Introduction
Atherosclerosis is a complex chronic inflammatory
condition that involves an excessive inflammatory response and
lipid accumulation (1). Reduction of
low-density lipoprotein (LDL) levels by treatment with a statin is
an established method for the prevention of cardiovascular disease.
Statins are able to reduce mortality and morbidity by lowering
blood lipid levels and inhibiting the inflammatory response.
Previous studies have indicated that statin use is associated with
a reduction in the levels of serum high-sensitivity C-reactive
protein (hsCRP) that occurs independently of the reduction in
LDL-cholesterol (LDL-C) (2–4).
Ezetimibe is a cholesterol absorption inhibitor that
prevents the absorption of dietary and biliary cholesterol from the
small intestine. Previous studies indicate that when used in
combination with statin therapy, ezetimibe produces a further
23–24% reduction in LDL-C and 9–10% reduction in hsCRP compared
with statin therapy alone (5–8). The
Improved Reduction of Outcomes: Vytorin Efficacy International
Trial (IMPROVE-IT) demonstrated that a significantly greater
proportion of patients treated with ezetimibe plus simvastatin met
the specified targets of LDL-C <70 mg/dl and hsCRP <2 mg/l
when compared with simvastatin monotherapy (9). In that trial, achievement of these two
targets was associated with improved clinical outcomes following
multivariable adjustment, which may indicate a strategy in which
the lipoprotein and inflammatory profile are monitored (9).
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is
excreted predominantly from atherosclerotic plaques by macrophages
and neutrophils and subsequently circulates in the blood stream
(10). It has been demonstrated that
Lp-PLA2 is a potentially important pathogenic factor participating
in the progression of atherosclerosis (11). In an animal model of hyperlipidemia
and hyperglycemia, an Lp-PLA2 inhibitor reduced macrophage
accumulation, and diminished the necrotic lipid-core volume and
fibrous cap of coronary atherosclerotic plaques (12). Furthermore, the mass and activity of
Lp-PLA2 is indicated to be positively correlated with an increased
risk of coronary artery disease and stroke (13). Statins also have been shown to reduce
Lp-PLA2 levels by up to 33% (14,15). The
Long-term Intervention with Pravastatin in Ischemic Disease (LIPID)
study demonstrated that the reduction in Lp-PLA2 activity during
the first year of treatment was a highly significant predictor of
coronary heart disease events, independent of any change in LDL-C,
and may account for over half of the benefits of pravastatin
(16).
Taking previous studies into consideration, the
present study sought to investigate: i) Whether the addition of
ezetimibe to rosuvastatin would further reduce LDL-C and hsCRP as
compared with rosuvastatin monotherapy; ii) whether the addition of
ezetimibe to rosuvastatin would further reduce Lp-PLA2 in
comparison with rosuvastatin alone and iii) if so, whether the
reduction of Lp-PLA2 differed for treatment with ezetimibe and
rosuvastatin vs. rosuvastatin alone across prespecified subgroups;
iv) the possible alterations of Lp-PLA2 and hsCRP levels during the
development of acute myocardial infarction (AMI) and their
relationship with LDL-C and other lipid parameters.
Materials and methods
Study population and procedures
All the subjects in the study were inpatients at the
Department of Cardiology, Nanjing First Hospital Affiliated with
Nanjing Medical University (Nanjing, China) from January 2015 to
June 2016. Inclusion criteria were that patients aged within the
range of 18 to 80 years were eligible if hospitalized within the
preceding 24 h for AMI, including ST-segment elevation myocardial
infarction (STEMI) with or without ST-segment elevation myocardial
infarction (NSTEMI). STEMI was defined as an AMI with dynamic
changes in the electrocardiogram and at least one instance of
elevated levels of cardiac enzymes or myocardial necrosis
biomarkers, defined as total creatine phosphokinase or creatine
kinase major basic fraction >2-fold the upper limit of the
normal range and/or positive troponin I or troponin T. The
exclusion criteria were: i) Contraindications for the intervention;
ii) statin use was contraindicated, for example, due to the patient
having active hepatitis or being allergic to statins; iii) severe
cardiac dysfunction (Killip class III or IV); iv) severe renal
insufficiency; and v) other comorbidities, including infection,
systemic immune diseases, pericarditis and malicious tumor.
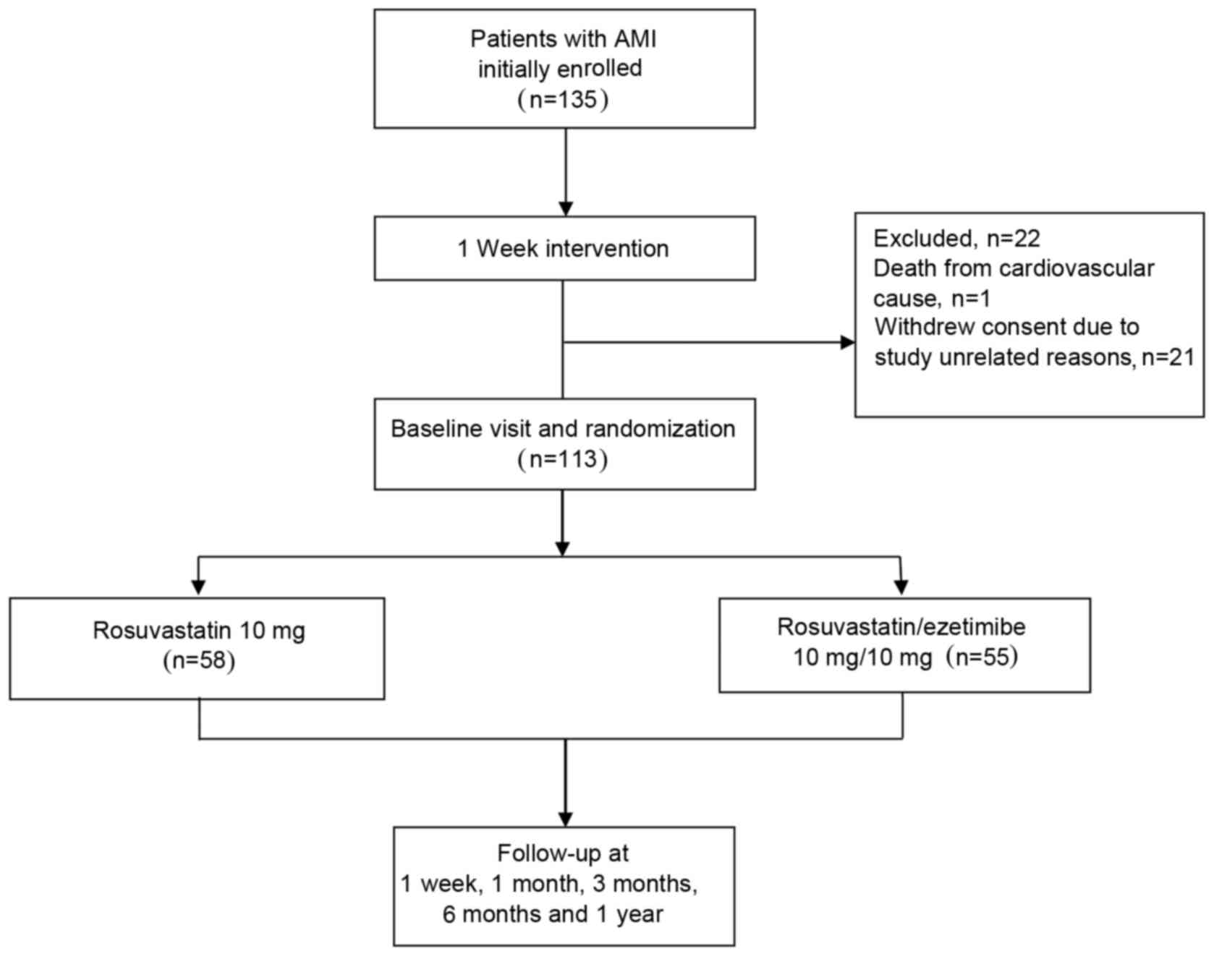
Initially, 135 patients (age: 62.7±3.8 years; male:
female, 109:26) were enrolled (Fig.
