Introduction
Renal cell carcinoma (RCC), accounting for 2–3% of
all tumor malignancies in humans, is the most lethal urologic tumor
(1). Despite increased early
detection of RCC and more frequent surgery or other therapy, the
prognosis remains poor due to metastasis of RCC and its low
response to chemotherapy and radiotherapy (2,3). Thus,
there is an urgent need to elucidate the mechanisms of RCC
progression to provide useful information for the clinical
management of this disease.
MicroRNA (miR) are a class of short, single
stranded, non-coding RNA molecules, of 19–25 nucleotides in length,
that act as important regulators of gene expression by binding to
the 3′-untranslated region (UTR) of specific target mRNA (4). Study has demonstrated that miR are
involved in various biological processes, such as cell growth,
migration, invasion, apoptosis, metabolism and cellular
differentiation (5,6). Accumulating evidence suggests that miR
could have oncogenic or tumor-suppressive roles in the regulation
of cell growth, migration and invasion by repressing their target
genes (7,8). Recently, some miR have been reported to
be involved in RCC procession, and may serve as diagnostic markers
or therapy agents for RCC (9,10).
miR-338-3p, located on the 7th intron of the
apoptosis-associated tyrosine kinase gene, has been reported to be
downregulated in many types of cancer, such as hepatocellular
carcinoma (11,12), colorectal cancer (13), ovarian cancer (14), gastric cancer (15,16) and
breast cancer (17). However, little
is known about the biological role and underlying mechanism of
miR-338-3p in RCC. The present study therefore investigated the
biological functions and the mechanism of miR-338-3p in RCC
progression. It was demonstrated that miR-338-3p was downregulated
in RCC tissues and cell lines, and miR-338-3p expression was
associated with clinicopathological characteristics in RCC. It was
also observed that miR-338-3p overexpression inhibited cell
proliferation, colony formation, migration and invasion of RCC by
repressing sex-determining region Y-box 4 (SOX4). Studies such as
these may contribute to the understanding of the molecular
mechanisms underlying RCC development.
Materials and methods
Clinical samples
A total of 48 RCC samples and adjacent normal
tissues were collected from patients (mean age, 52.5±4.5 years; age
range, 43.2–75.4 years; 21 males and 27 females) who underwent
resection of their primary RCC at the Department of General
Surgery, The Affiliated Hospital, Changchun University of Chinese
Medicine (Changchun, China) between April 2012 and December 2014.
All participants provided written informed consent prior to
participating in the study. The present study was approved by the
Medicine Ethics Committee of Changchun University of Chinese
Medicine. All tissue samples were immediately stored in liquid
nitrogen until use. The clinicopathological information of the
patients, including age, sex, tumor size, TNM stage and lymph node
metastasis was recorded (Table I).
No patients had received chemotherapy or radiotherapy prior to
surgery.
 | Table I.Correlation between
clinicopathological features and miR-338-3p expression in RCC
tissues. |
Table I.
Correlation between
clinicopathological features and miR-338-3p expression in RCC
tissues.
|
|
| miR-338-3p
expression, (n, %) |
|
|---|
|
|
|
|
|
|---|
| Variables | n | Low | High | P-value |
|---|
| Age (years) |
|
|
| >0.05 |
|
<55 | 23 | 13 (56.5) | 10 (43.5) |
|
| ≥55 | 25 | 15 (60) | 10 (40) |
|
| Sex |
|
|
| >0.05 |
| Male | 21 | 12 (57.1) | 9 (42.9) |
|
|
Female | 27 | 16 (59.3) | 11 (40.7) |
|
| TNM stage |
|
|
| <0.01 |
|
T1-T2 | 34 | 15 (44.1) | 19 (55.9) |
|
|
T3-T4 | 14 | 13 (92.9) | 1 (7.1) |
|
| Tumor size (cm) |
|
|
| >0.05 |
|
<5 | 31 | 17 (45.7) | 14 (54.3) |
|
| ≥5 | 17 | 11 (64.7) | 6 (35.6) |
|
| Lymph node
metastasis |
|
|
| <0.01 |
| No | 35 | 16 (37.1) | 19 (62.9) |
|
|
Yes | 13 | 12 (92.3) | 1 (7.7) |
|
Cell lines and transfection
Four RCC cell lines (786-O, ACHN, Caki-1 and Caki-2)
and a human renal proximal tubule epithelial cell line (HK-2) were
purchased from the Type Culture Collection of the Chinese Academy
of Sciences (Shanghai, China). Cells were cultured in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(FBS; Invitrogen; Thermo Fisher Scientific, Inc.), 100 IU/ml
penicillin and 100 IU/ml streptomycin at 37°C in a 5%
CO2 humidified incubator.
miR-338-3p mimic (miR-338-3p, GUU GUU UUA GUG AC U
ACG ACC U) and corresponding negative control (NC, GUC CTU GCU CGA
GCG AGG UGA) mimic (miR-NC) were purchased from GeneCopoeia, Inc.
(Rockville, MD, USA). A SOX4 overexpression plasmid was purchased
from OriGene Technologies, Inc., (Beijing, China). miR-338-3p (100
nM), miR-NC (100 nM) or the SOX4 plasmid (100 ng) were transfected
into RCC cells using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's
instructions.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from tissues and cells was extracted using
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.). To detect
miR-338-3p expression, the RNA was reverse transcribed into cDNA
using a TaqMan miRNA Reverse Transcription kit (Thermo Fisher
Scientific, Inc.), and were quantified using a TaqMan Human
MicroRNA Assay kit (Thermo Fisher Scientific, Inc.). Both steps
were conducted according to the manufacturer's instructions.
