Introduction
As the most common cause of cancer-related
mortality, lung cancer is divided into small cell lung cancer
(SCLC) and non-small cell lung cancer (NSCLC) (1). It is estimated that 75–80% of patients
with primary lung cancer present with NSCLC (2,3). At
present, surgery is the most effective treatment method for NSCLC.
However, the postoperative prognosis remains poor and the 5-year
survival rate for NSCLC is reported to be <20% (4). Thus, it is necessary to develop novel
prevention and treatment strategies that effectively manage
NSCLC.
More recently, autophagy has become established as
an important cellular process involved in the development of NSCLC
(5,6). Autophagy primarily refers to
degradation of cytoplasmic content, superfluous or damaged
organelles and engulfment of pathogens (7–9). The
mechanism by which autophagy regulates cancer is complex and
principally depends on the tumor type and stage (10). Under homeostatic conditions,
autophagy generally suppresses the progression of cancer, and
abnormal activation of autophagy may lead to malignancy, as
observed for NSCLC (11–13). Therefore, insight into the regulation
of homeostasis may aid to identify effective therapeutic methods
that prevent the progression of NSCLC.
Curcumin is a natural polyphenol that is extracted
from the spice turmeric, and is characterized by anti-inflammatory,
anti-oxidative, anti-carcinogenic and immuno-regulatory activities
(14). In mice, it has been observed
that treatment with curcumin decreased Helicobacter pylori
infection and suppressed infection-induced gastric damage (15). In a clinical trial, an average
curcumin dose of 500 mg for 7 days markedly decreased the level of
serum lipid peroxide, as a biomarker of oxidative stress (16). Furthermore, an enhanced therapeutic
effect against cancer has been observed when curcumin was used
alone or in combination with chemotherapeutic agents (17,18).
However, to the best of our knowledge, no previous studies have
investigated the potential protective effects of curcumin against
the activation of autophagy in NSCLC. This was the focus of the
present study.
The present study explored the effects of curcumin
on the proliferation and migration of lung cancer cells. The
findings indicated that curcumin reduced cell growth and suppressed
colony formation capacity in human NSCLC cells. Furthermore,
abnormal activation of autophagy was inhibited following curcumin
treatment, indicating that curcumin may be a useful anticancer
agent for the treatment of NSCLC.
Materials and methods
Lung cancer cell lines and cell
culture
The human lung cancer cells lines, A549 and H1299
(American Type Culture Collection, Manassas, VA, USA) were cultured
in RPMI-1640 medium and Dulbecco's modified Eagle's medium (DMEM)
both supplemented with 10% fetal bovine serum (FBS) (all from
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
respectively. The cells were incubated under humidified conditions
at 37°C and 5% CO2.
Cell proliferation assay
Curcumin was purchased from Cayman Chemical Company
(Ann Arbor, MI, USA). To determine the effects of curcumin on cell
proliferation, A549 and H1299 cells were seeded into 96-well tissue
culture plates at a density of 5×103 cells/well. Cells
were administered with medium only (containing 0.01% dimethyl
sulfoxide as a negative control) or incubated with 0.5, 1, 5, 10
and 20 µM curcumin. Following incubation for 24, 48 and 72 h at
37°C, cell viability was determined with an
3-(4,5-dimethylthiazol-2-yl)-2,5-di-phenyltetrazolium bromide (MTT)
assay (Sigma-Aldrich; Merck KGaA; Darmstadt, Germany). Following
treatment, the cells were cultured in fresh media containing 0.5
mg/ml MTT for 4 h. Dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck
KGaA) was then added to the wells to dissolve the formazan products
and the absorbance was measured spectrophotometrically at a
wavelength of 550 nm. Three replicates were performed and
analyzed.
