Introduction
Ginsenosides, which are extracted from ginseng
(Panax ginseng), American ginseng (Panax
quinquefolium) and notoginseng (Panax notoginseng),
exhibit a variety of pharmacological activities, including
cardiovascular protective (1–7),
neuroprotective (8–10) and anti-tumor effects (11–16).
Ginsenoside Rb1 (Rb1) is one of the monomers contained in total
ginsenosides (extracted from sun-cured ginseng) whereas ginsenoside
Rg3 (Rg3), a particularly rare ginsenoside, is obtained from other
ginsenosides by heat treatment during ginseng processing (17). Chemical (17) or biological (18,19)
methods have also been used to transform other ginsenosides,
including Rb1, into Rg3.
Previous studies have demonstrated that Rb1 exhibits
beneficial effects on the cardiovascular system. It is able to
attenuate myocardial ischemia, reperfusion injury (2) and ventricular remodeling (1,5). The
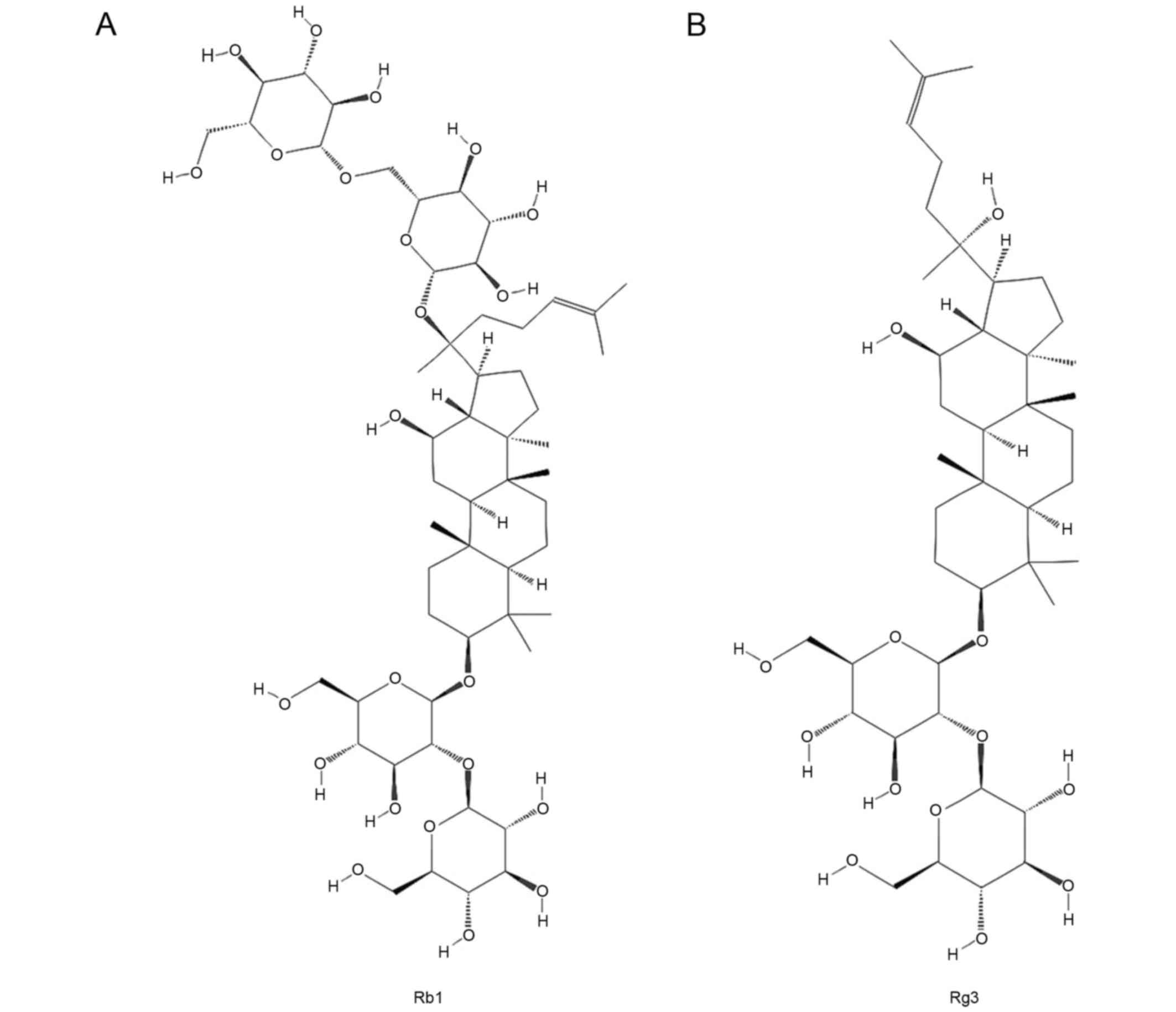
chemical structure of Rg3 is similar to that of Rb1 (Fig. 1); however Rg3 exhibits strong
anti-tumor activity (12,13,15,16),
which Rb1 does not, meaning that it may be used to treat patients
with tumors. The Shenyi Capsule, which is produced by Jilin Yatai
Pharmaceutical Co., Ltd., is a widely used anti-tumor medication in
China and its principal component is Rg3. Although the anti-tumor
activity of Rg3 has been well documented, to the best of our
knowledge, it remains unknown whether Rg3 induces the same
beneficial effects on the cardiovascular system that Rb1 does. Our
group is currently undertaking a long-term study in which the
effects of various ginsenosides, including Rb1 and Rg3, are
assessed in various animal models of chronic disease, such as
hypertension, hyperlipemia and diabetes.
The spontaneously hypertensive rat (SHR) is a widely
used animal model of hypertension. Angiotensin II (Ang II) levels
in the blood and myocardial tissue are abnormally higher in SHR
than in healthy Wistar-Kyoto (WKY) rats. Increased Ang II levels
cause progressive hypertension, myocardial fibrosis and may even
induce ventricular remodeling, resulting in heart failure (20,21). The
aim of the present study was to assess and compare the effects of
Rb1 and Rg3 and determine whether Rg3 exhibits protective effects
on the cardiovascular system in SHR rats.
Materials and methods
Reagents
Rb1 (95% purity) was obtained from Dr. Yanping Chen
at the Department of Natural Medicinal Chemistry, School of
Chemistry, Jilin University (Jilin, China) and dissolved in
double-distilled water (ddH2O) prior to use. Rg3 (95%
purity) was obtained from Jilin Yatai Pharmaceutical Co., Ltd.