Primers for miR-338-3p and U6 (GeneCopoeia, Inc.) were used as
follows: miR-338-3p, 5′-CCGCTCGAGGCCTGCAGAGCAGGACCTGGG-3′ (sense)
and 5′-ATAAGAATGCGGCCGCATACAAAAACCTTTTATTGACTGTATTTCTTCTC-3′
(antisense); U6, 5′-TGCGGGTGCTCGCTTCGGCAGC-3′ and
5′-CCAGTGCAGGGTCCGAGGT-3′ (antisense). U6 was used as an internal
control. For detection of SOX4, cDNA was synthesized using a
PrimerScript RT reagent kit (Takara Biotechnology Co., Ltd.,
Dalian, China), and was quantified using a SYBR Green PCR Master
Mix (Takara Biotechnology Co., Ltd.) on an ABI 7900HT Fast
Real-Time PCR system (Thermo Fisher Scientific, Inc.). The primers
used for SOX4 and GAPDH were as previously described (18). GAPDH was used as an internal control.
The following PCR conditions were used: Denaturation at 94°C for 5
min, followed by 40 cycles of amplification (denaturation at 94°C
for 10 sec, annealing at 58°C for 30 sec and extension at 72°C for
30 sec). The comparative 2−∆∆Cq method was used for
relative quantification (19).
Cell proliferation and colony
formation
The proliferation of RCC cell lines (786-O and
Caki-1) was examined by MTT assay (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). Briefly, transfected cells (5×103
cells/well) were seeded into 24-well plates, and cultured in DMEM
containing 10% FBS for 24 to 72 h at 37°C. At 24, 48 and 72 h, 100
µl of fresh DMEM medium containing 0.5 mg/ml MTT and 10% FBS, was
added into the plate, and incubated at 37°C for 4 h. Subsequently,
150 µl dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA) was added to
each well for 10 min at 37°C. Proliferation of cells was evaluated
using a Synergy HT Multi-Mode Microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA) at a wavelength of 490
nm.
For the colony formation assay, 786-O and Caki-1
transfected cells were seeded into 6-well plates at a density of
500 cells/well, and cultured in DMEM medium containing 10% FBS at
37°C for 2 weeks. Cells were subsequently washed twice with
phosphate-buffered saline (PBS), and fixed with 4% paraformaldehyde
for 20 min at room temperature (20–25°C) and stained with 1%
crystal violet (Sigma-Aldrich; Merck KGaA) for 5 min at room
temperature (20–25°C). Colony formation was imaged and counted in
five randomly selected fields under a light microscope (Olympus,
Tokyo, Japan).
Migration and invasion assays
For the migration assay of 768-O and Caki-1 cells, a
wound healing assay was performed in 6-well plates at a density of
2×104 cells/well. After 24 h of transfection, a linear
wound in the cellular monolayer was created by scraping the
confluent cell monolayer with a 200-µl sterile pipette tip and
washing twice with PBS. The migration of cells was observed at 0
and 24 h after wounding and then photographed under a light
microscope (magnification, ×100).
For the invasion assay, 1.0×105 786-O and
Caki-1 transfected cells in serum-free medium were seeded into the
upper chamber of Transwell inserts (Corning, Inc., Corning, NY,
USA) pre-coated with Matrigel (BD Biosciences, San Jose, CA, USA).
The lower chamber was filled with 600 µl DMEM supplemented with 10%
FBS as a chemoattractant. Following incubation for 48 h at 37°C,
non-invading cells on the upper surface of the membrane were
removed using cotton swabs, while the invasive cells that attached
to the lower surface of the membrane insert were fixed with 4%
paraformaldehyde for 20 min at room temperature (20–25°C), stained
with 1% crystal violet solution for 5 min at room temperature
(20–25°C), then counted in five random fields per well under a
light microscope.
Dual-luciferase reporter assay
Prediction of miR-338-3p targets was performed using
three publicly available algorithms: TargetScan (http://www.targetscan.org), miRanda (http://www.microrna.org) and PicTar (http://www.pictar.org). SOX4 was selected as a target
gene of miR-338-3p. The 3′-UTR of SOX4 that contained the wild-type
or mutant putative binding site of miR-338-3p was inserted into the
psiCHECK2 vector (Promega). For the dual-luciferase reporter assay,
2.0×104 786-O and Caki-1 cells/well were seeded into
24-well plates and co-transfected with 200 ng of wild-type or
mutant SOX4 reporter plasmid and 100 nM of miR-338-3p mimic or
miR-NC, and the pRL-TK plasmid (Promega), which was used for
internal normalization, using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
instructions. After 48 h of transfection, a luciferase reporter
gene assay was implemented using the Dual-Luciferase Reporter Assay
system (Promega), according to the manufacturer's instructions.
Renilla luciferase acitivity was used for normalization.
Western blot analysis
Protein from the 786-O and Caki-1 transfected cells
was extracted on ice in radioimmunoprecipitation assay lysis buffer
supplemented with protease inhibitor (both from Beyotime Institute
of Biotechnology, Shanghai, China), and protein concentrations were
determined using the Bradford assay (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Proteins (20 µg of each sample) were separated
by 10% SDS-PAGE and transferred to nitrocellulose membranes (EMD
Millipore, Billerica, MA, USA) at 80 V for 2 h at 4°C. Following
blocking in 5% non-fat milk in PBS for 2 h at 37°C, the membranes
were probed with primary antibodies overnight at 4°C as follows:
Mouse monoclonal anti-human SOX4 (1:1,000, cat. no. sc-365964;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and mouse
monoclonal anti-human GAPDH (1:5,000, cat. no. sc-365062; Santa
Cruz Biotechnology, Inc.). Subsequently, the membranes were
incubated with polyclonal goat anti-mouse horseradish
peroxidase-conjugated immunogloblin G (1:10,000, cat. no. sc-2005;
Santa Cruz Biotechnology, Inc.) for 2 h at room temperature.