Cell apoptosis assay
Following 10 µM curcumin or DMSO control treatment
for 48 h, the cells were washed three times with cold
phosphate-buffered saline (PBS). An Annexin V-fluorescein
isothiocyanate (FITC)-propidium iodide (PI) Apoptosis kit
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to measure
cell apoptosis. Briefly, cells were washed three times with 1X PBS
and suspended at a density of 2–3×106 cells/ml in 1X
Annexin V-binding buffer (10 mM HEPES/NaOH, pH 7.4, 140 mM NaCl,
2.5 mM CaCl2). Annexin V-FITC and PI buffer was
administered to the cells, which were then incubated for 15 min at
room temperature in the dark. Cells lacking treatment with curcumin
were used as an internal control. Following incubation, the cells
were filtered with a 200-mesh filter screen and analyzed with a
FACScan flow cytometer (BD Biosciences, San Jose, CA, USA) within 1
h of staining. Cell apoptosis was analyzed using BD CellQuest Pro
software (BD Biosciences). A total of 10,000 cells were evaluated
in each sample.
Western blotting
Following 10 µM curcumin or DMSO control treatment
for 48 h, cell protein was extracted using radioimmunoprecipitation
assay buffer (Solarbio Science & Technology Co., Ltd., Beijing,
China) and was collected after centrifugation at 10,000 × g at 4°C
for 20 min. A bicinchoninic protein assay kit (Pierce; Thermo
Fisher Scientific, Inc.) was used to determine the protein
concentration. A total of 15 µg protein was loaded per lane and
separated by 10% SDS-PAGE and transferred to polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). The
membranes were blocked with 8% nonfat dry milk at 4°C overnight.
Following three washes with PBS with Tween-20 (5 min/wash), the
membranes were incubated with primary antibodies at 4°C overnight.
The blots were then incubated with horseradish peroxidase
(HRP)-conjugated anti-immunoglobulin G (all 1:5,000; Zhongshan Gold
Bridge Biological Technology Co., Beijing, China) for 2 h at room
temperature and then washed. Protien detection was performed with
enhanced chemiluminescent substrate (EMD Millipore). Primary
antibodies against microtubule-associated protein 1 light chain 3
II/I (LC3II/I; L8918, 1:1,000, Sigma-Aldrich; Merck KGaA), beclin-1
(cat no. 3495; 1:1,000), mechanistic target of rapamycin (mTOR; cat
no. 2983; 1:1,000), phosphorylated (p)-mTOR (cat no. 5536;
1:1,000), ribosomal protein S6 (cat no. 2217; 1:1,000), p-S6 (cat
no. 4858; 1:1,000), phosphoinositide 3-kinase (PI3K; cat no. 4249;
1:1,000), p-PI3K (cat no. 4228; 1:1,000), AKT (protein kinase B;
cat no. 9840; 1:1,000), p-AKT (cat no. 8200; 1:1,000) and β-actin
(cat no. 4970; 1:1,000) (all from Cell Signaling Technology, Inc.,
Boston, MA, USA). β-actin was used as an internal control. ImageJ
software (National Institutes of Health, Bethesda, MD, USA) was
used for density analysis.
Colony formation assay
Cells were suspended in 0.3% agar (Sigma-Aldrich;
Merck KGaA) in RPMI-1640 or DMEM with or without 10 µM curcumin
treatment for 48 h and plated at a density of 1×105
cells/dish into a 10-cm dish, which was preloaded with a thin layer
of 1.0% agar. Cells were maintained in RPMI-1640 or DMEM
supplemented with 10% FBS during the assay and monitored for colony
formation. After culturing for 7 days, the colony formation was
observed. The clones were stained with trypan blue (Sigma-Aldrich;
Merck KGaA) at room temperature for 15 min to evaluate colony
formation.
Electron microscopy
Following 10 µM curcumin or DMSO control treatment
for 48 h, cells were centrifuged at 800 × g for 10 min at room
temperature and the cell pellets were fixed at room temperature in
2.3% glutaraldehyde for 1 h, postfixed in 2% osmium tetroxide
(O5500; Sigma-Aldrich; Merck KGaA) at 4°C for 30 min and 0.5%
uranyl acetate (EMD Millipore) at room temperature for 15 min,
dehydrated and embedded in Spurr epoxy resin (Shanghai Huake Co.,
Ltd., Shanghai, China) at 4°C overnight. Ultrathin sections (90 nm)
were cut and double-stained with 3% uranyl acetate and lead citrate
(Solarbio, Science & Technology Co., Ltd.) at room temperature
for 20 min, and viewed with a Philips CM10 transmission electron
microscope (Phillips Electronics, Amsterdam, The Netherlands).
