Introduction
Leptin, almost exclusively synthesized and secreted
by white adipocytes, is a protein hormone encoded by the obesity
(OB) gene. Its main function is to target the hypothalamic arcuate
nucleus, resulting in the suppression of appetite and increase of
energy consumption so as to regulate the energy balance (1).
Leptin receptors are widely expressed in
cardiovascular tissues, including endothelial cells (ECs) (2). Therefore, leptin also targets arterial
ECs, and is involved in the occurrence and development of
hypertension (3), which it may
achieve through causing EC dysfunction (4) and inflammation (5) as well as increasing the expression of
endothelin-1 (6). In addition,
leptin enhances endothelial-dependent vasorelaxation by
upregulating the expression of neuronal nitric oxide synthase
(nNOS) (7) and endothelium-derived
hyperpolarizing factor (EDHF) (8).
These opposite roles of leptin make it necessary to further study
the expression of factors by ECs in association with hypertension
regulated by leptin.
Cyclooxygenases (COXs) are rate-limiting enzymes,
which catalyze free arachidonic acid and synthetize prostaglandin
(PG)H2, which is further converted to prostacyclin
(PGI2) and thromboxane (TX)A2. Among the
COXs, COX-2 is an inducible enzyme whose expression is low in ECs
under physiological conditions. The expression of COX-2 increases
during pathological conditions of inflammation, such as
hypertension, a lower-grade inflammatory disease. The downstream
products of COX-2, PGI2 and TXA2, affect
vasomotion and platelet aggregation (9). However, the expression of COX-2 and its
downstream products involved in hypertension induced by leptin has
remained to be clarified.
The purpose of the present study was to investigate
the expression of COX-2 and its downstream products PGI2
and TXA2 (represented by 6-keto PGF1α and
TXB2, respectively) induced by leptin in vitro,
so as to further understand the mechanisms of the involvement of
leptin in hypertension, or to obtain information on the influence
of leptin on systolic and/or diastolic function of blood vessels.
The findings partially explained the mechanisms by which leptin
mediates hypertension and put forward precautions from the aspect
of medication use in hypertension.
Materials and methods
Experimental animals
A total of 8 male Wistar rats were purchased from
the Experimental Animal Center of Sunyat-sen University (Guangzhou,
China). All rats were between 4 and 5 weeks of age and fed normal
chow in specific pathogen-free facilities. All animal protocols
were approved by the Institutional Animal Care and Use Committee of
the First Affiliated Hospital of Guangzhou Medical University
(Guangzhou, China).
Isolation and culture of rat aortic
endothelial cells (RAECs)
Wistar rats were anesthetized by an intraperitoneal
injection of pentobarbital (300 mg/100 g; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) resulting in euthanasia. Aortic
separation was performed as described previously (10). RAECs were isolated from aortic strips
by the method of mixed enzyme digestion [0.1% type II collagenase
(4 ml) and 0.1% trypsin (4 ml; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) digested for 20 min separately]. Cells were
grown in Dulbecco's modified Eagle's medium containing 10% fetal
bovine serum (both from Gibco; Thermo Fisher Scientific, Inc.), 1%
penicillin/streptomycin and 100 µg/ml endothelial cell growth
supplement (Sigma-Aldrich; Merck KGaA) and passaged as described
previously (10).
Characterization of RAECs by
immunofluorescence analysis
When the cells reached 80% confluence, the medium
was drained, the cells were washed twice with PBS, fixed with 4%
methanol for 30 min, permeabilized with 0.1% Triton X-100 for 15
min and washed three times with PBS successively. After incubation
with 1% bovine serum albumin (BSA) (Beyotime Institute of
Biotechnology, Haimen, China) for 1 h. All above procedures were
performed at room temperature. The cells were incubated overnight
at 4°C with rabbit anti-rat factor VIII antibody (1:150 dilution;
65707; Cell Signaling Technologies, Inc., Danvers, MA, USA),
followed by incubation with fluorescein isothiocyanate-labeled goat
anti-rabbit immunoglobulin G antibody (A0562; 1:100 dilution;
Beyotime Institute of Biotechnology) for 2 h at room temperature.
