Introduction
LNT (lentinan) is a polysaccharide isolated from
Shiitake (Lentinula edodes), which has pharmaceutical
properties, including immunomodulatory, antitumor, anti-viral and
anti-bacterial effects, with high efficacy and minimal side effects
(1). LNT is efficient in the
treatment of gastric, colon, breast and lung cancer, leading to
prolonged survival time of patients. LNT is often used as immune
enhancer in clinical applications and enhances curative effects
and/or reduces side effects of other drugs if given in combination
(2). For instance, LNT significantly
enhanced the anticancer activity of cisplatin in the treatment of
colon cancer (3). Furthermore, LNT
increased the activation of bacillus Calmette-Guérin (BCG)
pulmonary macrophages in guinea pigs and reduced systemic adverse
reactions of BCG vaccine (4).
Oridonin is an ent-kaurene diterpenoid compound
mainly isolated from Rhodamnia rubescens. A previous study
has demonstrated that oridonin induces tumor cell apoptosis
(5); furthermore, it sensitized
liver cancer cell lines to radiotherapy in vitro, rendering
it unique as a naturally occurring compound with a
radiosensitization effect (6).
An ideal anticancer agent is a drug that induces
differentiation and apoptosis of tumor cells. Previous clinical
studies have focused on preparations based on their toxic effects
on cancer cells, while the focus has shifted towards the
development of substances which induce differentiation and
apoptosis of cancer cells in recent years (7,8).
Unspecific cytotoxic effects of anticancer drugs are frequently
associated with a significant negative impact on normal cells,
while an apoptosis-inducing effect on cancer cells allows for
selective targeting whilst avoiding any influence on normal cells.
Certain biologically active chemical substances contained in plants
have strong apoptosis-inducing effects on cancer cells, such as
oridonin, an active component of Shiitake mushrooms (6).
Certain substances with cancer cell
apoptosis-inducing effects that were extracted from plants have low
toxicity and are safe to use, which enhances the quality of life of
patients during treatment. While numerous natural anticancer drugs
contained in plants have a lower efficacy than certain synthetic
drugs, the combination of various natural products may have an
improved therapeutic effect (9).
Identification of a reasonable combination has become an important
task in the field of natural product-based as anticancer
treatments. The present study assessed how oridonin improves the
anticancer effect of LNT in vitro with the aim of developing
a novel combined anticancer treatment. By using various methods
such as MTT, flow cytometry, reverse-transcription quantitative
polymerase chain reaction (RT-qPCR) and western blot analysis the
enhancing effect of oridonin on growth inhibition and apoptosis
induction by LNT on hepatocellular carcinoma cells was assessed
in vitro, in order to further establish a theoretical basis
for animal experiments and even clinical application.
Materials and methods
Cell preparation
The L02 normal human liver cell line and the
SMMC-7721 human hepatoma cell line were purchased from the
Conservation Genetics CAS Kunming Cell Bank (Kunming, China). The
cells were cultured in RPMI-1640 medium with 10% fetal bovine serum
(FBS) (both from Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) in a humidified atmosphere containing 5% CO2 at
37°C. The medium was replaced 2–3 times a week and cells were
passaged every 6–7 days.
MTT assay
The cells were seeded into 96-well culture plates
(180 µl of a 1×104 cells/ml suspension per well) and
cultured for 24 h for attachment. Subsequently, oridonin or LNT
(Shanghai Yuanye Biotechnology Co., Ltd., Shanghai, China) at the
indicated concentrations were added to each well in a volume of 20
µl, followed by incubation for 48 h. A total of 20 µl MTT reagent
solution (5 mg/ml; Amresco, Solon, OH, USA) was then added.
Following 4 h of further incubation, the supernatant fluid was
aspirated and 150 µl dimethyl sulfoxide was added per well.
Subsequent to agitation for 30 min, the optical density value of
each well was determined at a wavelength of 540 nm by using an
ELISA plate reader and the cell proliferation as well as inhibition
rate were calculated (10).
Flow cytometric assay
After culture for 24 h with Sp-cyclic 3′,5′-hydrogen
phosphorothioate adenosine hydrate (Thermo Fisher Scientific,
Inc.), cancer cells were incubated with 0.25% pancreatic enzymes
(Thermo Fisher Scientific, Inc.) to digest and disperse cells.
After cells were detached, they were added into medium with serum
to neutralize the pancreatic enzymes. Subsequent to centrifugation
at 1,500 × g for 5 min, supernatant fluid was abandoned and the
cell precipitate was collected and washed with PBS, followed by
addition of 1 ml 75% ethanol to fix cells and incubation at 4°C
overnight. The concentration of cancer cells was adjusted to
5×105/ml and the cells were washed with PBS 3 times,
after which 500 µl PBS containing 50 µg/ml ethidium bromide (Gibco;
Thermo Fisher Scientific, Inc.), 100 µg/ml RNase and 0.2% Triton
X-100 (CycleTEST™ PLUS kit; Becton Dickinson, Franklin Lakes, NJ,
USA) were added. Subsequent to incubation for 30 min at 4°C in the
dark, cells were detected with a flow cytometry instrument (Accuri
C6; BD Biosciences, Franklin Lakes, NJ, USA) (11).
RT-qPCR assay
Different groups of cells treated using the same
method as MTT assay (100 or 200 µg/ml LNT and/or 20 µg/ml oridonin)
were collected, total RNA was extracted with TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and complementary
(c)DNA was prepared by using a reverse transcription kit (GE
Healthcare, Little Chalfont, UK). PCR amplification
(SuperScript® III Platinum®
SYBR−Green® One-Step qRT-PCR kit; Thermo
Fisher Scientific, Inc.) of cDNA was performed and GAPDH was used
as a housekeeping gene. The primer sequences are shown in Table I. The reaction conditions were 50°C
for 2 min, then 40 cycles of 95°C for 30 sec, 95°C for 5 sec and
60°C for 34 sec (12,13).
 | Table I.Sequences of primers used for
polymerase chain reaction. |
Table I.
