Introduction
Osteoarthritis (OA) is a progressively degenerative
joint disorder affecting ~10% of males and ~18% of females aged
>60 years old worldwide (1). Risk
factors of OA include obesity, bone mass, joint injury and
deformity, trauma and age (2).
Although OA influences the adjoining bone, synovial lining and
periarticular muscle, the cause may be primarily attributed to a
progressive structural and functional compromise of the cartilage
(3). Typical symptoms include
activity-related or mechanical pain and brief morning stiffness
(3). Therapeutic approaches
currently available are limited for patients with OA prior to
prosthetic joint replacement, which may be required (4).
Sirtuin 1 (Sirt1), the homolog of silence
information regulator 2, is a highly conserved and
well-characterized nicotinamide adenine dinucleotide
(NAD)-dependent class III histone deacetylase in mammalian cells
(5). Increased cellular ionized NAD
(NAD+) as a substrate induces the activation of Sirt1,
whereas a high concentration of nicotinamide (NAM) inhibits the
activity of Sirt1. Numerous studies have demonstrated that Sirt1
has a central role in the regulation of cell proliferation,
apoptosis and inflammation (6–8).
Abnormal Sirt1 expression has been considered to be involved in OA
pathogenesis. Compared with normal cartilage from cadavers, Sirt1
protein was highly expressed in OA cartilage (9). Sirt1 mRNA expression was also higher in
severely degenerated cartilage, when compared with less damaged
cartilage (10). Previous studies
have demonstrated that Sirt1 induced abnormal sclerostin expression
in human osteoarthritis subchondral osteoblasts (11) and regulated the osteoarthritic gene
expression changes in human chondrocytes (12).
Resveratrol (RES) is a polyphenolic compound
commonly identified in grape skin, berries and peanuts that has
multiple functions, including anti-apoptosis, anti-inflammation and
anti-oxidation effects (13). It has
been indicated that RES may be an activator of Sirt1 in articular
chondrocytes (14) and RES has been
demonstrated to have a positive effect against OA (15). Although Sirt1 activation induced by
RES appears to improve the survival and metabolism of OA
chondrocytes (9,10), the precise molecular mechanism of RES
and the potential link between Sirt1 and RES remain to be
elucidated (16).
The present study aimed to examine the effect of
Sirt1 on the Wnt/β-catenin signaling pathway. To do this, the
present study detected the expression levels of a number of pivotal
genes and corresponding translated proteins implicated in
apoptosis, extracellular matrix (ECM) degradation and the
Wnt/β-catenin signaling pathway in RES-treated osteoarthritis
chondrocyte.
Materials and methods
Cartilage sample
Cartilage samples were harvested from 30 individual
patients with OA (69.0±14 years; 14 males and 16 females) between
March 2015 and March 2016. Patients were admitted to the Department
of Orthopaedics, Tianyou Hospital (Wuhan, China) and samples were
collected during surgery. An OA diagnosis in these patients was
determined according to the criteria of the American College of
Rheumatology (17). The present
study was performed following approval from the Experimental Animal
Ethics Committee of Tianyou Hospital Affiliated to Wuhan University
of Science and Technology and informed consent was provided by all
participants.
Preparation of chondrocytes
OA cartilage tissue was washed with sterile
phosphate-buffered saline (PBS; pH 7.2) and sliced into
1-mm3 sections with ophthalmic scissors. OA chondrocytes
were acquired via incubation at 37°C in 0.25% trypsin for 30 min
and 0.2% type II collagenase (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) for 2 h. Chondrocytes were filtered,
centrifuged at 37°C at 1,500 × g for 10 min and resuspended in
Dulbecco's modified Eagle's medium (DMEM)/F-12 supplemented with
10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.) at
37°C and 5% CO2. Chondrocytes began to converge
following 4–5 h incubation. Second generation chondrocytes were
used in the present experiment.
Groups
Log phase chondrocytes were randomly divided into
three groups: Control group without any stimulus, the RES group
administered the ultimate concentration of 10 µM RES, and the NAM
group administered the ultimate concentration of 20 µM NAM.
Chondrocytes were cultivated for 48 h separately prior to
subsequent experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Following three washes with PBS for 90 sec, total
RNA was extracted from OA chondrocytes using a RNAprep pure kit
(DP430; Tiangen Biotech, Co., Ltd., Beijing, China) according to
the manufacturer's instructions. RNA was quantified using a
NanoDrop 2000 (Thermo Fisher Scientific, Inc.). Isolated RNA was
transcribed into cDNA using a PrimeScript One Step miRNA cDNA
Synthesis kit (Takara Bio, Inc., Otsu, Japan) according to the
manufacturer's instructions. qPCR (2X SYBR Green qPCR kit; Beijing
Solarbio Science and Technology Co., Ltd., Beijing, China) was
subsequently performed with a reaction mixture of 20 µl containing:
10 µl 2X SYBR Fast qPCR Mix, 0.8 µl PCR forward and reverse
primers, 0.4 µl 50X ROX Reference Dye II and 2 µl cDNA template.