Finally, the cells were washed three times with PBS and stained
with DAPI (Sigma-Aldrich; Merck KGaA). Cells were observed and
images were captured under an inverted fluorescence microscope.
Leptin treatment of RAECs
RAECs at passage 3 were cultured in a 6-well plate
at 5×105 cells/well in complete culture medium for 12 h.
Leptin (P50596; R&D Systems, Inc., Minneapolis, MN, USA) in a
stock solution in PBS was added to the complete culture medium to
achieve final concentrations of 0, 10−10,
10−9 or 10−8 M, followed by incubation for 36
or 48 h. Each experimental condition was set up in triplicate.
Reverse-transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was obtained from RAECs by lysis with
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) for 10 min and
extraction with chloroform, isopropanol and 75% ethanol.
Complementary DNA was synthesized by using a PrimeScript RT Master
mix kit (RR064A; Takara Bio Inc., Otsu, Japan). The primers used
for PCR were as follows: Rat COX-2 forward,
5′-GCTTAAAGACCGCATCGAGGGTT-3′ and reverse,
5′-GCATTGAGAGATGGGCTGTTGTGT-3′; rat 18S-ribosomal (r)RNA forward,
5′-GAATTCCCAGTAAGTGCGGGTCAT-3′ and reverse,
5′-CGAGGGCCTCACTAAACCATC-3′. PCR reactions were performed using a
SYBR Premix Ex Taq II kit (RR82LR, Takara Bio, Inc.) in an ABI 7500
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Thermocyling conditions were as follows: 95°C for 30 sec; 40
cycles of 95°C for 5 sec and 62°C for 30 sec; 95°C for 15 sec, 60°C
for 1 min and 95°C for 15 sec. COX-2 mRNA was normalized to the
18S-rRNA and relatively quantified by standard curve analysis; the
2−∆∆Cq method was used for quantification, as described
previously (11).
Western blot analysis
Protein from RAECs was lysed with RIPA lysis buffer
(WB0101; Biotech Well, Shanghai, China). The protein concentration
was determined using a BCA protein quantification kit (23225;
Shanghai Yu Bo Biological Technology Co., Ltd., Shanghai, China).
Protein (50 µg per lane) was separated by 12% SDS-PAGE and then
transferred onto a polyvinylidene difluoride membrane. After
incubation with 3% BSA for 1 h at room temperature. 2 membranes
(corresponding to 2 gels) were blotted with polyclonal rabbit
anti-rat COX-2 antibody (1:1,000 dilution; 12282; Cell Signaling
Technology, Inc.) and polyclonal rabbit anti-β-actin antibody
(1:1,000 dilution; 4970; Cell Signaling Technology, Inc.) as a
control overnight at 4°C. The membranes were washed with
Tris-HCl-buffered saline and re-blotted with secondary antibody
(HRP-conjugated goat anti-rabbit antibody; 1:5,000; sc-2004; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) at room temperature for
2 h. Bands were displayed using a chemiluminescent reagent (P0018;
Beyotime Institute of Biotechnology). Densitomtric analysis was
performed with ImageJ software (version 1.46; National Institutes
of Health, Bethesda, MD, USA).
ELISA for assessment of 6-keto
PGF1α and TXB2 protein secretion
The concentrations of 6-keto PGF1α and
TXB2 in the cell culture supernatant were detected by
competitive ELISA (6-keto Prostaglandin F1α ELISA kit; 515211;
Cayman Chemical Co., Ann Arbor, MI, USA); and TXB2 ELISA
kit (KGE011; R&D Systems, Inc.) according to the manufacturer's
instructions. Optical density (OD) values of 6-keto
PGF1α and TXB2 were read using an ELISA
reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 405 or
450 nm, respectively. According to the OD values and the formulas
included in the instructions of the ELISA kits, the percentage of
sample bound vs. maximum binding were calculated for standard and
test samples.