Sequences of primers used for
polymerase chain reaction.
| Gene | Forward primer | Reverse primer |
|---|
| Caspase-3 |
5′-CAAACTTTTTCAGAGGGGATCG-3′ |
5′-GCATACTGTTTCAGCATGGCA-3′ |
| Caspase-8 | 5′-CCC CAC CCT CAC
TTT GCT-3′ |
5′-GGAGGACCAGGCTCACTTA-3′ |
| Caspase-9 |
5′-GGCCCTTCCTCGCTTCATCTC-3′ |
5′-GGTCCTTGGGCCTTCCTGGTAT-3′ |
| Bax |
5′-AAGCTGAGCGAGTGTCTCCGGCG-3′ |
5′-CAGATGCCGGTTCAGGTACTCAGTC-3′ |
| Bcl-2 |
5′-CTCGTCGCTACCGTCGTGACTTGG-3′ |
5′-CAGATGCCGGTTCAGGTACTCAGTC-3′ |
| Bcl-xL |
5′-CCCAGAAAGGATACAGCTGG-3′ |
5′-GCGATCCGACTCACCAATAC-3′ |
| p53 |
5′-GCTCTGACTGTACCACCATCC-3′ |
5′-CTCTCGGAACATCTCGAAGCG-3′ |
| p21 | 5′-CTC AGA GGA GGC
GCC ATG-3′ | 5′-GGG CGG ATT AGG
GCT TCC-3′ |
| EGF |
5′-CAGGCCAGCCTCGTCTCAT-3′ |
5′-GCCAAGCTCAGAAGGCTAC-3′ |
| EGFR |
5′-TTTCTGGCAGTTGCTCCTC-3′ | 5′-TCG GTG CTG TGC
GAT TTA-3′ |
| GAPDH |
5′-AGCCTTCTCCATGGTCGTGA-3′ |
5′-CGGAGTCAACGGATTTGGTC-3′ |
Western blot assay
Cells were collected and total protein was extracted
using lysis buffer (Thermo Fisher Scientific, Inc.), followed by
determination of the protein concentration via the bicinchoninic
acid method (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Protein (30 µg) was subjected to SDS-PAGE (0.4%) and transferred
onto polyvinylidene difluoride membranes activated by methanol.
Following blocking with 10% skimmed milk powder for 1 h at 25°C,
the membranes were incubated with the primary antibodies of
caspase-3 (ab13847), caspase-8 (ab25901), caspase-9 (ab52298), Bax
(ab32503), Bcl-2 (ab59348), Bcl-xL (ab32370), p53 (ab1431), p21
(ab188224), EGF (ab9695) and EGFR (ab52894) (all from Abcam,
Cambridge, MA, USA) at a dilution of 1:1,500 at 4°C overnight,
followed by washing 3 times with Tris-buffered saline containing
Tween-20 (TBST) for 10 min each. Subsequently, the corresponding
secondary antibodies (1:2,000 dilution, ab131368; Abcam) were added