The PCR reaction was performed as follows: 95°C for 30 sec,
followed by 40 cycles of 95°C for 3 sec and 60°C for 13 sec. The
reaction was performed using an Applied Biosystems® 7500
Fast Dx Real-Time PCR instrument (Thermo Fisher Scientific, Inc.).
Expression of Sirt1 was normalized to GAPDH, as a reference
control. Primer sequences were as follows: Sirt1 forward,
5′-TGGACTCCACGACGTACT-3′ and reverse, 5′-TCTCCTGGGAGGCATAGACC-3′
(total length, 122 bp); and GAPDH forward, 5′-AGCCACATCGCTCAGACA-3′
and reverse, 5′-TCTCCTGGGAGGCATAGACC-3′ (total length, 314 bp).
Primers were synthesized by Sangon Biotech Co., Ltd. (Shanghai,
China). Relative target gene expression was calculated as a fold
change of 2−ΔΔCq value, in which ΔCt =
Cttarfet gene - Ctendogenous control
(18). The experiments were
performed in triplicate on three independent occasions.
MTT assay
The three groups of OA chondrocytes were seeded at a
density of 5×103 cells/ml into 96-well plates. Following
24, 48 and 72 h incubations at 37°C, 20 µl 5 mg/ml cells were
combined with 10 µl sterile
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Beyotime Institute of Biotechnology, Haimen, China) for 4 h at
37°C. The solution containing MTT was removed and cells were mixed
with 150 µl dimethyl sulfoxide for 10 min. The plate was read
spectrophotometrically at a wavelength of 560 nm by a Bio-Rad model
550 microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) to detect cell viability.
Apoptosis detection
Apoptosis of OA chondrocytes was detected using
fluorescein isothiocyanate (FITC)-labeled Annexin V and propidium
iodide (PI) kit (cat. no. C1063; BestBio, Co., Shanghai, China),
according to the manufacturer's instructions. Briefly, the
chondrocytes were collected following digestion in EDTA-free 0.25%
trypsin for 1 h at 37°C. Following centrifugation at room
temperature (1,000 × g; 10 min), 8 ml Annexin-FITC and 10 ml PI
were added to the cell suspension and incubated for 15 min at room
temperature. The rate of apoptosis was analyzed using a FACSCalibur
flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and
inbuilt software.
Western blot analysis
Protein expression levels of Sirt1, B-cell lymphoma
2 (Bcl-2), Bcl-2 associated X protein (Bax), procaspase-3, −9,
matrix metalloproteinase 1(MMP1), MMP3, MMP13, Wnt3a, Wnt5a, Wnt7a
and GAPDH were determined by western blot analysis. Briefly, the
proteins were extracted from the cells in each group using an
EpiQuik Total Histone Extraction kit (Epigentek, Farmingdale, NY,
USA) according to manufacturer instructions and quantified using a
BCA Protein Quantification kit (Vazyme Biotech Co., Ltd., Nanjing,
China) according to the manufacturer's instructions. Subsequently,
~50 mg total protein extracts were separated by 12% SDS-PAGE prior
to being transferred to a polyvinylidene fluoride membrane (EMD
Millipore, Billerica, MA, USA). The membrane was blocked using 5%
milk solution for 2 h at room temperature and washed three times
with Tris-buffered saline with Tween-20 (TBST; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany). The membrane was respectively incubated
with the primary rabbit monoclonal antibodies of Sirt1 (cat. no.
ab110304; Abcam, Cambridge, MA, USA), Bax, Bcl-2, procaspase-3, −9
(cat. nos. 1063-1, 1071-1, 1061-1 and 1084-1; Epitomics; Abcam),
MMP1, MMP3, MMP13, Wnt3a, Wnt5a, Wnt7a, β-catenin and GAPDH (cat.
nos. EP1247Y, ab53015, ab39012, ab28472, ab72583, ab100792, ab32572
and ab8245; Abcam) at 4°C overnight at a dilution of 1:100. This
incubation was followed by a further three TBST washes for 5 min.
The membrane was subsequently incubated at room temperature with
secondary goat anti-rabbit antibody conjugated with horseradish
peroxidase (cat. no. A0208; Beyotime Institute of Biotechnology) at
a dilution of 1:500 for 90 min and washed with TBST. Strip gray
levels were quantified using Quantity One software (version 4.62;
Bio-Rad Laboratories, Inc.).
Statistical analysis
Experiments were performed in triplicate on three
independent occasions. Data were presented as the mean ± standard
deviation and analyzed using a two-tailed Student's t-test.