Statistical analysis
Values are expressed as the mean ± standard error of
the mean and were analyzed by SPSS 17.0 statistical software (SPSS,
Inc., Chicago, IL, USA). The statistical significance of
differences among ≥3 groups was determined by one-way analysis of
variance followed by Dunn's post hoc analysis. Student's 2-tailed
test was performed when only 2 groups were being compared.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Isolation, culture and identification
of RAECs
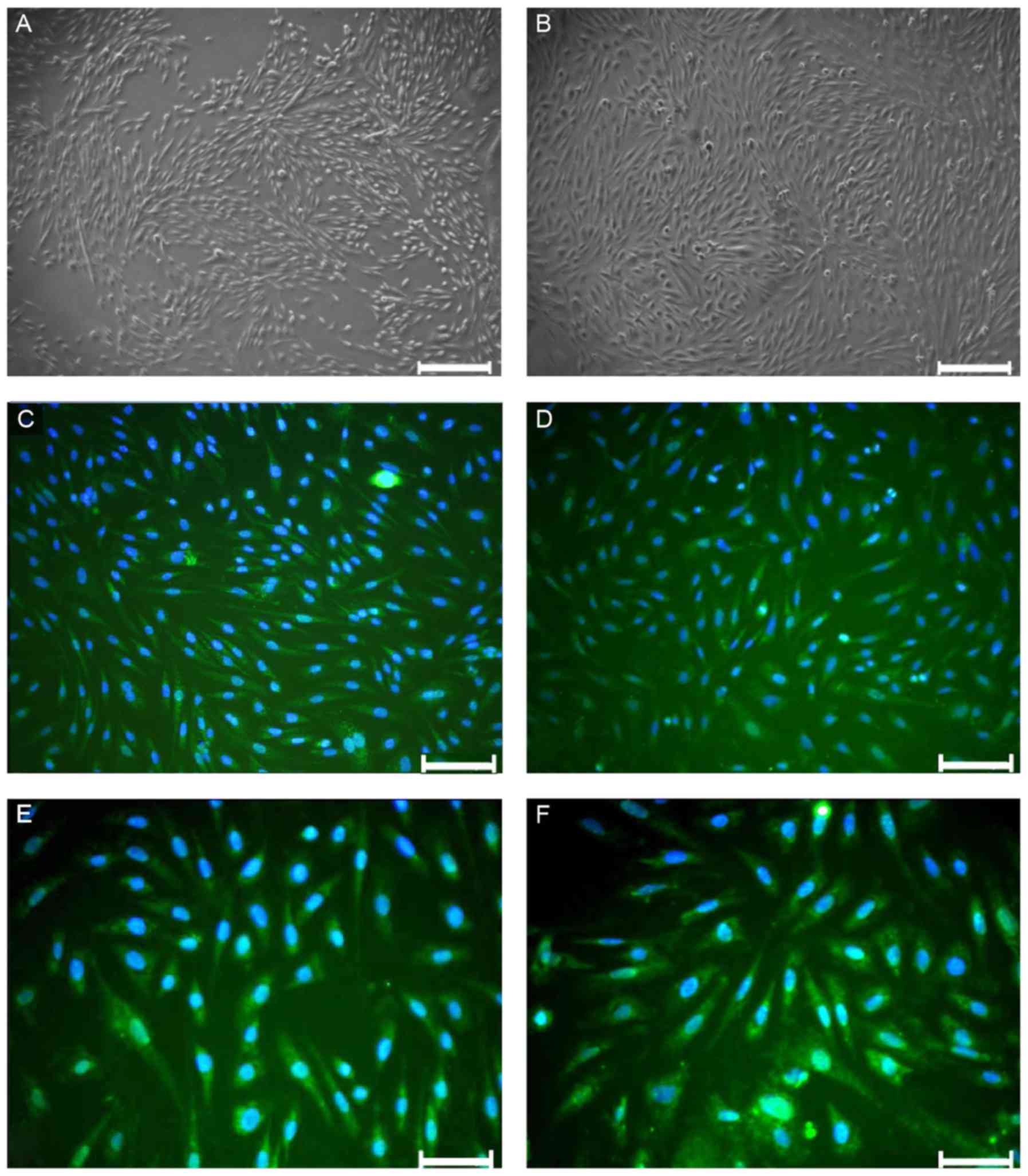
At 6 days of primary culture, RAECs presented as
shuttle-like or polygonal structures (Fig. 1A), and subsequently grew and
integrated into a monolayer. After culture for 10 days, RAECs had a
cobblestone-like appearance (Fig.