(Changchun, China) and suspended in 0.5% sodium carboxymethyl
cellulose solution for use. All other chemicals were analytical
reagents.
Animals and treatments
A total of 24 SHR and 8 WKY rats (male,
16-17-week-old, 250-300 g) were purchased from Vital River
Laboratories Co., Ltd. (Beijing, China). All rats were kept in a
specific pathogen-free experimental animal workshop (25°C, 10/14-h
light/dark cycle), and had free access to food and water.
Experiments were performed in accordance with the Guide for the
Care and Use of Laboratory Animals of Jilin University and approved
by the Ethics Committee of Jilin University.
The rats were randomly divided into 4 groups (each,
n=8): i) A WKY group, consisting of WKY rats orally administered
ddH2O; ii) a SHR group, consisting of SHR rats orally
administered ddH2O; iii) a Rb1 group consisting of SHR
rats orally administered 20 mg/kg Rb1; and iv) a Rg3 group Rg3
consisting of SHR rats orally administered 20 mg/kg Rg3. All
treatments were administered once a day over 42 consecutive
days.
After treatment for 6 weeks with ddH2O or
ginsenosides, all rats were anesthetized with chloral hydrate (300
mg/kg, intraperitoneally), and blood samples were obtained from the
abdominal aorta before the rats were sacrificed. Hearts were
obtained and weighed after the rats were sacrificed, and myocardium
tissue samples were then fixed in 4% buffered paraformaldehyde
solution (25°C, 24 h) for histopathological examination or frozen
in liquid nitrogen and stored at −80°C prior to reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
Blood pressure measurement
The systolic blood pressure (SBP), diastolic blood
pressure (DBP) and pulse pressure (PP) of rats were measured using
the tail-cuff method and a small animal sphygmomanometer (BP-2010A;
Softron Biotechnology Ltd., Beijing, China) following a previously
described protocol (22) on the
first and the last days of the 6-week treatment.
Echocardiography
On the first and last days of the 6-week treatment,
transthoracic echocardiography was performed as previously
described (23), using a standard
setting with a 10S transducer (Vivid-i; GE Healthcare, Chicago, IL,
USA). Animals were anesthetized with chloral hydrate (300 mg/kg,
intraperitoneally) and two-dimensional and M-mode echocardiographic
measurements were conducted. A short-axis two-dimensional image of
the left ventricle was obtained at the position of the papillary
muscles. Subsequently, M-mode images were acquired at a sweep speed
of 100 mm/s and digitally stored. The left ventricular internal
dimension at diastole (LVIDd) and left ventricular internal
dimension at systole (LVIDs) were acquired from M-mode images;
subsequently, left ventricular fractional shortening (FS) and left
ventricular ejection fraction (EF) were calculated automatically by
the equipment. The parameters were measured by an experienced
echocardiographer blinded to the treatment groups.
Assay of the angiotensin converting
enzyme (ACE) and Ang II levels in the serum
Blood samples were collected and left at room
temperature for 2 h to allow complete clotting and then centrifuged
at 1,500 × g, 4°C for 15 min. The serum was removed and stored at
−80°C prior to ELISA. ACE (CSB-E04490r) and Ang II (CSB-E04494r)
ELISA kits were purchased from Cusabio Biotech Co., Ltd. (Wuhan,
China) and the assays were completed by this company.
Histopathological examination
Myocardial tissue samples were fixed in 4% buffered
paraformaldehyde solution and then embedded in paraffin.
Paraffin-embedded sections 4-μm thick were stained with hematoxylin
and eosin (H&E) and Masson trichrome stain. Sections were
examined using a Nikon E100 light microscope (Nikon Corporation,
Tokyo, Japan) and photomicrograph images were captured.
Immunohistochemistry (IHC)
Primary antibodies against ACE (bs-0439R), Ang II
(bs-0587R), Ang II receptor type 1 (AT1, bs-0438R) and transforming
growth factor β1 (TGF-β1, bs-0103R) were purchased from Bioss
Antibodies (Beijing, China). Peroxidase-conjugated goat anti-rabbit
IgG (ZB-2301), DAB kit (ZLI-9018) and two step rabbit IHC kit
(PV-6001) were purchased from ZSGB-BIO (Beijing, China). IHC was
performed following the manufacturer's protocols of the IHC kit and
DAB kit. Photomicrograph images were then captured, and Image Pro
Plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA) was used for
image analysis.
RNA preparation and RT-qPCR
Total RNA was isolated from frozen myocardium tissue
samples using TRIzol reagent (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) following the manufacturer's protocol. Total RNA
was reverse-transcribed and qPCR was conducted using the
TransScript Green Two-Step qRT-PCR SuperMix (TransGen Biotech Co.,
Ltd., Beijing, China) on the Stratagene Mx3000P (Agilent
Technologies, Inc., Santa Clara, CA, USA) and following the
manufacturer's protocol (94°C for 5 sec, 60°C for 15 sec and 72°C
for 10 sec, 40 cycles). The relative fold changes in the mRNA
levels of the target genes were determined using the
2−ΔΔCq method (24) and
β-actin was used as a housekeeping gene. Primer sequences are
provided in Table I.
 | Table I.Primer sequences of TNF-α, IL-6,
IL-1β, ET-1 and β-actin. |
Table I.
Primer sequences of TNF-α, IL-6,
IL-1β, ET-1 and β-actin.
| Primer name | Sequences |
|---|
| β-actin | Forward:
5′-GATCAAGATCATTGCTCCTCCTG-3′ |
|
| Reverse:
5′-AGGGTGTAAAACGCAGCTCA-3′ |
| TNF-α | Forward:
5′-GTCGTAGCAAACCACCAAGC-3′ |
|
| Reverse:
5′-TGTGGGTGAGGAGCACGTAG-3′ |
| IL-6 | Forward:
5′-TGTATGAACAGCGATGATG-3′ |
|
| Reverse:
5′-AGAAGACCAGAGCAGATT-3′ |
| IL-1β | Forward:
5′-GCAATGGTCGGGACATAGTT-3′ |
|
| Reverse:
5′-AGACCTGACTTGGCAGAGG-3′ |
| ET-1 | Forward:
5′-GCTCCTCCTTGATGGACAA-3′ |
|
| Reverse:
5′-TTTGGTGAGCACACTGGC-3′ |
Statistical analysis
SPSS 15.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis. All data are expressed
as the mean ± standard deviation. One-way analysis of variance with
Tukey's post hoc test was used to analyze differences among groups
and P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of Rb1 and Rg3 on cardiac
structure and function
The effects of Rb1 and Rg3 on cardiac structure and
function were evaluated using echocardiography. As depicted in
Fig. 2, compared with the WKY group
prior to the 6-week treatment, the three groups of SHR rats
exhibited slight cardiac function injury; they had a significantly
lower FS and EF compared with the WKY group (P<0.05; Fig. 2D and E). However, LVIDd and LVIDs
were similar among all groups, indicating that the cardiac
structure of SHR rats was unaffected prior to the 6-week
treatment.