Statistical analysis
Data are presented as the mean ± standard deviation
for the indicated number of separate experiments. Three independent
experiments were performed for each study. Data were statistically
evaluated with an unpaired Student's t-test using GraphPad Prism
software (version 6.0; GraphPad Software, Inc., LaJolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
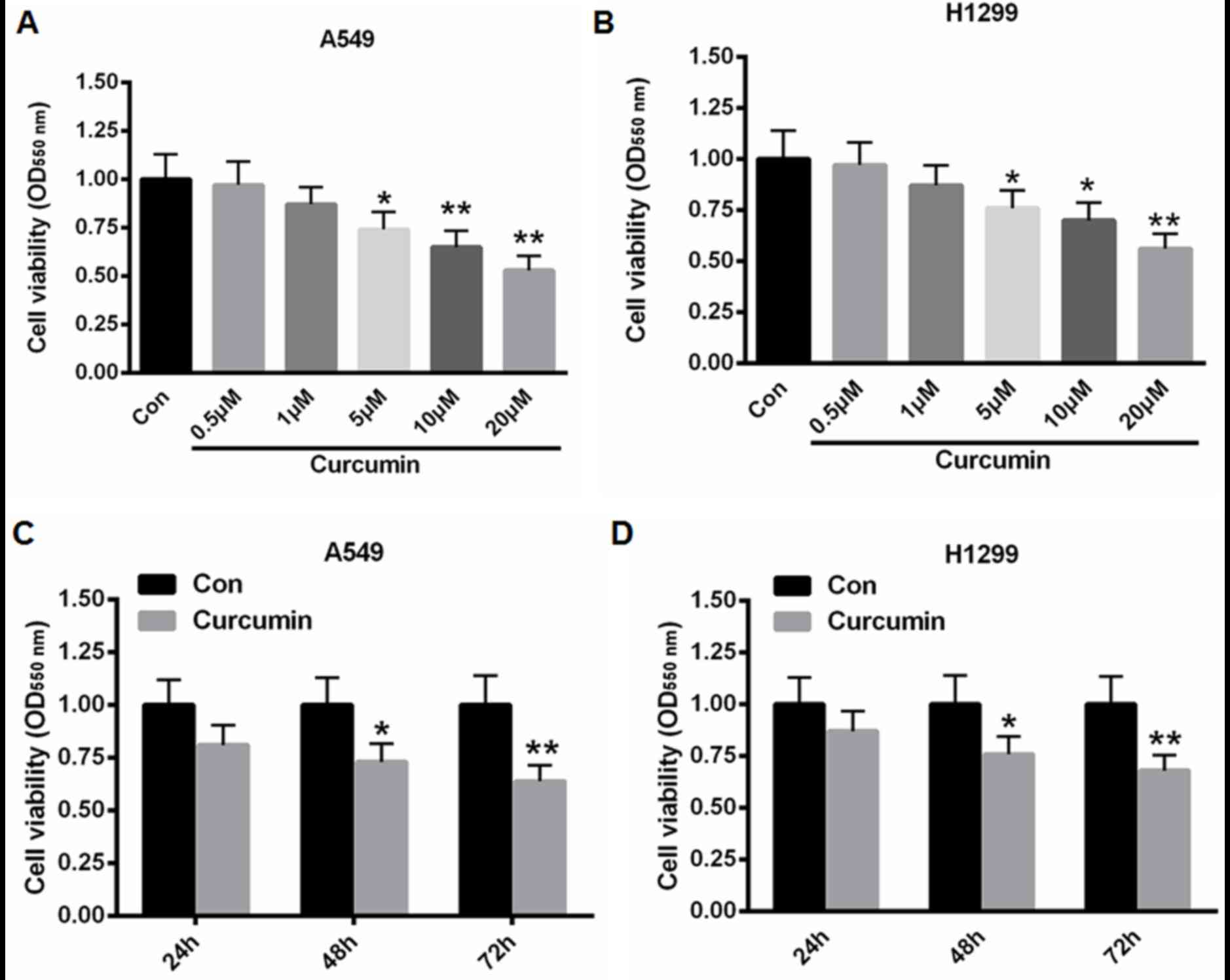
Curcumin decreases human lung cancer
cell viability in a time- and dose-dependent manner
A549 and H1299 cells were treated with 0.5, 1, 5, 10
and 20 µM curcumin for 48 h and cell viability was determined using
an MTT assay. As depicted in Fig. 1A and
B, treatment with 5, 10 and 20 µM curcumin significantly
suppressed cell proliferation when compared with control cells (for
A549 cells: P<0.05 for 5 µM curcumin and P<0.01 for 10 and 20
µM curcumin; for H1299 cells: P<0.05 for 5 and 10 µM curcumin
and P<0.01 for 20 µM curcumin). Furthermore, when A549 and H1299
cells were incubated with 10 µM curcumin for 24, 48, and 72 h, cell
proliferation was significantly reduced by 27 and 36% for A549
cells, and 24 and 32% for H1299 cells at 48 and 72 h, respectively
(Fig. 1C and D). These data
suggested that curcumin decreased the viability of A549 and H1299
cells in a time- and dose-dependent manner.
