into a control (CON) group, a high-fat diet group (HFD)
group and a TII A treatment (TAN) group (n=30 per group). Rats in
the CON group were fed a regular balanced diet, while those in the
HFD group and TAN group were fed a high-fat diet (6.0% sucrose,
1.0% sodium glutamate, 5.0% yolk powder, 8.0% peanut oil, 1.5%
cholesterol, 0.4% methylthiouracil, 0.2% sodium cholate and 77.9%
regular balanced diet) for 3 months. Furthermore, rats in the TAN
group received 1.2 ml sodium TIIA sulfonate (10 mg/kg) by
intraperitoneal injection once a day in the last 2 months, while
those in the control group and the HFD group received the same
quantity of normal saline for the same duration of time. At 3
months, the rats were sacrificed by cervical dislocation under 10%
choral hydrate anesthesia (TCI Development Co, Ltd., Shanghai,
China), and small portions of hepatic tissue were excised from the
right lobe of the liver.
High-resolution ultrasound
imaging
Rats were anaesthetized with isoflurane (Beijing
Zhongsheng Ruitai Technology Co., Ltd., Beijing, China) (1.5–3.0%),
together with continuous oxygen (1 l/min). Lipid deposition in the
liver was observed using a Vevo 2100 High-Resolution Imaging System
(FUJIFILM VisualSonics Inc., Toronto, Canada) according to the
manufacturer's instructions.
Serum LDH, MDA, POD, T-SOD, CK and
GSH-PX content
Rats were anaesthetized with 10% chloral hydrate.
Aortic blood was collected and the samples were left to stand for 2
h at room temperature prior to centrifugation at 4°C at 1,000 × g
for 20 min. Serum was collected and stored at −80°C. Serum LDH,
MDA, POD, T-SOD, CK, and GSH-PX content were estimated using
commercial assay kits (A020-1, A003-1, A084-3, A001-1, A032 and
A005, all Nanjing Jiancheng Bioengineering Institute).
Lipid detection
A liver sample of 0.1 g was homogenized in 1 ml
normal saline, supplemented with 50 ml of a 2:1 chloroform (≥99.7%)
to methanol (≥99.0%) mixture and left to stand for 10 min.
Subsequently, the sample was centrifuged at 4°C at 3,000 × g for 10
min and the upper layer was removed. The remaining sample was dried
using a Termovap sample concentrator, resuspended in 0.5 ml
methanol (≥99.0%) and examined using an automatic biochemical
analyzer (Toshiba Medical Systems Corp., Otawara-Shi, Japan). TC
and TG levels were measured by the COD-CE-PAP and GPO-PAP methods
(22), respectively. Serum TG, TC,
HDL-C, LDL-C levels were determined by corresponding assay kits
(0615021, 0815031, 0915051 and 0915061, all Sichuan Maker
Biotechnology Co., Ltd., Sichuan, China).
Hematoxylin and eosin staining of
liver tissues
Liver samples were fixed in 4% paraformaldehyde
solution at room temperature for 24 h and stained with hematoxylin
and eosin using standard techniques. The samples were embedded in
paraffin, and 5-µm thick paraffin sections were cut. The paraffin
was removed with xylene, rehydrated through an alcohol gradient,
and stained with hematoxylin and eosin and observed using light
microscopy at magnification, ×200. Images were captured using light
microscope linked to a digital CCD camera (Olympus Corp., Tokyo,
Japan).
Oil Red O staining of liver
tissue
Oil Red O staining was performed following a
standard protocol. Frozen liver sections (6-µm thick) were cut and
dried. Samples were washed with 50% ethanol, stained with Oil Red O
for 8 min, differentiated with 50% ethanol, rinsed with tap water,
stained with hematoxylin, rinsed with tap water and mounted with
glycerin jelly. Sections were subsequently examined and images were
captured using a light microscope linked to a digital CCD camera at
magnification, ×200.
Hoechst staining
Liver samples that had been fixed with 4%
paraformaldehyde were cut into 5-µm thick sections, deparaffinized
and rehydrated. The tissue was permeabilized with Triton X-100 for
15 min. Nuclei were counterstained with Hoechst 33258, according to
the manufacturer's instructions using an Apoptotic cell Hoechst
33258 Detection kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China). Slides were examined for Hoechst using a fluorescence
microscope at 350 nm and magnification, ×200.
RNA isolation and RT-qPCR
Total RNA was isolated from liver tissue using
TRIzol reagent and reverse transcribed into cDNA using the miScript
II RT kit (Qiagen GmbH, Hiden, Germany). Primers were chosen to
detect the amplification of the chosen gene and were designed as
follows: B cell lymphoma 2 associated protein X (Bax), forward
5′-GCAAACTGGTGCTCAAGG-3′ and reverse 5′-CGTCCCGAAGTAGGAAAGG-3′; B
cell lymphoma-2 (Bcl-2), forward 5′-CGGGAGAACAGGGTATGA-3′ and
reverse 5′-CAGGCTGGAAGGAGAAGAT-3′; caspase 3, forward
5′-CTGGACTGCGGTATTGAG-3′ and reverse 5′-GGGTGCGGTAGAGTAAGC-3′; and
GAPDH, forward 5′-TGTGTCCGTCGTGGATCTGA-3′ and reverse
5′-TTGCTGTTGAAGTCGCAGGAG-3′. The reaction mix consisted of 2 µl
cDNA, 0.5 µl forward and reverse primer mix (10 µM of each), 12.5
µl SYBR Premix Ex Taq II (Takara Biotechnology Co., Ltd.) in a
final volume of 25 µl. The following thermal conditions were used
for all PCR reactions: 2 min at 95°C, followed by 40 cycles of 30
sec at 95°C and 30 sec at 60°C. RT-qPCR was performed in triplicate
using SYBR Green Master Mix (Takara Biotechnology Co., Ltd.) on an
ABI 7500 PCR Sequence Detector (Applied Biosystems; Thermo Fisher
Scientific Inc., Waltham, MA, USA). Data were analyzed using the
SDS software (version 2.0.6; Applied Biosystems; Thermo Fisher
Scientific, Inc.). The fold-change in gene expression was
determined by the 2ΔΔCq (23) method with GAPDH (housekeeping gene)
as an internal control. The size of the amplified products were
indicated to be 149, 149 and 102 bp for Bax, Bcl-2 and caspase 3,
respectively.