Protein bands were visualized on X-ray film using a
chemiluminescent detection system (Beyotime Institute of
Biotechnology).
Statistical analysis
Quantitative data were presented as the mean ±
standard deviation from at least three independent experiments with
similar results. Student's t-tests or one-way analysis of variance
with Tukey's post hoc tests were used to assess the differences
between different groups using SPSS v19.0 (IBM Corp., Armonk, NY,
USA). The correlations between miR-338-3p expression and SOX4 mRNA
in patients with RCC were analyzed using Spearman's rank test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-338-3p expression is downregulated
in RCC tissues and cell lines
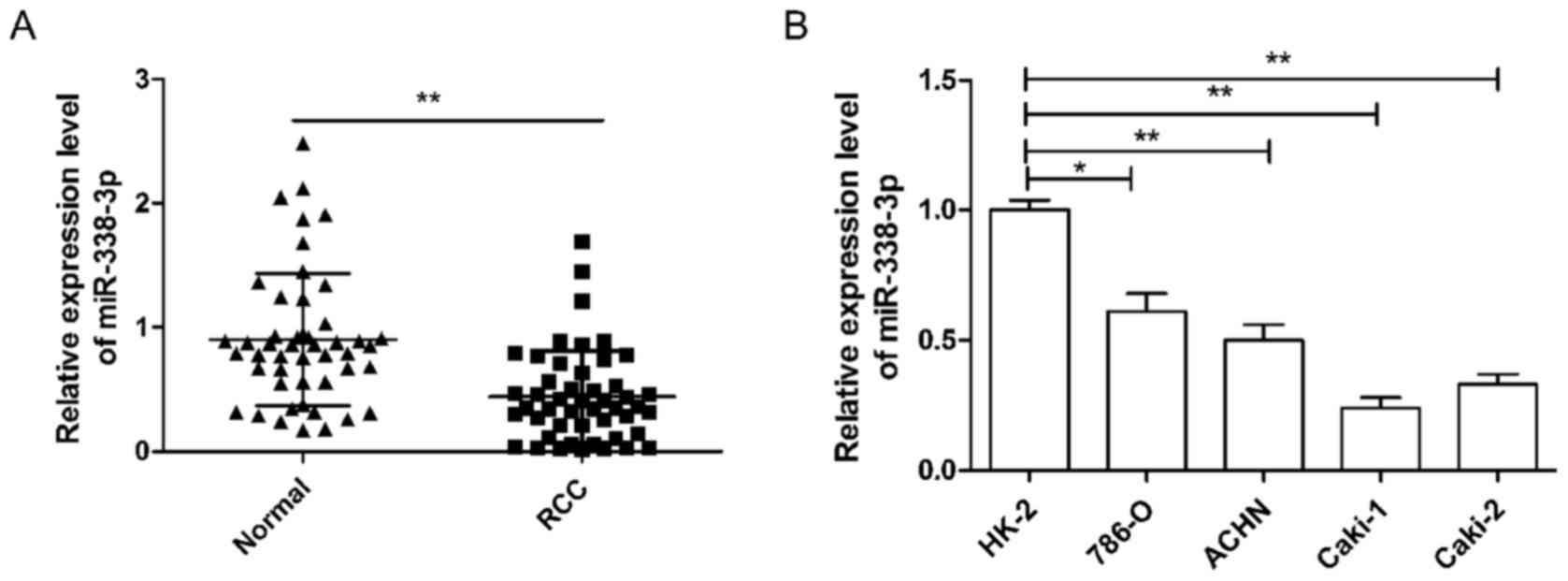
To investigate miR-338-3p expression in RCC, RT-qPCR
was performed in 48 pairs of human RCC and their adjacent normal
tissue samples. As demonstrated in Fig.
1A, the expression of miR-338-3p was significantly lower in RCC
tissues than in the corresponding adjacent normal tissues
(P<0.01). In addition, the association between miR-338-3p
expression and the clinicopathological parameters of the patients
were analyzed. It was observed that miR-338-3p expression was
significantly associated with TNM stage and lymph node metastasis
(both P<0.01), but not with age, sex or tumor size (Table I). Next, miR-338-3p expression in
four human RCC cell lines was analyzed. It was demonstrated that
the expression of miR-338-3p in the RCC cell lines, 786-O, ACHN,
Caki-1 and Caki-2, was significantly lower than that observed in
the human renal proximal tubule epithelial cell line, HK-2
(P<0.05; Fig. 1B). Cell line
786-O, which had the highest expression of miR-338-3p of the four
RCC cell lines, and Caki-1, which had the lowest expression of
miR-338-3p of the four cell lines, were selected as representatives
to perform subsequent experiments.