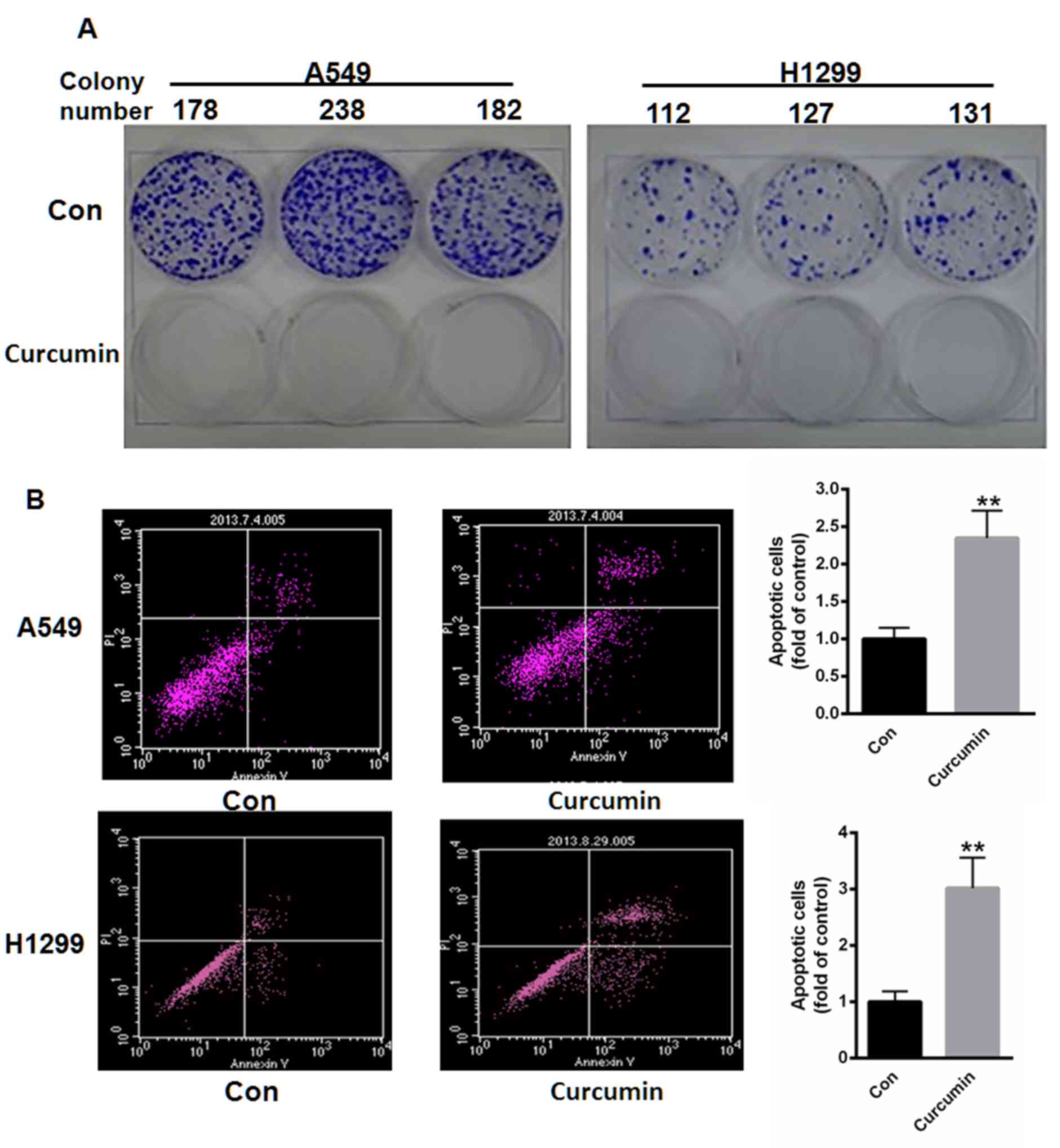
Curcumin inhibits colony formation and
promotes apoptosis
A549 and H1299 cells were subsequently treated with
10 µM curcumin for 48 h and the colony formation capacity of cells
was determined. As depicted in Fig.
