Introduction
Cirrhosis is a chronic, progressive, diffuse disease
caused by a variety of factors, including hepatitis B virus,
alcoholic liver disease and autoimmune liver disease (1–3). Liver
cirrhosis mortality increased from 1.54% of global mortality in
1980 to 1.95% in 2010 (4). Ascites
is a common complication of cirrhosis decompensation. Approximately
50% of patients with compensated cirrhosis develop ascites within a
period of 10 years (5). The
emergence of ascites predicts a poor prognosis of decompensated
cirrhosis, with a mortality of 15% after 1 year and 44% at the 2
year follow-up. (6). In addition,
the quality of life of patients with cirrhosis decreases following
the formation of ascites and the 5-year survival rate drops to 50%
(7). When ascites progress to
refractory ascites, if a liver transplant is not conducted, the
prognosis worsens and the 2-year survival rate falls to 35–50%
(8). Treatment of ascites not only
improves the quality of life of patients, but also reduces the risk
of progression to spontaneous bacterial peritonitis, which is the
most common fatal complication of liver cirrhosis (9). An improved understanding of the
pathophysiological mechanism for ascites in patients with liver
cirrhosis is necessary to improve patient treatment and to assist
the use of targeted therapies.
Ascites formation is the result of the combined
action of many factors; however, the mechanism underlying the
formation of cirrhotic ascites has not been fully elucidated. The
formation of ascites is a complex process that involves the liver,
kidney, hemodynamics and neuro-hormonal factors. The main
pathophysiological theories of ascites formation include the
underfill theory, the overfill theory and the peripheral artery
expansion theory (10). The
underfill theory (11) is a
derivation of Starling's liquid equilibrium theory (12), which is based on the balance between
the internal and external vessel hydrostatic pressure and colloid
osmotic pressure. According to this theory, liver cirrhosis leads
to increased portal pressure. The increase of portal vein capillary
bed hydrostatic pressure and/or the drop of plasma colloid osmotic
pressure disrupt the Starling balance of the capillary bed and
endovascular liquid spills into the abdominal cavity. However,
blocking and congestion reduce circulatory system resistance, and
effective renal blood flow is reduced. In addition, ascites
formation reduces effective renal blood flow. The activation of the
renin-angiotensin-aldosterone system (RAAS), norepinephrine system
and arginine vasopressin system then induce the absorption of
sodium and water by the renal tubules, further promoting the
formation of ascites. Therefore, sodium and water retention is
secondary (13,14). The overfill theory (14) is based on the association between
portal hypertension and low blood volume. Levy and Wexler (15) suggested that low pressure receptors
in the liver send signals to the renal tubules indicating sodium
retention. In cirrhotic portal hypertension, liver function changes
and high hepatic sinus internal pressure lead to renal sodium
retention by neuro-humoral factors (16), and the expansion of blood volume then
results in the formation of ascites. The peripheral artery
expansion theory (17) states that
patients with liver cirrhosis first develop sodium and water
retention, followed by the formation of ascites. This theory was
first proposed in 1988 by Schrier et al (17), who hypothesized that the formation of
cirrhosis-related ascites was preceded by peripheral artery
expansion resulting in the activation of vaso-excitor material,
including 5-hydroxytryptamine and thromboxane A2, the sodium and
water retention system, the sympathetic nerve, RAAS and
vasopressin, leading to renal vasoconstriction, sodium and water
retention, and ascites formation. The three theories are not
completely conflicting, and have the same pathological
physiological principles at certain levels; that is, when the body
senses that the effective arterial blood volume has decreased, it
is able to activate the sympathetic nerve, arginine-vasopressin
feedback system and RAAS, resulting in renal vasoconstriction, and
increased sodium and water absorption by the renal tubules leading
to ascites formation or aggravation. Studies have suggested that
renal artery resistance increases significantly in patients with
cirrhosis and massive ascites (18–20).
Therefore, the process of formation of ascites as a complication of
liver cirrhosis involves changes in the functional status of the
liver, renal function, circulatory system disorders and
neuro-hormonal activation. These findings indicate that renal
dysfunction serves an important role in the formation and
progression of cirrhotic ascites.
Retinol-binding protein (RBP) is a small protein
present at low levels in human urine. The ~90% of normal serum RBP
that is combined with thyroxine-binding protein is not filtered by
the glomeruli, and the ~10% unbound RBP is absorbed by renal
tubules following glomerular filtration (21,22).
Urinary RBP level remains relatively stable at a pH of 4.5, and is
not affected by gender or age (21,22).
Urinary RBP is a sensitive marker of early renal tubular function
damage, which is increased due to the disabsorption of RBP in the
presence of renal tubular damage (23). Current research on urinary RBP in
patients with cirrhosis is limited and there is no consensus with
regard to its clinical value for the detection of kidney damage in
patients with cirrhosis. The aim of the present study was to
investigate renal injury in patients with liver cirrhosis and
ascites, the association between renal injury and ascites
classification, and the correlation of urinary RBP with urinary
microalbumin (mAlb), serum urea nitrogen (urea), serum creatinine
(Cr) and estimated glomerular filtration rate (eGFR). In addition,
the association between urinary RBP and the curative effect of
treatment was investigated by recording the changes in urinary RBP
that occurred following treatment in patients with liver cirrhosis
and ascites.