miR-338-3p inhibits RCC cell
proliferation, colony formation, migration and invasion
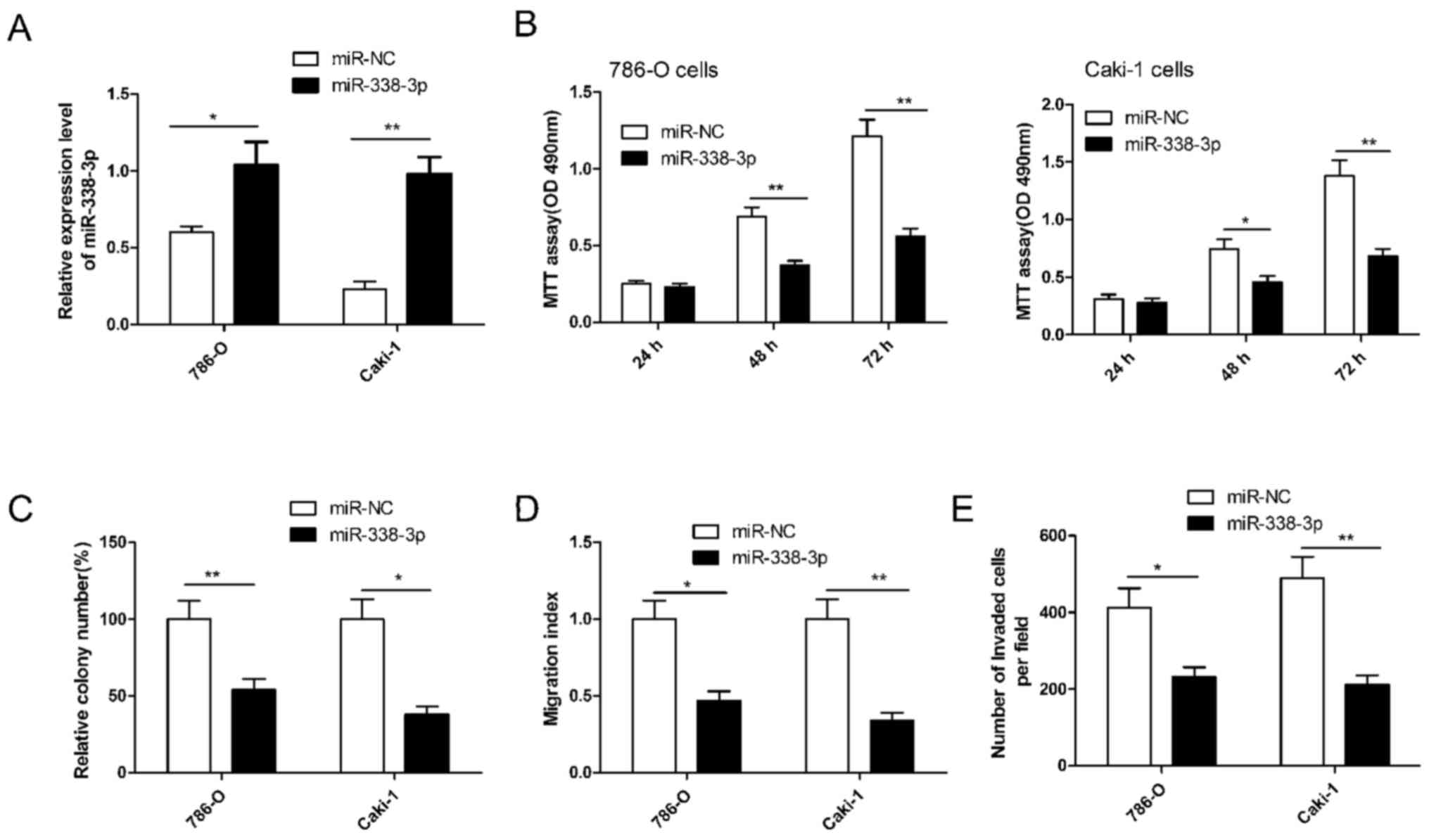
To investigate the potential role of miR-338-3p in
regulating RCC cell growth and metastasis, miR-338-3p was
overexpressed in 786-O and Caki-1 cells by transfection with an
miR-338-3p mimic. RT-qPCR demonstrated that the miR-338-3p mimic
significantly upregulated the level of miR-338-3p expression in the
two cell lines compared with cells transfected with miR-NC
(P<0.05; Fig. 2A). Subsequently,
cell proliferation, colony formation, migration and invasion were
determined in RCC cells transfected with miR-338-3p. It was
demonstrated that miR-338-3p significantly inhibited RCC cell
proliferation (P<0.01), colony formation (P<0.05), migration
(P<0.05) and invasion (P<0.05) in both cells lines compared
with cells transfected with miR-NC (Fig.
2B-E). These results implied that miR-338-3p has a suppressive
role in RCC cells.