and allowed to react for 1 h at 25°C, followed by washing three
times with TBST for 10 min each, visualization of bands with
enhanced chemiluminescence reagent (Thermo Fisher Scientific, Inc.)
and capturing of X-ray film images. The same amount of β-actin
(Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was taken as the
comparing standard used as a reference for probing of membranes
(13).
Statistical analysis
Values are expressed as the mean ± standard
deviation. Significant differences between groups were determined
by Duncan's multiple range test using SAS version 9.2 (SAS
Institute Inc., Cary, NC, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Oridonin and LNT inhibit the growth of
SMMC-7721, but not L02 cells
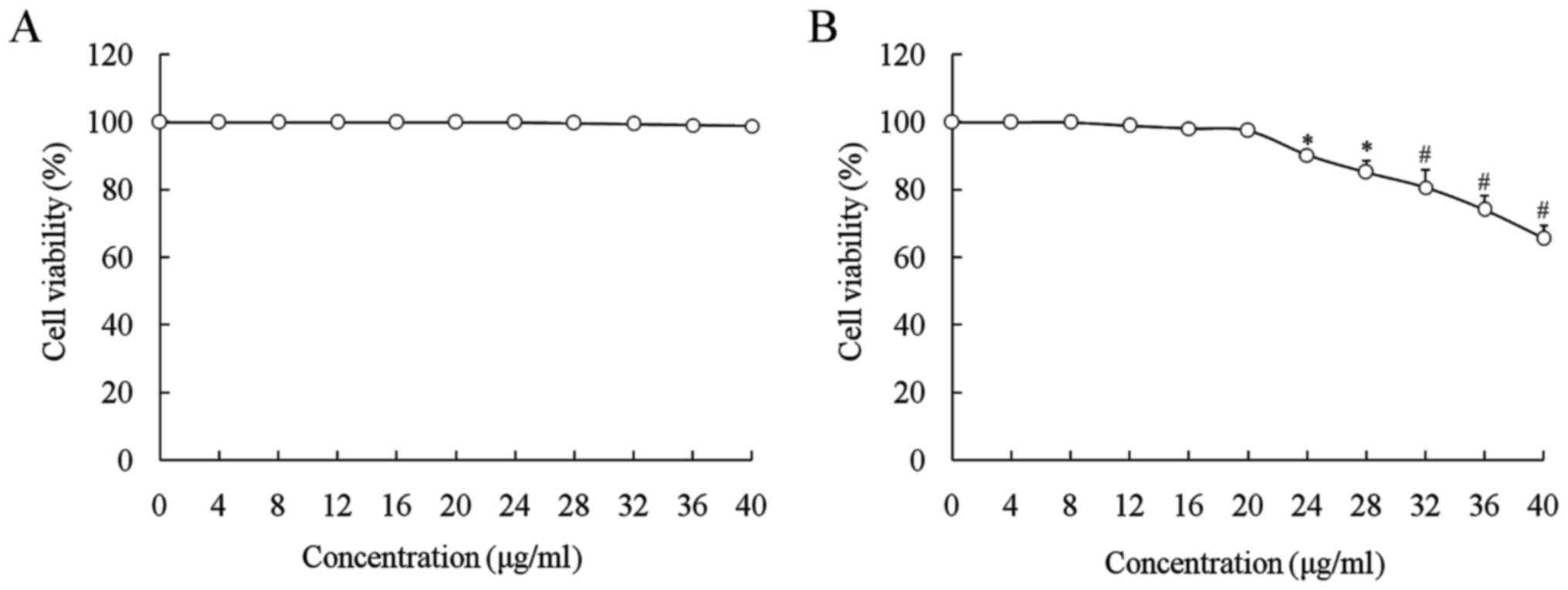
The growth inhibitory effects of oridonin and LNT in
L02 and SMMC-7721 cells were determined by an MTT assay (Figs. 1 and 2). Oridonin significantly decreased the
growth of SMMC-7721 cells at concentrations of >20 µg/ml
(P<0.05), but not on L02 cells, while oridonin at 0–20 µg/ml had
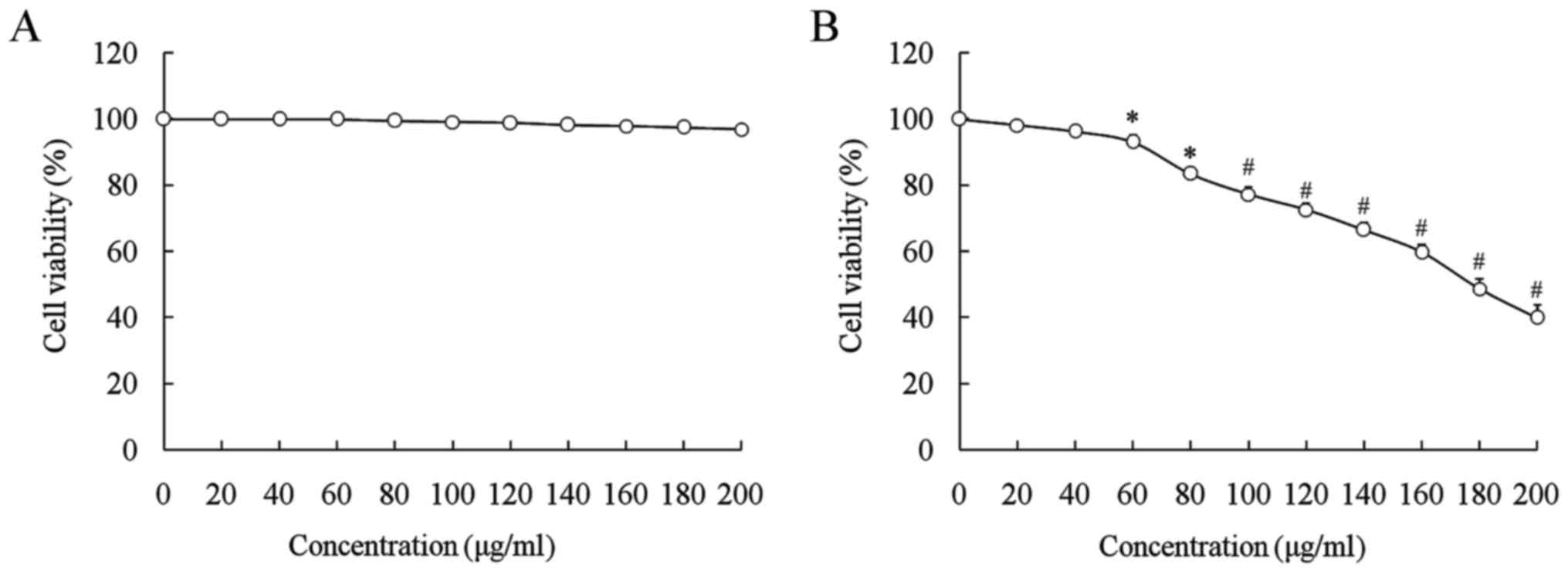
no toxic effect on normal or cancer cells (Fig. 1). LNT at 0–200 µl/ml decreased the
growth of SMMC-7721 cells in a significantly
concentration-dependent manner (60–200 µg/ml; P<0.05), while it
did not affect the growth of L02 normal cells (Fig. 2). Based on these results, oridonin
was selected as a complementary drug to enhance the anticancer
effect of LNT. LNT (100 and 200 µg/ml) inhibited the proliferative
rate of SMMC-7721 cells, and in the presence of oridonin (20
µg/ml), these inhibitory effects were substantially increased
(Table II).
 | Table II.Growth inhibition of SMMC-7721 human
hepatoma cells by oridonin and lentinan by an MTT assay. |
Table II.
Growth inhibition of SMMC-7721 human
hepatoma cells by oridonin and lentinan by an MTT assay.