Subjects and methods
Study subjects
A total of 90 patients with liver cirrhosis and
ascites hospitalized in Shanghai Tenth People's Hospital of Tongji
University (Shanghai, China) between May 2011 and January 2012 were
enrolled in the present study. They all conformed to the standard
diagnosis of cirrhosis (24). The
exclusion criteria used for the present study was as follows:
Patients with diabetes and/or high blood pressure; ascites formed
7–10 days after upper gastrointestinal bleeding; malignant ascites;
and ascites were caused by right cardiac insufficiency and renal
insufficiency. They were divided into three groups as follows: Mild
ascites (ascites only detectable by ultrasound; n=27), moderate
ascites (moderate symmetry of abdominal distension; n=45) and
severe ascites (a large amount of ascites, apparent abdominal
distension; n=18), according to the guidelines of the European
Association for the Study of the Liver (EASL) (25). The patients comprised 58 women and 32
men with a mean age of 46.6±7.15 years (range, 36–75 years). There
were 75 patients with cirrhosis caused by chronic hepatitis B, 10
patients with alcoholic cirrhosis and 5 patients with unexplained
liver cirrhosis. A control group was also enrolled in the study,
and consisted of 30 healthy subjects including 20 women and 10 men
with a mean age of 44.2±6.89 years. The study was approved by the
ethics committee of the Shanghai Tenth People's Hospital of Tongji
University. Written informed consent was obtained from the
participants prior to the study.
Methods
The morning urine of the control group was collected
and 5 ml of this was used for the measurement of urinary RBP and
urinary mAlb. In patients with cirrhosis, 24-h urine samples were