SOX4 is a direct downstream target of
miR-338-3p in RCC cells
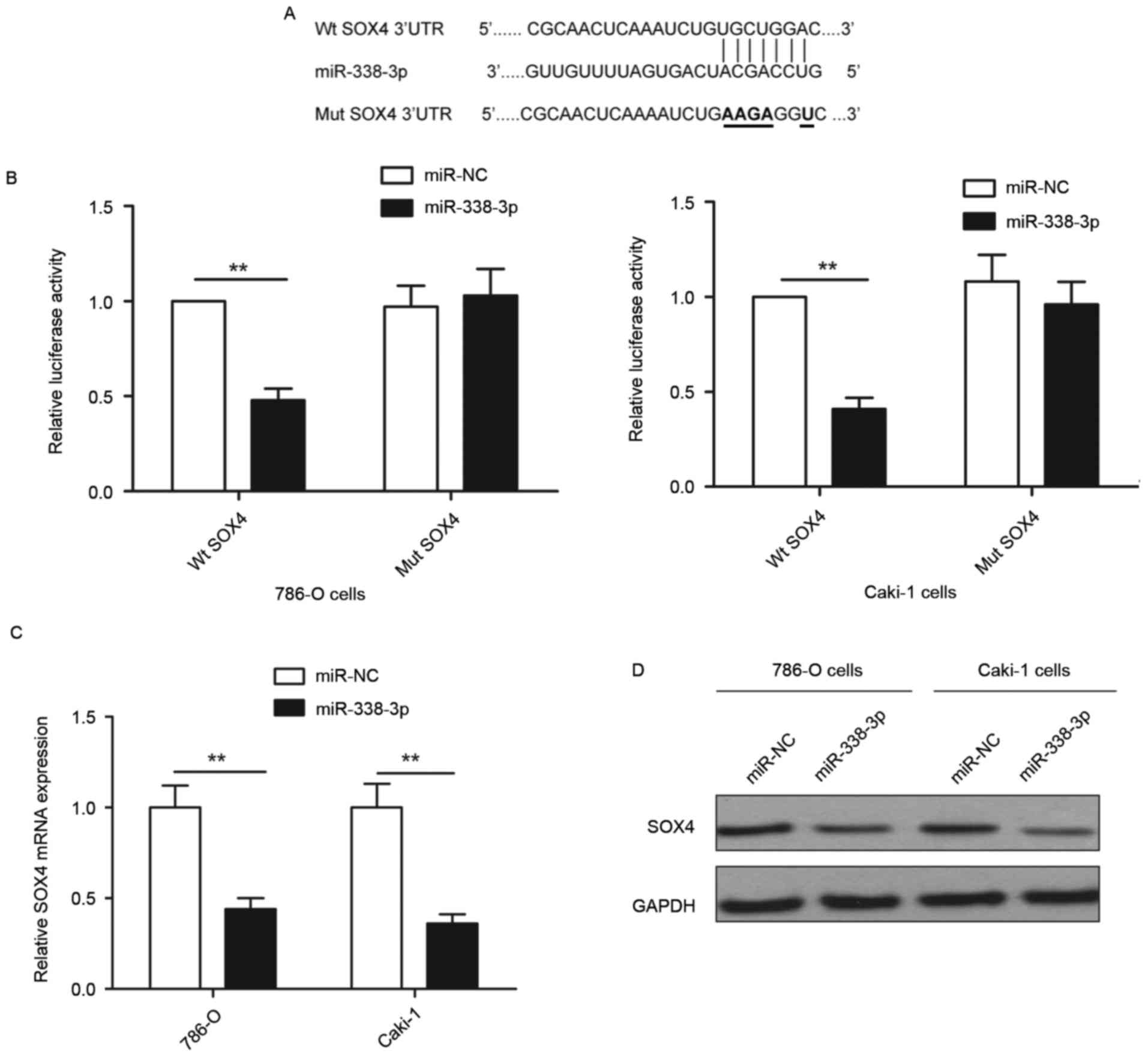
To investigate the molecular mechanisms by which
miR-338-3p inhibits RCC progression, bioinformatics database
(TargetScan, PicTar and miRanda) analyses were used to predict
putative miR-338-3p targets. SOX4 was selected as a direct target
of miR-338-3p based on putative target sequences at 1289–1295 bp of
SOX4 (Fig. 3A). To further confirm
targeting of SOX4 by miR-338-3p, a luciferase activity assay was
performed. As demonstrated in Fig.
3B, miR-338-3p overexpression significantly inhibited wild-type
SOX4 3′-UTR reporter activity compared with the cells transfected
with miR-NC (P<0.01), while had no inhibition effect on the
mutant SOX4 3′-UTR reporter activity. In addition, it was observed
that overexpression of miR-338-3p induced significant
downregulation of SOX4 expression at the mRNA level (P<0.01) and
marked downregulation of SOX4 at the protein level compared with
cells transfected with miR-NC (Fig. 3C
and D) in both 786-O and Caki-1 cells. These results implied
that miR-338-3p suppressed SOX4 expression by binding to the 3′-UTR
of SOX4 mRNA.
SOX4 is upregulated and inversely
correlated with miR-338-3p in RCC tissues