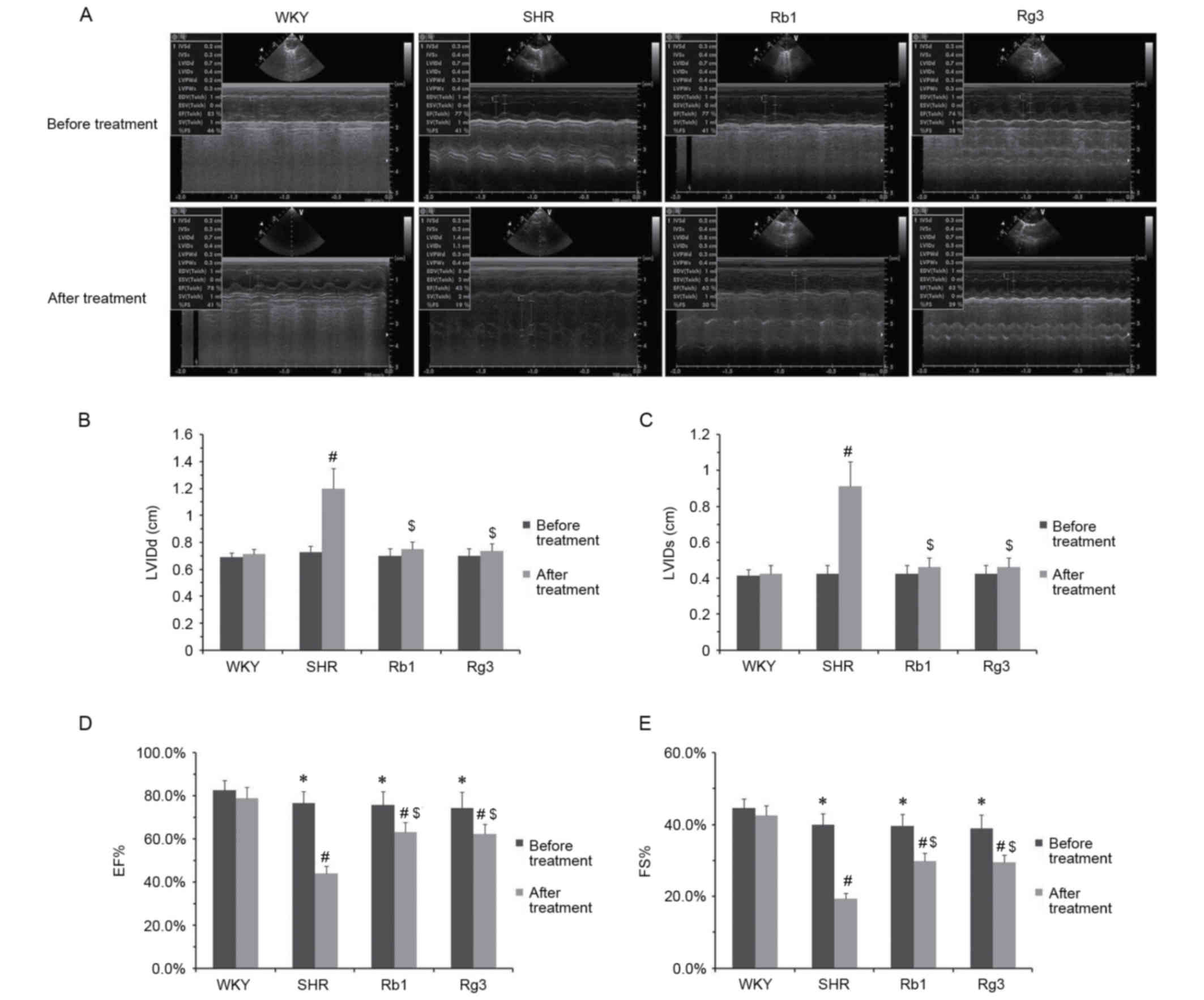 | Figure 2.Effects of Rb1 and Rg3 on cardiac
structure and function. (A) Representative echocardiographic M-mode
records. (B) LVIDd, (C) LVIDs, (D) EF and (E) FS of rats prior to
and following 6 weeks treatment. Data are presented as the mean ±
standard deviation, where n=8 for each group. *P<0.05 vs. WKY
group prior the treatment; #P<0.05 vs. WKY group
following treatment; $P<0.05 vs. SHR group following
treatment. Rb1, ginsenoside Rb1; Rg3, ginsenoside Rg3; LVIDd, left
ventricular internal dimension at diastole; LVIDs, left ventricular
internal dimension at systole; EF, left ventricular ejection
fraction; FS, left ventricular fractional shortening; WKY,
Wistar-Kyoto rats; SHR, spontaneously hypertensive rats. |
Following the 6-week treatment, the FS and EF of the
three SHR groups were all significantly reduced compared with the
WKY group (P<0.05). However, the FS and EF of the Rb1 and Rg3
groups were significantly higher than those of the SHR group
(P<0.05; Fig. 2D and E). Rb1 and
Rg3 also exhibited protective effects on cardiac structure.
Following treatment, the LVIDd and LVIDs of the SHR group were
significantly higher than those of the WKY group (P<0.05);
however, those of the Rb1 and Rg3 groups were significantly lower
than the SHR group and did not differ significantly between those
of the WKY group (Fig. 2B and C).
Notably, the cardiac protective effects of Rb1 and Rg3 were
comparable.