2A, treatment with curcumin suppressed the colony formation
capacities of both A549 and H1299 cells. Cell apoptosis was also
measured using flow cytometry. Following incubation with 10 µM
curcumin for 48 h, cell apoptosis was significantly increased by
2.35- and 3.02-fold in A549 and H1299 cells, respectively, when
compared with controls (P<0.01; Fig.
2B).
Curcumin enhances autophagy in human
lung cancer cells
It was also determined whether curcumin induced cell
autophagy in lung cancer cells. Following treatment with 10 µM
curcumin for 48 h, western bolt analysis demonstrated that the
ratio of LC3-II to LC3-I and protein level of beclin-1 were
significantly increased in A549 and H1299 cells (P<0.05;
Fig. 3A). Electronic
microphotography was also used to assess the autophagosomes in each
group. As depicted in Fig. 3B,
curcumin markedly increased the number and volume of autophagosomes
in curcumin-treated cells when compared with controls, indicating
that curcumin may induce cell autophagy in human lung cancer
cells.
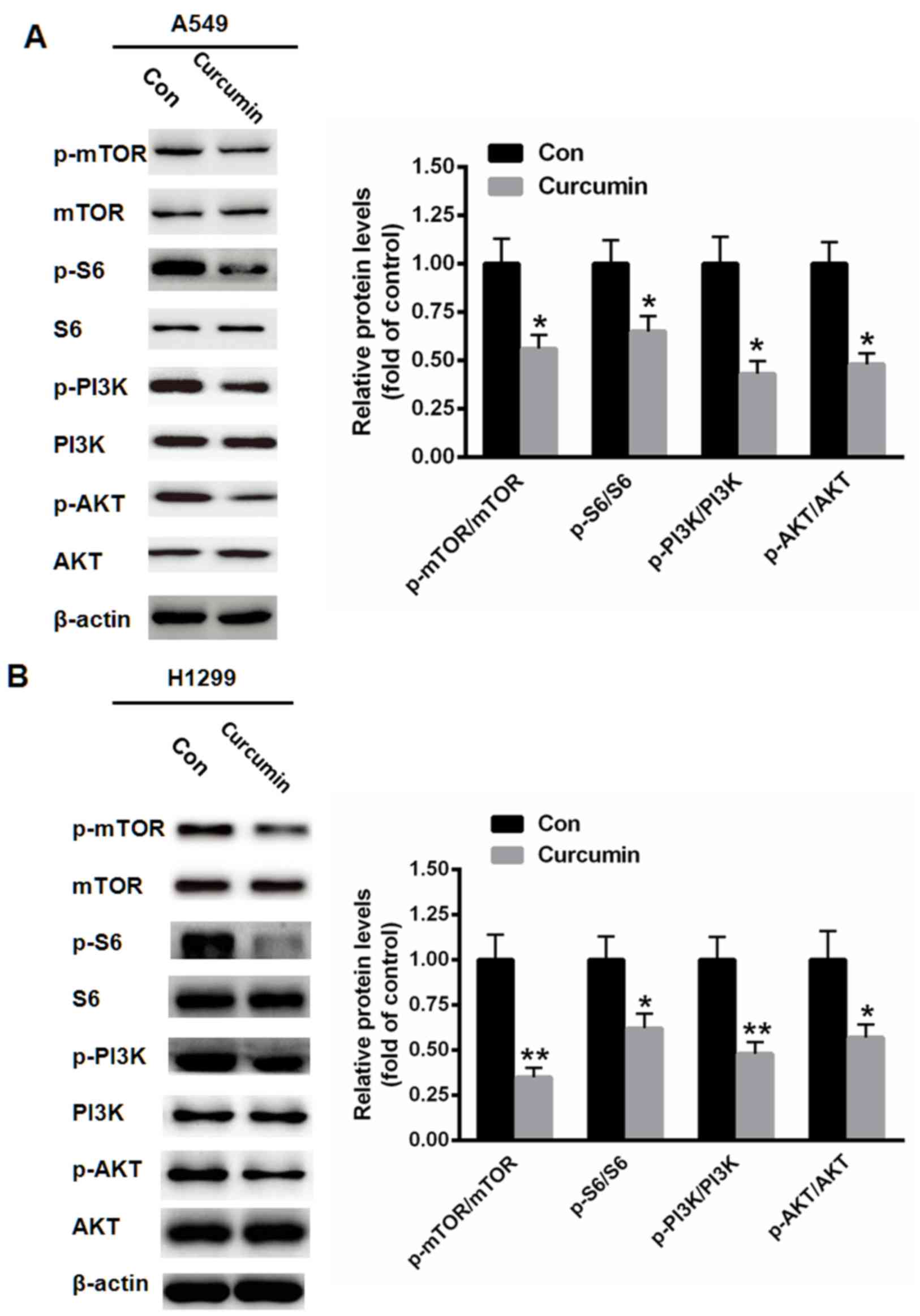
Curcumin suppresses PI3K/mTOR
activation
It has been documented that P13K/mTOR signaling
serves a key inhibitory role in the autophagy of various types of
human cancer (19). Therefore, the
activation of P13K/mTOR signaling following curcumin treatment was
evaluated. As depicted in Fig. 4,
treatment with 10 µM curcumin for 48 h markedly reduced the
phosphorylation levels of mTOR, S6, PI3K and AKT in A549 (all
P<0.05) and H1299 (P<0.05 for S6 and AKT, P<0.01 for mTOR
and PI3K) cells.
Discussion
Lung cancer has been reported as the most common
cause of cancer-related mortality (20). Therefore, detailed studies into the
oncological mechanisms of lung cancer are required for the
development of improved therapeutics. The majority of lung
carcinomas present as NSCLC (20).
In signaling pathways related to cell growth and proliferation, the