Statistical analyses were conducted using SPSS version 17 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
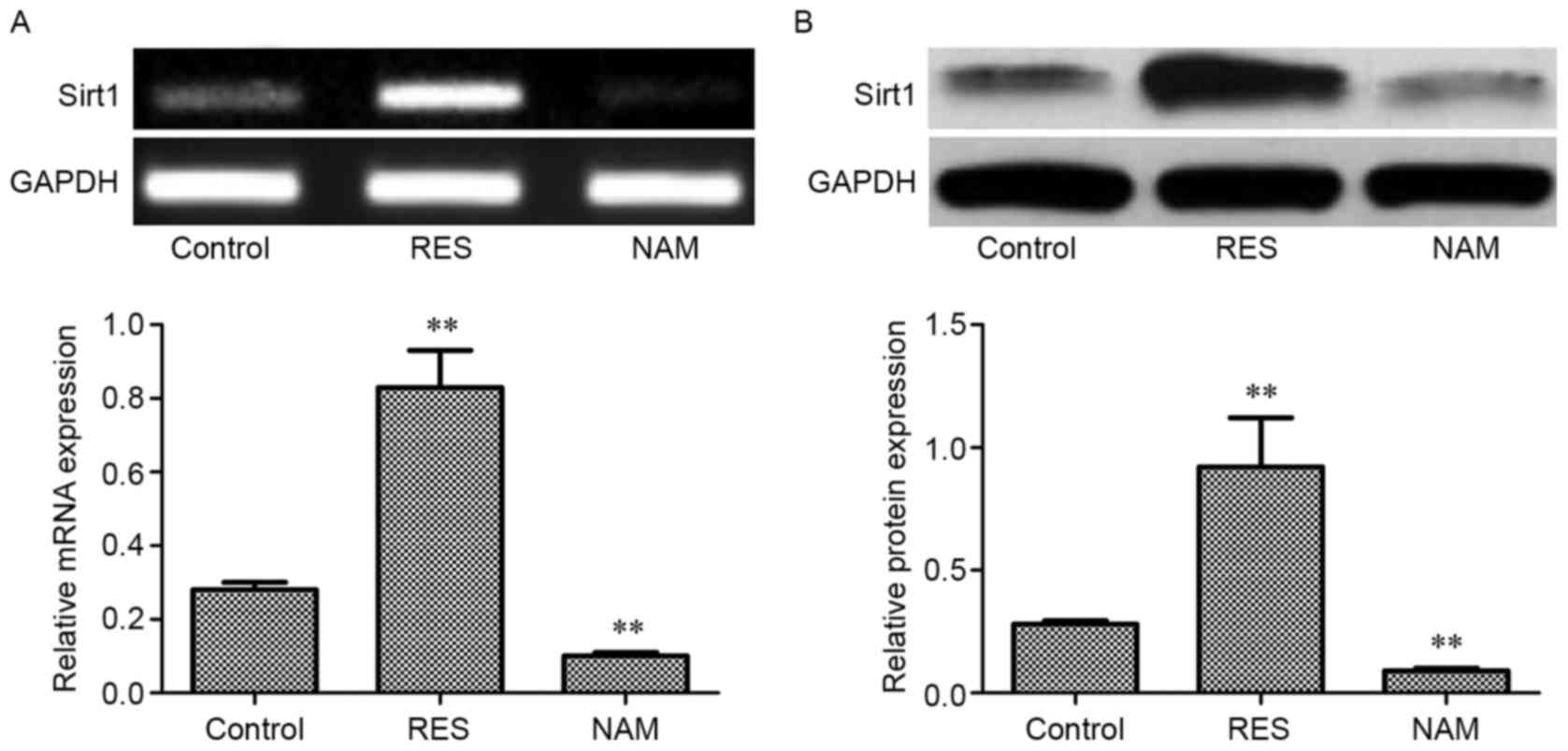
Expression of Sirt1 mRNA and protein
in OA chondrocytes
Compared with the control, the expression levels of
Sirt1 mRNA and protein in the RES group were significantly
increased (P<0.01; Fig. 1A and B,
respectively), but decreased significantly in the NAM group
(P<0.01).