collected and 15 ml of each sample was used to measure urinary RBP
and urinary mAlb. The urinary RBP (cat. no. E016) and urinary mAlb
(cat. no. E038) were measured using ELISA kits (both Nanjing
Jiancheng Bioengineering Institute, Nanjing, China). A total of 3
ml fasting venous blood was collected from the patients each
morning; serum urea and serum Cr were measured using an automatic
biochemistry analyzer (7180; Hitachi, Ltd., Tokyo, Japan) and its
built-in measuring function in clinical laboratory. eGFR was
calculated by Modification of Diet in Renal Disease Study equation
(26). Ascites treatment was
performed according to the 2010 EASL clinical practice guidelines
on the management of ascites in cirrhosis (25). Urinary RBP was then measured 1, 2 and
4 weeks after treatment. After 1 month, ultrasound of the ascites
was performed and patients were divided into two groups according
to the response of the ascites to treatment as follows: Responsive
group (ascites could not be detected by ultrasound after 1 month)
and unresponsive group (no evident reduction in ascites after 1
month, or the development of refractory ascites).
Statistical analysis
All data are expressed as the mean ± standard
deviation, and the Student's unpaired t-test was used to analyze
differences between two groups. One-way analysis of variance was
used to compare data among the groups and Bonferroni correction was
used for post hoc tests. The Pearson's test was used for
correlation analysis of urinary RBP and urine mAlb with serum urea,
serum Cr and eGFR. P<0.05 was considered to indicate a
statistically significant result.
Results
General information
The present study included 90 patients with liver