Effects of Rb1 and Rg3 on blood
pressure
Rb1 and Rg3 did not significantly affect the blood
pressure of SHR rats. The SBP and DBP of the three groups of SHR
rats were all significantly higher than the WKY group prior to and
following 6-week treatment (P<0.05) and there was no significant
difference in blood pressure between the SHR group and the Rb1 or
Rg3 groups (Fig. 3A and B).
Regarding PP, there were no significant differences between any
groups prior to or following treatment (Fig. 3C).
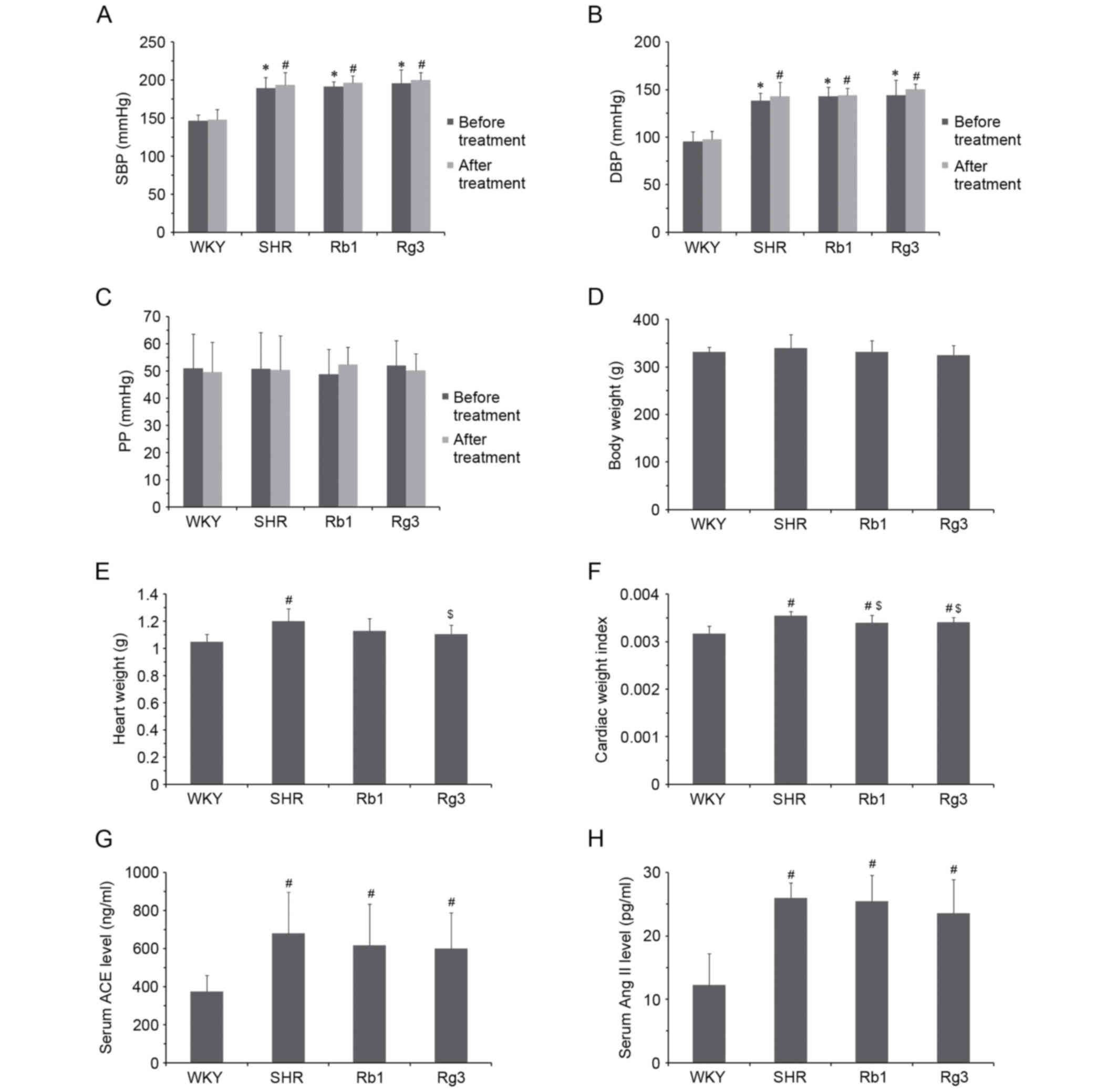 | Figure 3.Effects of Rb1 and Rg3 on blood
pressure, CWI and RAS activity in the serum. (A) SBP, (B) DBP and
(C) PP of rats prior to and following 6 weeks treatment. (D) Body
and (E) heart weights and (F) CWI. Serum (G) ACE and (H) Ang II
levels. Data are presented as the mean ± standard deviation, n=8
(A-F) or n=6 (G-H) for each group. *P<0.05 vs. WKY group prior
to treatment; #P<0.05 vs. WKY group following
treatment; $P<0.05 vs. SHR group following treatment.
Rb1, ginsenoside Rb1; Rg3, ginsenoside Rg3; CWI, cardiac weight
index; RAS, renin angiotensin system; SBP, systolic blood pressure;
DBP, diastolic blood pressure; PP, pulse pressure; ACE, angiotensin
converting enzyme; Ang II, angiotensin II; WKY, Wistar-Kyoto; SHR,
spontaneously hypertensive rats. |
Effects of Rb1 and Rg3 on the cardiac
weight index (CWI)
The body weights and heart weights of all four
groups are presented in Fig. 3D and
E. CWI was subsequently calculated using the following formula:
Heart weight/body weight. The CWI of the SHR group was
significantly higher than that of the WKY group (P<0.05), while
those of groups Rb1 and Rg3 were significantly lower than the SHR
group (P<0.05; Fig. 3F). This
result demonstrated that hypertension induces cardiac structural
changes and that Rb1 and Rg3 significantly attenuate these
changes.
Effects of Rb1 and Rg3 on renin
angiotensin system (RAS) activity
Rb1 and Rg3 had no significant effects on RAS
activity in the serum. ACE and Ang II levels of the three SHR
groups were all significantly higher than those of the WKY group
(P<0.05) and there were no significant differences between ACE
and Ang II levels between the three SHR groups (Fig. 3G and H). This may explain why Rb1 and
Rg3 did not significantly reduce blood pressure in SHR rats.