1B). Green fluorescence was observed in the cytoplasm under a
fluorescence microscope after immunofluorescence staining with
anti-factor VIII antibody, indicating EC-specific factor VIII
expression in the cytoplasm. Expression of factor VIII was present
in ~90% of the cells (Fig.
1C-F).
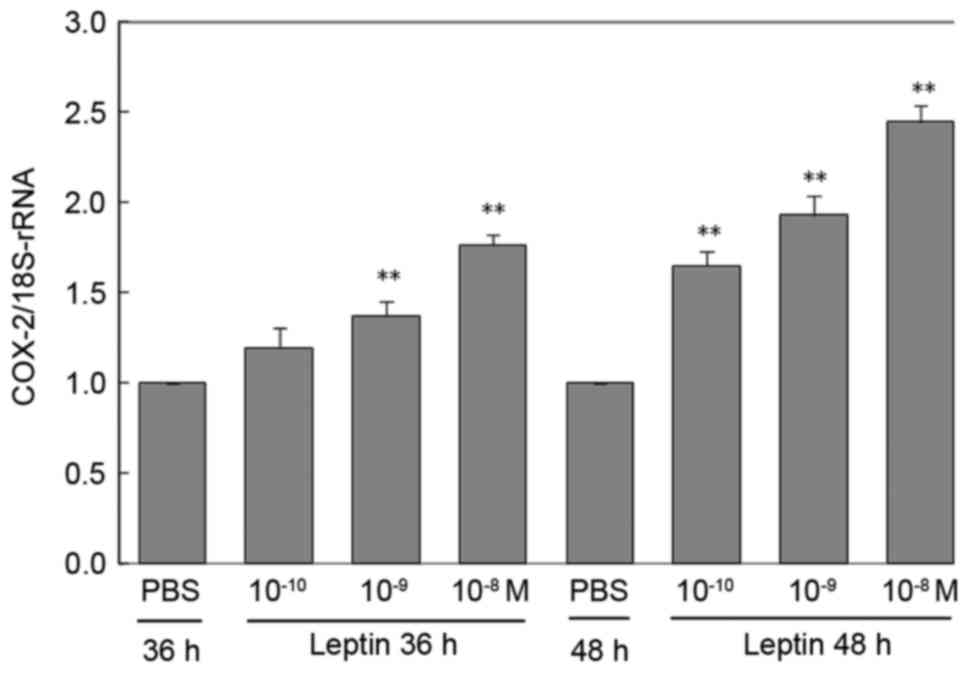
Effects of leptin on COX-2 mRNA
expression
RAECs treated with PBS were used as controls and
they expressed low levels of COX-2 mRNA (Fig. 2). However, when RAECs were treated
with different concentrations of leptin for different durations,
the expression levels of COX-2 mRNA were increased. Although there
was no statistical significance between the PBS group and that
treated with the lowest concentration of leptin (10−10
M) at 36 h (P=0.14), all other groups of RAECs treated with
different concentrations of leptin for different durations
exhibited a significantly increased expression of COX-2 mRNA
(P<0.01; P=0.003 for 10−9 or 10−8 M leptin
vs. PBS at 36 h). Furthermore, these increases were dependent on
the concentration of leptin and the incubation time (Fig. 2). Particularly after treatment with
the high concentration of leptin (10−8 M) for 48 h, the
expression of COX-2 mRNA was significantly increased by up to
1.65-fold compared with that in the PBS group (P<0.01; P=0.002,
leptin 10−10 M vs. PBS; P<0.001, leptin
10−9 M vs. PBS; P=0.004, leptin 10−8 M vs.
PBS at 48 h).