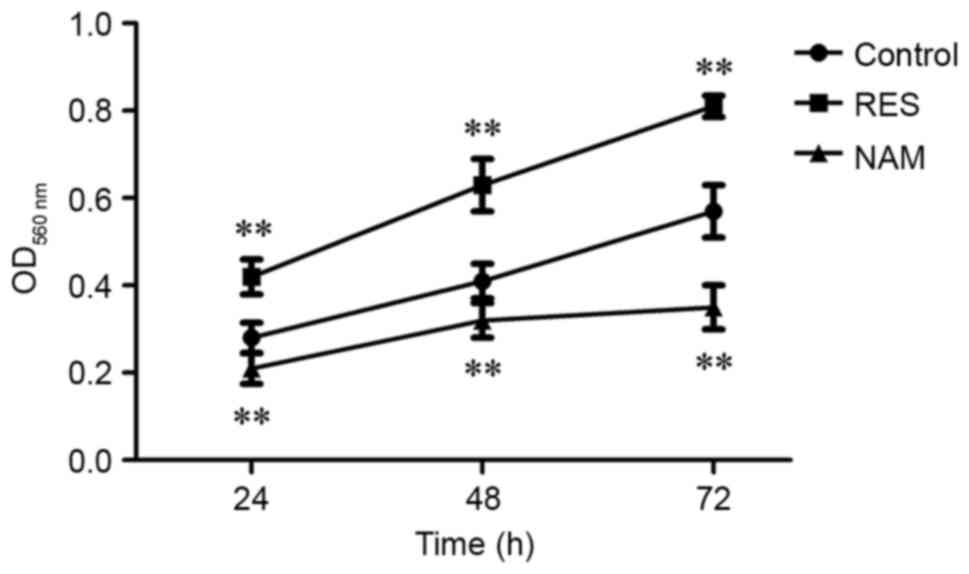
Cell viability and apoptosis in OA
chondrocytes
Compared with the control, the cell viabilities were
significantly improved at 24, 48 and 72 h in the RES group
(P<0.01; Fig. 2), and
significantly suppressed in the NAM group despite the slow growth
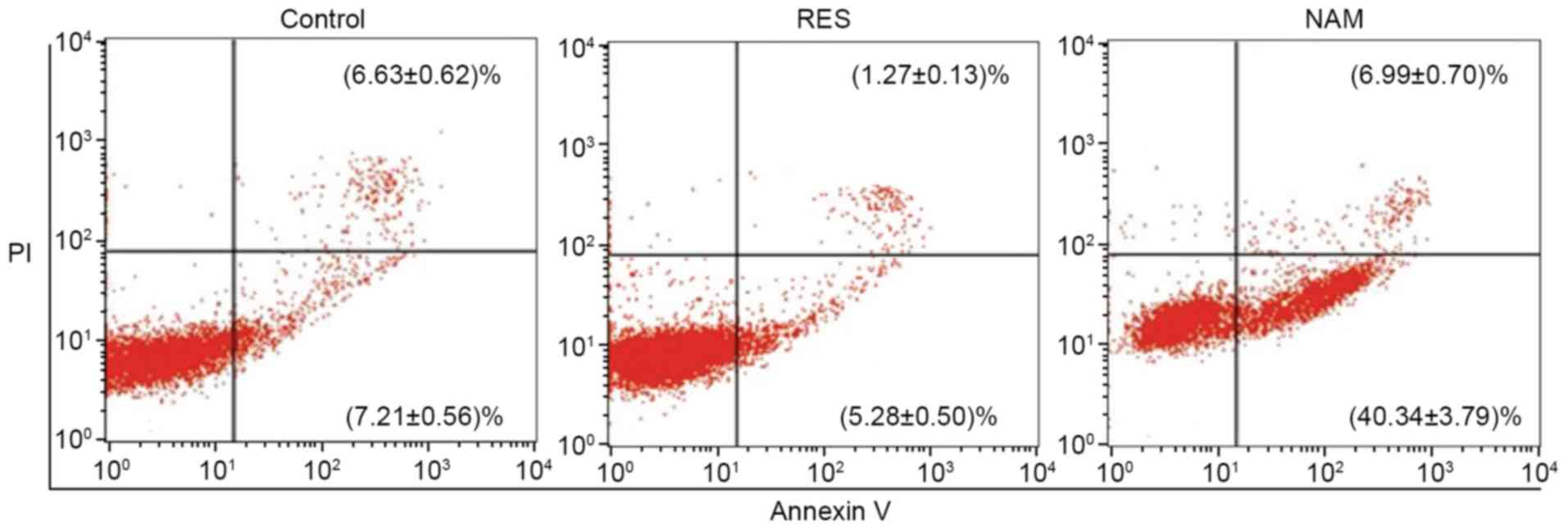
of chondrocytes (P<0.01). The rate of cell apoptosis was reduced
from 13.84 to 6.55% in response to 10 µM RES, but increased from
13.84 to 47.33% in response to 20 µM NAM (P<0.01; Fig. 3).