Effects of Rb1 and Rg3 on myocardium
histology
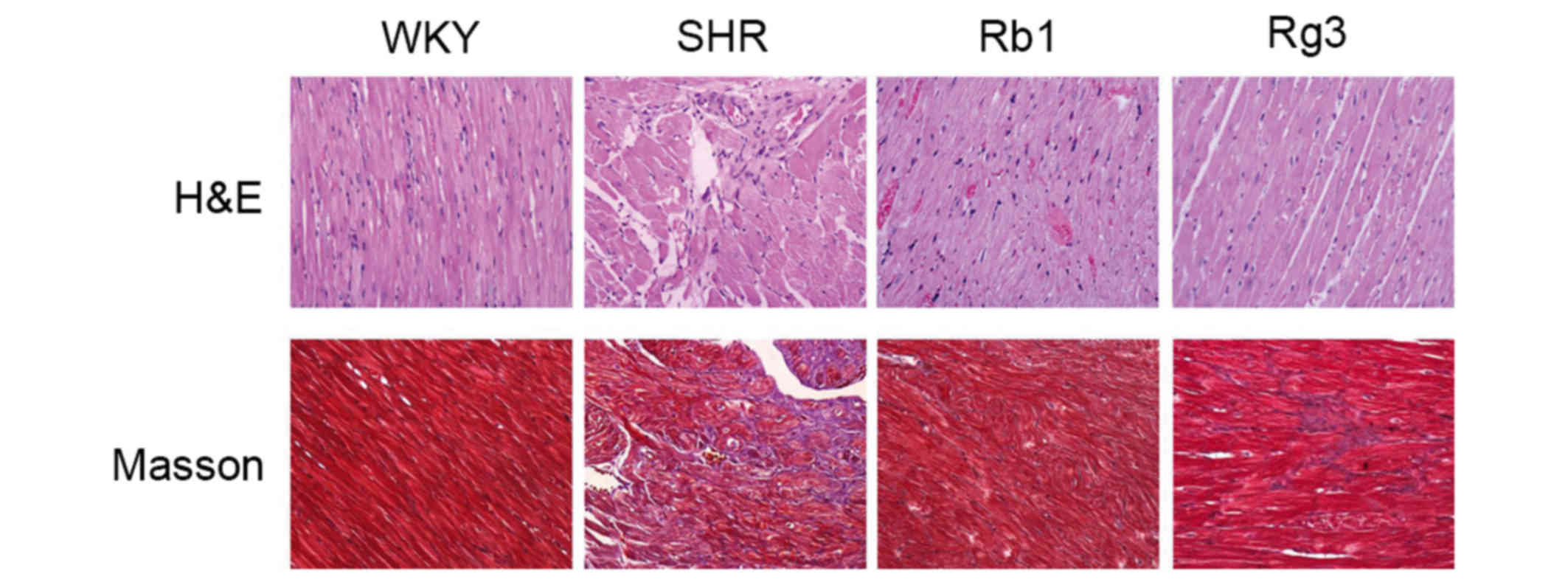
Representative H&E and Masson staining histology
photomicrographs are presented in Fig.
4. According to the H&E photomicrographs, myocardium
tissues from the SHR group exhibited increased myocardial cell
size, myocardial structural disorder and intercellular space
dilatation, which are the typical pathological changes of
ventricular remodeling induced by hypertension. Inflammatory cell
infiltration was also observed. The Masson photomicrographs
indicated increased collagen deposition (blue area) in the SHR
group compared with the WKY group. However, treatment with Rb1 and
Rg3 markedly improved all these histopathological changes.
Effects of Rb1 and Rg3 on RAS activity
and TGF-β1 levels in the myocardium
The expression of ACE, Ang II, AT1 and TGF-β1 in the
myocardium was evaluated using IHC. Representative photomicrographs
are presented in Fig. 5A and
quantitative results are presented in Fig. 5B-E. Compared with the WKY group,
levels of ACE, Ang II, AT1 and TGF-β1 were significantly increased
in myocardium samples from the SHR group (all P<0.05). However,
these increases were significantly attenuated following treatment
with Rb1 and Rg3 (all P<0.05). Indeed, the expression of Ang II
and TGF-β1 in the Rb1 and Rg3 groups did not differ significantly
between that of the WKY group (Fig. 5D
and E). The downregulation of local RAS activity in the
myocardium reduced the expression of TGF-β1, which is also a key
factor to myocardial fibrosis.
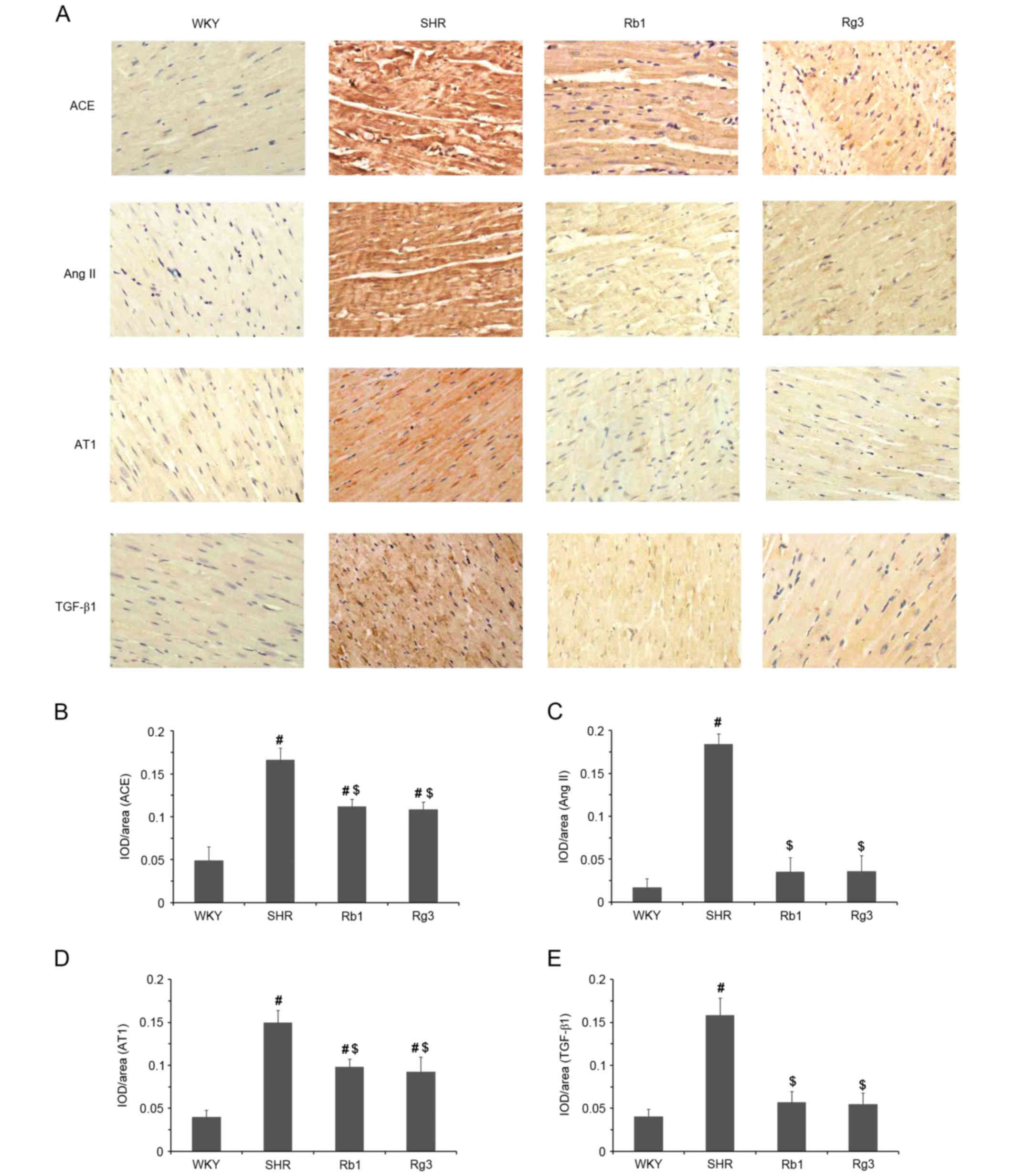 | Figure 5.Effects of Rb1 and Rg3 on RAS and
TGF-β1 levels in the myocardium. (A) Representative IHC staining
photomicrographs of myocardium tissue. (Magnification, ×400).
Antibodies against ACE, Ang II, AT1 and TGF-β1 were used as the
primary antibodies. (B-E) Quantitative results of IHC staining,
which were presented as IOD/Area and were proportional to the