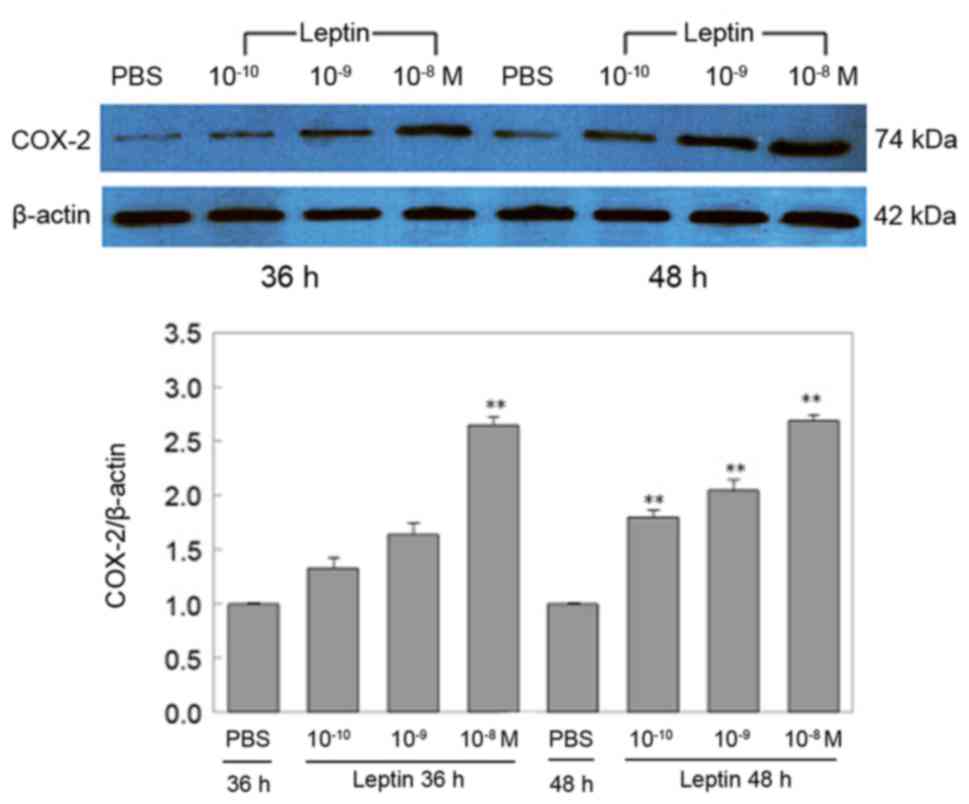
Effects of leptin on COX-2 protein
expression
In agreement with the COX-2 mRNA results above, the
expression of COX-2 protein was consistently decreased by RAECs
treated with PBS (Fig. 3).
Furthermore, the expression levels of COX-2 protein were
concentration- and time-dependently increased by leptin. However,
the increases at the protein level were not as significant as those
at the mRNA level at 36 h, as there was no statistical significance
between the PBS group and the 10−10 or 10−9 M
leptin group (P>0.05); only at the high concentration, leptin
(10−8 M) significantly upregulated COX-2 protein, namely
by up to 2.64-fold of that of the PBS group (P<0.05; P=0.135,
leptin 10−10 M vs. PBS; P=0.072, leptin 10−9
M vs. PBS; P=0.002, leptin 10−8 M vs. PBS at 36 h). At
48 h, the expression of COX-2 protein was similar to that of COX-2
mRNA, as all three concentrations of leptin led to a significant
upregulation of COX-2 protein expression (P=0.007, leptin
10−10 M vs. PBS; P=0.004 leptin 10−9 M vs.
PBS; P<0.001, leptin 10−8 M vs. PBS at 48 h; Fig. 3).
Effects of leptin on 6-keto
PGF1α and TXB2
To explore the effects of leptin on the secretion of
COX-2 products 6-keto PGF1α and TXB2, these
proteins were detected in the cell culture supernatant by ELISA
(Table I). At 36 and 48 h, the low
concentration of leptin (10−10 M) had no significant
effect on the levels of 6-keto PGF1α. However, the
higher concentrations of leptin (10−9 and
10−8 M), particularly at the longer incubation time (48
h), produced significantly elevated levels of 6-keto
PGF1α (P<0.05; Table
I). No significant effect on TXB2 was observed.