cirrhosis and 30 healthy individuals. Table I presents the gender composition and
the mean age of the two groups in detail, and neither parameter
differed significantly between the groups.
 | Table I.Characteristics of the control group
and the cirrhotic ascites group. |
Table I.
Characteristics of the control group
and the cirrhotic ascites group.
| Group | Sex
(male/female) | Age (years) |
|---|
| Control | 10/20 | 44.2±6.89 |
| Cirrhotic
ascites | 32/58 | 46.6±7.15 |
Results of the analysis of urinary
RBP, urinary mAlb, serum urea, serum Cr and eGFR in the liver
cirrhosis and control groups
The results presented in Table II show that urinary RBP, urinary
mAlb, serum urea and serum Cr in the liver cirrhosis group were
significantly higher compared with those in the control group
(P<0.05). Furthermore, eGFR was significantly lower in the
cirrhosis group compared with the control group (P<0.01). It is
evident that renal injury exists in the patients with liver
cirrhosis and ascites, and is associated with the formation of the
ascites.
 | Table II.Urinary RBP, urinary mAlb, serum urea,
serum Cr and eGFR results. |
Table II.
Urinary RBP, urinary mAlb, serum urea,
serum Cr and eGFR results.
| Group | n | Urinary RBP
(mg/l) | Urinary mAlb
(mg/l) | eGFR (ml/min/1.73
m2) | Serum urea
(mmol/l) | Serum Cr
(µmol/l) |
|---|
| Control | 30 |
0.27±0.08 |
12.47±5.12 |
100.01±20.32 |
5.22±1.73 |
82.21±15.82 |
| Cirrhotic
ascites | 90 |
2.02±1.03a |
18.56±6.87b |
70.52±15.39a |
6.20±1.93b |
94.45±17.01b |
| Mild ascites | 27 |
0.91±0.41a |
13.68±4.31 |
77.21±15.52a |
5.11±1.51 |
81.21±11.31 |
| Moderate
ascites | 45 |
2.21±0.72a |
17.44±4.34a |
68.43±16.52a |
6.49±2.04b |
90.99±13.35b |
| Severe ascites | 18 |