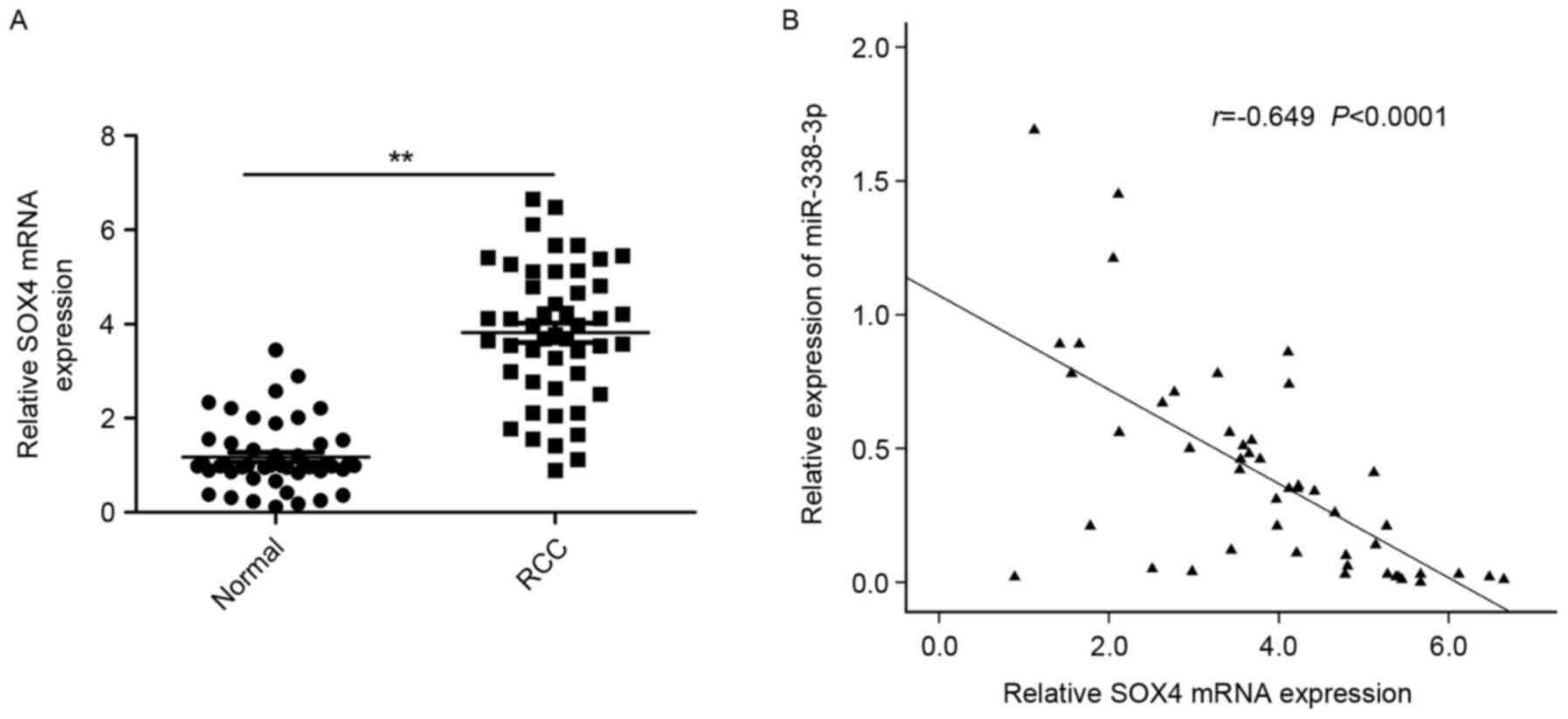
To further explore the relationship between
miR-338-3p and SOX4 in RCC, the expression of SOX4 at the mRNA
level in RCC tissues and adjacent normal tissues was examined by
RT-qPCR. It was demonstrated that the expression of SOX4 was
significantly increased in RCC tissues compared with adjacent
normal tissues (P<0.01; Fig. 4A).
A statistically significant inverse correlation was observed
between expression levels of miR-338-3p and SOX4 mRNA in RCC
tissues through Spearman's correlation analysis (r=−0649,
P<0.0001; Fig. 4B).
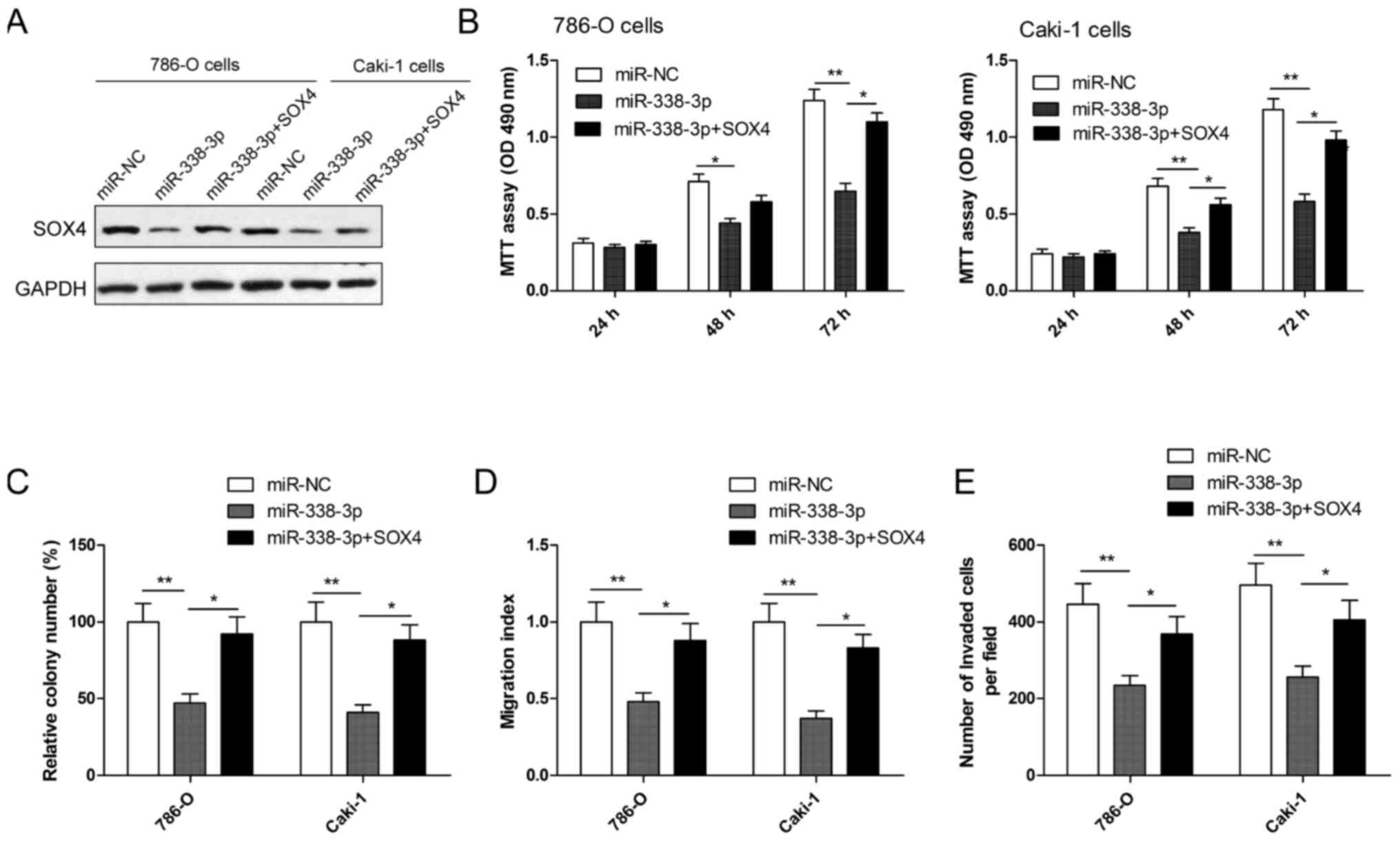
Overexpression of SOX4 reverses the
inhibition effect induced by miR-338-3p mimics in RCC cells
To evaluate whether SOX4 is responsible for the
functional effects of miR-338-3p in RCC cells, RCC cells were
co-transduced with miR-338-3p mimics or miR-NC and SOX4
overexpression plasmid. Following this, cell proliferation, colony
formation, migration and invasion assays were performed. Western
blotting demonstrated that RCC cells transfected with miR-338-3p
mimic had markedly decreased SOX4 expression, while cells
co-transfected with miR-338-3p mimics plus SOX4 plasmid restored
SOX4 protein expression (Fig. 5A).