PI3K/AKT/mTOR pathway serves a key role (21,22).
Aberrant regulation of the PI3K/AKT/mTOR pathway has been
identified in various types of tumor, including prostate, breast,
lung and liver cancer (22,23). Recent studies have investigated the
potential of mTOR targeting as a molecular-targeting therapy for
the treatment of human cancers (24,25).
The present study focused on curcumin, as a natural
polyphenol isolated from the spice turmeric (26). Previous studies have demonstrated
that curcumin suppresses cytotoxicity and DNA injury by suppressing
oxidative damage caused by reactive oxygen species (27,28). The
current study observed that curcumin decreased the viability of
human lung cancer cells in an apparent dose- and time-dependent
manner. Furthermore, cell apoptosis was increased and colony
formation capacity was inhibited by curcumin treatment, as
determined by annexin V-PI staining and evaluation of colony
numbers, respectively. These results indicated that curcumin
reduced the viability of lung cancer cells by promoting cell
apoptosis and reducing colony formation capacity.
Autophagy is a complex process and is under fine
regulation, which depends upon the physiological and pathological
conditions of the cellular environment (29). As such, autophagy has become
established as a potential anti-cancer therapeutic (24). The process of autophagy may stimulate
the degradation of cytoplasmic content in the lysosomal compartment
of cells at a cellular level (30).
The maintenance of autophagy at a steady level is a possible
therapeutic target for anti-cancer therapy. In the process of
autophagy, the conversion of LC3-I into LC3-II form is regarded as
a hallmark of autophagy, and prompts the formation of the
autophagosome (29). In the present
study, it was observed that curcumin enhanced autophagy in lung
cancer cells, potentially by acting as an mTOR complex 1/2
inhibitor.