1). Following 1 week of the intervention, 113 patients (age:
59.0±2.0 years; male: female, 94:19) continued to meet the
inclusion criteria and were randomly divided into two groups:
Ezetimibe (10 mg; Merck KGaA, Darmstadt, Germany) plus rosuvastatin
(10 mg; IPR Pharmaceuticals, Inc., Canovanas, PR, USA) group
(combination group; n=5) and rosuvastatin (10 mg) group (n=58).
Randomization was performed by means of a computer-generated
sequence of random numbers. Participants were instructed to take
tablets once daily in the evening and to pay attention to the side
effects of rosuvastatin and ezetimibe. The patients’ age, sex,
medical history, smoking history, echocardiography parameters,
blood lipid levels, Lp-PLA2 and hsCRP were recorded at admission
within the first 24 h after the onset of AMI. All patients received
treatment according to common guidelines, including appropriate use
of antiplatelet agents, anticoagulants, statins, β-blockers and
revascularization. The therapies administered were identical in the
two groups (Fig. 1). This study was
approved by the Ethics Committee of Nanjing First Hospital. All
subjects provided written informed consent.
Clinical data collection
Visits to hospital took place at baseline, and at 1,
3 and 12 months after drug treatment commenced. Blood samples were
obtained at each visit and echocardiography reexamination was
arranged at 12 months. Serum levels of total cholesterol (TC),
triglycerides (TG), high-density lipoprotein cholesterol (HDL-C),
LDL-C, hsCRP and Lp-PLA2 were measured using standard enzymatic
methods in the hospital laboratory as previously described
(17).
Statistical analysis
All data were analyzed using SPSS 24.0 software (IBM
Corp., Armonk, NY, USA). Numerical data are expressed as n (%).
Measurement data are expressed as the mean ± standard deviation.
Means of the two groups were compared using an independent sample
t-test. Means in a group prior to and following treatment were
compared using a paired t-test. Pearson correlation coefficients
were used to investigate the correlation of changes in plasma
biomarkers and lipids from baseline to 12 months (bivariate
correlation analysis). The results were presented using PRISM
version 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05
(two-tailed) was considered to indicate a statistically significant
result.
Results
Demographic characteristics
Initially, 135 patients were enrolled (Fig. 1). Following 1 week of intervention,
21 patients withdrew consent and were excluded from the study. A
total of 113 patients continued to participate and were randomized
to receive either 10 mg rosuvastatin (n=58) or the combination of
10 mg rosuvastatin plus 10 mg ezetimibe (n =55). All randomized
subjects completed the study. Baseline characteristics (Table I), lipid and biomarkers (Table II) were generally consistent in the
two treatment groups and were not significantly different.
 | Table I.Baseline clinical characteristics. |
Table I.
Baseline clinical characteristics.
| Characteristic | R10 (n=58) | R10+E10 (n=55) | P-value |
|---|
| Males, n (%) | 46 (79.3) | 48 (87.3) | 0.258 |
| Age, years | 60.7±1.3 | 57.3±1.5 | 0.123 |
| Current smoker, n
(%) | 38 (65.5) | 39 (70.9) | 0.539 |
| Hypertension, n
(%) | 35 (60.3) | 31 (56.4) | 0.668 |
| Diabetes mellitus, n
(%) | 10 (17.2) | 10 (18.2) | 0.896 |
| Dyslipidemia, n
(%) | 22 (37.9) | 20 (36.4) | 0.863 |
| Previous MI, n
(%) | 1 (1.7) | 2 (3.6) | 0.527 |
| Previous PCI, n
(%) | 3 (5.3) | 5 (9.1) | 0.432 |
| Previous medication,
n (%) |
|
|
|
|
Statins | 6 (10.5) | 5 (9.1) | 0.799 |
|
ASA | 10 (17.5) | 12 (21.8) | 0.569 |
|
Thienopyridine | 3 (5.3) | 5 (9.1) | 0.432 |
|
CCB | 11 (19.3) | 5 (9.1) | 0.123 |
|
Diuretics | 3 (5.3) | 4 (7.3) | 0.660 |
|
ACEI/ARB | 4 (7.0) | 9 (16.4) | 0.123 |
 | Table II.Parameters at baseline and during the
follow-up period. |
Table II.