Determination of hepatic ROS
Levels of ROS in liver slices were determined by the
oxidation of dihydroethidium (DHE). Frozen sections of liver (5-µm
thick) were incubated for 30 min at 37°C in 1 ml of
4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid buffer
containing 50 µM of DHE. Frozen sections were subsequently washed
three times in phosphate-buffered saline. DHE was oxidized by ROS
to produce fluorescent ethidium, which is able to bind to nucleic
acids, thus further staining the nucleus a bright fluorescenct red.
The formation of ethidium was monitored by fluorescence inversion
microscopy (magnification, ×200) with excitation and emission
wavelengths of 480 and 590 nm, respectively.
Protein extraction and western
blotting
Total cellular protein was extracted from liver
tissues using RIPA lysis buffer (Beyotime Institute of
Biotechnology). Protein concentration was measured using a BCA
protein assay kit (Beijing Dingguo Changsheng Biotechnology Co.,
Ltd., Beijing, China). To assess the protein expression levels of
Bax, Bcl-2, and caspase 3 proteins, 80 µg of protein samples were
separated by 10% SDS-PAGE and transferred onto polyvinylidene
difluoride membranes (Merck Millipore, Darmstadt, Germany). The
membranes were incubated at 4°C overnight with primary antibodies
against caspase 3 (1:800, 9662, Cell Signaling Technology, Inc.,
Danvers, MA, USA), Bax, Bcl-2 and GAPDH (1:500; sc-70407, sc-23960
and sc-32233; all Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
and subsequently with a secondary horseradish peroxidase-conjugated
goat anti-rabbit and anti-mouse antibody (1:4,000, both Santa Cruz
Biotechnology, Inc.) at room temperature for 1 h. Protein bands
were visualized using an enhanced chemiluminescence detection kit
(Thermo Fisher Scientific, Inc.) and exposed to X-ray film. Protein
expression was normalized to the levels of GAPDH.
Statistical analysis
Data are presented as the mean + standard deviation.
Differences among the groups were evaluated with the analysis of
variance test for multiple groups, using GraphPad Prism 5.0
software (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Serum lipid levels and lipid
deposition in the liver
Compared with the CON group, the rats in the HFD
group exhibited significantly increased levels of serum TG
(P<0.05), TC (P<0.01) and LDL-C (P<0.01), and
significantly decreased levels of serum HDL-C (P<0.01; Fig. 1). However, the TAN group did not
indicate any significant difference in lipid profiles when compared
with the HFD group (Fig. 1).