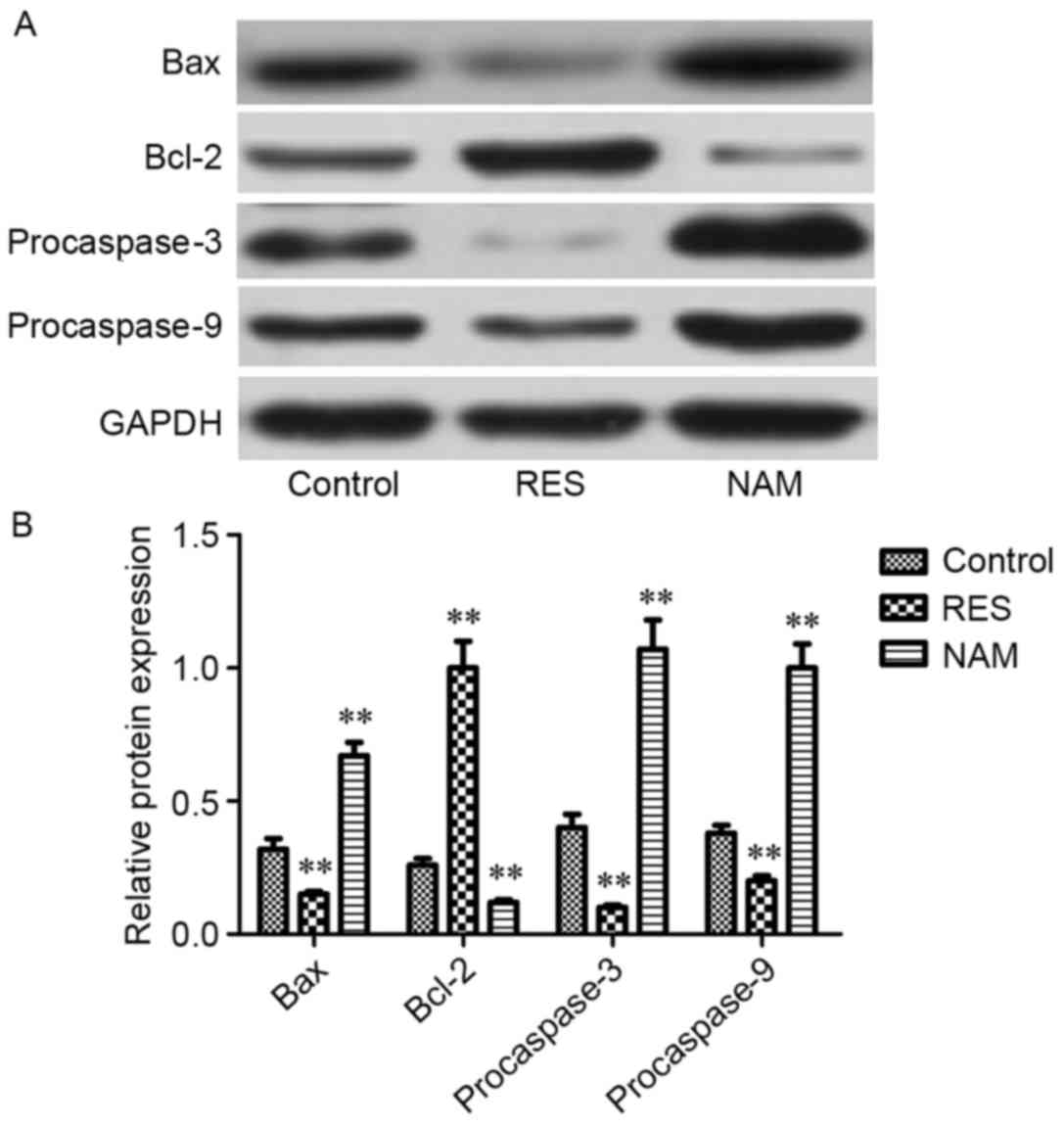
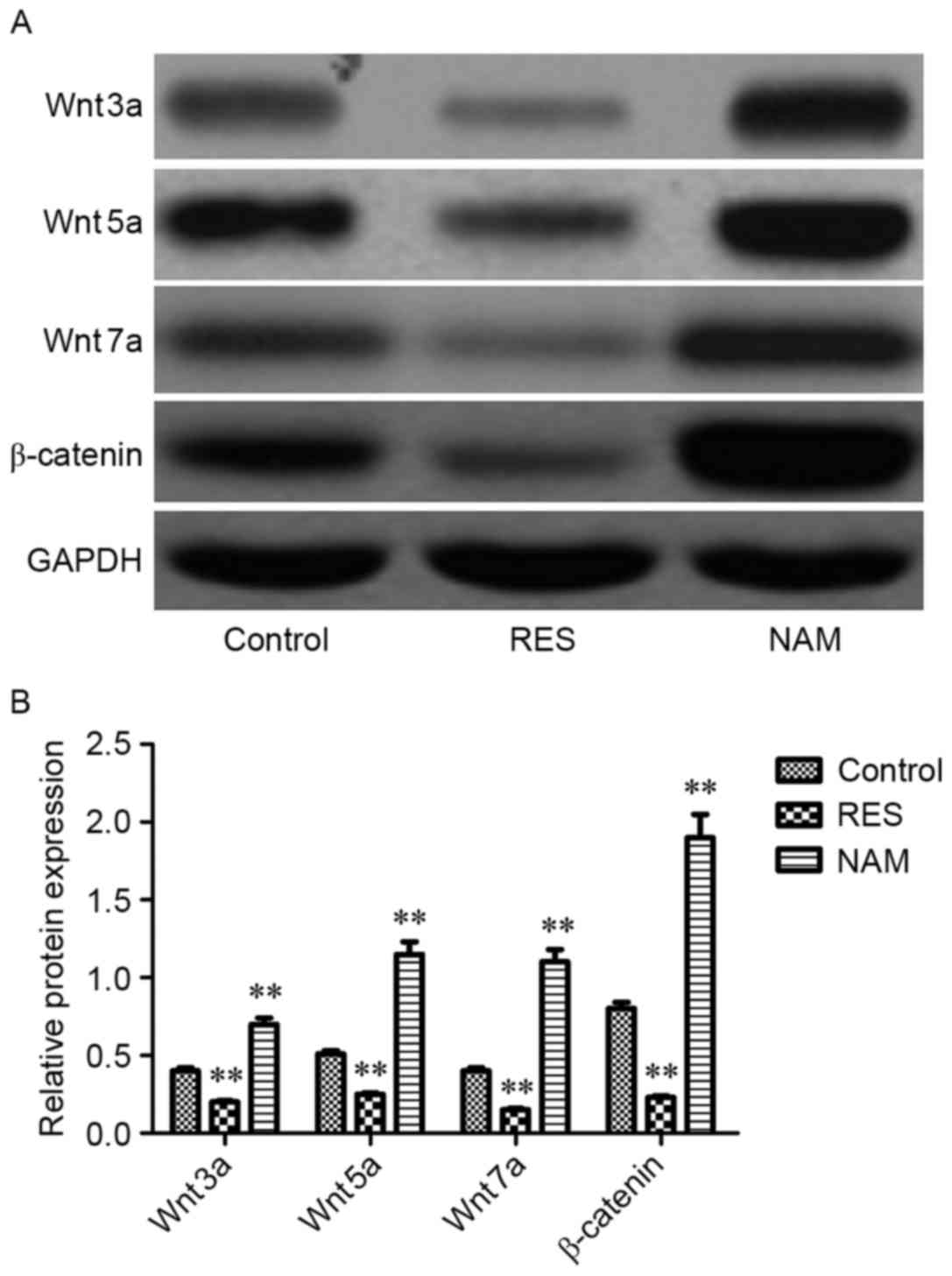
Protein expression of critical genes
implicated in apoptosis, ECM degradation and the Wnt/β-catenin
signaling pathway
To assess the effect of Sirt1 on apoptosis, ECM
degradation and Wnt/β-catenin signaling in RES-treated OA
chondrocytes, the protein expression levels of Bax, Bcl-2,
procaspase-3, −9, MMP1, MMP3, MMP13, Wnt3a, Wnt5a, Wnt7a and
β-catenin were determined. It was observed that the expression
levels of Bax, procaspase-3 and −9, MMP1, MMP3, MMP13, Wnt3a,
Wnt5a, Wnt7a, and β-catenin were significantly inhibited following
treatment with RES (P<0.01; Figs.
4–6). However, Bcl-2 expression
levels were significantly increased in RES-treated OA chondrocytes
(P<0.01; Fig. 4). These variation
tendencies were the opposite in NAM-treated OA chondrocytes; when
the expression was significantly increased in the RES group, it was
significantly decreased in the NAM group (P<0.01; Figs. 4–6).
Discussion
RES is a natural phytoalexin and has been
demonstrated to be an effective stimulator of Sirt1 expression in
response to OA chondrocyte metabolism, apoptosis and proliferation
(15,16,19).
Consistent with previous studies, the present study observed that
the levels of Sirt1 mRNA and protein were significantly increased
in OA chondrocytes treated with RES. Sirt1 has been confirmed in
vitro and in vivo to be essential in the prevention of
apoptosis in human chondrocytes, which is associated with cartilage
degeneration in OA, via the mitochondrial-related pathway (20,21).
Following exposure to RES, the present findings demonstrated that
increased Sirt1 expression had a lower apoptotic rate compared with
the control, indicating that Sirt1 may promote OA chondrocyte
survival. Further experiments evaluated the association of Sirt1
with the mitochondrial-related apoptotic pathway. As in
mitochondria, the balance between proapoptotic members (such as
Bax) and antiapoptotic members (such as Bcl-2) in the Bcl family
determines cellular apoptosis and survival. Therefore, the effect
of Sirt1 on the protein expression levels of Bax, Bcl-2,
procaspase-3 and −9 was surveyed. In line with previous findings,
Sirt1 inhibited the expression of Bax, procaspase-3 and
procaspase-9 and increased Bcl-2 expression (20), indicating that Sirt1 induced the
apoptosis of OA chondrocytes treated with RES through the
mitochondria pathway.