| Treatment | OD540
value | Inhibitory rate
(%) |
|---|
| Control |
0.430±0.003a | – |
| LNT (100 µg/ml) |
0.332±0.014b | 22.8±2.2c |
| Oridonin + LNT (100
µg/ml) |
0.227±0.012c | 47.2±4.1b |
| LNT (200 µg/ml) |
0.172±0.007d |
60.0±3.8a |
| Oridonin + LNT (200
µg/ml) |
0.083±0.005e | 80.7±4.4 |
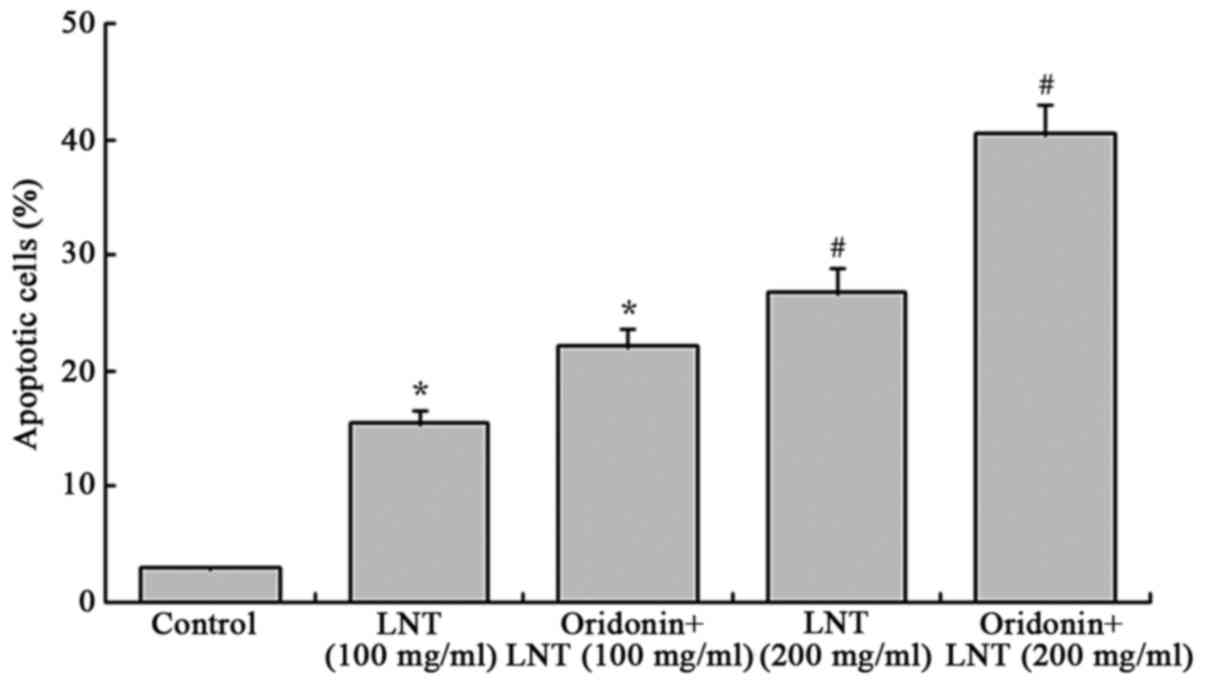
Oridonin and LNT induce apoptosis of
SMMC-7721 cells
After treatment with LNT, the apoptotic rate (sub-G1
population of cells stained for their DNA content) of SMMC-7721
cancer cells was enhanced as compared with that of the control
cells (3.0±0.2%; Fig. 3). Treatment
with 100 and 200 µg/ml LNT led to an apoptotic rate of 15.5±1.2 and
26.8±2.2%, respectively, which was further increased by
co-treatment with oridonin (20 µg/ml) to 22.1±1.7 and 40.5±2.5%,
respectively.
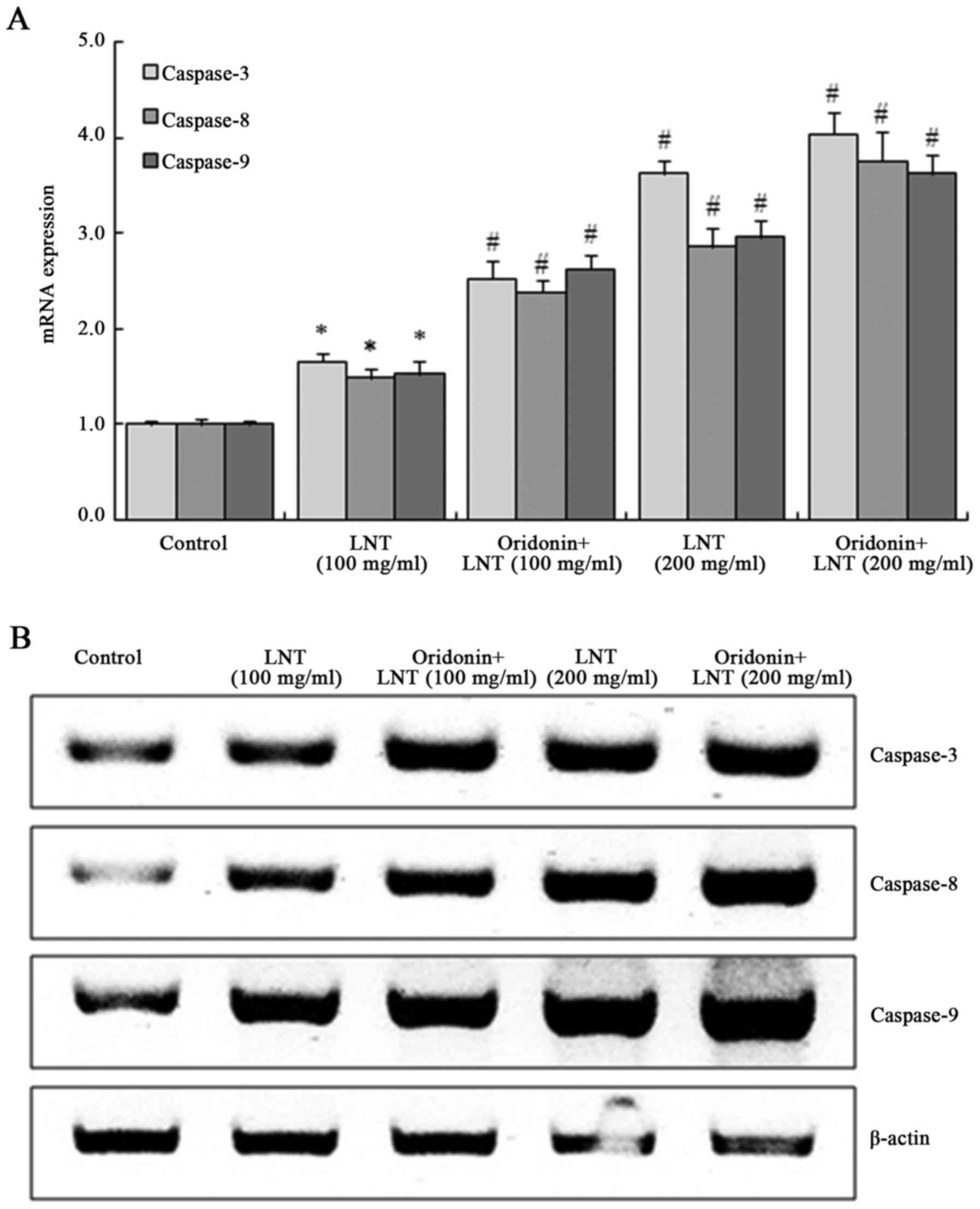
Oridonin and LNT induce caspase-3, −8
and −9 mRNA and protein expression in SMMC-7721 cells
The expression of caspase-3, −8 and −9 in SMMC-7721
cells was significantly increased after treatment with 100 and 200
µg/ml LNT, and was further enhanced by co-treatment with oridonin
(20 µg/ml). SMMC-7721 cells treated with oridonin (20 µg/ml) and
200 µg/ml LNT showed the highest increase in caspase-3, −8 and −9
mRNA and protein expression (Fig.