Furthermore, hematoxylin and eosin-stained liver sections indicated
severe hepatic steatosis in the HFD group (Fig. 2A). Compared with the CON group, the
hepatocytes from the HFD group were swollen and round with a
smaller cytoplasm and contained large lipid droplets. Furthermore,
lipid vacuoles of different sizes and inflammatory cells were
observed in hematoxylin and eosin-stained sections from the HFD
group. In several hepatocytes, the nucleus appeared to be pushed to
the side towards the cell membrane. The TAN group exhibited a
reduction in hepatic steatosis, cell swelling and hepatocyte size
in a number of lipid vacuoles when compared with HFD group
(Fig. 2A). Oil Red O staining
revealed that the hepatocytes in the HFD group contained a markedly
increased number of lipid droplets when compared with the CON
group. However, the number of lipid droplets was markedly decreased
in the hepatocytes of the TAN group when compared with the HFD
group. Compared with the homogeneous liver parenchyma of the HFD
group, ultrasound examination of the liver of HFD rats indicated
increased echogenicity of the liver parenchyma, characteristic of
liver steatosis and heterogeneous parenchyma (24). The echo level of the liver parenchyma
was revealed to be decreased in the TAN group when compared with
the HFD group (Fig. 2A).
Additionally, when compared with the CON group, the livers of rats
in the HFD group exhibited significantly increased levels of TG
(P<0.05) and TC (P<0.01); however, significantly decreased
levels of TG (P<0.01) and TC (P<0.05) were indicated in the
TAN group when compared with the HFD group (Fig. 2B).
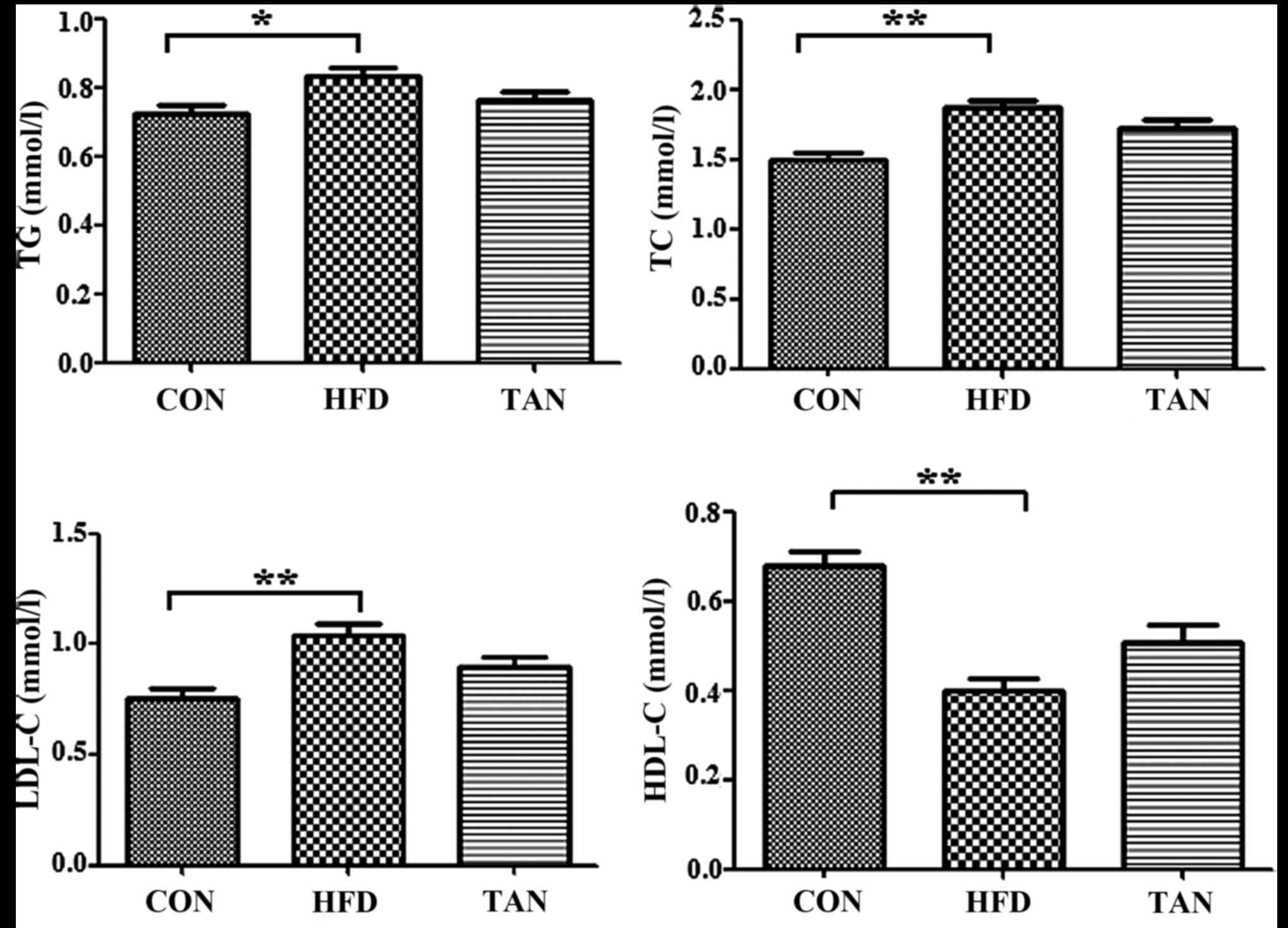 | Figure 1.Serum lipid profile. TG, TC, HDL-C
and LDL-C levels were determined using corresponding test kits in
the CON, HFD and TAN groups. Data are presented as the mean +
standard deviation (n=6). *P<0.05; **P<0.01. CON, control
group; HFD, high-fat diet group; TAN, tanshinone II A treatment
group; TG, thyroglobulin; TC, total cholesterol; HDL-C, high
density lipoprotein cholesterol; LDL-C, low density lipoprotein
cholesterol. |
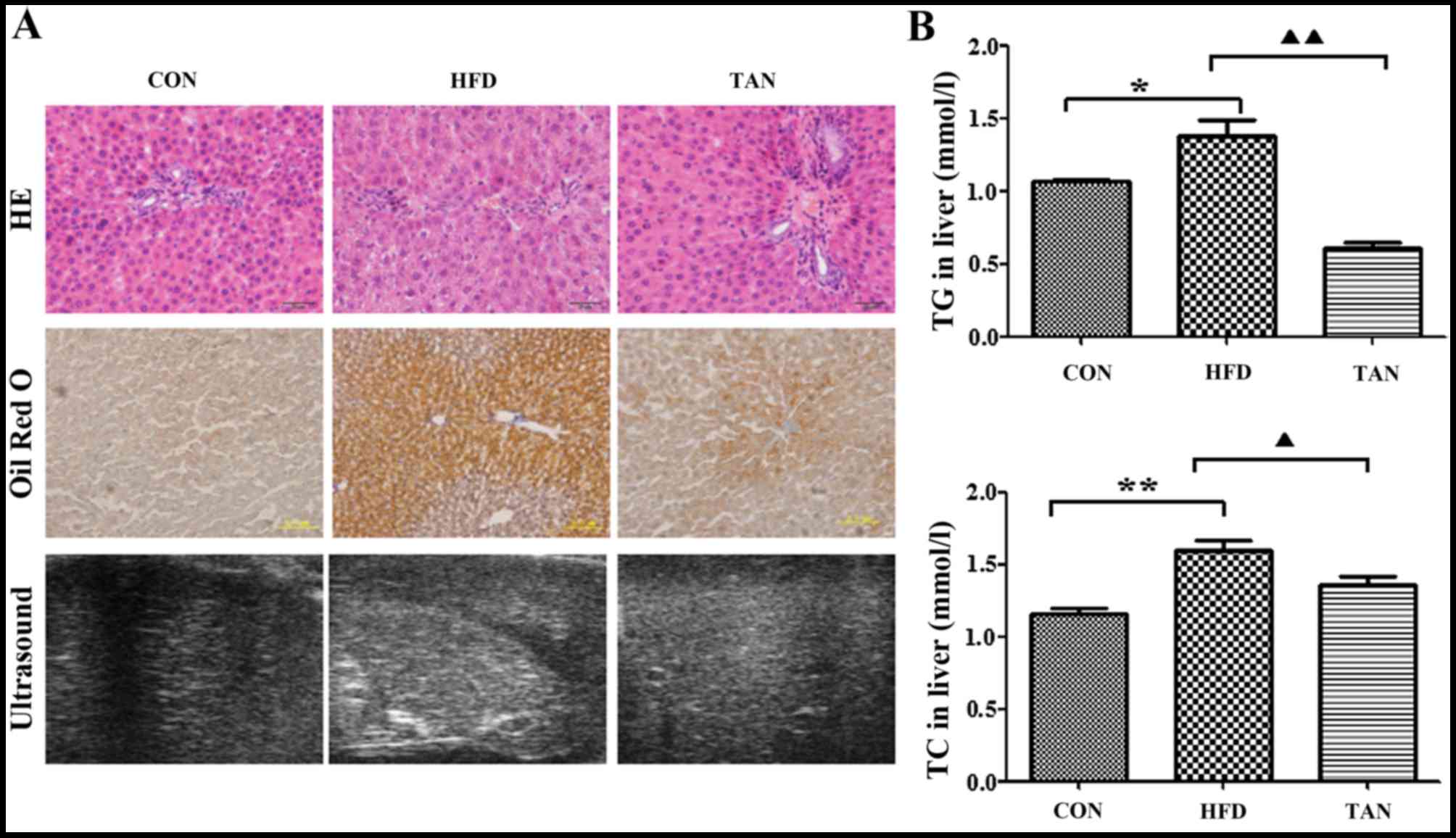 | Figure 2.Differences in morphology and lipid
deposition in rats from the CON, HFD and TAN groups. (A)
Morphological observation of rat hepatocytes in CON, HFD and TAN
groups by HE staining, Oil Red O staining and high-resolution
ultrasound. (B) TG and TC were measured by the COD-CE-PAP and
GPO-PAP methods, respectively, in the livers of CON, HFD and TAN
rats. Data are presented as the mean + standard deviation (n=3).