Parameters at baseline and during the
follow-up period.
|
| Baseline | 1 week | 1 month | 3 months | 6 months | 1 year |
|---|
|
|
|
|
|
|
|
|
|---|
| Parameter | R10
(n=58)a | R10+E10 (n=55) | R10 (n=58) | R10+E10 (n=55) | R10 (n=57) | R10+E10 (n=54) | R10
(n=55)a | R10+E10 (n=53) | R10
(n=54)a | R10+E10 (n=51) | R10
(n=53)a | R10+E10 (n=50) |
|---|
| TC (mmol/l) |
4.28±1.27 |
4.55±1.05 |
3.50±1.02b |
3.26±0.82b |
3.12±0.61b |
2.95±0.82b |
3.09±0.64b |
2.89±0.75b |
3.07±0.60b |
2.87±0.82b |
3.03±0.64b |
2.82±1.00b |
| TG (mmol/l) |
1.77±1.04 |
1.92±1.14 |
1.61±0.54b |
1.60±0.91b |
1.45±0.66b |
1.55±0.74b |
1.51±0.66b |
1.47±0.73b |
1.43±0.73b |
1.30±0.73b |
1.39±1.08b |
1.08±0.54b |
| LDL-C (mmol/l) |
2.93±1.02 |
3.00±0.96 |
2.38±0.73b |
2.14±0.75b |
1.76±0.78b |
1.48±0.70b |
1.60±0.51b |
1.35±0.61b |
1.52±0.42b |
1.24±0.48b |
1.49±0.51b |
1.19±0.43b |
| HDL-C (mmol/l) |
1.06±0.23 |
1.04±0.26 |
0.94±0.19 |
0.97±0.25 |
1.08±0.25 |
1.08±0.29 |
1.13±0.31 |
1.18±0.34 |
1.17±0.35 |
1.27±0.46 |
1.28±0.43 |
1.46±0.55 |
| hsCRP (mg/l) |
4.33±3.47 |
5.15±3.85 |
6.05±4.64 |
6.45±6.41 |
3.02±3.73b |
2.57±3.22b |
1.67±2.01b |
1.33±1.14b |
1.55±1.12b |
1.33±1.37b |
1.49±1.97b |
0.68±0.35b |
| Lp-PLa2
(ng/ml) |
327.95± |
333.13± |
393.46± |
413.92± |
196.18± |
139.64± |
187.68± |
121.69± |
132.56± |
93.88± |
123.62± |
79.07± |
|
| 106.79 | 110.29 | 92.75 | 78.66 | 96.84b | 93.09b | 48.47b | 82.80b | 37.66b | 78.19b | 51.60b | 68.34b |
| LVDd (mm) |
50.07±5.82 |
50.37±5.16 | – | – | – | – | – | – | – | – |
49.82±3.50 |
49.91±5.77 |
| LVEF (%) |
52.41±9.86 |
55.72±8.67 | – | – | – | – | – | – | – | – |
57.05±6.62 |
61.89±5.55 |
Effect on lipids
All lipid parameters were obtained at 1 week, and 1,
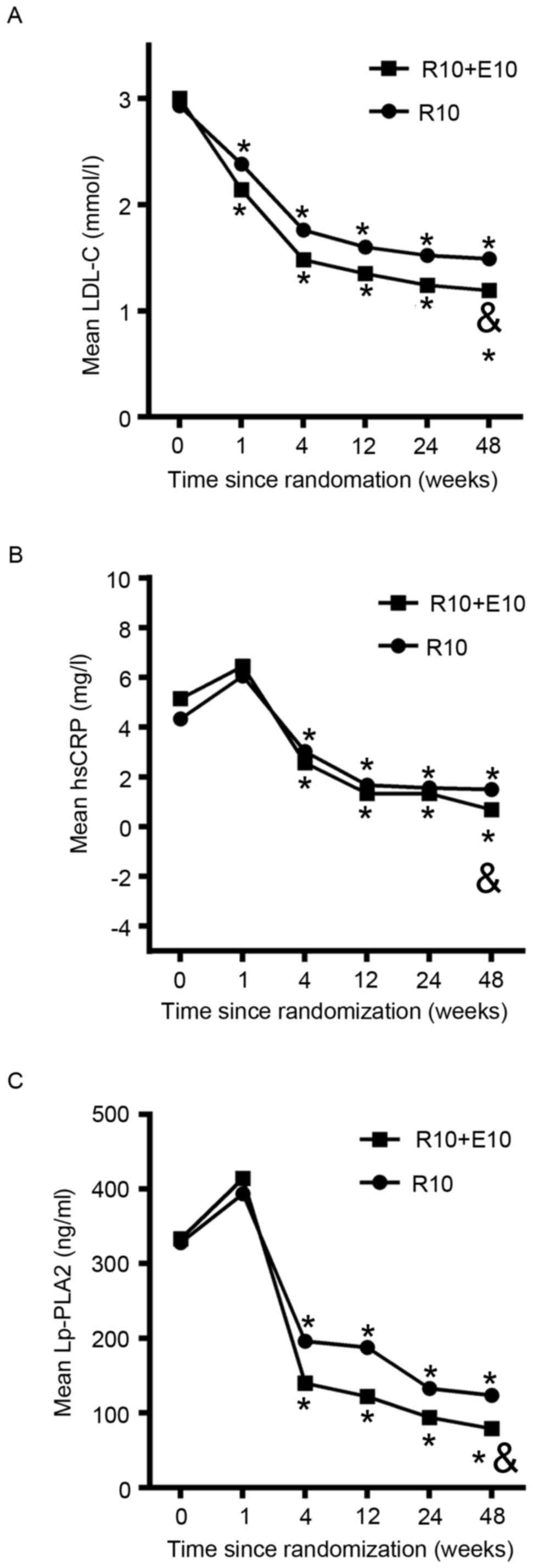
3, 6 and 12 month, after randomization (Table II and Fig. 2) and changes from baseline were
evaluated (Table III and Fig. 3). Compared with baseline, significant
reductions were obtained in the two groups for TC, LDL-C and TG
over the duration of the study (Table
II). During the study duration, marked increases in HDL-C from
baseline were also observed in the two groups, although there was a
slight reduction at 1 week (Table
II). During the 12 months of observation, LDL-C significantly
decreased from 3.00 to 1.19 mmol/l in the combination group and
from 2.93 to 1.49 mmol/l in the rosuvastatin group. The addition of
ezetimibe to rosuvastatin resulted in further reductions in mean
LDL-C over the study duration in comparison with rosuvastatin alone
(Fig. 2A). Net changes from baseline
in TC and LDL-C were significantly greater in the combination group
compared with the rosuvastatin group; however, no significant
difference in the change of TG levels was observed between the two
groups (Table III; Fig. 3A).