However, the leptin concentrations of 10−9 or
10−8 M significantly reduced the TXB2/6-keto
PGF1α ratio at 48 h (P<0.01; Table I). The purpose of calculating the
ratio was to predict the effects of leptin on vascular systolic and
diastolic function, as vasomotor function partly depends on this
ratio.
 | Table I.Effects of leptin treatment at
different concentrations and for different durations on
6-keto-PGF1α and TXB2 in the supernatant of
cultured rat aortic endothelial cells. |
Table I.
Effects of leptin treatment at
different concentrations and for different durations on
6-keto-PGF1α and TXB2 in the supernatant of
cultured rat aortic endothelial cells.
|
Time-point/group | 6-Keto
PGF1α (pg/ml) | TXB2
(ng/ml) |
TXB2/6-keto
PGF1α |
|---|
| 36 h |
|
|
|
|
PBS |
255.78±18.06 |
15.07±3.47 |
59.48±12.89 |
|
10−10 M leptin |
259.11±30.22 |
16.03±1.45 |
62.76±12.38 |
|
10−9 M leptin |
321.73±10.65a |
15.74±3.10 |
49.18±11.15 |
|
10−8 M leptin |
420.19±17.54b |
16.8±2.39 |
39.99±5.60 |
| 48 h |
|
|
|
|
PBS |
257.48±11.42 |
16.85±2.03 |
65.48±7.79 |
|
10−10 M leptin |
264.67±15.03 |
15.98±1.55 |
60.38±4.61 |
|
10−9 M leptin |
444.69±20.07b |
14.35±3.30 |
32.38±7.79b |
|
10−8 M leptin |
625.34±17.59b |
17.43±3.95 |
27.77±5.61b |
Discussion
Leptin is an endocrine hormone; in addition to
inhibiting food intake and increasing energy consumption, it has a
broad role in regulating biological functions, including immune,
inflammation and hematopoietic functions. Studies have found that
leptin participates in the occurrence and development of
hypertension by activating the sympathetic nerve (12) as well as increasing renal
Na+-K+-adenosine triphosphatase activity
(13) and oxidative stress (14) in vivo. In vitro, leptin
was found to act on endothelial cells by causing cell dysfunction
(4), inflammatory injury (5) and endothelin-1 expression (6) to presumably participate in hypertension
(3).
COX-2 is mainly expressed in ECs, macrophages and
fibroblasts. It is generally accepted that when ECs are in
inflammatory or pathological states, they increase the expression
of COX-2 and therefore the production of its downstream products
PGI2 and TXA2, which regulate vascular
tension and platelet aggregation. The synthesis and release of
PGI2 are mainly from ECs. PGI2 has a role in
vasodilation and anti-platelet aggregation. The stable metabolite
of PGI2 is 6-keto PGF1α. TX is an arachidonic
hormone, which may occur in two major forms: TXA2 and
TXB2. In vivo, TXA2 is mainly
synthesized and secreted by the platelet microsomes and ECs, and
has a strong effect on promoting vascular contraction and platelet
aggregation (9). However, the
biological half-life of TXA2 is only 30 sec, and is
quickly converted into the inactive and stable metabolite
TXB2. Therefore, the levels of 6-keto PGF1α
and TXB2 reflect the levels of PGI2 and
TXA2; studying the effects of leptin on the expression
of COX-2 and it downstream products may help to explain the
pathogenesis of hypertension mediated by leptin. At the same time,
it may also provide evidence for understanding the mechanisms of
obesity-associated diseases.
In order to explore the effect of leptin on the
expression of COX-2 and its downstream products PGI2 and
TXA2, ECs were separated from rat aortas and cultured.
Factor VIII as a marker of ECs was detected by immunofluorescence
staining. The results suggested that the purity of RAECs reached
~90%. After treatment of the RAECs, the expression levels of COX-2
mRNA and protein were significantly as well as leptin
concentration- and time-dependently increased. The levels of 6-keto
PGF1α were increased by relatively high concentrations
of leptin for the longer incubation time. Although the expression
of TXB2 was not affected by leptin, the TXB2/6-keto
PGF1α ratio was increased after incubation with leptin at high
concentrations and the longer incubation time. Thus, leptin
upregulated the expression levels of the inflammation marker COX-2
and increased the vasodilator PGI2, while decreasing the
ratio of TXB2 (vasoconstrictor substance) to
PGI2. These results implied that leptin is associated
with inflammation, while it enhanced endothelium-dependent
vasorelaxation.