4).
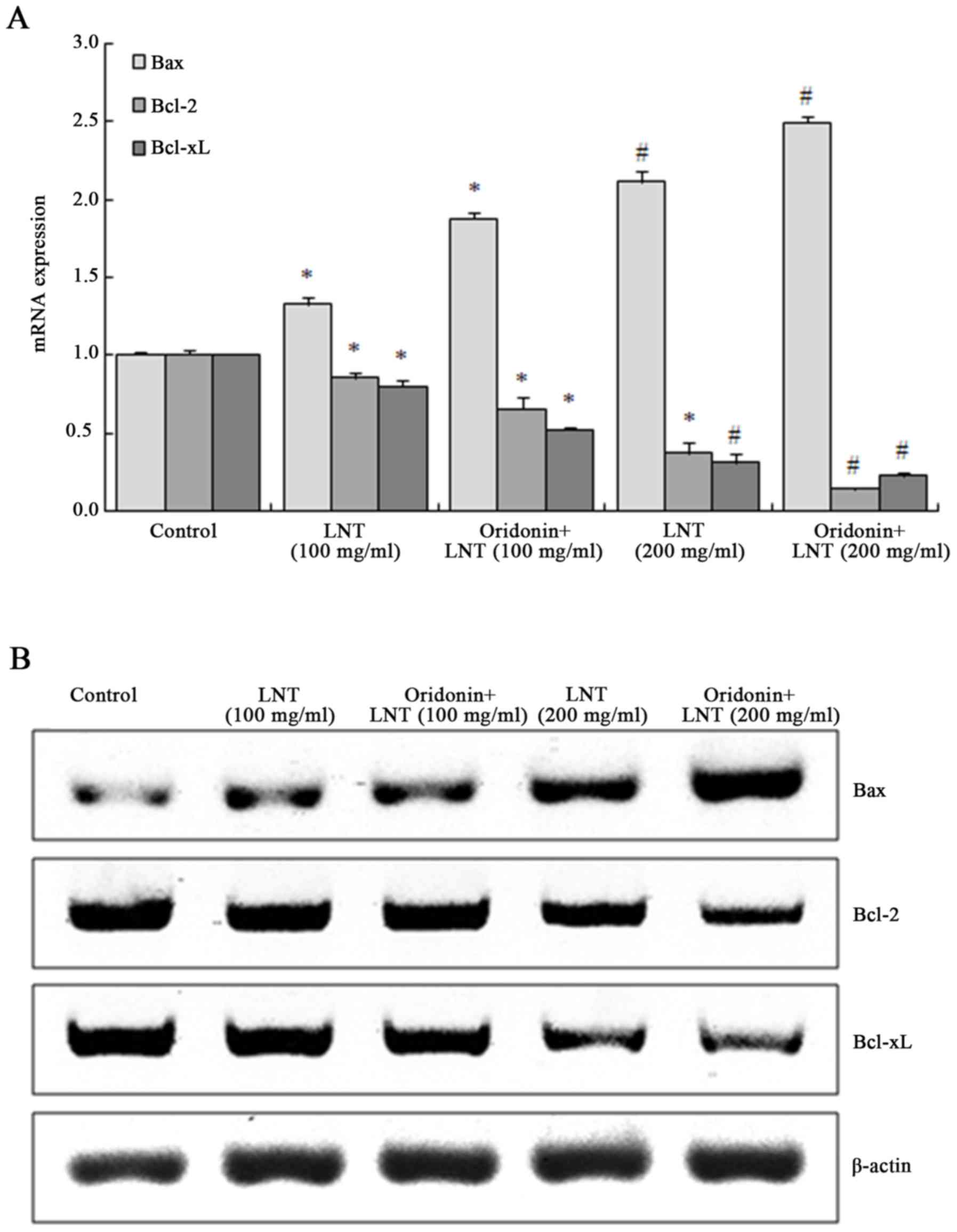
Oridonin and LNT shift the balance of
B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax) and Bcl
extra large protein (Bcl-xL) in SMMC-7721 cells
The mRNA and protein expression of Bax in SMMC-7721
cells was significantly increased after treatment with 100 and 200
µg/ml LNT, which was further enhanced in the presence of oridonin.
By contrast, the expression of Bcl-2 and Bcl-xL were decreased
after treatment with 100 and 200 µg/ml LNT, and was further
suppressed by co-treatment with oridonin (Fig. 5).