In conclusion, the present findings demonstrated
novel data that curcumin suppressed mTOR/PI3K/AKT signaling,
thereby inducing lung cancer cell apoptosis and autophagy. Thus,
curcumin is a potential therapeutic for the treatment of human
NSCLC. However, the present study lacks in vivo experiments
and further clinical investigation is required.
References
|
1
|
Clark PA: Puberty: When it comes too soon
guidelines for the evaluation of sexual precocity. J Ky Med Assoc.
96:440–447. 1998.PubMed/NCBI
|
|
2
|
Lv X, Liu F, Shang Y and Chen SZ: Honokiol
exhibits enhanced antitumor effects with chloroquine by inducing
cell death and inhibiting autophagy in human non-small cell lung
cancer cells. Oncol Rep. 34:1289–1300. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao R, Chen M, Jiang Z, Zhao F, Xi B,
Zhang X, Fu H and Zhou K: Platycodin-D induced autophagy in
non-small cell lung cancer cells via PI3K/Akt/mTOR and MAPK
signaling pathways. J Cancer. 6:623–631. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu W, Zhang H, Niu Y, Wu Y, Sun W, Li H,
Kong J, Ding K, Shen HM, Wu H, et al: Long non-coding RNA linc00673
regulated non-small cell lung cancer proliferation, migration,
invasion and epithelial mesenchymal transition by sponging
miR-150-5p. Mol Cancer. 16:1182017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Choi JY, Hong WG, Cho JH, Kim EM, Kim J,
Jung CH, Hwang SG, Um HD and Park JK: Podophyllotoxin acetate
triggers anticancer effects against non-small cell lung cancer
cells by promoting cell death via cell cycle arrest, ER stress and
autophagy. Int J Oncol. 47:1257–1265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giatromanolaki A, Kalamida D, Sivridis E,
Karagounis IV, Gatter KC, Harris AL and Koukourakis MI: Increased
expression of transcription factor EB (TFEB) is associated with
autophagy, migratory phenotype and poor prognosis in non-small cell
lung cancer. Lung Cancer. 90:98–105. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hwang KE, Kim YS, Jung JW, Kwon SJ, Park
DS, Cha BK, Oh SH, Yoon KH, Jeong ET and Kim HR: Inhibition of
autophagy potentiates pemetrexed and simvastatin-induced apoptotic
cell death in malignant mesothelioma and non-small cell lung cancer
cells. Oncotarget. 6:29482–29496. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Izdebska M, Klimaszewska-Wiśniewska A,
Hałas M, Gagat M and Grzanka A: Green tea extract induces
protective autophagy in A549 non-small lung cancer cell line.
Postepy Hig Med Dosw (Online). 69:1478–1484. 2015.PubMed/NCBI
|
|
9
|
Lee JG, Shin JH, Shim HS, Lee CY, Kim DJ,
Kim YS and Chung KY: Autophagy contributes to the chemo-resistance
of non-small cell lung cancer in hypoxic conditions. Respir Res.
16:1382015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu JT, Li WC, Gao S, Wang F, Li XQ, Yu
HQ, Fan LL, Wei W, Wang H and Sun GP: Autophagy inhibition
overcomes the antagonistic effect between gefitinib and cisplatin
in epidermal growth factor receptor mutant non-small-cell lung
cancer cells. Clin Lung Cancer. 16:e55–e66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei J, Ma Z, Li Y, Zhao B, Wang D and Jin
Y and Jin Y: miR-143 inhibits cell proliferation by targeting
autophagy-related 2B in non-small cell lung cancer H1299 cells. Mol
Med Rep. 11:571–576. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu JH, Yang HP, Zhou XD, Wang HJ, Gong L
and Tang CL: Autophagy accompanied with
bisdemethoxycurcumin-induced apoptosis in non-small cell lung
cancer cells. Biomed Environ Sci. 28:105–115. 2015.PubMed/NCBI
|
|
13
|
Zhang L, Dai F, Sheng PL, Chen ZQ, Xu QP
and Guo YQ: Resveratrol analogue 3,4,4′-trihydroxy-trans-stilbene
induces apoptosis and autophagy in human non-small-cell lung cancer
cells in vitro. Acta Pharmacol Sin. 36:1256–1265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sankar P, Telang AG, Ramya K, Vijayakaran
K, Kesavan M and Sarkar SN: Protective action of curcumin and
nano-curcumin against arsenic-induced genotoxicity in rats in vivo.