Studies have indicated that endothelium-dependent
vasorelaxation mainly includes three pathways (15): i) Release of nitric oxide by
activation of endothelial (e)NOS in the aorta; ii) release of
endothelium-derived hyperpolarization factor in arteries with low
resistance; and iii) stimulation of COX-2 to produce
PGI2. Regarding the first two pathways associated with
leptin, it has been confirmed that leptin promotes the expression
of eNOS via phosphatidylinositol 3-kinase (16) and increases the release of EDHF
(8). The latter pathway associated
with leptin has remained to be fully elucidated, although leptin
has been found to increase the expression of COX-2 (16,17).
Manuel-Apolinar et al (17)
found that leptin is involved in the inflammatory response by
increasing the expression of intercellular adhesion molecules and
COX-2 on murine aorta tissue mediated by the long leptin receptor.
Garonna et al (18) found
that the pro-angiogenic actions of leptin required a functional
endothelial p38 mitogen-activated protein kinase/Akt/COX-2
signaling axis. To the best of our knowledge, the effects of
different doses of leptin on COX-2 and its downstream products
6-keto PGF1α and TXB2 have remained elusive.
The results of the present study provided direct evidence to answer
this question.
As selective COX-2 inhibitors, non-steroidal
anti-inflammatory drugs, such as aspirin and celecoxib, are widely
used, which have anti-inflammatory analgesic effects. At different
stages of hypertension and atherosclerotic plaque formation, ECs
may highly express COX-2. A selective COX-2 inhibitor was able to
reduce inflammation and platelet aggregation, resulting in a
decrease of the incidence of cardiovascular events and a protective
effect on the cardiovascular system (19,20).
However, when taking such medication at large dosages for a long
time, patients present with various types of complications, the
most common of which is digestive tract damage. Importantly,
adverse effects on the cardiovascular system have been reported for
prostanoid inhibition by COX-2 inhibitors: Certain studies have
demonstrated that COX-2 inhibitors may increase the risk of
cardiovascular events, such as myocardial infarction and stroke
(21). Therefore, the effects of
COX-2 inhibitors on the cardiovascular system require re-analysis;
consistently with the results of the present study, COX-2
inhibitors may increase the risk of hypertension.
In conclusion, the present study investigated the
effects of leptin on the expression of COX-2 and it downstream
products 6-keto PGF1α and TXB2 from RAECs.
Treatment with leptin, a mediator of hypertension, was identified
to significantly upregulate the expression of COX-2, a mediator of
inflammation, and the levels of its vasodilator product 6-keto
PGF1α, while downregulating the ratio of the
vasoconstrictor TXB2 to 6-keto PGF1α,
suggesting that leptin may promote cardiovascular diseases by
increasing the expression of COX-2. However, elective COX-2
inhibitors may not provide a benefit for leptin-mediated
cardiovascular diseases, as they may rather increase the occurrence
of hypertension due to inhibiting vasodilator 6-keto
PGF1α, a downstream product of COX-2, as well.
Acknowledgements
The present study was funded by grants from the
Science and Technology Plan Project of Guangzhou city in China
(grant no. 201510010181), the Science and Technology Plan Project
of Guangdong Province in China (grant nos. 2014A020212364 and
2013B021800282) and the Guangdong Natural Science Foundation in
China (grant nos. 2015A030313467 and S2013010015962).
References
|
1
|
Münzberg H and Morrison CD: Structure,
production and signaling of leptin. Metabolism. 64:13–23. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wada N, Hirako S, Takenoya F, Kageyama H,
Okabe M and Shioda S: Leptin and its receptors. J Chem Neuroanat.
61-62:1–199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kang YS: Obesity associated hypertension:
New insights into mechanism. Electrolyte Blood Press. 11:46–52.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Artwohl M, Roden M, Hölzenbein T,
Freudenthaler A, Waldhäusl W and Baumgartner-Parzer SM: Modulation
by leptin of proliferation and apoptosis in vascular endothelial
cells. Int J Obes Relat Metab Disord. 26:577–580. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singh P, Hoffmann M, Wolk R, Shamsuzzaman
AS and Somers VK: Leptin induces C-reactive protein expression in
vascular endothelial cells. Arterioscler Thromb Vasc Biol.