3.17±0.64a |
26.17±7.58a |
60.11±13.27a |
7.53±2.11b |
99.15±17.51b |
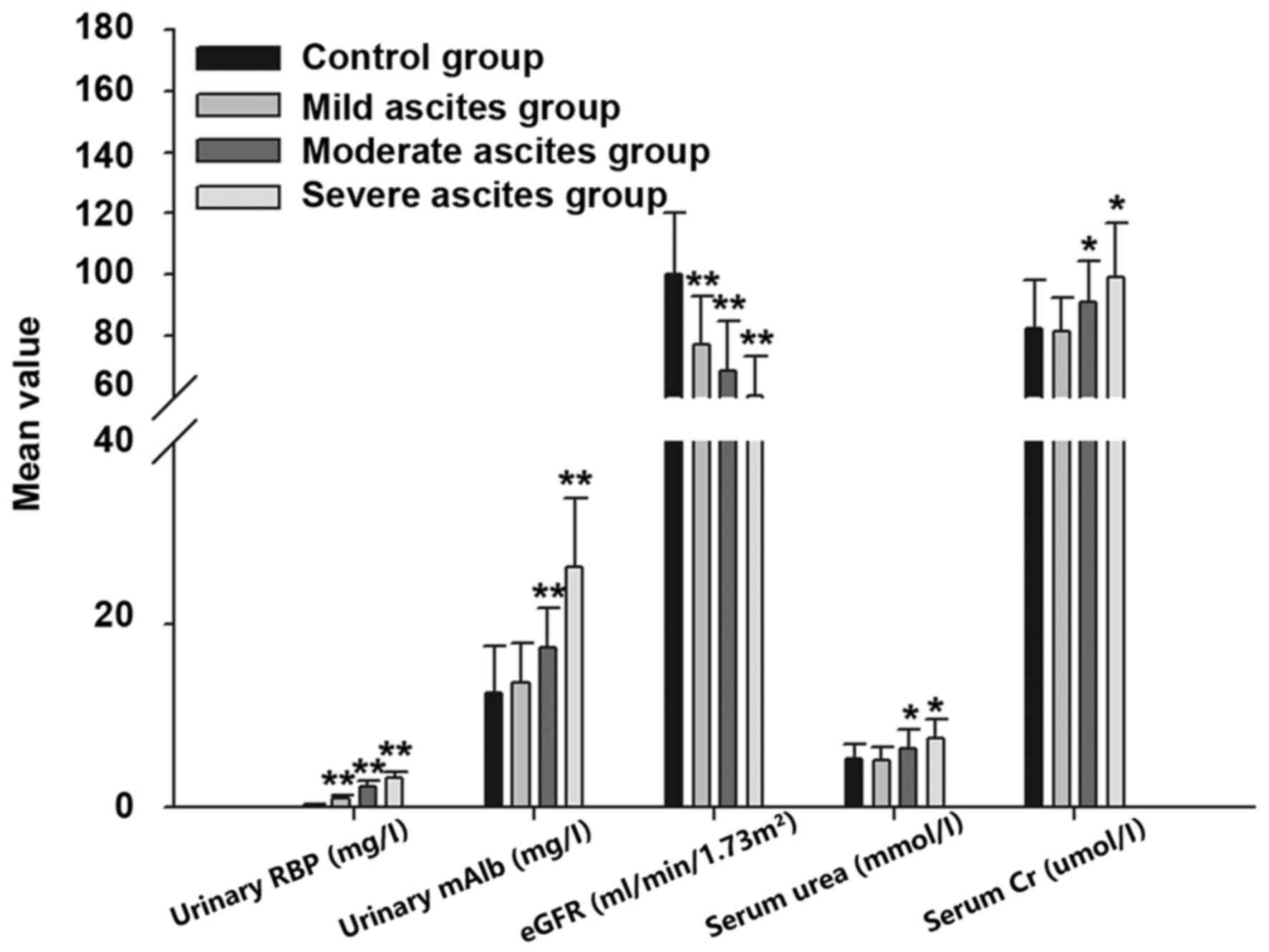
Comparison of the results in the mild, moderate and
severe ascites groups (Table III
and Fig. 1) demonstrated that
urinary RBP, urinary mAlb, serum urea and serum Cr increased and
eGFR decreased as the severity of the ascites increased. This
indicates that the degree of ascites in liver cirrhosis is
proportional to the renal injury.
 | Table III.Comparison of urinary RBP, urinary
mAlb, serum urea, serum Cr and eGFR among the cirrhotic ascites
groups. |
Table III.
Comparison of urinary RBP, urinary
mAlb, serum urea, serum Cr and eGFR among the cirrhotic ascites
groups.
| Variable | Statistics | Mild vs. moderate
ascites | Mild vs. severe
ascites | Moderate vs. severe
ascites |
|---|
| Urinary RBP | Z | −4.25 | −3.46 | −1.17 |
|
| P-value | <0.01 | <0.01 | <0.05 |
| Urinary mAlb | Z | −3.92 | −3.59 | −2.11 |
|
| P-value | <0.01 | <0.01 | <0.05 |
| eGFR | Z | −3.00 | −2.98 | −1.99 |
|
| P-value | <0.01 | <0.01 | <0.05 |
| Serum urea | Z | −3.20 | −2.94 | −1.36 |
|
| P-value | <0.01 | <0.01 | <0.05 |
| Serum Cr | Z | −4.31 | −3.07 | −1.152 |
|
| P-value | <0.01 | <0.01 | <0.05 |
Correlation of urinary RBP with
urinary mAlb, eGFR, serum urea and serum Cr, and of urinary mAlb
and eGFR, serum urea and serum Cr
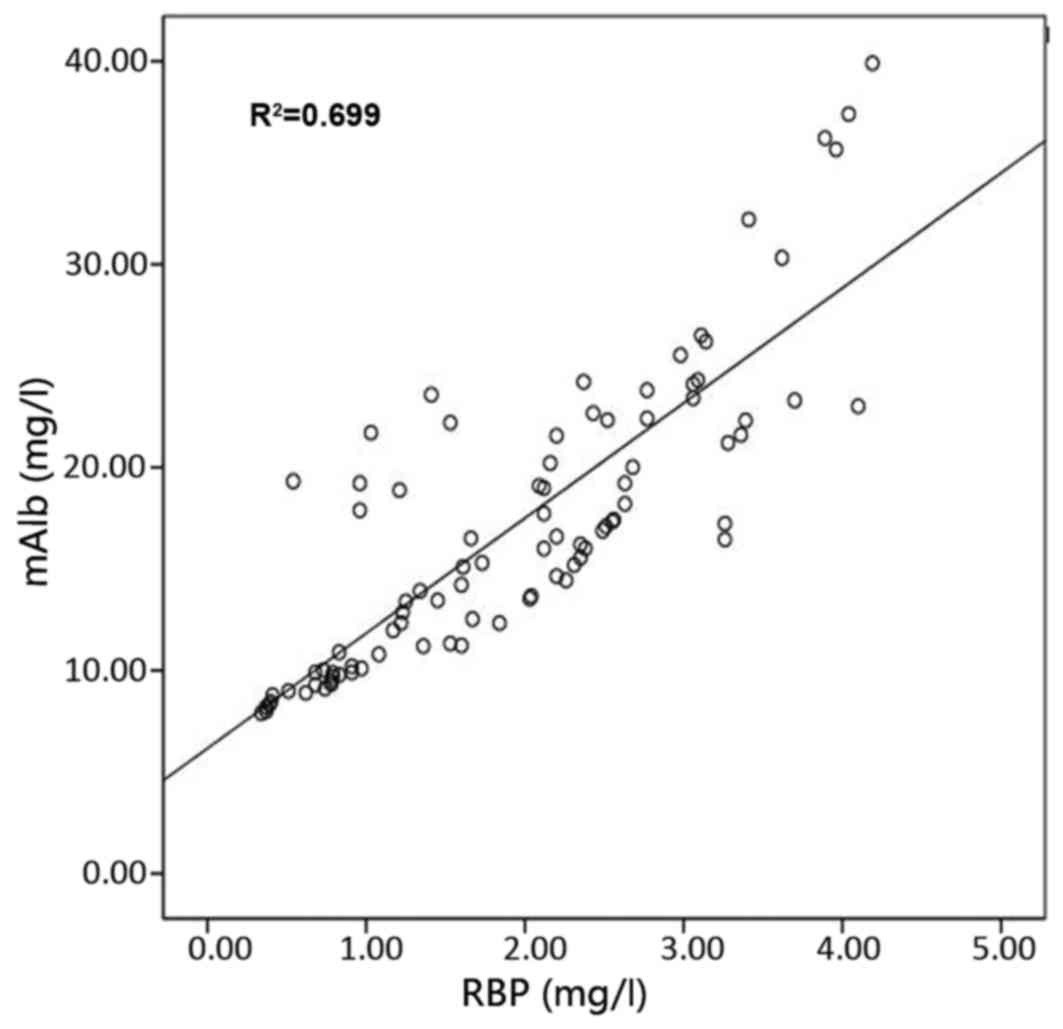
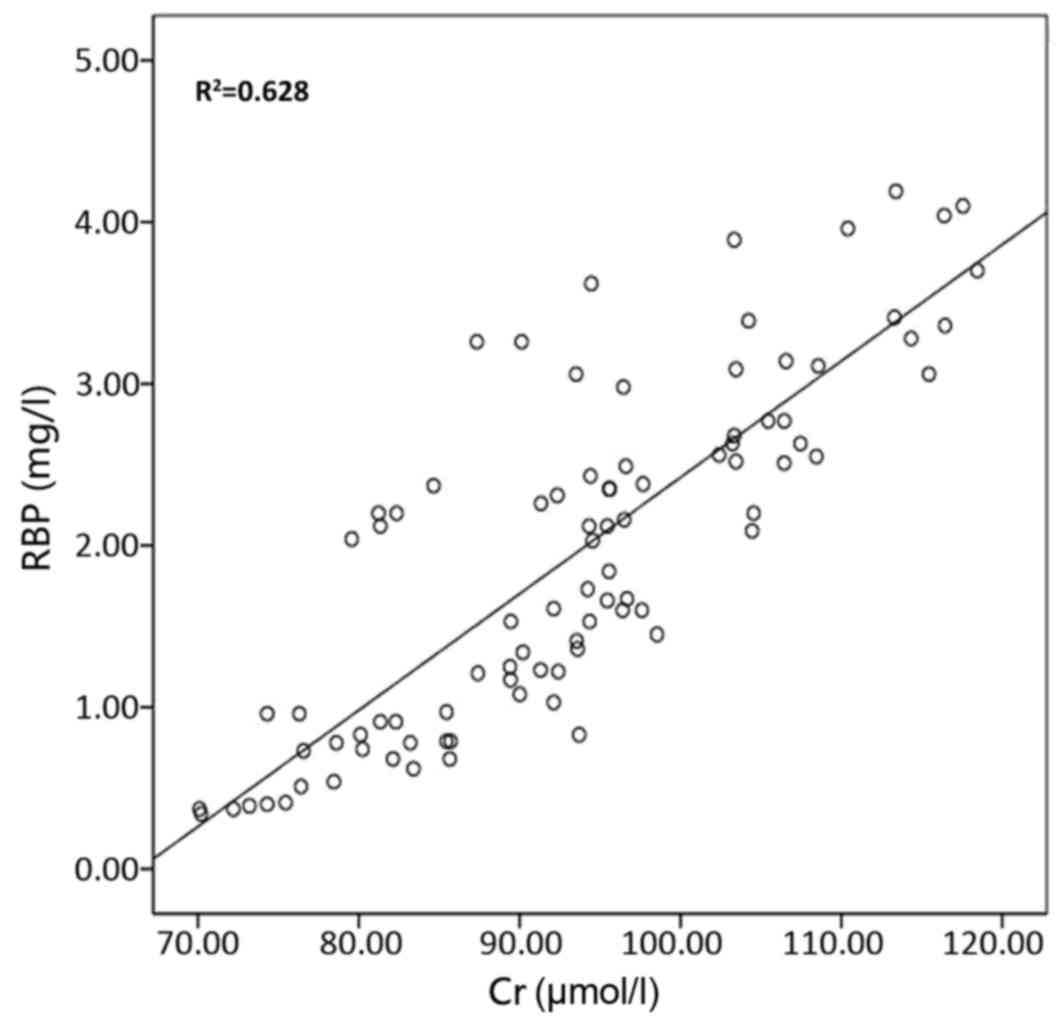
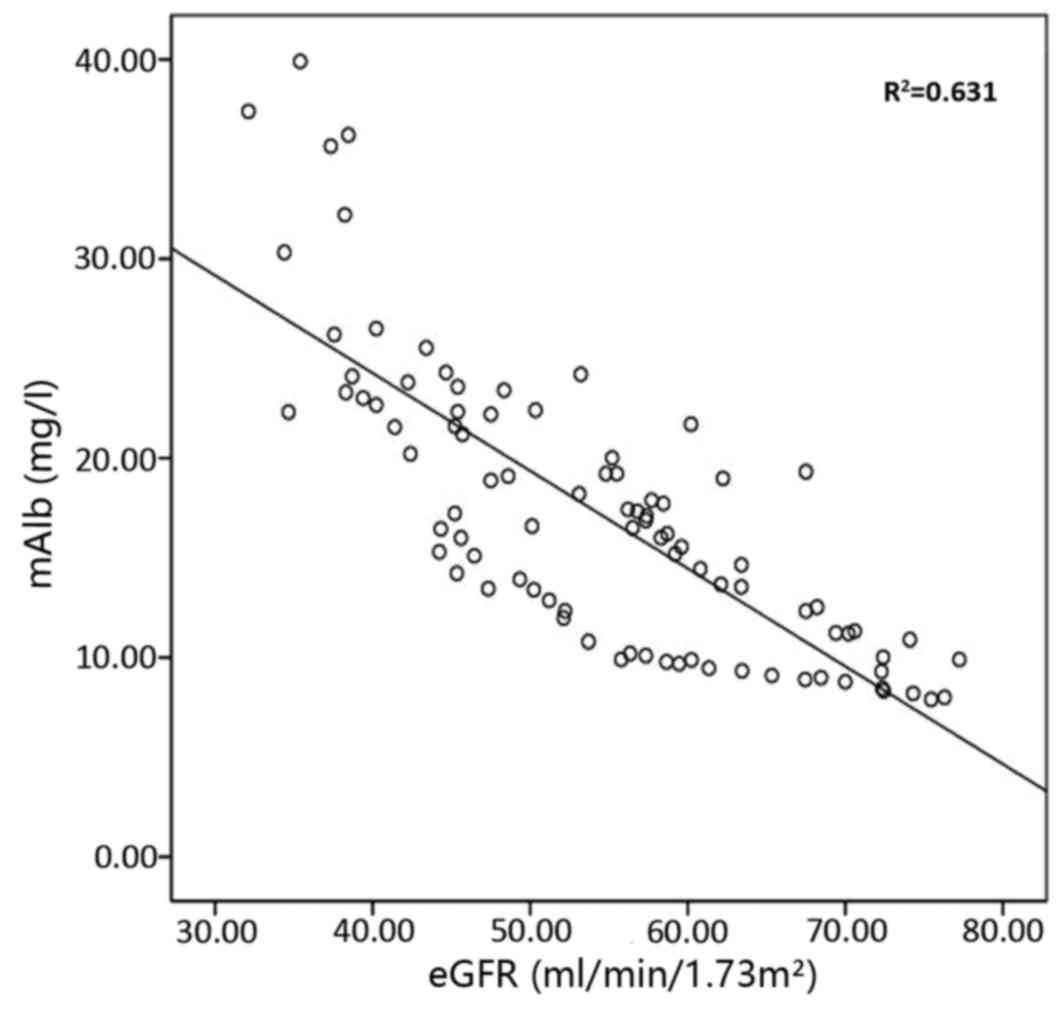
The correlation of urinary RBP with urinary mAlb,
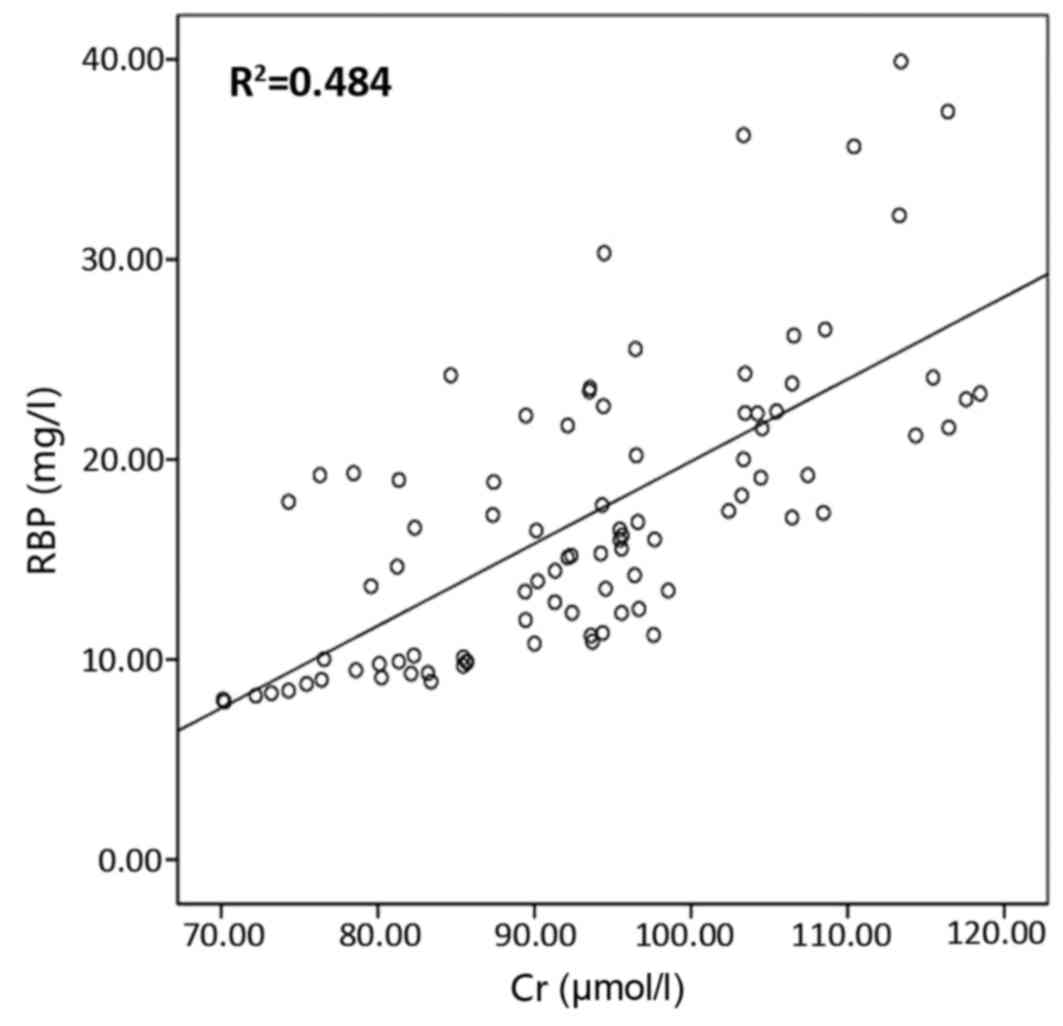
eGFR, serum urea and serum Cr is presented in Figs. 2–5,
respectively, and the correlation of urinary mAlb with eGFR, serum
urea and serum Cr is presented in Figs.