Mol Biol Rep. 41:7413–7422. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De R, Kundu P, Swarnakar S, Ramamurthy T,
Chowdhury A, Nair GB and Mukhopadhyay AK: Antimicrobial activity of
curcumin against Helicobacter pylori isolates from India and during
infections in mice. Antimicrob Agents Chemother. 53:1592–1597.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Soni KB and Kuttan R: Effect of oral
curcumin administration on serum peroxides and cholesterol levels
in human volunteers. Indian J Physiol Pharmacol. 36:273–275.
1992.PubMed/NCBI
|
|
17
|
Betts JW, Sharili AS, La Ragione RM and
Wareham DW: In vitro antibacterial activity of curcumin-polymyxin B
combinations against multidrug-resistant bacteria associated with
traumatic wound infections. J Nat Prod. 79:1702–1706. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu DJ, Huang YF, Chen XW, Luo ZT, Wang
GX, Liu CC, Zhang WJ and Ouyang MZ: Curcumin partly ameliorates
irinotecan-induced diarrhea and synergistically promotes apoptosis
in colorectal cancer through mediating oxidative stress.
Oncotarget. Jul 14–2016.(Epub ahead of print).
|
|
19
|
Bjelogrlić SK, Srdić T and Radulović S:
Mammalian target of rapamycin is a promising target for novel
therapeutic strategy against cancer. J BUON. 11:267–276.
2006.PubMed/NCBI
|
|
20
|
Micke P, Mattsson JS, Djureinovic D, Nodin
B, Jirström K, Tran L, Jönsson P, Planck M, Botling J and
Brunnström H: The impact of the fourth edition of the WHO
classification of lung tumours on histological classification of
resected pulmonary NSCCs. J Thorac Oncol. 11:862–872. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Laplante M and Sabatini DM: mTOR signaling
in growth control and disease. Cell. 149:274–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Efeyan A and Sabatini DM: mTOR and cancer:
Many loops in one pathway. Curr Opin Cell Biol. 22:169–176. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Salmena L, Carracedo A and Pandolfi PP:
Tenets of PTEN tumor suppression. Cell. 133:403–414. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Benjamin D, Colombi M, Moroni C and Hall
MN: Rapamycin passes the torch: A new generation of mTOR
inhibitors. Nat Rev Drug Discov. 10:868–880. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baehrecke EH: Autophagy: Dual roles in
life and death? Nat Rev Mol Cell Biol. 6:505–510. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shi J, Wang Y, Jia Z, Gao Y, Zhao C and
Yao Y: Curcumin inhibits bladder cancer progression via regulation
of b-catenin expression. Tumour Biol. 39:10104283177025482017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Granados-Castro LF, Rodríguez-Rangel DS,
Fernández-Rojas B, León-Contreras JC, Hernández-Pando R,
Medina-Campos ON, Eugenio-Pérez D, Pinzón E and Pedraza-Chaverri J:
Curcumin prevents paracetamol-induced liver mitochondrial
alterations. J Pharm Pharmacol. 68:245–256. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Trujillo J, Molina-Jijón E, Medina-Campos
ON, Rodríguez-Muñoz R, Reyes JL, Loredo ML, Barrera-Oviedo D,
Pinzón E, Rodríguez-Rangel DS and Pedraza-Chaverri J: Curcumin
prevents cisplatin-induced decrease in the tight and adherens
junctions: Relation to oxidative stress. Food Funct. 7:279–293.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kabeya Y, Mizushima N, Ueno T, Yamamoto A,
Kirisako T, Noda T, Kominami E, Ohsumi Y and Yoshimori T: LC3, a
mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang S, Shu L, Dilling MB, Easton J,
Harwood FC, Ichijo H and Houghton PJ: Sustained activation of the
JNK cascade and rapamycin-induced apoptosis are suppressed by
p53/p21(Cip1). Mol Cell. 11:1491–1501. 2003. View Article : Google Scholar : PubMed/NCBI
|