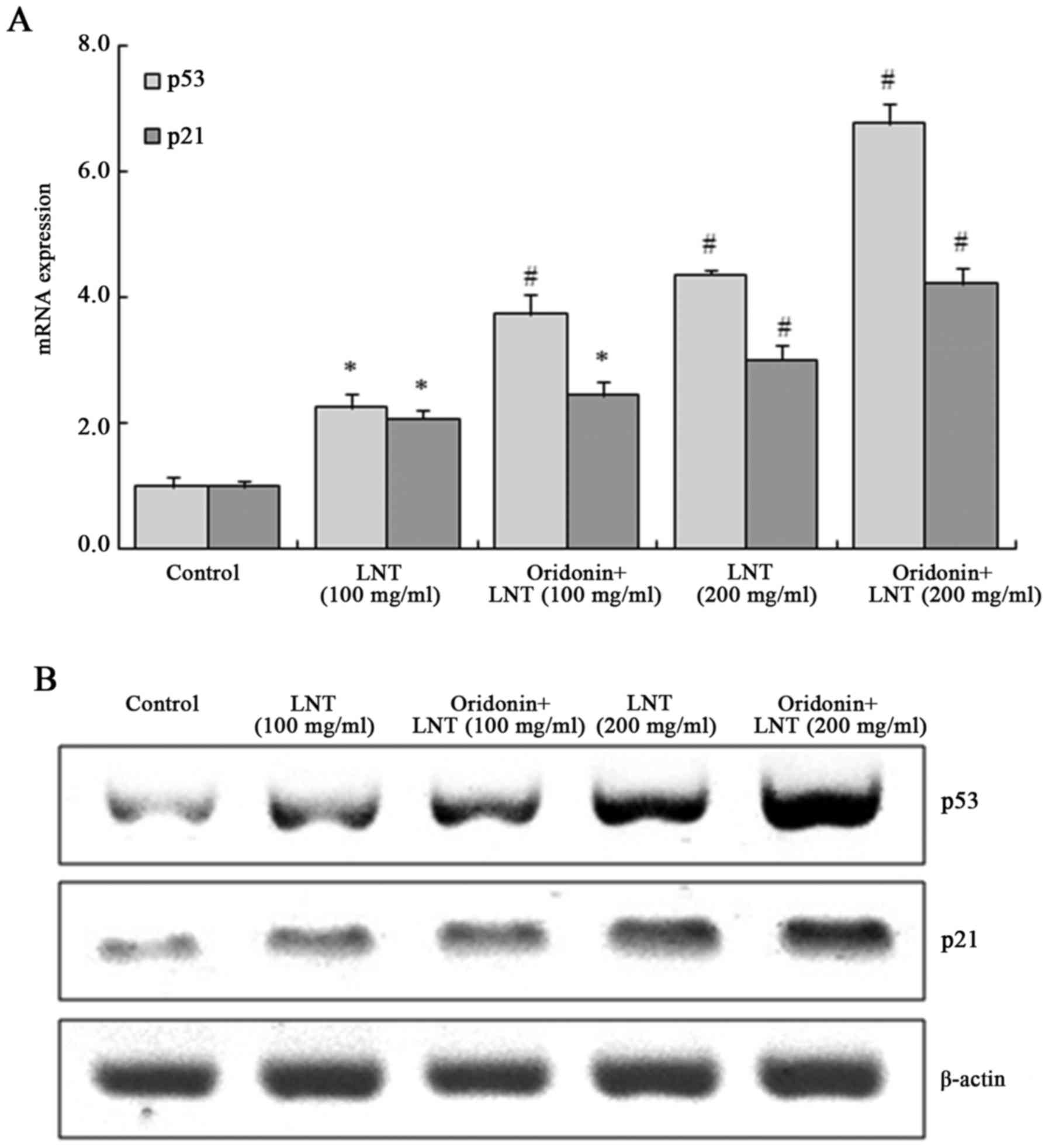
Oridonin and LNT increase p53 and p21
mRNA and protein expression in SMMC-7721 cells
The mRNA and protein expression of p53 and p21 in
100 µg/ml LNT-treated cells were significantly enhanced compared to
those in the control group and further increased in the 200 µg/ml
LNT-treated cells; this effect was enhanced by co-treatment with
oridonin (Fig. 6).
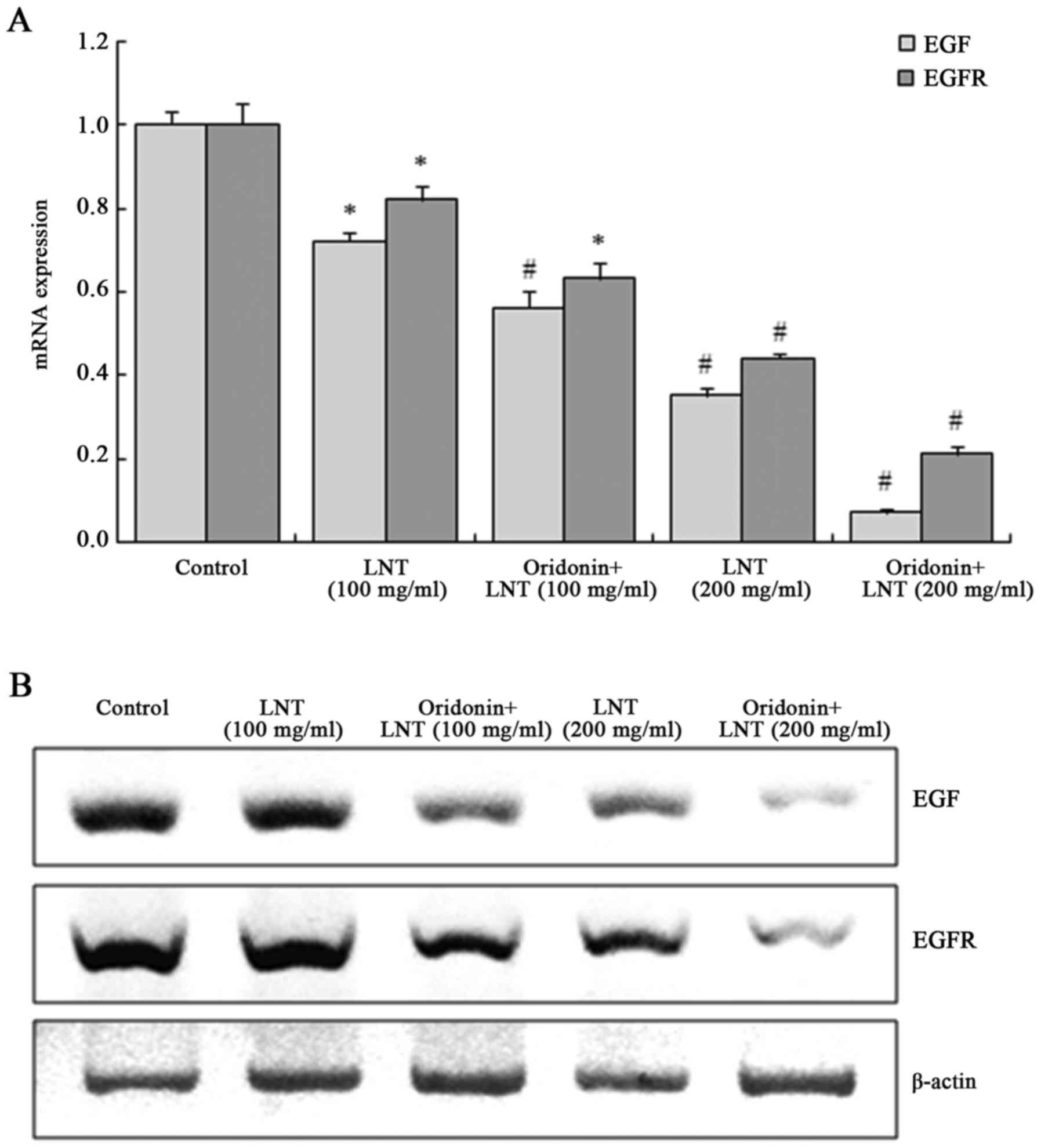
Oridonin and LNT decrease epidermal
growth factor (EGF) and EGF receptor (EGFR) mRNA and protein
expression in SMMC-7721 cells
LNT at 100 and 200 µg/ml dose-dependently decreased
the EGF and EGFR mRNA and protein expression in SMMC-7721 cells,
which was further suppressed by oridonin treatment (Fig. 7).