*P<0.05; **P<0.01; ▲P<0.05;
▲▲P<0.01. CON, control group; HFD, high-fat diet
group; TAN, tanshinone II A treatment group; TG, thyroglobulin; TC,
total cholesterol; HE, hematoxylin and eosin. |
Effect of TSIIA on antioxidant enzymes
and the activity of CK and LDH
Compared with the CON group, MDA content in the HFD
group was significantly elevated (P<0.01; Fig. 3A), whereas the TAN group exhibited
significantly decreased MDA content when compared with the HFD
group (P<0.05). A significant decrease in T-SOD (P<0.05) and
GSH-PX (P<0.01) was observed in the HFD group when compared with
the CON group; however, significantly increased levels of T-SOD
(P<0.05) and GSH-PX (P<0.01) were exhibited in the TAN group
compared with the HFD group, indicating treatment with TSIIA
reversed this effect. The level of POD activity was not
significantly altered in all three groups. A comparison of the
serum-marker enzymes LDH and CK, revealed a significant increase in
CK activity (P<0.01) in the HFD group when compared with the TAN
group; however, LDH activity was unchanged across all three
groups.
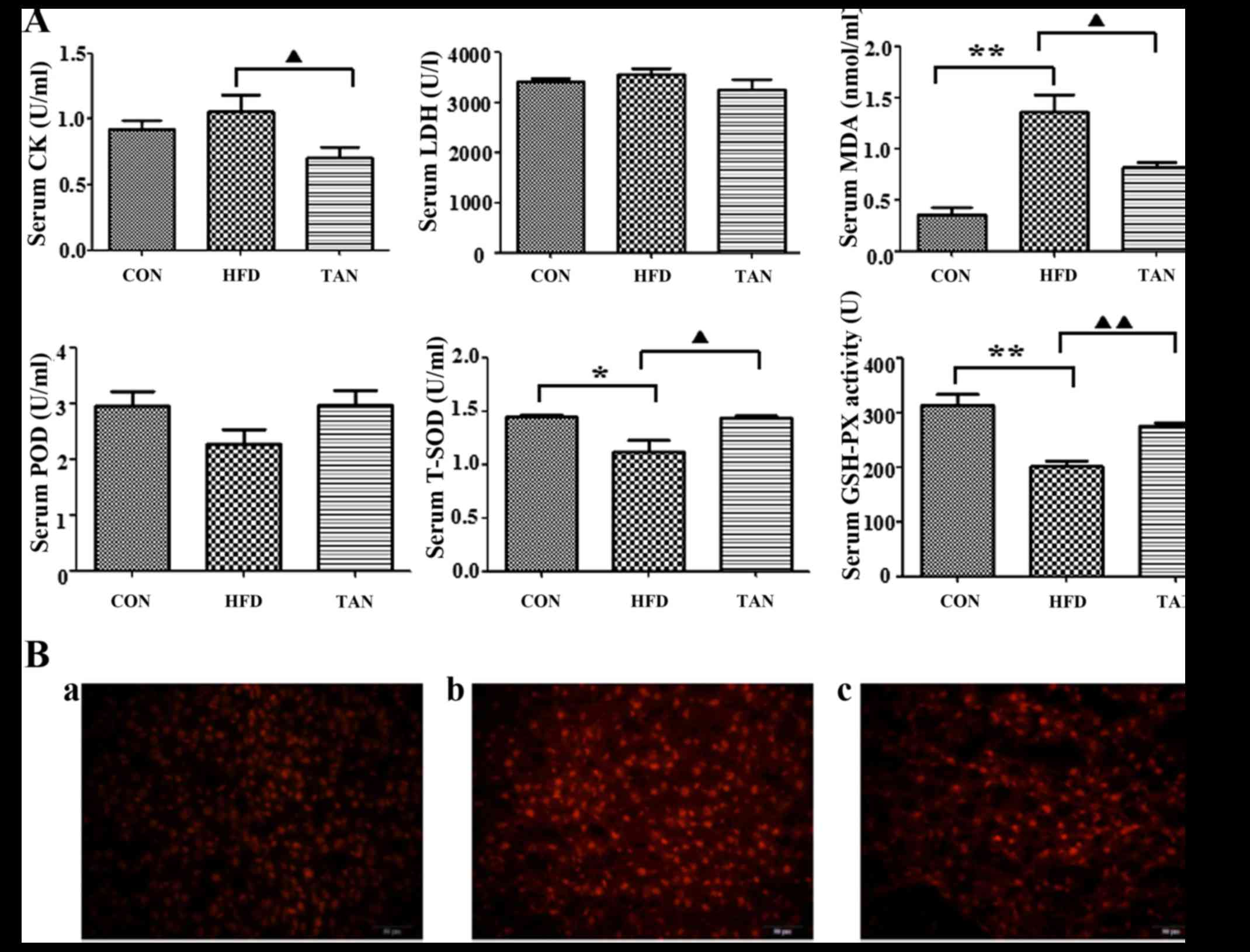 | Figure 3.Effects of tanshinone IIA on high-fat
diet-induced oxidative stress in the liver of rats. (A)
Quantification of CK, LDH, MDA, POD, T-SOD and GSH-PX in the serum
of rats from the CON, HFD and TAN groups. Rats were treated and the
contents were subsequently assayed. Data are presented as the mean
+ standard deviation (n=3). *P<0.05; **P<0.01;
▲P<0.05; ▲▲P<0.01. (B) Detection of ROS
in the liver of rats from the (a) CON group; (b) HFD group; and (c)
TAN group. Rats were treated and dihydroethidium fluorescence was
detected. Red color fluorescence represents ROS. CK, creatine
kinase; LDH, lactate dehydrogenase; MDA, malondialdehyde; POD,
peroxidase, T-SOD, total superoxide dismutase; GSH-PX, glutathione
peroxidase; CON, control group; HFD, high-fat diet group; TAN,
tanshinone II A treatment group; ROS, reactive oxygen species. |
Inhibitory effect of TSIIA on
HFD-induced oxidative stress in the liver
Effects of TSIIA on HFD-induced oxidative stress in
the liver of rats were indicated by fluorescence microscopy.