27:e302–e307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Quehenberger P, Exner M, Sunder-Plassmann
R, Ruzicka K, Bieglmayer C, Endler G, Muellner C, Speiser W and
Wagner O: Leptin induces endothelin-1 in endothelial cells in
vitro. Circ Res. 90:711–718. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Benkhoff S, Loot AE, Pierson I, Sturza A,
Kohlstedt K, Fleming I, Shimokawa H, Grisk O, Brandes RP and
Schröder K: Leptin potentiates endothelium-dependent relaxation by
inducing endothelial expression of neuronal NO synthase.
Arterioscler Thromb Vasc Biol. 32:1605–1612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lembo G, Vecchione C, Fratta L, Marino G,
Trimarco V, d'Amati G and Trimarco B: Leptin induces direct
vasodilation through distinct endothelial mechanisms. Diabetes.
49:293–297. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Santovito D, Mezzetti A and Cipollone F:
Cyclooxygenase and prostaglandin synthases: Roles in plaque
stability and instability in humans. Curr Opin Lipidol. 20:402–408.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kobayashi M, Inoue K, Warabi E, Minami T
and Kodama T: A simple method of isolating mouse aortic endothelial
cells. J Atheroscler Thromb. 12:138–142. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shek EW, Brands MW and Hall JE: Chronic
leptin infusion increases arterial pressure. Hypertension.
31:409–414. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Beltowski J: Leptin and the regulation of
renal sodium handling and renal na-transporting ATPases: Role in
the pathogenesis of arterial hypertension. Curr Cardiol Rev.
6:31–40. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wojcicka G, Jamroz-Wiśniewska A, Widomska
S, Ksiazek M and Bełtowski J: Role of extracellular
signal-regulated kinases (ERK) in leptin-induced hypertension. Life
Sci. 82:402–412. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Durand MJ and Gutterman DD: Diversity in
mechanisms of endothelium-dependent vasodilation in health and
disease. Microcirculation. 20:239–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vecchione C, Maffei A, Colella S, Aretini
A, Poulet R, Frati G, Gentile MT, Fratta L, Trimarco V, Trimarco B
and Lembo G: Leptin effect on endothelial nitric oxide is mediated
through Akt-endothelial nitric oxide synthase phosphorylation
pathway. Diabetes. 51:168–173. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Manuel-Apolinar L, Lopez-Romero R, Zarate
A, Damasio L, Ruiz M, Castillo-Hernández C, Guevara G and
Mera-Jiménez E: Leptin mediated ObRb receptor increases expression
of adhesion intercellular molecules and cyclooxygenase 2 on murine
aorta tissue inducing endothelial dysfunction. Int J Clin Exp Med.
6:192–196. 2013.PubMed/NCBI
|
|
18
|
Garonna E, Botham KM, Birdsey GM, Randi
AM, Gonzalez-Perez RR and Wheeler-Jones CP: Vascular endothelial
growth factor receptor-2 couples cyclo-oxygenase-2 with
pro-angiogenic actions of leptin on human endothelial cells. PLoS
One. 6:e188232011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rahme E, Pilote L and Lelorier J:
Association between naproxen use and protection against acute
myocardial infarction. Arch Intern Med. 162:1111–1115. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
De Vecchis R, Baldi C, Di Biase G, Ariano
C, Cioppa C, Giasi A, Valente L and Cantatrione S: Cardiovascular
risk associated with celecoxib or etoricoxib: A meta-analysis of
randomized controlled trials which adopted comparison with placebo
or naproxen. Minerva Cardioangiol. 62:437–448. 2014.PubMed/NCBI
|
|
21
|
Mcgettigan P and Henry D: Cardiovascular
risk with non-steroidal anti-inflammatory drugs: systematic review
of population-based controlled observational studies. PLoS Med.
8:e10010982011. View Article : Google Scholar : PubMed/NCBI
|