In addition, it was also observed that SOX4 overexpression
significantly reversed the effect on cell proliferation, colony
formation, migration and invasion (all P<0.05) in RCC cells
induced by the miR-338-3p mimic (Fig.
5B-E).
Discussion
miR, as important regulators, have been documented
to have crucial roles in renal cancer development by regulating
cell proliferation, apoptosis and metastasis (9,10). Data
from the present study provided evidence that miR-338-3p is
deficient in RCC tissues and cell lines, and is associated with TNM
stage and lymph node metastasis. The in vitro experiments
demonstrated that restoration of miR-338-3p repressed cell
proliferation, colony formation, migration and invasion of RCC
cells. Regarding the underlying mechanisms, the present results
indicated that the direct targeting of SOX4 may contribute to the
functions of miR-338-3p in the above processes. Although
substantial study is required to further explore the underlying
mechanisms of miR-338-3p in growth and metastasis, the present
study has provided vital clues to characterize the key roles of
miR-338-3p in RCC and facilitate its application for future
treatment strategies for RCC.
miR-338-3p, located at chromosome 17q25.3, has been
reported to function as a tumor suppressor in many types of cancer,
such as hepatocellular carcinoma (11,12),
colorectal cancer (13), ovarian
cancer (14), gastric cancer
(15,16) and breast cancer (17). It has important roles in cell
proliferation, migration and invasion by targeting multiple genes,
such as smoothened, cyclin D1,
phosphatidylinositol-3,4,5-trisphosphate-dependent Rac exchange
factor 2a, SSX family member 2 interacting protein and zinc finger
E-box binding homeobox 2 (11–17,20,21).
However, the biological function of miR-338-3p and its related
molecular pathways involved in the progression of RCC have not been
fully elucidated. The present study demonstrated that the
expression miR-338-3p was significantly lower in RCC tissues and
cell lines than in the adjacent normal tissues and normal renal
cells. Cellular function of miR-338-3p in RCC demonstrated that
miR-338-3p overexpression inhibited cell proliferation, colony
formation, migration and invasion of RCC cells. These results
suggested that miR-338-3p may serve as tumor suppressor in RCC.
SOX4, a member of the SOX family of transcription
factors, has been demonstrated to be overexpressed in several types
of human cancer, including esophageal squamous cell carcinoma
(22), prostate cancer (23), colon cancer (24), breast cancer (25), hepatocellular carcinoma (26) and non-small cell lung cancer
(27). SOX4 also contributes to cell
survival and metastasis in many cancer types by regulating
epithelial-to-mesenchymal transition (23,28–30). It
is also well recognized that miR-338-3p overexpression leads to
downregulation of SOX4 in non-small cell lung cancer (31) and breast cancer (17); however, prior to the present study,
this had not been demonstrated in RCC. As expected, the present
results indicated that miR-338-3p was able to bind directly to the
3′-UTR of SOX4 mRNA and suppress SOX4 mRNA and protein expression
in RCC cells. It was also demonstrated that SOX4 expression was
upregulated, and inversely correlated with miR-338-3p expression in
RCC tissue specimens. Furthermore, SOX4 was responsible for
miR-338-3p-induced modulation of proliferation, colony formation,
migration and invasion of RCC cells. These results indicated that
miR-338-3p may exert biological functions in growth and metastasis
of RCC cells by targeting SOX4.
In summary, the present study provided more evidence
that miR-338-3p expression was downregulated in RCC tissues and
cell lines, was associated with TNM stage and lymph node
metastasis, and that miR-338-3p was able to inhibit cell
proliferation, colony formation, migration and invasion of RCC
cells by repressing SOX4. These findings suggested that miR-338-3p
may provide a novel therapeutic strategy for the treatment of
patients with advanced RCC.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pantuck AJ, Zisman A and Belldegrun AS:
The changing natural history of renal cell carcinoma. J Urol.
166:1611–1623. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chow WH, Dong LM and Devesa SS:
Epidemiology and risk factors for kidney cancer. Nat Rev Urol.
7:245–257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo H, Ingolia NT, Weissman JS and Bartel
DP: Mammalian microRNAs predominantly act to decrease target mRNA
levels. Nature. 466:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fabian MR, Sonenberg N and Filipowicz W:
Regulation of mRNA translation and stability by microRNAs. Annu Rev
Biochem. 79:351–379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Farazi TA, Spitzer JI, Morozov P and
Tuschl T: miRNAs in human cancer. J Pathol. 223:102–115. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garzon R and Marcucci G: Potential of
microRNAs for cancer diagnostics, prognostication and therapy. Curr
Opin Oncol. 24:655–659. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kurozumi A, Goto Y, Okato A, Ichikawa T
and Seki N: Aberrantly expressed microRNAs in bladder cancer and
renal cell carcinoma. J Hum Genet. 62:49–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gu L, Li H, Chen L, Ma X, Gao Y, Li X,
Zhang Y, Fan Y and Zhang X: MicroRNAs as prognostic molecular
signatures in renal cell carcinoma: A systematic review and
meta-analysis. Oncotarget. 6:32545–32560. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang XH, Chen JS, Wang Q, Chen XL, Wen L,
Chen LZ, Bi J, Zhang LJ, Su Q and Zeng WT: miR-338-3p suppresses
invasion of liver cancer cell by targeting smoothened. J Pathol.