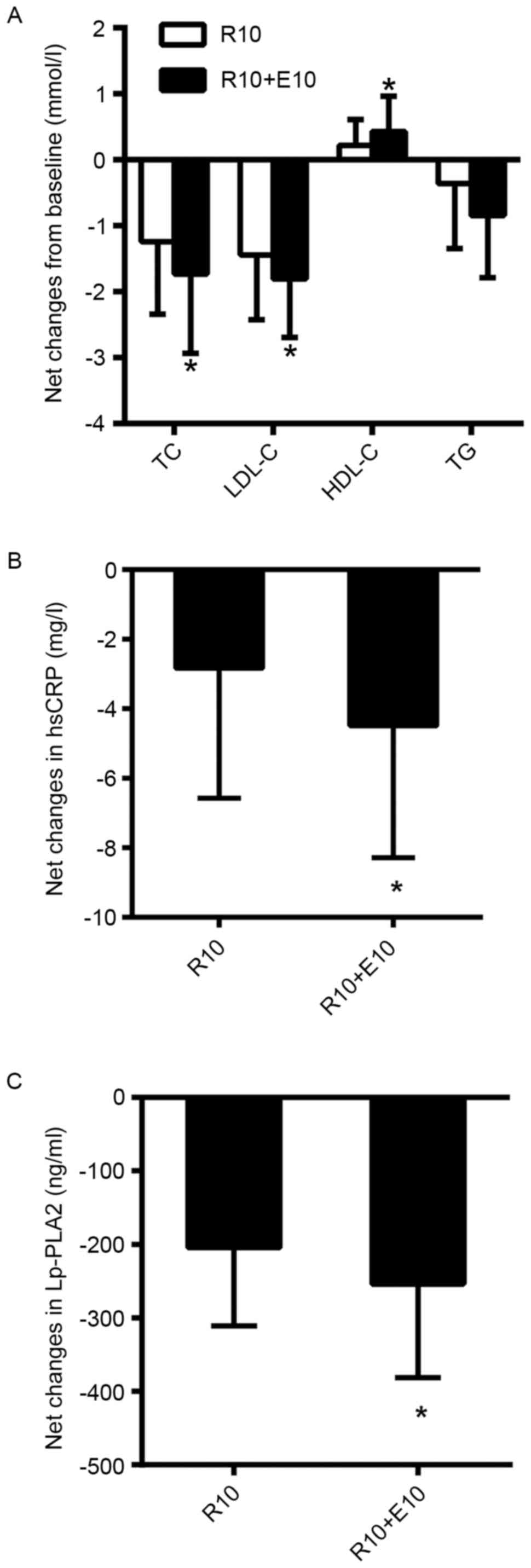 | Figure 3.Net changes from baseline to 12 months
in (A) TC, LDL-C, HDL-C and TG, (B) hsCRP and (C) Lp-PLA2 following
randomized treatment. *P<0.05 vs. R10. TC, total cholesterol;
LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density
lipoprotein cholesterol; TG, triglycerides; hsCRP, high-sensitivity
C-reactive protein; Lp-PLA2, lipoprotein-associated phospholipase
A2; R10, 10 mg rosuvastatin; R10+E10, 10 mg rosuvastatin plus 10 mg
ezetimibe. Numerical values and standard deviations for the results
in these plots are shown in Table
III. *P<0.05 vs. R10 group. |
 | Table III.Changes of assessed parameters from
baseline to follow-up period. |
Table III.
Changes of assessed parameters from
baseline to follow-up period.
|
| D1 | D2 | D3 | D4 | D5 |
|---|
|
|
|
|
|
|
|
|---|
| Parameter | R10
(n=58)a | R10+E10
(n=55)a | P-value | R10+E10
(n=57)a | R10
(n=54)a | P-value | R10
(n=55)a | R10+E10
(n=53)a | P-value | R10
(n=54)a | R10+E10
(n=51)a | P-value | R10
(n=53)a | R10+E10
(n=50)a | P-value |
|---|
| TC (mmol/l) |
−0.78±0.85b |
−1.29±0.92b | 0.003 |
−1.16±1.07b |
−1.60±1.22b | 0.044 |
−1.19±1.14b |
−1.67±1.10b | 0.019 |
−1.21±0.98b |
−1.68±1.09b | 0.019 |
−1.24±1.10b |
−1.73±1.20b | 0.042 |
| TG (mmol/l) |
−0.15±0.66 |
−0.32±0.86c | 0.375 |
−0.31±
1.07c |
−0.37±0.92c | 0.773 |
−0.25±1.00 |
−0.46±0.91c | 0.381 |
−0.33±0.89c |
−0.62±1.08d | 0.229 |
−0.36±0.99c |
−0.85±0.94b | 0.720 |
| LDL-C (mmol/l) |
−0.55±0.57b |
−0.86±0.74b | 0.015 |
−1.18±0.67b |
−1.53±0.99b | 0.028 |
−1.34±0.81b |
−1.65±0.87b | 0.048 |
−1.41±0.83b |
−1.76±0.89b | 0.033 |
−1.44±0.98b |
−1.81±0.88b | 0.045 |
| HDL-C (mmol/l) |
−0.12±0.20b |
−0.07±0.13b | 0.108 |
0.02±0.23 |
0.04±0.20 | 0.526 |
0.07±0.26c |
0.14±0.26b | 0.145 |
0.11±0.36c |
0.23±0.48b | 0.130 |
0.22±0.39b |
0.43±0.53b | 0.027 |
| hsCRP (mg/l) |
1.72±4.55c |
1.29±7.75 | 0.880 |
−1.31±4.65 |
−3.29±4.28b | 0.161 |
−2.66±3.28b |
−3.81±3.88b | 0.908 |
−2.79±3.09b |
−3.82±3.54b | 0.218 |
−2.84±3.73b |
−4.49±3.79b | 0.010 |
| Lp-PLA2
(ng/ml) |
65.51±140.71c |
80.79±131.70d | 0.683 |
−131.76±158.30b |
−193.49±152.36b | 0.424 |
−140.27±97.29b |
−211.44±134.62b | 0.046 |
−195.39±96.38b |
−239.25±97.07b | 0.072 |
−204.33±106.75b |
−254.06±127.29b | 0.043 |
| LVDd (mm) | – | – | – | – | – | – | – | – | – | – | – | – |
−0.25±4.98 |
−0.46±4.87 | 0.616 |
| LVEF (%) | – | – | – | – | – | – | – | – | – | – | – | – |
4.64±8.55 |
6.17±7.48d | 0.882 |
Effect on inflammatory markers
Inflammatory markers, hsCRP and Lp-PLA2, were
evaluated at 1 week, and 1, 3, 6 and 12 months, after
randomization. Compared with baseline, significant reductions were
obtained in the two groups for hsCRP and Lp-PLA2 over the study
duration (Table II). The values of
hsCRP and Lp-PLA2 increased substantially 1 week after
randomization, and then dropped steeply within the next 3 weeks,
after which the values remained stable (Fig. 2B and C). During the 12 months of
observation, hsCRP decreased significantly from 5.15 to 0.68 mg/l
in the combination group and from 4.33 to 1.49 mg/l in the
rosuvastatin alone group (P=0.01; Table
II). The addition of ezetimibe to rosuvastatin resulted in a
further reduction in mean hsCRP over the study duration in
comparison with rosuvastatin alone (Fig.