Compared with the CON group, the HFD group exhibited markedly
increased dihydroethidium fluorescence, which indicated increased
ROS content in the liver. However, the level of fluorescence from
livers of TAN group rats was indicated to be decreased when
compared with the HFD group (Fig.
3B).
Effect of TIIA on liver cell
apoptosis
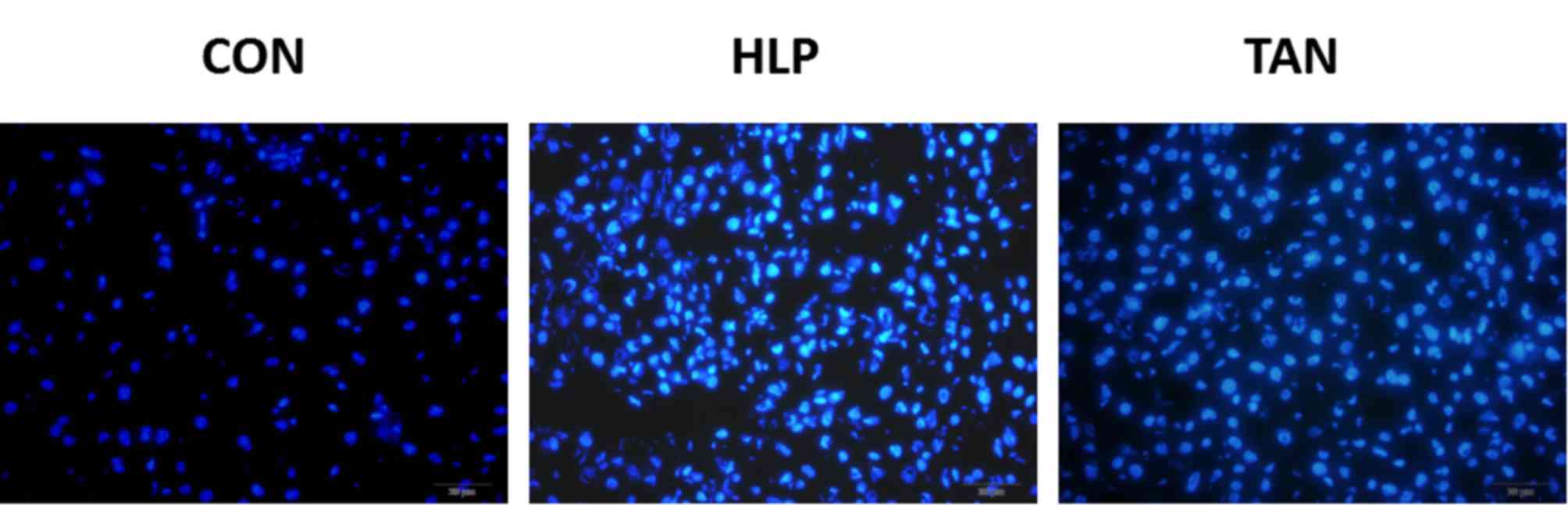
To determine whether HFD induces apoptosis in liver
cells and to explore whether apoptosis may be ameliorated by TSIIA
treatment, rates of liver cell apoptosis were quantified via
Hoechst assay analysis. The Hoechst assay revealed that the HFD
group displayed a markedly increased rate of apoptosis when
compared with the CON group. Furthermore, the TAN group exhibited a
markedly decreased rate of apoptosis when compared with the HFD
group, which indicated that TSIIA treatment was able to markedly
reduce the rate of apoptosis, almost to the level observed in the
CON group (Fig. 4).
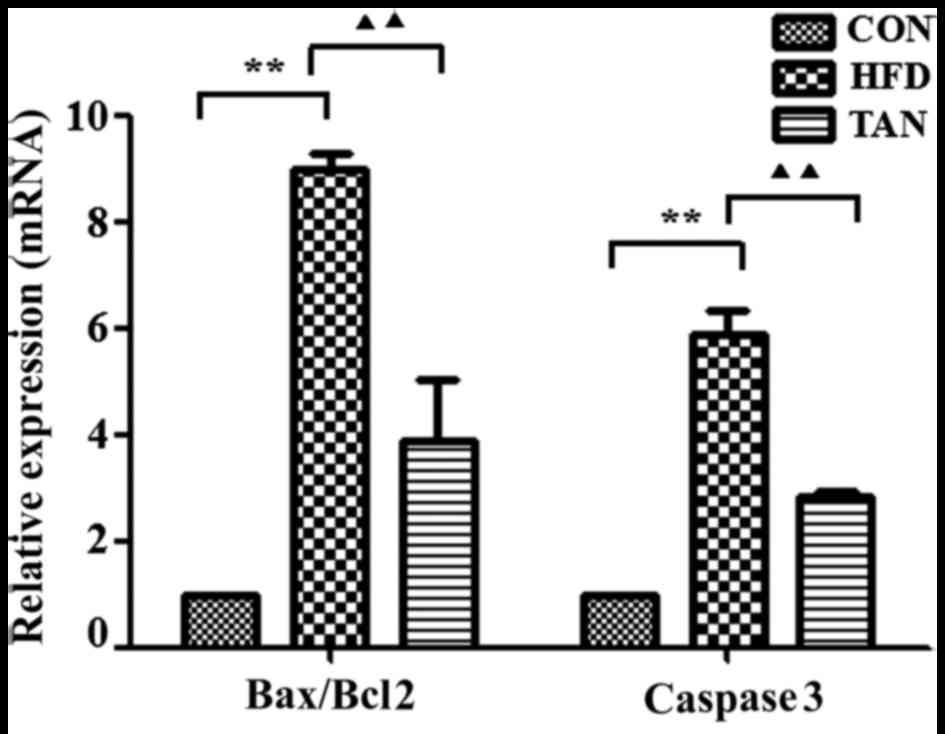
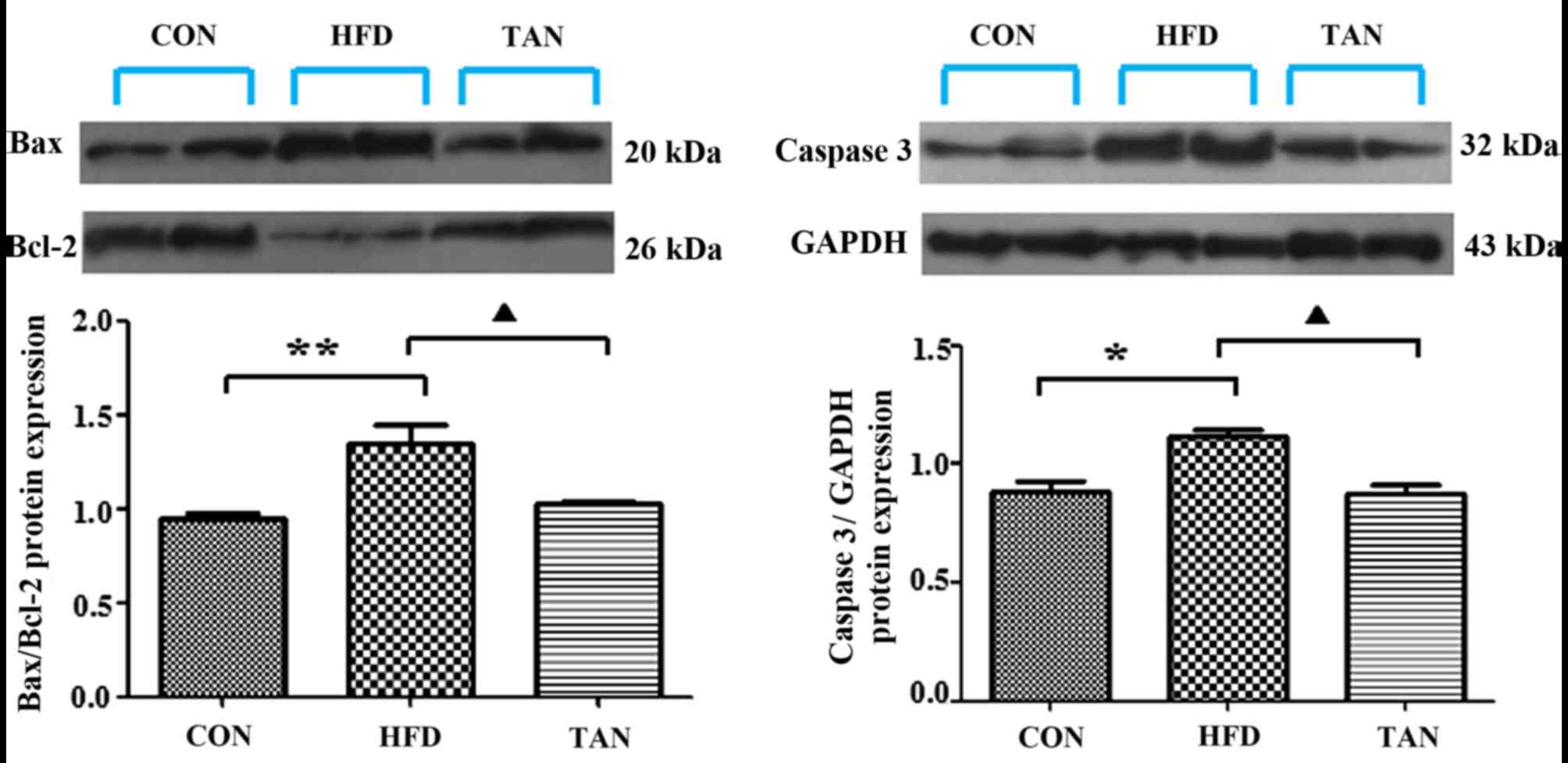
Apoptosis-related gene and protein
expression levels in the liver of rats
The ratio between the pro-apoptotic protein, Bax,
and the anti-apoptotic protein, Bcl-2, in addition to the
expression levels of caspase 3, are important apoptotic indicators
(25,26). The results of RT-qPCR and western
blotting showed that Bax/Bcl-2 mRNA and protein expression levels
in the liver were significantly increased in the HFD group when
compared with the CON group; however, these mRNA and protein
expression levels were significantly decreased in the TAN group