6–8, respectively. The
correlation coefficients for the correlation of urinary RBP with
urinary mAlb, serum urea, serum Cr and eGFR were 0.836, 0.79, 0.826
and −0.768, respectively; and those for the correlation of urinary
mAlb with urinary RBP, serum urea, serum Cr and eGFR were 0.836,
0.666, 0.696 and −0.794, respectively. The results of these
correlation analyses indicate a significant correlation of urinary
RBP and urine mAlb with serum urea, serum Cr and eGFR.
Comparison of urinary RBP prior to and
following treatment
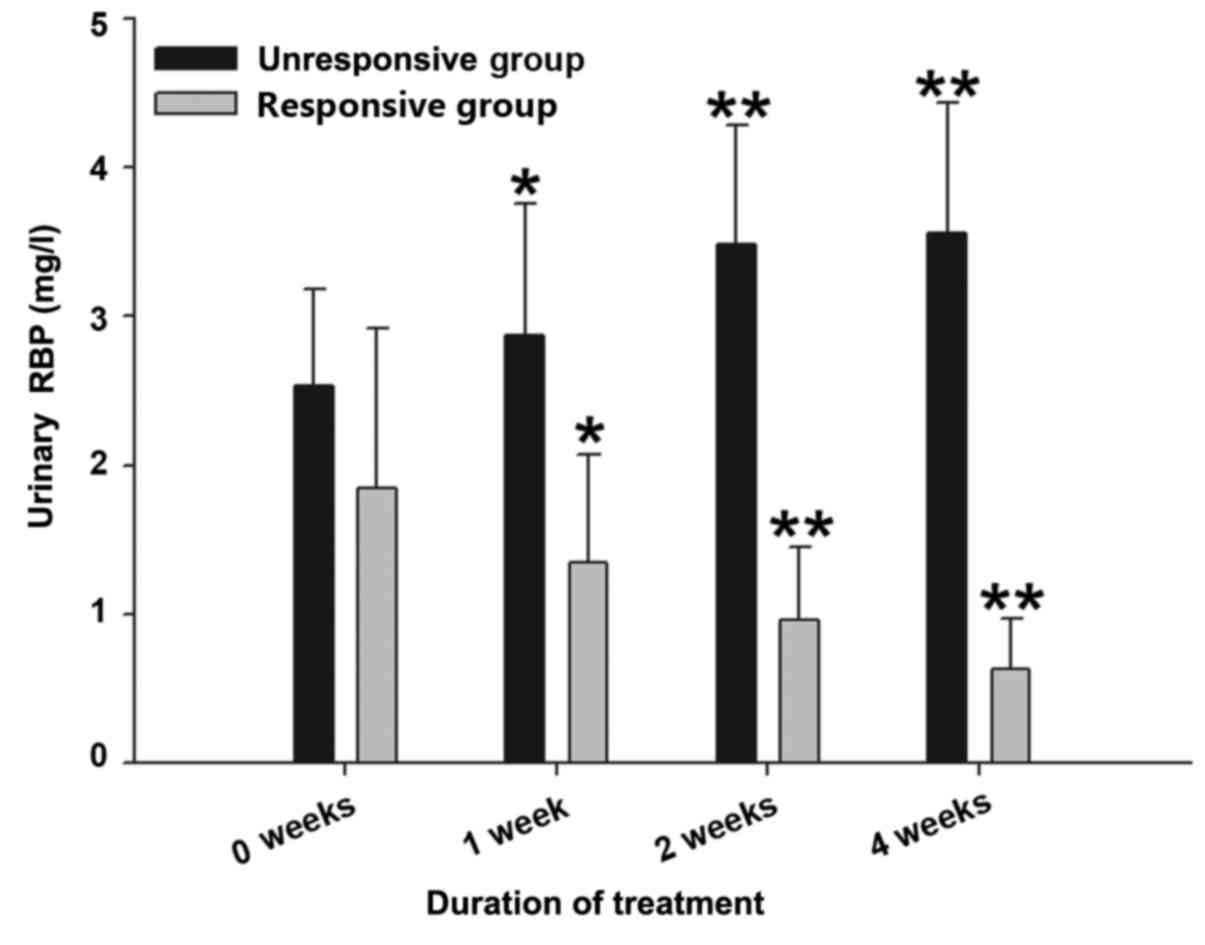
The results presented in Table IV show that the urinary RBP of the
responsive group with cirrhotic ascites was reduced significantly
at 2 and 4 weeks after treatment (P<0.01) and the urinary RBP of
the unresponsive group with cirrhotic ascites increased
significantly at 2 and 4 weeks after treatment (P<0.01) compared
with the urinary RBP prior to treatment. The results presented in
Fig. 9 show that the urinary RBP
level of the unresponsive group with cirrhotic ascites exhibited a
gradual increase over time at 1, 2 and 4 weeks after treatment
(P<0.05 vs. 0 weeks in the unresponsive group), whereas the
urinary RBP level of the responsive group with cirrhotic ascites
exhibited a gradual reduction over time at 1, 2 and 4 weeks after
treatment (P<0.05 vs. 0 weeks in the responsive group).
 | Table IV.Urinary RBP prior to and following 1,
2 and 4 weeks of treatment. |
Table IV.