Discussion
Induction of cancer cell apoptosis is an important
strategy for the treatment of liver cancer. It is well known that a
variety of cell signals mediated by receptors and signaling
molecules are responsible for triggering liver cancer cell
apoptosis, and a variety of proteases are involved in the signal
conduction; it is a strictly regulated process involving a large
variety of genes. The protease caspase-3 has a core role in signal
conduction and execution of apoptosis (14). As a downstream event of the apoptotic
cascade, its activation may occur via multiple pathways. Upon its
activation through cleavage, e.g. by initiator caspase-9, caspase-3
carries out the programmed cell death by dismantling the
cytoskeleton, and its activation is therefore desirable for
inducing cancer cell apoptosis (15). Caspase-8 is another core protease
that may cause the caspase cascade reaction of cell apoptosis. In
liver cancer cells, apoptosis via caspase-3 and −8 activation has a
central role and represents a downstream target for anticancer
drugs, while these pathways may be deactivated in certain types of
cancer cell (16). In the present
study, caspase-3, −8 and −9 expression in cancer cells was
increased by oridonin and LNT treatment, which was consistent with
previous results (14).
The Bcl-2 family of apoptotic proteins has an
important regulatory role in the process of caspase-3 activation
(16,17). As an anti-apoptotic member of the
Bcl-2 family, Bcl-xL inhibits oligomers of apoptotic protease
activating factor 1 (Apaf-1) molecules, resulting in loss of
function of Apaf-1 and inhibition of the activation of caspase-9,
which depends on Apaf-1 (18).
Anti-apoptotic members of Bcl-2 family, which mainly exist in the
outer membrane of mitochondria, may prevent mitochondria from
releasing cytochrome c, thus inhibiting the activation of
pro-caspase-9 (19). All
pro-apoptotic Bcl-2 family members form miscellaneous dimers with
Bcl-2, Bcl-xL, A1 and myeloid cell leukemia-1 via their BH3 domain,
and therefore at least partly function by influencing
anti-apoptotic Bcl-2 family members. Once pro-apoptotic Bcl-2
family members outnumber their anti-apoptotic counterparts, they
induce the activation of caspases (20). In the present study, the drug
combination could also raise Bax and reduce Bcl-2 and Bcl-xL
expression compared with untreated cancer cells.
p53 causes apoptosis through the activation of
caspases (21,22). Following restoration of wild-type
p53, activation of caspase-3 and characteristic changes of cell
apoptosis occurred in cells with a Fas defect, indicating that
p53-dependent apoptosis directly activates caspases without
mediation via Fas (23).
Apoptosis-inhibiting factor Bcl-2 regulates apoptosis through
forming a homodimer or heterodimers with Bax protein. When the
Bcl-2/Bax ratio is high, apoptosis is inhibited, while apoptosis is
induced if the ratio decreases below a certain threshold. The
mechanism of Bcl-xL is similar to that of Bcl-2 (24). Silencing of Bcl-2 induces the
activation of the p53-dependent apoptotic pathway. In p53 wild-type
cancer cells, after p53 is activated, the expression of Bax
increases with the quantity of cancer inhibitors, while Bcl-2 and
Bcl-xL are simultaneously reduced, which shows that in the process
of killing cancer cells by inhibitors, p53 induces apoptosis mainly
through Bax/Bcl-2 and Bax/Bcl-xL pathways (25). In cancer cells, certain active
substances may change the expression of p21 through p53, thus
inducing the stagnation of the cell cycle. Increased expression of
p21 and p53 is one of the indicators of apoptosis induction in
cancer cells by certain substances (25). In the present study, oridonin and LNT
combination exhibited a good anticancer effect, based on increased
p53 and p21 expression.
Studies have shown that in the process of
proliferation, stem cells secrete growth factors such as EGF and
vascular endothelial growth factor to maintain their own growth and
inhibit apoptosis. EGF and EGFR take part in the process of
inducing cancer cell apoptosis (26). EGF is a growth factor with a large
variety of functions and acts by combining with EGFR. Studies have
shown that growth factors such as EGF promote the proliferation of
stem cells and maintain the balance of normal cells (27). EGFR is a member of the ErbB receptor
family on the cell surface, which is involved in cellular
processes, including proliferation, growth, migration and invasion
(28). Studies have also found that
EGFR adjusts EGF-mediated cancer cell proliferation through saliva
acidification (28,29). In addition, EGFR inhibitors were
shown to induce cancer cells to express UL16 binding protein 1 in
order to enhance their sensitivity to natural killer cells
(29). In the present study,
oridonin and LNT were observed to inhibit growth of cancer cells by
reducing EGF and EGFR expression.
In the present study MTT, flow cytometry, RT-qPCR
and western blot assays revealed that LNT had a potent and
dose-dependent anticancer effect on SMMC-7721 cancer cells, while a
non-toxic concentration of oridonin increase these in vitro
anticancer effects of LNT. It is concluded that oridonin may be
used as a drug to increase the anticancer effect of LNT.
References
|
1
|
Kupfahl C, Geginat G and Hof H: Lentinan
has a stimulatory effect on innate and adaptive immunity against
murine Listeria monocytogenes infection. Int Immunopharmacol.
6:686–696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hou XJ and Chen W: Optimization of
extraction process of crude polysaccharides from wild edible BaChu
mushroom by response surface methodology. Carbohyd Polym. 72:67–74.