2B). Similarly, during the 12 months of observation, Lp-PLA2
decreased significantly from 333.13 to 79.07 mg/l in the
combination group and from 327.95 to 123.62 mg/l in the
rosuvastatin alone group (P=0.04; Table
II). The addition of ezetimibe to rosuvastatin resulted in a
further reduction in mean Lp-PLA2 over the study duration in
comparison with rosuvastatin monotherapy (Fig. 2C). Net changes from baseline in hsCRP
and Lp-PLA2 were significantly greater in the combination group
compared with the rosuvastatin alone group (Table III; Fig.
3B and C). Treatment effects for each of the treatments were
similar for net changes from baseline to 12 months in Lp-PLA2
across prespecified subgroup categories of age, gender, smoking and
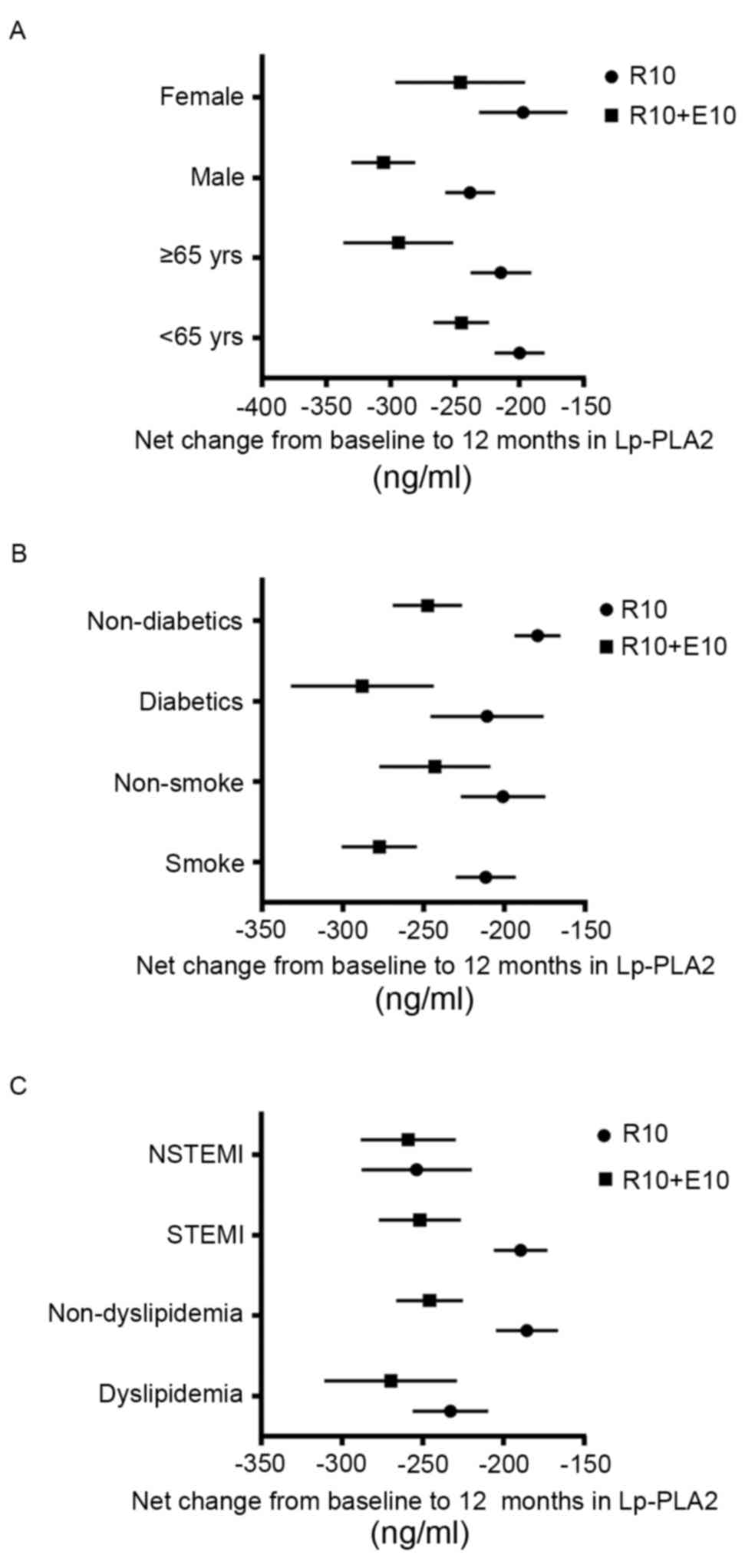
diabetic status (Fig. 4).
Correlation between lipids and
inflammatory markers
Table IV shows
correlations between lipids and inflammatory markers. Although
LDL-C and Lp-PLA2 levels decreased from baseline, the correlation
in achieved net changes from baseline to 12 months for LDL-C and
Lp-PLA2 values was weak in all subjects (r=0.367; P=0.002)
and not significant for the combination treatment and the
rosuvastatin monotherapy respectively (data not shown).