225:463–472. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fu X, Tan D, Hou Z, Hu Z, Liu G, Ouyang Y
and Liu F: The effect of miR-338-3p on HBx deletion-mutant
(HBx-d382) mediated liver-cell proliferation through CyclinD1
regulation. PloS One. 7:e432042012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun K, Deng HJ, Lei ST, Dong JQ and Li GX:
miRNA-338-3p suppresses cell growth of human colorectal carcinoma
by targeting smoothened. World J Gastroenterol. 19:2197–2207. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wen C, Liu X, Ma H, Zhang W and Li H:
miR3383p suppresses tumor growth of ovarian epithelial carcinoma by
targeting Runx2. Int J Oncol. 46:2277–2285. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li P, Chen X, Su L, Li C, Zhi Q, Yu B,
Sheng H, Wang J, Feng R, Cai Q, et al: Epigenetic silencing of
miR-338-3p contributes to tumorigenicity in gastric cancer by
targeting SSX2IP. PloS One. 8:e667822013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo B, Liu L, Yao J, Ma R, Chang D, Li Z,
Song T and Huang C: miR-338-3p suppresses gastric cancer
progression through a PTEN-AKT axis by targeting P-REX2a. Mol
Cancer Res. 12:313–321. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jin Y, Zhao M, Xie Q, Zhang H, Wang Q and
Ma Q: MicroRNA-338-3p functions as tumor suppressor in breast
cancer by targeting SOX4. Int J Oncol. 47:1594–1602. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen X, Ruan A, Wang X, Han W, Wang R, Lou
N, Ruan H, Qiu B, Yang H and Zhang X: miR-129-3p, as a diagnostic
and prognostic biomarker for renal cell carcinoma, attenuates cell
migration and invasion via downregulating multiple
metastasis-related genes. J Cancer Res Clin Oncol. 140:1295–1304.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang G and Sun Y, He Y, Ji C, Hu B and Sun
Y: MicroRNA-338-3p inhibits cell proliferation in hepatocellular
carcinoma by target forkhead box P4 (FOXP4). Int J Clin Exp Pathol.
8:337–344. 2015.PubMed/NCBI
|
|
21
|
Huang N, Wu Z, Lin L, Zhou M, Wang L, Ma
H, Xia J, Bin J, Liao Y and Liao W: miR-338-3p inhibits
epithelial-mesenchymal transition in gastric cancer cells by
targeting ZEB2 and MACC1/Met/Akt signaling. Oncotarget.
6:15222–15234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han R, Huang S, Bao Y, Liu X, Peng X, Chen
Z, Wang Q, Wang J, Zhang Q, Wang T, et al: Upregulation of SOX4
antagonizes cellular senescence in esophageal squamous cell
carcinoma. Oncol Lett. 12:1367–1372. 2016.PubMed/NCBI
|
|
23
|
Wang L, Zhang J, Yang X, Chang YW, Qi M,
Zhou Z, Zhang J and Han B: SOX4 is associated with poor prognosis
in prostate cancer and promotes epithelial-mesenchymal transition
in vitro. Prostate Cancer Prostatic Dis. 16:301–307. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin CM, Fang CL, Hseu YC, Chen CL, Wang
JW, Hsu SL, Tu MD, Hung ST, Tai C, Uen YH and Lin KY: Clinical and
prognostic implications of transcription factor SOX4 in patients
with colon cancer. PloS One. 8:e671282013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song GD, Sun Y, Shen H and Li W: SOX4
overexpression is a novel biomarker of malignant status and poor
prognosis in breast cancer patients. Tumour Biol. 36:4167–4173.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hur W, Rhim H, Jung CK, Kim JD, Bae SH,
Jang JW, Yang JM, Oh ST, Kim DG, Wang HJ, et al: SOX4
overexpression regulates the p53-mediated apoptosis in
hepatocellular carcinoma: Clinical implication and functional
analysis in vitro. Carcinogenesis. 31:1298–1307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang D, Hao T, Pan Y, Qian X and Zhou D:
Increased expression of SOX4 is a biomarker for malignant status
and poor prognosis in patients with non-small cell lung cancer. Mol
Cell Biochem. 402:75–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu CC, Chen PN, Peng CY, Yu CH and Chou
MY: Suppression of miR-204 enables oral squamous cell carcinomas to
promote cancer stemness, EMT traits and lymph node metastasis.
Oncotarget. 7:20180–20192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shi S, Cao X, Gu M, You B, Shan Y and You
Y: Upregulated expression of SOX4 is associated with tumor growth
and metastasis in nasopharyngeal carcinoma. Dis Markers.
2015:6581412015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Parvani JG and Schiemann WP: Sox4, EMT
programs, and the metastatic progression of breast cancers:
Mastering the masters of EMT. Breast Cancer Res. 15:R722013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Y, Chen P, Zu L, Liu B, Wang M and Zhou
Q: MicroRNA-338-3p suppresses metastasis of lung cancer cells by
targeting the EMT regulator Sox4. Am J Cancer Res. 6:127–140.
2016.PubMed/NCBI
|