2008. View Article : Google Scholar
|
|
3
|
Murata T, Hatayama I, Kakizaki I, Satoh K,
Sato K and Tsuchida S: Lentinan enhances sensitivity of mouse colon
26 tumor to cis-diamminedichloroplatinum (II) and decreases
glutathione transferase expression. Jpn J Cancer Res. 87:1171–1178.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Drandarska I, Kussovski V, Nikolaeva S and
Markova N: Combined immunomodulating effects of BCG and Lentinan
after intranasal application in guinea pigs. Int Immunopharmacol.
5:795–803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang HL, Weng HY, Wang LQ, Yu CH, Huang
QJ, Zhao PP, Wen JZ, Zhou H and Qu LH: Triggering Fbw7-mediated
proteasomal degradation of c-Myc by oridonin induces cell growth
inhibition and apoptosis. Mol Cancer Ther. 11:1155–1165. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang H, Yu HS and Xue HW: Radio
sensitization effect of oridonin on HepG2 in vitro. Med J Qi Lu.
22:339–342. 2007.
|
|
7
|
Mullard A: Pioneering apoptosis-targeted
cancer drug poised for FDA approval. Nat Rev Drug Discov.
15:147–149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Denisenko TV, Sorokina IV, Gogvadze V and
Zhivotovsky B: Mitotic catastrophe and cancer drug resistance: A
link that must to be broken. Drug Resist Updat. 24:1–12. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O'Connor SE: Plant biochemistry. Fighting
cancer while saving the mayapple. Science. 349:1167–1168. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao X, Wang Q, Li GJ, Chen F, Qian Y and
Wang R: In vitro antioxidant, anti-mutagenic, anti-cancer and
anti-angiogenic effects of Chinese bowl tea. J Funct Food.
7:590–598. 2014. View Article : Google Scholar
|
|
11
|
Zhao X, Qian Y, Zhou YL, Wang R, Wang Q
and Li GJ: Pu-erh tea has in vitro anticancer activity in TCA8113
cells and preventive effects on buccal mucosa cancer in U14 cells
injected mice in vivo. Nutr Cancer. 66:1059–1069. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suo H, Zhao X, Qian Y, Sun P, Zhu K, Li J
and Sun B: Lactobacillus fermentum Suo attenuates HCl/ethanol
induced gastric injury in mice through its antioxidant effects.
Nutrients. 8:1552016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
O'Donovan N, Crown J, Stunell H, Hill AD,
McDermott E, O'Higgins N and Duffy MJ: Caspase 3 in breast cancer.
Clin Cancer Res. 9:738–742. 2003.PubMed/NCBI
|
|
16
|
Rodríguez-Berriguete G, Galvis L, Fraile
B, de Bethencourt FR, Martínez-Onsurbe P, Olmedilla G, Paniagua R
and Royuela M: Immunoreactivity to caspase-3, caspase-7, caspase-8,
and caspase-9 forms is frequently lost in human prostate tumors.
Hum Pathol. 43:229–237. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsujimoto Y: Role of Bcl-2 family proteins
in apoptosis: Apoptosomes or mitochondria? Gene Cell. 3:697–707.
1998. View Article : Google Scholar
|
|
18
|
Swanton E, Savory P, Cosulich S, Clarke P
and Woodman P: Bcl-2 regulates a caspase-3/caspase-2 apoptotic
cascade in cytosolic extracts. Oncogene. 18:1781–1787. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park HJ, Jeon YK, You DH and Nam MJ:
Daidzein causes cytochrome c-mediated apoptosis via the Bcl-2
family in human hepatic cancer cells. Food Chem Toxicol.
60:542–549. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Willis SN, Chen L, Dewson G, Wei A, Naik
E, Fletcher JI, Adams JM and Huang DC: Proapoptotic Bak is
sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by
BH3-only proteins. Gene Dev. 19:1294–1305. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu SY, Xiao DJ, Luan YZ, Wang YS, Wang L
and Shi K: Regulatory mechanism of cell cycle block and apoptosis
in p53 mutated gastric cancer cells during cisplatin stress. J
Shandong Univ (Health Sci). 46:478–480. 2008.
|
|
22
|
Moulin M, Carpentier S, Levade T and
Arrigo AP: Potential roles of membrane fluidity and ceramide in
hyperthermia and alcohol stimulation of TRAIL apoptosis. Apoptosis.
12:1703–1720. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fuchs EJ, Mckenna KA and Bedi A:
p53-dependent DNA damage-induced apoptosis requires
Fas/APO-1-independent activation of CPP32beta. Cancer Res.
57:2550–2554. 1997.PubMed/NCBI
|
|
24
|
Klostergaard J, Leroux ME, Auzenne E,
Khodadadian M, Spohn W, Wu JY and Donato NJ: Hyperthermia engages
the intrinsic apoptotic pathway by enhancing upstream caspase
activation to overcome apoptotic resistance in MCF-7 breast
adenocarcinoma cells. J Cell Biochem. 98:356–369. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Woo SM, Choi YK, Kim AJ, Cho SG and Ko SG:
p53 causes butein-mediated apoptosis of chronic myeloid leukemia
cells. Mol Med Rep. 13:1091–1096. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hollmann G, Linden R, Giangrande A and
Allodi S: Increased p53 and decreased p21 accompany apoptosis
induced by ultraviolet radiation in the nervous system of a
crustacean. Aquat Toxicol. 173:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deng W, Gu L, Li X, Zheng J, Zhang Y, Duan
B, Cui J, Dong J and Du J: CD24 associates with EGFR and supports
EGF/EGFR signaling via RhoA in gastric cancer cells. J Transl Med.
14:322016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Appert-Collin A, Hubert P, Crémel G and
Bennasroune A: Role of ErbB receptors in cancer cell migration and
invasion. Front Pharmacol. 6:2832015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yen HY, Liu YC, Chen NY, Tsai CF, Wang YT,
Chen YJ, Hsu TL, Yang PC and Wong CH: Effect of sialylation on EGFR
phosphorylation and resistance to tyrosine kinase inhibition. Proc
Natl Acad Sci USA. 112:pp. 6955–6960. 2015; View Article : Google Scholar : PubMed/NCBI
|