Furthermore, Lp-PLA2 did not correlate with hsCRP in all subjects
combined (r=0.264; P=0.512), nor in the combination treatment or
rosuvastatin monotherapy groups respectively (date not shown). In
addition, no significant correlation was identified between Lp-PLA2
and total cholesterol or HDL-C. Similarly, no significant
correlation was observed between hsCRP and lipid values. Even
though Lp-PLA2 had positive correlations with LDL-C, the
correlations were weak.
 | Table IV.Correlations between net changes from
baseline to 12 months in inflammatory markers and lipids. |
Table IV.
Correlations between net changes from
baseline to 12 months in inflammatory markers and lipids.
|
| Change in
hsCRP | Change in
Lp-PLA2 |
|---|
|
|
|
|
|---|
| Variables | r | P-value | r | P-value |
|---|
| Change in
Lp-PLA2 | 0.264 | 0.512 | – | – |
| Change in
hsCRP | – | – | 0.264 | 0.512 |
| Change in TC | −0.173 | 0.115 | 0.204 | 0.500 |
| Change in TG | −0.090 | 0.571 | 0.243 | 0.790 |
| Change in
LDL-C | −0.082 | 0.473 | 0.367 | 0.002 |
| Change in
HDL-C | −0.061 | 0.579 | −0.150 | 0.150 |
Change of cardiac function
As shown in Tables
II and III, left ventricular
end diastolic dimension (LVDd) and left ventricular ejection
fraction (LVEF) were measured at baseline and 12 months after
randomization. Although there was greater reduction in LVDd and
further increase in LVEF with the addition of ezetimibe to
rosuvastatin in comparison with rosuvastatin alone, the differences
were not found to be significant (P=0.616 and P=0.882,
respectively).
Discussion
In the present study, the results suggested that the
combined treatment led to a greater reduction of LDL-C, as well as
significantly reduced levels of hsCRP and Lp-PLA2 compared with
rosuvastatin monotherapy. Moreover, it was found that the reduction
of Lp-PLA2 had a positive correlation with the change of LDL-C,
although the correlation was weak. In addition, no significant
correlation was detected between changes in Lp-PLA2 and hsCRP.
Although the addition of ezetimibe to the treatment resulted in a
greater reduction of Lp-PLA2, the increases did not achieve
statistical significance in prespecified subgroups. To clarify the
alterations of Lp-PLA2 and hsCRP levels during the development of
AMI, blood samples were obtained from the patients and assessed
within the first 24 h of admission and patients had follow-up
visits at 1 week, and 1, 3, 6, and 12 months thereafter. It was
observed that the values of hsCRP and Lp-PLA2 appeared to increase
at week 1 after randomization, then dropped steeply to a lower
level and remain stable after that. The findings of the present
study are novel in that they extend previous observations with
statin therapy to non-statin-based therapeutic augmentation with
ezetimibe, an agent with no observed safety concerns (18). In the analysis conducted in the
present study, reductions in Lp-PLA2 were observed to be
significantly greater with combination therapy compared with
monotherapy. These results are consistent with previous studies
that demonstrated the degree of Lp-PLA2 reduction with combination
therapy was consistent with the extent of LDL-C lowering efficacy
(19–22). Furthermore, it has been verified that
the addition of ezetimibe to rosuvastatin or simvastatin provides
additional reductions in Lp-PLA2 mass and activity in patients
already receiving statin monotherapy (20). It was observed in the present study
that the addition of ezetimibe to rosuvastatin further reduced
LDL-C and hsCRP levels, as compared with the levels achieved using
rosuvastatin monotherapy. These findings are consisted with the
IMPROVE-IT trial, and extend the superiority of combination therapy
with ezetimibe from simvastatin to rosuvastatin (9).
The mechanisms by which statins and ezetimibe
influence Lp-PLA2 levels are not well defined. However, simvastatin
has been shown to reduce Lp-PLA2 expression and activity in
lipopolysaccharide (LPS)-stimulated human myocyte-derived
macrophages through inhibition of the mevalonate-geranylgeranyl
pyrophosphate-RhoA-p38 mitogen-activated protein kinase pathway
(23). Ezetimibe, rosuvastatin and
fenofibrate monotherapy have been shown to primarily reduce the
activity and mass of Lp-PLA2, which are associated with LDL
lipoprotein subfractions via receptor-mediated uptake (24). Furthermore, it has also been reported
that non-statin lipid-lowering therapies reduce Lp-PLA2 through a
receptor-independent clearance mechanism and that Lp-PLA2 changes
are weakly correlated with LDL-C changes, indicating that Lp-PLA2
reduction is only partly explained by LDL-C lowering (25). Likewise, in the present study, the
results indicated that reductions in Lp-PLA2 with statins or
combination therapy were proportionate to the extent of LDL-C
lowering, along with weak correlations between reductions in
Lp-PLA2 and changes of LDL-C.
The data regarding the correlation of Lp-PLA2
changes and hsCRP changes with statin or non-statin therapy
obtained in the present study are in line with those in other
reports. Ostadal et al reported that no association was
observed between the alterations in the levels of Lp-PLA2 and
C-reactive protein (CRP; r=0.06, P=0.70) (26). Another study also reported a lack of
correlation between Lp-PLA2 and CRP (27). In the Pravastatin Inflammation/CRP
Study (PRINCE) study, a weak, but statistically significant
correlation between changes in Lp-PLA2 levels and CRP change
(r=−0.13, P=0.05) was observed (15). While the reason for these results is
unclear, data concerning markers of inflammation and Lp-PLA2 levels
are conflicting. Some data suggest that pro-inflammatory-mediators,
such as LPS, tumor necrosis factor (TNF)-α and interleukin (IL)-1,
−6 and −8, result in the downregulation of Lp-PLA2 by macrophages
(28). However, the opposite
observation was noted when TNF-α and IL-1 were administered to
experimental animals (29).
Although the addition of ezetimibe to rosuvastatin
resulted in a greater reduction of Lp-PLA2, the difference between
the monotherapy and combination treatment did not achieve
statistical significance in prespecified subgroups. Consistent with
these results, a previous study reported that correlations between
non-smokers and smokers and Lp-PLA2 levels were small and did not
significantly affect the change in Lp-PLA2 when evaluated using
multivariate analyses (15).
However, as only 113 patients were examined in the present study,
the results may have been due to chance. The results require
confirmation in a larger study. However, these results possibly
indicate that a high baseline Lp-PLA2 level may not be associated
with a further reduction of Lp-PLA2 following treatment.