levels of ACE, Ang II, AT1 and TGF-β1. Data are presented as the
mean ± standard deviation, n=4. #P<0.05 vs. the WKY
group following treatment; $P<0.05 vs. the SHR group
following treatment. Rb1, ginsenoside Rb1; Rg3, ginsenoside Rg3;
RAS, renin angiotensin system; TGF-β1, transforming growth factor
β1; IHC, immunohistochemistry; ACE, angiotensin converting enzyme;
Ang II, angiotensin II; AT1, Ang II receptor type 1; IOD,
integrated optical density; WKY, Wistar-Kyoto; SHR, spontaneously
hypertensive rats. |
Effects of Rb1 and Rg3 on levels of
inflammatory factors and ET-1 in the myocardium
Levels of tumor necrosis factor-α (TNF-α),
interleukin-6 (IL-6), interleukin-1β (IL-1β) and ET-1 mRNA were
measured (Fig. 6). In the SHR group,
the expression of TNF-α, IL-6, IL-1β and ET-1 mRNA were all
significantly higher than in the WKY group (all P<0.05).
However, in the Rb1 and Rg3 groups, the levels of all four mRNAs
were all significantly lower than the SHR group (P<0.05). These
results suggest that Rb1 and Rg3 may attenuate inflammation and
endothelial injury in the myocardium.
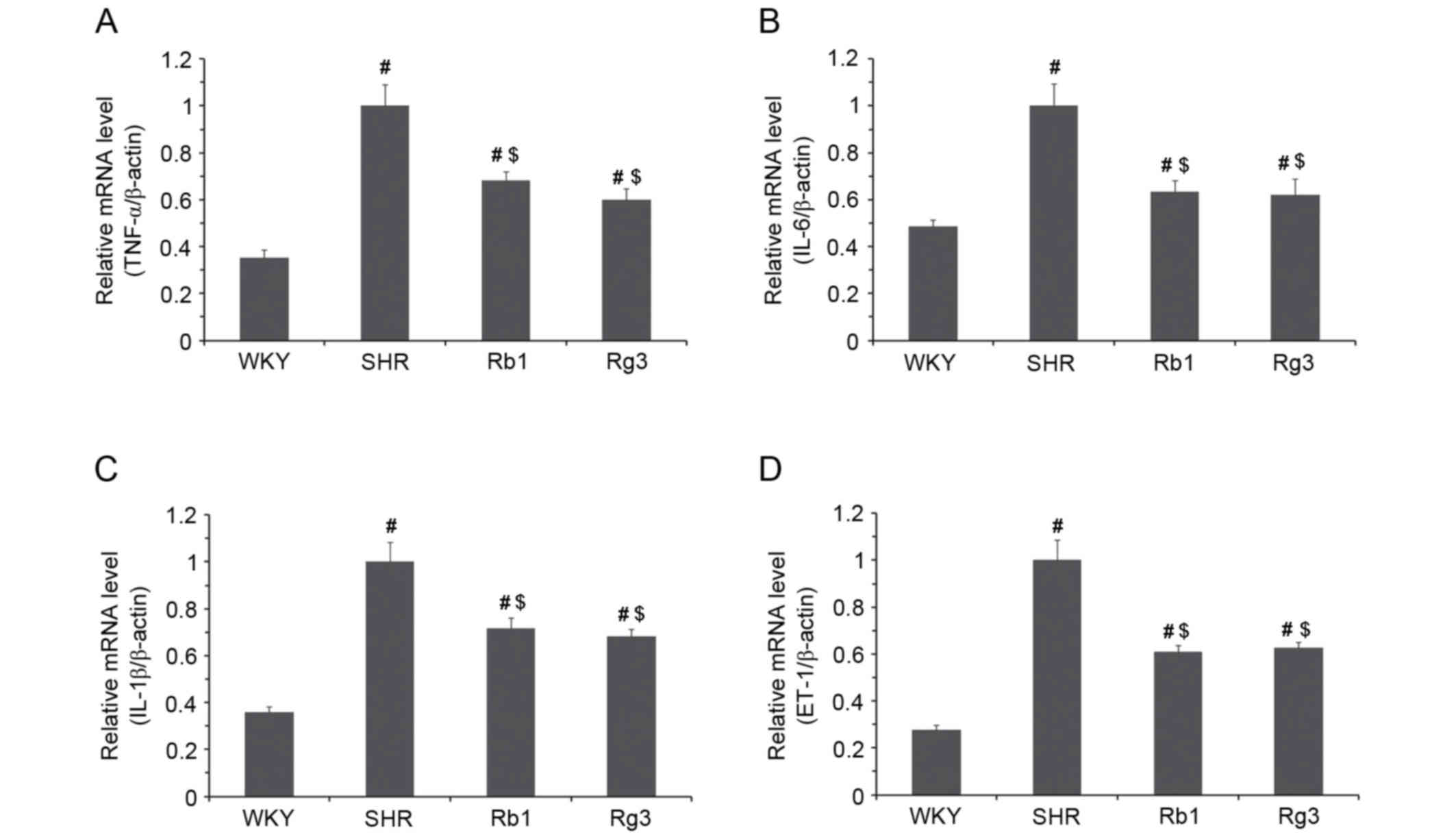 | Figure 6.Effects of Rb1 and Rg3 on the mRNA
levels of inflammatory factors and ET-1 in the myocardium. (A)
TNF-α, (B) IL-6, (C) IL-1β and (D) ET-1 mRNA expression. β-actin
was used as a housekeeping gene. Data are presented as the mean ±
standard deviation, n=4. #P<0.05 vs. the WKY group
following treatment; $P<0.05 vs. the SHR group
following treatment. Rb1, ginsenoside Rb1; Rg3, ginsenoside Rg3;
TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; IL-1β,
interleukin-1β; ET-1, endothelin-1; WKY, Wistar-Kyoto; SHR,
spontaneously hypertensive rats. |
Discussion
One of the main mechanisms by which hypertension
arises in SHR is congenital abnormal RAS activation, which is
similar to what occurs in human essential hypertension (25). Therefore, ACE inhibitors and AT1
receptor blockers are currently the most popular antihypertensive