when compared with the HFD group (P<0.01; Figs. 5 and 6). Furthermore, caspase 3 mRNA and protein
expression levels were significantly increased in the HFD group
when compared with the CON group and these expression levels were
significantly decreased in the TAN group when compared with the HFD
group (P<0.05; Figs. 5 and
6).
Discussion
TSIIA is a major component of Salvia
miltiorrhiza Bge (14). Previous
studies have indicated that TSIIA possesses a range of biological
properties (15,16,27),
predominantly based on its anti-oxidative effects (18). In the present study, the effect of
TSIIA on liver steatosis was assessed in vivo. TSIIA was
identified to reduce lipid deposition in the liver and ameliorate
liver steatosis; however, this effect was not based on alterations
of serum lipid levels, despite 3 months of treatment. This suggests
that actions other than the lipid-lowering effect of TSIIA are
responsible for the reduction in liver steatosis exhibited in the
present study.
It has been previously reported that oxidative
stress is associated with the pathogenesis of NAFLD (28,29).
Similarly, the present results indicated that the ROS levels, which
are typically generated in a HFD (30), increased in rats in the HFD group.
Correlating with increased ROS, the present findings demonstrated
increased levels of lipid peroxide products, in the form of MDA,
and decreased T-SOD and GSH-PX were exhibited in rats of the HFD
group. As a result, the present findings indicated that the
hepatocytes of fatty liver rats were damaged, through increased
liver steatosis and apoptosis.