It was observed that the values of hsCRP and Lp-PLA2
increased at 1 week after randomization, and then dropped steeply
to a lower level and remain stable thereafter. These results are
consistent with those of previous studies, which have found that
CRP exhibited a more marked increase during the first 24–96 h from
symptom onset and markedly decreased over 30 days (30,31).
Notably, the transient elevation of CRP levels might indicate a
propensity for a pronounced inflammatory response and is associated
with increased mortality (32,33). In
contrast with the results of the present study, serum levels of
Lp-PLA2 have been reported to decrease significantly following
acute vascular events (median, 5 days; range 2–40 days), such as
stroke and myocardial infarction (34). In the present study, baseline values
of Lp-PLA2 were obtained and assessed within the first 24 h after
the onset of symptoms. Therefore, it is possible that the baseline
levels of Lp-PLA2 were obtained during the reduction phase
following an acute event, and the high level of Lp-PLA2 observed at
1 week was a return toward the usual baseline levels that existed
prior to the acute coronary syndrome.
The present study has several limitations, mostly
attributable to the limited size of the study population. It may
also be argued that the inclusion of the two types of AMI (ST
elevation and non-ST elevation) may have increased the
heterogeneity of the study group. As only 113 patients were
enrolled in the study, the results may have been due to chance.
Therefore, the results require confirmation in a larger study.
Also, the study subjects were patients with AMI, so it is not known
whether the results are applicable to other populations. Further
studies with additional populations are needed.
In conclusion, the preset study confirmed that the
addition of ezetimibe to rosuvastatin further reduced LDL-C and
hsCRP, and also resulted in further reductions in Lp-PLA2, as
compared with rosuvastatin monotherapy. The alterations in the
levels of Lp-PLA2 were weakly but significantly correlated with LDL
changes, which indicated that Lp-PLA2 reduction is only partly
explained by LDL-C lowering. These findings provide evidence for
the debate concerning the value of adjunctive LDL-C reduction in
combination with a statin. In addition, reductions of Lp-PLA2
levels may be considered as target for the suppression of
inflammation.
References
|
1
|
Ross R: The pathogenesis of
atherosclerosis: A perspective for the 1990s. Nature. 362:801–809.
1993. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Lemos JA, Blazing MA, Wiviott SD, Lewis
EF, Fox KA, White HD, Rouleau JL, Pedersen TR, Gardner LH,
Mukherjee R, et al: Early intensive vs a delayed conservative
simvastatin strategy in patients with acute coronary syndromes:
Phase Z of the A to Z trial. JAMA. 292:1307–1316. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ridker PM, Cannon CP, Morrow D, Rifai N,
Rose LM, McCabe CH, Pfeffer MA and Braunwald E: Pravastatin or
Atorvastatin Evaluation and Infection Therapy-Thrombolysis in
Myocardial Infarction 22 (PROVE IT-TIMI 22) Investigators:
C-reactive protein levels and outcomes after statin therapy. N Engl
J Med. 352:20–28. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ridker PM, Danielson E, Fonseca FA, Genest
J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ,
MacFadyen JG, et al: Rosuvastatin to prevent vascular events in men
and women with elevated C-reactive protein. N Engl J Med.
359:2195–2207. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ballantyne CM, Blazing MA, King TR, Brady
WE and Palmisano J: Efficacy and safety of ezetimibe
co-administered with simvastatin compared with atorvastatin in
adults with hypercholesterolemia. Am J Cardiol. 93:1487–1494. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morrone D, Weintraub WS, Toth PP, Hanson
ME, Lowe RS, Lin J, Shah AK and Tershakovec AM: Lipid-altering
efficacy of ezetimibe plus statin and statin monotherapy and
identification of factors associated with treatment response: A
pooled analysis of over 21,000 subjects from 27 clinical trials.
Atherosclerosis. 223:251–261. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pearson TA, Ballantyne CM, Veltri E, Shah
A, Bird S, Lin J, Rosenberg E and Tershakovec AM: Pooled analyses
of effects on C-reactive protein and low density lipoprotein
cholesterol in placebo-controlled trials of ezetimibe monotherapy
or ezetimibe added to baseline statin therapy. Am J Cardiol.
103:369–374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pearson T, Ballantyne C, Sisk C, Shah A,
Veltri E and Maccubbin D: Comparison of effects of
ezetimibe/simvastatin versus simvastatin versus atorvastatin in
reducing C-reactive protein and low-density lipoprotein cholesterol
levels. Am J Cardiol. 99:1706–1713. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bohula EA, Giugliano RP, Cannon CP, Zhou
J, Murphy SA, White JA, Tershakovec AM, Blazing MA and Braunwald E:
Achievement of dual low-density lipoprotein cholesterol and
high-sensitivity C-reactive protein targets more frequent with the
addition of ezetimibe to simvastatin and associated with better
outcomes in IMPROVE-IT. Circulation. 132:1224–1233. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Macphee CH, Nelson J and Zalewski A: Role
of lipoprotein-associated phospholipase A2 in atherosclerosis and
its potential as a therapeutic target. Curr Opin Pharmacol.
6:154–161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ragab SM, Safan MA, Obeid OM and Sherief
AS: Lipoprotein-associated phospholipase A2 (Lp-PLA2) and tumor
necrosis factor-alpha (TNF-α) and their relation to premature
atherosclerosis in β-thalassemia children. Hematology. 20:228–238.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wilensky RL, Shi Y, Mohler ER III,
Hamamdzic D, Burgert ME, Li J, Postle A, Fenning RS, Bollinger JG,
Hoffman BE, et al: Inhibition of lipoprotein-associated
phospholipase A2 reduces complex coronary atherosclerotic plaque
development. Nat Med. 14:1059–1066. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lp-PLA(2) Studies Collaboration; Thompson
A, Gao P, Orfei L, Watson S, Di Angelantonio E, Kaptoge S,
Ballantyne C, Cannon CP, Criqui M, et al: Lipoprotein-associated
phospholipase A(2) and risk of coronary disease, stroke, and
mortality: Collaborative analysis of 32 prospective studies.
Lancet. 375:1536–1544. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
O'Donoghue M, Morrow DA, Sabatine MS,
Murphy SA, McCabe CH, Cannon CP and Braunwald E:
Lipoprotein-associated phospholipase A2 and its association with
cardiovascular outcomes in patients with acute coronary syndromes
in the PROVE IT-TIMI 22 (PRavastatin Or atorVastatin evaluation and
infection therapy-thrombolysis in myocardial infarction) trial.