Type II collagen is one of the primary ECM
macromolecules in cartilage. MMPs are a family of 23 enzymes with a
specific function to hydrolyze the triple helical structure in type
II collagen, maintaining the balance between synthesis and
degradation in normal cartilage ECM. It is generally hypothesized
that the abnormal expression of various MMP members may lead to
proteolysis and pathological cartilage breakdown in OA (22,23). The
primary function of three MMPs (MMP1, MMP3 and MMP13) has been
demonstrated to serve a role in chondrocyte-mediated cartilage
matrix degeneration in OA (24),
among which MMP13 has a central role in the regulation of OA
chondrocytes (22,25). In RES-treated OA chondrocytes, the
expression of MMP1, MMP3 and MMP13 decreased along with the
increase of Sirt1, and MMP13 exhibited a more evident decrease of
expression than MMP1 and 3. Notably, increased Sirt1 induced by RES
may inhibit MMP13 and suppress type II collagen degradation
(26,27). These observations suggest
Sirt1-mediated OA delay may benefit from the suppressions of a
number of critical MMP members, including MMP1, MMP3 and MMP13, in
RES-treated chondrocytes.
Wnt signaling is a conserved pathway that is
associated with the response to cell differentiation and fate
determination during embryogenesis and the late stages of
development (28). Activation of
Wnt/β-catenin, the canonical pathway, has been suggested to be
involved in OA aggravation (29).
Primary effectors implicated in OA development include three Wnt
proteins (Wnt3a, Wnt5a and Wnt7a) (30–32). As
described, MMP13, a target protein downstream of the Wnt/β-catenin
signaling pathway (2,33), exhibited reduced expression caused by
the elevated Sirt1. Notably, the present study demonstrated that an
increase in the expression of Sirt1 may effectively suppress the
expression of Wnt3a, Wnt5a, Wnt7a and β-catenin in RES-treated OA
chondrocytes, indicating that Sirt may reduce OA progression
through the Wnt/β-catenin signaling pathway.
In conclusion, the present study used RES-treated
chondrocytes to monitor a number of critical factors in the
progression of OA, and demonstrated that RES may increase Sirt1,
leading to a decrease in the expression levels of Bax, procaspase-3
and −9, MMP1, MMP3, MMP13, Wnt3a, Wnt5a, Wnt7a and β-catenin and an
increase in the level of Bcl-2. These results demonstrate that
Sirt1 may regulate the apoptosis and ECM degradation in RES-treated
osteoarthritis chondrocytes via the Wnt/β-catenin signaling
pathway.
References
|
1
|
Glyn-Jones S, Palmer AJ, Agricola R, Price
AJ, Vincent TL, Weinans H and Carr AJ: Osteoarthritis. Lancet.
386:376–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou X, Li W, Jiang L, Bao J, Tao L, Li J
and Wu L: Tetrandrine inhibits the Wnt/β-catenin signalling pathway
and alleviates osteoarthritis: An in vitro and in vivo study. Evid
Based Complement Alternat Med. 2013:8095792013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gelber AC: In the clinic. Osteoarthritis.
Ann Intern Med. 161:ITC1–ITC16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takamatsu A, Ohkawara B, Ito M, Masuda A,
Sakai T, Ishiguro N and Ohno K: Verapamil protects against
cartilage degradation in osteoarthritis by inhibiting Wnt/β-catenin
signaling. PLoS One. 9:e926992014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blander G and Guarente L: The Sir2 family
of protein deacetylases. Annu Rev Biochem. 73:417–435. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seo JS, Moon MH, Jeong JK, Seol JW, Lee
YJ, Park BH and Park SY: SIRT1, a histone deacetylase, regulates
prion protein-induced neuronal cell death. Neurobiol Aging.
33:1110–1120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Michan S and Sinclair D: Sirtuins in
mammals: Insights into their biological function. Biochem J.
404:1–13. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamamoto H, Schoonjans K and Auwerx J:
Sirtuin functions in health and disease. Mol Endocrinol.
21:1745–1755. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dvir-Ginzberg M, Gagarina V, Lee EJ and
Hall DJ: Regulation of cartilage-specific gene expression in human
chondrocytes by SirT1 and nicotinamide phosphoribosyltransferase. J
Biol Chem. 283:36300–36310. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fujita N, Matsushita T, Ishida K, Kubo S,
Matsumoto T, Takayama K, Kurosaka M and Kuroda R: Potential
involvement of SIRT1 in the pathogenesis of osteoarthritis through
the modulation of chondrocyte gene expressions. J Orthop Res.
29:511–515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abed É, Couchourel D, Delalandre A, Duval
N, Pelletier JP, Martel-Pelletier J and Lajeunesse D: Low sirtuin 1
levels in human osteoarthritis subchondral osteoblasts lead to
abnormal sclerostin expression which decreases Wnt/β-catenin
activity. Bone. 59:28–36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsushita T, Sasaki H, Takayama K, Ishida
K, Matsumoto T, Kubo S, Matsuzaki T, Nishida K, Kurosaka M and
Kuroda R: The overexpression of SIRT1 inhibited osteoarthritic gene
expression changes induced by interleukin-1β in human chondrocytes.