Apoptosis is an active process of cell suicide.
Under physiological conditions, it can clear senescent and injured
cells to maintain the normal liver volume. However, dysregulation
of apoptosis may result in liver diseases (31,32).
Feldstein et al (33,34) considered that hepatocyte apoptosis
was the distinguishing feature of NAFLD and increased with the
development of fatty liver. Furthermore, Murakawa et al
(35) and other previous studies
(36,37) demonstrated that antioxidants had
anti-apoptotic effects and implied that TSIIA may inhibit apoptosis
due to its antioxidant activity. The present study identified that
the rate of apoptosis was significantly increased in HFD group rats
when compared to the CON group, by Hoechst staining. Moreover, the
present findings further supported these effects at the molecular
level by investigating Bax and Bcl-2 mRNA and protein expression.
Bax and Bcl-2 are apoptosis-related genes; Bax is a pro-apoptotic
factor, whereas Bcl-2 has anti-apoptotic activity (38). Xie et al (39) and McClintock et al (40) suggested that the survivability of
cells post-apoptosis stimulation is dependent on the ratio of
Bax/Bcl-2. Caspase 3, a cysteine protease, is able to promote
apoptosis as a key protease (41).
The present findings indicated an increase of the Bax/Bcl-2 ratio
and caspase 3 mRNA and protein expression levels in the HFD group.
However, these negative effects of a HFD on rat livers were
reversed with TSIIA treatment, as indicated by the results from the
TAN group. The present results indicate that enhanced
anti-oxidative and anti-apoptotic capability may partially explain
the protective effect of TSIIA on NAFLD.
Although TSIIA has no effect on serum lipid
profiles, we found that attenuation of oxidative stress by TSIIA
contributes to the amelioration of hepatic steatosis. In addition,
the enhanced antioxidant capacity may account for the inhibition of
apoptosis.
In summary, the present study showed that TSIIA
improved the antioxidant capacity of the liver, leading to
decreased apoptosis and improved hepatic steatosis in a rat model
of fatty liver. In future studies, we plan to focus on the
anti-apoptotic mechanisms of antioxidants, which may provide a
potential target in the prevention and treatment of NAFLD.
Acknowledgements
The present work was supported by the National
Program on Key Basic Research Project (973 Program) (grant no.
2013CB531704), the National Natural Science Foundation of China
(grant no. 81774022) and the Key Laboratory of Ministry of
Education for TCM Viscera-State Theory and Applications, Liaoning
University of Traditional Chinese Medicine of China (grant no.
2014348).
References
|
1
|
Sinha N and Dabla PK: Oxidative stress and
antioxidants in hypertension-A current review. Curr Hypertens Rev.
11:132–142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ibrahim W, Lee US, Yeh CC, Szabo J,
Bruckner G and Chow CK: Oxidative stress and antioxidant status in
mouse liver: Effects of dietary lipid, vitamin E and iron. J Nutr.
127:1401–1406. 1997.PubMed/NCBI
|
|
3
|
Delbosc S, Paizanis E, Magous R, Araiz C,
Dimo T, Cristol JP, Cros G and Azay J: Involvement of oxidative
stress and NADPH oxidase activation in the development of
cardio-vascular complications in a model of insulin resistance, the
fructose-fed rat. Atherosclerosis. 179:43–49. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shin MH, Moon YJ, Seo JE, Lee Y, Kim KH
and Chung JH: Reactive oxygen species produced by NADPH oxidase,
xanthine oxidase, and mitochondrial electron transport system
mediate heat shock-induced MMP-1 and MMP-9 expression. Free Radic
Biol Med. 44:635–645. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao ZQ: Oxidative stress-elicited
myocardial apoptosis during reperfusion. Curr Opin Pharmacol.
4:159–165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang K: Molecular mechanisms of hepatic
apoptosis. Cell Death Dis. 5:e9962014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sies H: What is oxidative stress?
Oxidative Stress Vascular Dis. 224:1–8. 2000. View Article : Google Scholar
|
|
8
|
Merker K, Stolzing A and Grune T:
Proteolysis, caloric restriction and aging. Mech Ageing Dev.
122:595–615. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smith BW and Adams LA: Non-alcoholic fatty
liver disease. Crit Rev Clin Lab Sci. 48:97–113. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Castelli WP, Garrison RJ, Wilson PW,
Abbott RD, Kalousdian S and Kannel WB: Incidence of coronary heart
disease and lipoprotein cholesterol levels. The Framingham study.
JAMA. 20:2835–2838. 1986. View Article : Google Scholar
|
|
11
|
Vendemiale G, Grattagliano I and Altomare
E: An update on the role of free radicals and antioxidant defense
in human disease. Int J Clin Lab Res. 29:49–55. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aboelwafa HR and Yousef HN: The
ameliorative effect of thymol against hydrocortisone-induced
hepatic oxidative stress injury in adult male rats. Biochem Cell
Biol. 93:282–289. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Duan L, Xiong XJ and Wang J: Activating
blood circulation to remove stasis and therapeutic angiogenesis of
coronary heart disease. Zhongguo Zhong Xi Yi Jie He Za Zhi.
33:1561–1566. 2013.(In Chinese). PubMed/NCBI
|
|
14
|
Chen FY, Guo R and Zhang BK: Advances in
cardiovascular effects of tanshinone II(A). Zhongguo Zhong Yao Za
Zhi. 40:1649–1653. 2015.(In Chinese). PubMed/NCBI
|
|
15
|
Gao S, Liu Z, Li H, Little PJ, Liu P and
Xu S: Cardiovascular actions and therapeutic potential of
tanshinone IIA. Atherosclerosis. 220:3–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shang Q, Xu H and Huang L: Tanshinone IIA:
A promising natural cardioprotective agent. Evid Based Complement
Alternat Med. 2012:7164592012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fu J, Huang H, Liu J, Pi R, Chen J and Liu
P: Tanshinone IIA protects cardiac myocytes against oxidative
stress-triggered damage and apoptosis. Eur J Pharmacol. 30:213–221.