Circulation. 113:1745–1752. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Albert MA, Glynn RJ, Wolfert RL and Ridker
PM: The effect of statin therapy on lipoprotein associated
phospholipase A2 levels. Atherosclerosis. 182:193–198. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
White HD, Simes J, Stewart RA, Blankenberg
S, Barnes EH, Marschner IC, Thompson P, West M, Zeller T, Colquhoun
DM, et al: Changes in lipoprotein-associated phospholipase A2
activity predict coronary events and partly account for the
treatment effect of pravastatin: Results from the long-term
intervention with pravastatin in ischemic disease study. J Am Heart
Assoc. 2:e0003602013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aydin MU, Aygul N, Altunkeser BB, Unlu A
and Taner A: Comparative effects of high-dose atorvastatin versus
moderate-dose rosuvastatin on lipid parameters, oxidized-LDL and
inflammatory markers in ST elevation myocardial infarction.
Atherosclerosis. 239:439–443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cannon CP, Blazing MA, Giugliano RP,
McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO,
Jukema JW, et al: Ezetimibe added to statin therapy after acute
coronary syndromes. N Engl J Med. 372:2387–2397. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Davidson MH, Ballantyne CM, Jacobson TA,
Bittner VA, Braun LT, Brown AS, Brown WV, Cromwell WC, Goldberg RB,
McKenney JM, et al: Clinical utility of inflammatory markers and
advanced lipoprotein testing: Advice from an expert panel of lipid
specialists. J Clin Lipidol. 5:338–367. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ballantyne CM, Hoogeveen RC, Raya JL, Cain
VA, Palmer MK and Karlson BW: GRAVITY Study Investigators:
Efficacy, safety and effect on biomarkers related to cholesterol
and lipoprotein metabolism of rosuvastatin 10 or 20 mg plus
ezetimibe 10 mg vs. simvastatin 40 or 80 mg plus ezetimibe 10 mg in
high-risk patients: Results of the GRAVITY randomized study.
Atherosclerosis. 232:86–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moutzouri E, Liberopoulos EN, Tellis CC,
Milionis HJ, Tselepis AD and Elisaf MS: Comparison of the effect of
simvastatin versus simvastatin/ezetimibe versus rosuvastatin on
markers of inflammation and oxidative stress in subjects with
hypercholesterolemia. Atherosclerosis. 231:8–14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Le NA, Tomassini JE, Tershakovec AM, Neff
DR and Wilson PW: Effect of switching from statin monotherapy to
ezetimibe/simvastatin combination therapy compared with other
intensified lipid-lowering strategies on lipoprotein subclasses in
diabetic patients with symptomatic cardiovascular disease. J Am
Heart Assoc. 4:e0016752015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song JX, Ren JY and Chen H: Simvastatin
reduces lipoprotein-associated phospholipase A2 in
lipopolysaccharide-stimulated human monocyte-derived macrophages
through inhibition of the mevalonate-geranylgeranyl
pyrophosphate-RhoA-p38 mitogen-activated protein kinase pathway. J
Cardiovasc Pharmacol. 57:213–222. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saougos VG, Tambaki AP, Kalogirou M,
Kostapanos M, Gazi IF, Wolfert RL, Elisaf M and Tselepis AD:
Differential effect of hypolipidemic drugs on
lipoprotein-associated phospholipase A2. Arterioscler Thromb Vasc
Biol. 27:2236–2243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang B, Fan P, Shimoji E, Itabe H, Miura
S, Uehara Y, Matsunaga A and Saku K: Modulating effects of
cholesterol feeding and simvastatin treatment on
platelet-activating factor acetylhydrolase activity and
lysophosphatidylcholine concentration. Atherosclerosis.
186:291–301. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ostadal P, Vondrakova D, Kruger A, Janotka
M, Psotova H and Prucha M: Alteration in lipoprotein-associated
phospholipase A2 levels during acute coronary syndrome and its
relationship to standard biomarkers. Lipids Health Dis. 11:1532012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li X, Kramer MC, Van der Loos CM, Koch KT,
de Boer OJ, Henriques JP, Baan J Jr, Vis MM, Piek JJ, Tijssen JG,
et al: A pattern of disperse plaque microcalcifications identifies
a subset of plaques with high inflammatory burden in patients with
acute myocardial infarction. Atherosclerosis. 218:83–89. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kawano Y, Narahara H and Johnston JM:
Inhibitory effect of interleukin-8 on the secretion of
platelet-activating factor acetylhydrolase by human decidual
macrophages. J Soc Gynecol Investig. 6:328–332. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Narahara H and Johnston JM: Effects of
endotoxins and cytokines on the secretion of platelet-activating
factor-acetylhydrolase by human decidual macrophages. Am J Obstet
Gynecol. 169:531–537. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin ZP, Shu PC, Liao ZJ, Wang XQ and Liu
Q: Rosuvastatin improves myocardial function and arteriosclerosis
plaque in patients with ST-segment elevation after acute myocardial
infarction and percutaneous coronary intervention. Nan Fang Yi Ke
Da Xue Xue Bao. 31:1789–1791. 2011.(In Chinese). PubMed/NCBI
|
|
31
|
Askevold ET, Gullestad L, Nymo S, Kjekshus
J, Yndestad A, Latini R, Cleland JG, McMurray JJ, Aukrust P and
Ueland T: Secreted frizzled related protein 3 in chronic heart
failure: Analysis from the controlled rosuvastatin multinational
trial in heart failure (CORONA). PLoS One. 10:e01339702015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
James SK, Oldgren J, Lindbäck J, Johnston
N, Siegbahn A and Wallentin L: An acute inflammatory reaction
induced by myocardial damage is superimposed on a chronic
inflammation in unstable coronary artery disease. Am Heart J.
149:619–626. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oldgren J, Wallentin L, Grip L, Linder R,
Nørgaard BL and Siegbahn A: Myocardial damage, inflammation and
thrombin inhibition in unstable coronary artery disease. Eur Heart
J. 24:86–93. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Elkind MS, Leon V, Moon YP, Paik MC and
Sacco RL: High-sensitivity C-reactive protein and
lipoprotein-associated phospholipase A2 stability before and after
stroke and myocardial infarction. Stroke. 40:3233–3237. 2009.
View Article : Google Scholar : PubMed/NCBI
|