medicines. Ang II, the key factor of the RAS, increases blood
pressure and induces inflammation, endothelial injury and fibrosis
in various organs (26).
Ang II is an independent factor that causes
myocardial fibrosis and ventricular remodeling, regardless whether
it has induced hypertension or not (27). A high amount of RAS activity causes
hypertension, whereas high Ang II levels in the myocardium increase
TGF-β1 expression, which may promote fibroblast proliferation and
collagen deposition (28). High
levels of Ang II may also increase levels of TNF-α, IL-6, IL-1β and
ET-1, which are all closely associated with cardiovascular injury
(26) and may contribute to
myocardial fibrosis and ventricular remodeling.
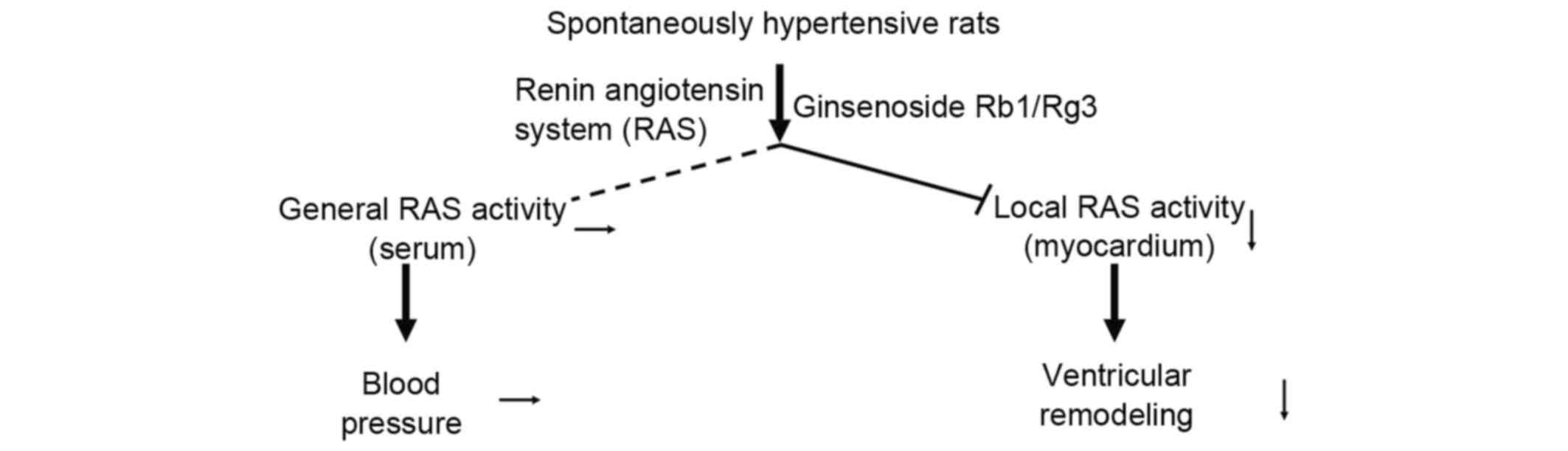
The results of the present study indicated that Rb1
and Rg3 attenuated cardiac function and structural changes in SHR
but did not reduce blood pressure (Fig.
7). RAS activity was attenuated in the myocardium of the Rb1
and Rg3 groups, whereas RAS activity in the serum remained high.
The mechanism of this action remains unknown; something in the
myocardium, for example, angiotensin-converting enzyme 2, an enzyme
that degrades Ang II into Ang 1–7 (27), may be activated by Rb1 or Rg3 to
degrade Ang II. However, further studies are required to determine
the exact mechanism of action by which this occurs.
As RAS activity was attenuated in the myocardium,
levels of TGF-β1, TNF-α, IL-6, IL-1β and ET-1 were all reduced
following treatment with Rg3 and Rb1, although blood pressure
remained high. Furthermore, Rb1 and Rg3 were able to protect
against myocardial fibrosis and ventricular remodeling in the
heart. According to the results of histopathology and
echocardiography, the cardiovascular protective effect of Rg3 is
similar to that of Rb1 in SHR. However, further studies are
required to determine the cardioprotective effects of Rg3 in other
models of cardiovascular injury, particularly in adriamycin induced
heart failure (29).