2007. View Article : Google Scholar
|
|
18
|
Niu XL, Ichimori K, Yang X, Hirota Y,
Hoshiai K, Li M and Nakazawa H: Tanshinone II-A inhibits low
density lipoprotein oxidation in vitro. Free Radic Res. 33:305–312.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang FT, Cao Y, Wang TQ, Wang LJ, Guo J,
Zhou XS, Xu SW, Liu WH, Liu PQ and Huang HQ: Tanshinone IIA
attenuates atherosclerosis in ApoE(−/−) mice through
down-regulation of scavenger receptor expression. Eur J Pharmacol.
650:275–284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jia LQ, Feng JY, Yang GL, Chen WN and Chen
Y: Effect of tanshinone IIA on TLR4 and TNF-α of endothelial cells
induced by LPS. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 27:733–735.
2011.(In Chinese). PubMed/NCBI
|
|
21
|
Jia LQ, Yang GL, Ren L, Chen WN, Feng JY,
Cao Y, Zhang L, Li XT and Lei P: Tanshinone IIA reduces apoptosis
induced by hydrogen peroxide in the human endothelium-derived
EA.hy926 cells. J Ethnopharmacol. 143:100–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jia LQ, Zhang N, Xu Y, Chen WN, Zhu ML,
Song N, Ren L, Cao HM, Wang JY and Yang GL: Tanshinone IIA affects
the HDL subfractions distribution not serum lipid levels: Involving
in intake and efflux of cholesterol. Arch Biochem Biophys.
592:50–59. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pereira ENGDS, Silvares RR, Flores EEI,
Rodrigues KL, Ramos IP, da Silva IJ, Machado MP, Miranda RA,
Pazos-Moura CC, Gonçalves-de-Albuquerque CF, et al: Hepatic
microvascular dysfunction and increased advanced glycation end
products are components of non-alcoholic fatty liver disease. PLoS
One. 12:e01796542017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jarskog LF, Selinger ES, Lieberman JA and
Gilmore JH: Apoptotic proteins in the temporal cortex in
schizophrenia: High Bax/Bcl-2 ratio without caspase-3 activation.
Am J Psychiatry. 161:109–115. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peiró G, Diebold J, Baretton GB, Kimmig R
and Löhrs U: Cellular apoptosis susceptibility gene expression in
endometrial carcinoma: Correlation with Bcl-2, Bax, and caspase-3
expression and outcome. Int J Gynecol Pathol. 20:359–367. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kimm HH, Kim JH, Kwak HB, Huang H, Han SH,
Ha H, Lee SW, Woo ER and Lee ZH: Inhibition of osteoclast
differentiation and bone resorption by tanshinone IIA isolated from
Salvia miltiorrhiza Bunge. Biochem Pharmacol. 67:1647–1656. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Day CP and James OF: Steatohepatitis: A
tale of two ‘hits’? Gastroenterology. 114:842–845. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu D, Zheng N, Qi K, Cheng H, Sun Z, Gao
B, Zhang Y, Pang W, Huangfu C, Ji S, et al: Exogenous hydrogen
sulfide mitigates the fatty liver in obese mice through improving
lipid metabolism and antioxidant potential. Med Gas Res. 5:12015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Das N, Ganguli D and Dey S: Moringa
oleifera Lam. seed extract prevents fat diet induced oxidative
stress in mice and protects liver cell-nuclei from hydroxyl radical
mediated damage. Indian J Exp Biol. 53:794–802. 2015.PubMed/NCBI
|
|
31
|
Rust C and Gores GJ: Apoptosis and liver
disease. Am J Med. 108:567–574. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Benedetti A and Marucci L: The
significance of apoptosis in the liver. Liver. 19:453–463. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Feldstein AE, Canbay A, Angulo P, Taniai
M, Burgart LJ, Lindor KD and Gores GJ: Hepatocyte apoptosis and fas
expression are prominent features of human nonalcoholic
steatohepatitis. Gastroenterology. 125:437–443. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feldstein AE, Canbay A, Guicciardi ME,
Higuchi H, Bronk SF and Gores GJ: Diet associated hepatic steatosis
sensitizes to Fas mediated liver injury in mice. J Hepatol.
39:978–983. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Murakawa M, Jung SK, Lijima K and Yonehara
S: Apoptosis-inducing protein, AIP, from parasite-infected fish
induces apoptosis in mammalian cells by two different molecular
mechanisms. Cell Death Differ. 8:298–307. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Emanuele S, Galvaruso G, Lauricella M,
Giuliano M, Bellavia G, D'Anneo A, Vento R and Tesoriere G:
Apoptosis induced in hepatoblastoma HepG2 cells by the proteasome
inhibitor MG132 is associated with hydrogen peroxide production,
expression of Bcl-XS and activation of caspase-3. Int J Oncol.
21:857–865. 2002.PubMed/NCBI
|
|
37
|
Woo SH, Park IC, Park MJ, Lee HC, Lee SJ,
Chun YJ, Lee SH, Hong SI and Rhee CH: Arsenic trioxide induces
apoptosis through a reactive oxygen species-dependent path way and
loss of mitochondrial membrane potential in HeLa cells. Int J
Oncol. 21:57–63. 2002.PubMed/NCBI
|
|
38
|
Walensky LD: BCL-2 in the crosshairs:
Tipping the balance of life and death. Cell Death Differ.
13:1339–1350. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xie Z, Koyama T, Suzuki J, Fujii Y,
Togashi H, Sawa H and Nagashima K: Coronary reperfusion following
ischemia: Different expression of bcl-1 and bax proteins, and
cardiomyocyte apoptosis. Jpn Heart J. 42:759–770. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
McClintock DS, Santore MT, Lee VY,
Brunelle J, Budinger GR, Zong WX, Thompson CB, Hay N and Chandel
NS: Bcl-2 family members and functional electron transport chain
regulate oxygen deprivation-induced cell death. Mol Cell Biol.
22:94–104. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nakagawa Y: Initiation of apoptotic signal
by the peroxidation of cardiolipin of mitochondria. Ann N Y Acad
Sci. 1011:177–184. 2004. View Article : Google Scholar : PubMed/NCBI
|