J Orthop Res. 31:531–537. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yadav M, Jain S, Bhardwaj A, Nagpal R,
Puniya M, Tomar R, Singh V, Parkash O, Prasad GB, Marotta F and
Yadav H: Biological and medicinal properties of grapes and their
bioactive constituents: An update. J Med Food. 12:473–484. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lei M, Wang JG, Xiao DM, Fan M, Wang DP,
Xiong JY, Chen Y, Ding Y and Liu SL: Resveratrol inhibits
interleukin 1β-mediated inducible nitric oxide synthase expression
in articular chondrocytes by activating SIRT1 and thereby
suppressing nuclear factor-κB activity. Eur J Pharmacol. 674:73–79.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang J, Gao JS, Chen JW, Li F and Tian J:
Effect of resveratrol on cartilage protection and apoptosis
inhibition in experimental osteoarthritis of rabbit. Rheumatol Int.
32:1541–1548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim H, Braun H and Dragoo J: The effect of
resveratrol on normal and osteoarthritic chondrocyte metabolism.
Bone Joint Res. 3:51–59. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Altman R, Asch E, Bloch D, Bole G,
Borenstein D, Brandt K, Christy W, Cooke T, Greenwald R, Hochberg
M, et al: Development of criteria for the classification and
reporting of osteoarthritis. Classification of osteoarthritis of
the knee. Diagnostic and Therapeutic Criteria Committee of the
American Rheumatism Association. Arthritis Rheum. 29:1039–1049.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie J, Zhang X and Zhang L: Negative
regulation of inflammation by SIRT1. Pharmacol Res. 67:60–67. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takayama K, Ishida K, Matsushita T, Fujita
N, Hayashi S, Sasaki K, Tei K, Kubo S, Matsumoto T, Fujioka H, et
al: SIRT1 regulation of apoptosis of human chondrocytes. Arthritis
Rheum. 60:2731–2740. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gabay O, Oppenhiemer H, Meir H, Zaal K,
Sanchez C and Dvir-Ginzberg M: Increased apoptotic chondrocytes in
articular cartilage from adult heterozygous SirT1 mice. Ann Rheum
Dis. 71:613–636. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kevorkian L, Young DA, Darrah C, Donell
ST, Shepstone L, Porter S, Brockbank SM, Edwards DR, Parker AE and
Clark IM: Expression profiling of metalloproteinases and their
inhibitors in cartilage. Arthritis Rheum. 50:131–141. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Murphy G, Knauper V, Atkinson S, Butler G,
English W, Hutton M, Stracke J and Clark I: Matrix
metalloproteinases in arthritic disease. Arthritis Res. 4 Suppl
3:S39–S49. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tetlow LC, Adlam DJ and Woolley DE: Matrix
metalloproteinase and proinflammatory cytokine production by
chondrocytes of human osteoarthritic cartilage: Associations with
degenerative changes. Arthritis Rheum. 44:585–594. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Davidson RK, Waters JG, Kevorkian L,
Darrah C, Cooper A, Donell ST and Clark IM: Expression profiling of
metalloproteinases and their inhibitors in synovium and cartilage.
Arthritis Res Ther. 8:R1242006. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu FC, Hung LF, Wu WL, Chang DM, Huang
CY, Lai JH and Ho LJ: Chondroprotective effects and mechanisms of
resveratrol in advanced glycation end products-stimulated
chondrocytes. Arthritis Res Ther. 12:R1672010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lei M: Resveratrol protects bone marrow
mesenchymal stem cell derived chondrocytes cultured on
chitosan-gelatin scaffolds from the inhibitory effect of
interleukin-1beta. Acta Pharmacol Sin. 29:1350–1356. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sassi N, Laadhar L, Allouche M, Achek A,
Kallel-Sellami M, Makni S and Sellami S: WNT signaling and
chondrocytes: From cell fate determination to osteoarthritis
physiopathology. J Recept Signal Transduct Res. 34:73–80. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yuasa T, Otani T, Koike T, Iwamoto M and
Enomoto-Iwamoto M: Wnt/β-catenin signaling stimulates matrix
catabolic genes and activity in articular chondrocytes: Its
possible role in joint degeneration. Lab Invest. 88:264–274. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nalesso G, Sherwood J, Bertrand J, Pap T,
Ramachandran M, De Bari C, Pitzalis C and Dell'accio F: WNT-3A
modulates articular chondrocyte phenotype by activating both
canonical and noncanonical pathways. J Cell Biol. 193:551–564.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huh YH, Ryu JH and Chun JS: Regulation of
type II collagen expression by histone deacetylase in articular
chondrocytes. J Biol Chem. 282:17123–17131. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sassi N, Laadhar L, Allouche M,
Zandieh-Doulabi B, Hamdoun M, Klein-Nulend J, Makni S and Sellami
S: The roles of canonical and non-canonical Wnt signaling in human
de-differentiated articular chondrocytes. Biotech Histochem.
89:53–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bernard NJ: Osteoarthritis: Repositioning
verapamil-for Wnt of an OA treatment. Nat Rev Rheumatol.
10:2602014. View Article : Google Scholar : PubMed/NCBI
|