Urinary RBP prior to and following 1,
2 and 4 weeks of treatment.
|
| Urinary RBP
(mg/l) |
|---|
|
|
|
|---|
| Group | 0 weeks | 1 week | 2 weeks | 4 weeks |
|---|
| Unresponsive |
2.53±0.65 |
2.87±0.89a |
3.48±0.80b |
3.56±0.87b |
| Responsive |
1.85±1.07 |
1.35±0.72a |
0.96±0.49b |
0.63±0.34b |
Discussion
Ascites is a common complication of liver cirrhosis
decompensation, and the prognosis of patients with cirrhosis and
ascites is poor, with 2-year mortality rates as high as 50%
(27). Therefore, the treatment of
liver cirrhosis-associated ascites is very important. However,
there currently are no clinical indicators for use in evaluation of
the treatment of this condition. The current study presents some
novel findings concerning urinary RBP in cirrhotic ascites.
In the group of 90 patients with cirrhotic ascites,
urinary mAlb, serum urea and serum Cr were significantly higher
compared with those in the healthy control group (P<0.05).
Furthermore, eGFR was significantly lower in the cirrhosis group
compared with the control group (P<0.01). This indicates that
renal injury is present in the patients with liver cirrhosis and
ascites, and may be involved in the formation of the ascites. As
shown in Fig. 1, urinary RBP, urine
mAlb, serum urea and serum Cr increased and eGFR gradually
decreased as the severity of the ascites increased. This suggests
that the degree of ascites is proportional to the renal injury. As
the ascites increase in severity, the Child-Pugh classification
will also increase; thus, it may be speculated that the Child-Pugh
classification is also proportional to the renal injury. The
correlation coefficients of urinary RBP with urinary mAlb, serum
urea, serum Cr and eGFR were 0.836, 0.79, 0.826 and −0.768,
respectively. The correlation coefficients of urinary mAlb with
urinary RBP, serum urea, serum Cr and eGFR were 0.836, 0.666,
0.696, and −0.794, respectively, suggesting that urinary RBP and
urinary mAlb are sensitive indicators of renal damage. Urinary RBP
showed a good correlation with eGFR, serum urea, and serum Cr. It
may be observed that in the mild ascites group, urinary RBP was
higher and eGFR was lower compared those in the control group
(P<0.01), whereas urinary mAlb, serum urea and serum Cr
exhibited no difference compared with the control group
(P>0.05), confirming that urinary RBP is a sensitive indicator
of early renal damage in liver cirrhosis with ascites. The
pathological classification of renal damage during the course of
liver cirrhosis is difficult to determine; hepatitis-related IgA
nephropathy and glomerular sclerosis are fairly common (28). A previous study including 65 cases
with proteinuria >0.5 g/day, microscopic hematuria or renal
damage of unknown causes (serum creatinine >1.5 mg/dl) in
patients with liver cirrhosis observed lesions of different degrees
in glomerular and non-glomerular structures, including renal blood
vessels, renal tubules and renal interstitial fibrosis (29). Therefore, hepatic dysfunction,
hemodynamic abnormalities, immune disorders and nervous system
dysfunction are closely associated with renal damage in cirrhotic
patients.
In the present study, urinary RBP was measured prior
to treatment and 1, 2 and 4 weeks after treatment, and patients
were divided into responsive ascites and unresponsive ascites
groups according to the change of the ascites observed following 1
month of treatment. Urinary RBP increased as the severity of the
ascites increased, and showed a tendency to increase in the
unresponsive group and a tendency to decline in the responsive
group. As shown in Table IV, an
increase in urinary RBP at 2 and 4 weeks after treatment indicated
a poor prognosis of ascites due to cirrhosis, which indicates the
potential of urinary RBP to serve as a prognostic indicator in the
clinical treatment of patients with liver cirrhosis and
ascites.
In summary, urine RBP is a sensitive indicator of
early renal damage in liver cirrhosis with ascites, which may be
used to monitor the curative effect of treatment, and serve as a
clinical indicator to evaluate the prognosis of patients with
ascites due to cirrhosis. In addition, the results of the present
study may prompt a novel method to study other complications of
cirrhosis, including portal hypertension (30), hepatic encephalopathy (31) and hepatocellular carcinoma (32,33).
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81500466).
References
|
1
|
Sivanathan V, Kittner JM, Sprinzl MF,
Weinmann A, Koch S, Wiltink J, Nguyen-Tat M, Marquardt JU, Wörns
MA, Zimmermann T, et al: Etiology and complications of liver
cirrhosis: Data from a German centre. Dtsch Med Wochenschr.
139:1758–1762. 2014.(In German). PubMed/NCBI
|
|
2
|
Silva MJ, Rosa MV, Nogueira PJ and Calinas
F: Ten years of hospital admissions for liver cirrhosis in
portugal. Eur J Gastroenterol Hepatol. 27:1320–1326. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qua CS and Goh KL: Liver cirrhosis in
Malaysia: peculiar epidemiology in a multiracial Asian country. J
Gastroenterol Hepatol. 26:1333–1337. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mokdad AA, Lopez AD, Shahraz S, Lozano R,
Mokdad AH, Stanaway J, Murray CJ and Naghavi M: Liver cirrhosis
mortality in 187 countries between 1980 and 2010: A systematic
analysis. BMC Med. 12:1452014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sandhu BS and Sanyal AJ: Management of
ascites in cirrhosis. Clin Liver Dis. 9:715–732, viii. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Runyon BA; AASLD Practice Guidelines
Committee, : Management of adult patients with ascites due to
cirrhosis: An update. Hepatology. 49:2087–2107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Planas R, Montoliu S, Ballesté B, Rivera
M, Miquel M, Masnou H, Galeras JA, Giménez MD, Santos J, Cirera I,
et al: Natural history of patients hospitalized for management of
cirrhotic ascites. Clin Gastroenterol Hepatol. 4:1385–1394. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salerno F, Cammà C, Enea M, Rössle M and
Wong F: Transjugular intrahepatic portosystemic shunt for
refractory ascites: A meta-analysis of individual patient data.
Gastroenterology. 133:825–834. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garcia-Tsao G: Current management of the
complications of cirrhosis and portal hypertension: Variceal
hemorrhage, ascites, and spontaneous bacterial peritonitis.
Gastroenterology. 120:726–748. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sabri M, Saps M and Peters JM:
Pathophysiology and management of pediatric ascites. Curr
Gastroenterol Rep. 5:240–246. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Witte MH, Witte CL and Dumont AE: Progress
in liver disease: Physiological factors involved in the causation
of cirrhotic ascites. Gastroenterology. 61:742–750. 1971.PubMed/NCBI
|
|
12
|
Levick JR and Michel CC: Microvascular
fluid exchange and the revised Starling principle. Cardiovasc Res.
87:198–210. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wongcharatrawee S and Garcia-Tsao G:
Clinical management of ascites and its complications. Clin Liver
Dis. 5:833–850. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schrier RW: Pathogenesis of sodium and
water retention in high-output and low-output cardiac failure,
nephrotic syndrome, cirrhosis, and pregnancy (2). N Engl J Med.
319:1127–1134. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Levy M and Wexler MJ: Hepatic denervation
alters first-phase urinary sodium excretion in dogs with cirrhosis.
Am J Physiol. 253:F664–F671. 1987.PubMed/NCBI
|
|
16
|
Kostreva DR, Castaner A and Kampine JP:
Reflex effects of hepatic baroreceptors on renal and cardiac
sympathetic nerve activity. Am J Physiol. 238:R390–R394.
1980.PubMed/NCBI
|
|
17
|
Schrier RW, Arroyo V, Bernardi M, Epstein
M, Henriksen JH and Rodés J: Peripheral arterial vasodilation
hypothesis: A proposal for the initiation of renal sodium and water
retention in cirrhosis. Hepatology. 8:1151–1157. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Donati G, Piscaglia F, Coli L, Silvagni E,
Righini R, Donati G, Pini P, Stefoni S and Bolondi L: Acute
systemic, splanchnic and renal haemodynamic changes induced by
molecular adsorbent recirculating system (MARS) treatment in
patients with end-stage cirrhosis. Aliment Pharmacol Ther.
26:717–726. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Catalina MV, Barrio J, Anaya F, Salcedo M,
Rincón D, Clemente G and Bañares R: Hepatic and systemic
haemodynamic changes after MARS in patients with acute on chronic
liver failure. Liver Int. 23 Suppl 3:S39–S43. 2003. View Article : Google Scholar
|
|
20
|
Jiang SM, Zhou GW, Shen C, Yan JQ, Wan L,
Li QY, Yang WP, Shen BY, Chen H, Peng CH and Li HW: A clinical
study on splanchnic hemodynamic changes after orthotopic liver
transplantation for patients with portal hypertension. Zhonghua Wai
Ke Za Zhi. 46:1699–1702. 2008.(In Chinese). PubMed/NCBI
|
|
21
|
Helen MN and Newcomer ME: The structure of
human retinol binding protein (RBP) with its carrier protein
transthyretin reveals an interaction with the carboxy terminus of
RBP. Biochemistry. 38:2647–2653. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Monaco HL, Rizzi M and Coda A: Structure
of a complex of two plasma proteins: Transthyretin and
retinol-binding protein. Science. 268:1039–1041. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chantrel F, Agin A, Offner M, Koehl C,
Moulin B and Hannedouche T: Comparison of cystatin C versus
creatinine for detection of mild renal failure. Clin Nephrol.
54:374–381. 2000.PubMed/NCBI
|
|
24
|
Fukui H, Saito H, Ueno Y, Uto H, Obara K,
Sakaida I, Shibuya A, Seike M, Nagoshi S, Segawa M, et al:
Evidence-based clinical practice guidelines for liver cirrhosis
2015. J Gastroenterol. 51:629–650. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
European Association for the Study of the
Liver, . EASL clinical practice guidelines on the management of
ascites, spontaneous bacterial peritonitis, and hepatorenal
syndromeincirrhosis. J Hepatol. 53:397–417. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Delanaye P and Mariat C: The applicability
of eGFR equations to different opulations. Nat Rev Nephrol.
9:513–522. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ghassemi S and Garcia-Tsao G: Prevention
and treatment of infections in patients with cirrhosis. Best Pract
Res Clin Gastroenterol. 21:77–93. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ozdamar SO, Gucer S and Tinaztepe K:
Hepatitis-B virus associated nephropathies: A clinicopathological
study in 14 children. Pediatr Nephrol. 18:23–28. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Trawalé JM, Paradis V, Rautou PE, Francoz
C, Escolano S, Sallée M, Durand F, Valla D, Lebrec D and Moreau R:
The spectrum of renal lesions in patients with cirrhosis: A
clinicopathological study. Liver Int. 30:725–732. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou Y, Dong Q, Zhang R, Zhou S, Li L,
Cheng K, Kong R, Yu Q, Xu S, Li J, et al: Cerebral hemodynamics and
cognitive function in cirrhotic patients with hepatic
encephalopathy. Gastroenterol Res Pract. 2016:84850322016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu L, Dai W, Li J, He L, Wang F, Xia Y,
Chen K, Li S, Liu T, Lu J, et al: Methylation-regulated miR-124-1
suppresses tumorigenesis in hepatocellular carcinoma by targeting
CASC3. Oncotarget. 7:26027–26041. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang J, Li J, Dai W, Wang F, Shen M, Chen
K, Cheng P, Zhang Y, Wang C, Zhu R, et al: Golgi protein 73 as a
biomarker for hepatocellular carcinoma: A diagnostic meta-analysis.
Exp Ther Med. 9:1413–1420. 2015.PubMed/NCBI
|
|
33
|
Liu Z, Wang J, Guo C and Fan X:
microRNA-21 mediates epithelial-mesenchymal transition of human
hepatocytes via PTEN/Akt pathway. Biomed Pharmacother. 69:24–28.
2015. View Article : Google Scholar : PubMed/NCBI
|