Rg3 is a widely used anti-tumor medicine in China
and it is important to determine whether it also exhibits
protective effects on the cardiovascular system. A number of
first-line chemotherapy agents, including adriamycin, are
cardiotoxic (30). As Rg3 exhibits
cardioprotective effects, it is worth determining whether Rg3
attenuates the cardiotoxicity of chemotherapeutic agents when they
are used together. This may facilitate the development of safer and
more efficient treatment protocols for chemotherapy.
In conclusion, the present study indicated that
hypertension and high RAS activity in the myocardium induce cardiac
structural and functional changes, which may be attenuated by Rg3
as well as Rb1, independent of reducing blood pressure.
Furthermore, the mechanism of these protective effects of Rg3 on
the cardiovascular system may be associated with the attenuation of
RAS activity in the myocardium. Therefore, Rg3 may also attenuate
inflammation, endothelial injury and fibrosis. Therefore, the
results of the present study demonstrated that Rg3 exhibits similar
cardiovascular protective effects to Rb1 independent of reducing
blood pressure in SHR rats.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81473378) and
Outstanding Doctoral Cultivation Program of Norman Bethune Health
Science Center of Jilin University (2014).
References
|
1
|
Jiang QS, Huang XN, Dai ZK, Yang GZ, Zhou
QX, Shi JS and Wu Q: Inhibitory effect of ginsenoside Rb1 on
cardiac hypertrophy induced by monocrotaline in rat. J
Ethnopharmacol. 111:567–572. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Z, Li M, Wu WK, Tan HM and Geng DF:
Ginsenoside Rb1 preconditioning protects against myocardial
infarction after regional ischemia and reperfusion by activation of
phosphatidylinositol-3-kinase signal transduction. Cardiovasc Drugs
Ther. 22:443–452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang T, Yu X, Qu S, Xu H, Han B and Sui D:
Effect of ginsenoside Rb3 on myocardial injury and heart function
impairment induced by isoproterenol in rats. Eur J Pharmacol.
636:121–125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang T, Yu XF, Qu SC, Xu HL and Sui DY:
Ginsenoside Rb3 inhibits angiotensin II-induced vascular smooth
muscle cells proliferation. Basic Clin Pharmacol Toxicol.
107:685–689. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao H, Lv D, Zhang W, Dong W, Feng J,
Xiang Z, Huang L, Qin C and Zhang L: Ginsenoside-Rb1 attenuates
dilated cardiomyopathy in cTnT(R141W) transgenic mouse. J Pharmacol
Sci. 112:214–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi Y, Han B, Yu X, Qu S and Sui D:
Ginsenoside Rb3 ameliorates myocardial ischemia-reperfusion injury
in rats. Pharm Biol. 49:900–906. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu X, Jiang Y, Yu X, Fu W, Zhang H and
Sui D: Ginsenoside-Rb3 protects the myocardium from
ischemia-reperfusion injury via the inhibition of apoptosis in
rats. Exp Ther Med. 8:1751–1756. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li F, Wu X, Li J and Niu Q: Ginsenoside
Rg1 ameliorates hippocampal long-term potentiation and memory in an
Alzheimer's disease model. Mol Med Rep. 13:4904–4910. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu H, Yu X, Qu S, Chen Y, Wang Z and Sui
D: Protective effect of Panax quinquefolium 20(S)-protopanaxadiol
saponins, isolated from Pana quinquefolium, on permanent focal
cerebral ischemic injury in rats. Exp Ther Med. 7:165–170. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Radad K, Gille G, Moldzio R, Saito H and
Rausch WD: Ginsenosides Rb1 and Rg1 effects on mesencephalic
dopaminergic cells stressed with glutamate. Brain Res. 1021:41–53.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qu X, Qu S, Yu X, Xu H, Chen Y, Ma X and
Sui D: Pseudo-G-Rh2 induces mitochondrial-mediated apoptosis in
SGC-7901 human gastric cancer cells. Oncol Rep. 26:1441–1446.
2011.PubMed/NCBI
|
|
12
|
Choi YJ, Lee HJ, Kang DW, Han IH, Choi BK
and Cho WH: Ginsenoside Rg3 induces apoptosis in the U87MG human
glioblastoma cell line through the MEK signaling pathway and
reactive oxygen species. Oncol Rep. 30:1362–1370. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim BM, Kim DH, Park JH, Na HK and Surh
YJ: Ginsenoside Rg3 induces apoptosis of human breast cancer
(MDA-MB-231) cells. J Cancer Prev. 18:177–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin G, Yu X, Wang J, Qu S and Sui D:
Beneficial effects of 20(S)-protopanaxadiol on antitumor activity
and toxicity of cyclophosphamide in tumor-bearing mice. Exp Ther
Med. 5:443–447. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang YH, Li HD, Li B, Jiang SD and Jiang
LS: Ginsenoside Rg3 induces DNA damage in human osteosarcoma cells
and reduces MNNG-induced DNA damage and apoptosis in normal human
cells. Oncol Rep. 31:919–925. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo Y, Zhang P, Zeng HQ, Lou SF and Wang
DX: Ginsenoside Rg3 induces apoptosis in human multiple myeloma
cells via the activation of Bcl-2-associated X protein. Mol Med
Rep. 12:3557–3562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vo HT, Cho JY, Choi YE, Choi YS and Jeong
YH: Kinetic study for the optimization of ginsenoside Rg3
production by heat treatment of ginsenoside Rb1. J Ginseng Res.
39:304–313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng LQ, Na JR, Bang MH, Kim MK and Yang
DC: Conversion of major ginsenoside Rb1 to 20(S)-ginsenoside Rg3 by
Microbacterium sp. GS514. Phytochemistry. 69:218–224. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Quan LH, Min JW, Yang DU, Kim YJ and Yang
DC: Enzymatic biotransformation of ginsenoside Rb1 to 20(S)-Rg3 by
recombinant β-glucosidase from Microbacterium esteraromaticum. Appl
Microbiol Biotechnol. 94:377–384. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pfeffer JM and Pfeffer MA: Angiotensin
converting enzyme inhibition and ventricular remodeling in heart
failure. Am J Med. 84:37–44. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pfeffer JM, Pfeffer MA, Mirsky I and
Braunwald E: Regression of left ventricular hypertrophy and
prevention of left ventricular dysfunction by captopril in the
spontaneously hypertensive rat. Proc Natl Acad Sci USA. 79:pp.
3310–3314. 1982; View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dias Da, Silva VJ, Viana PC Cavalcante, de
Melo Alves R, Salgado HC, Montano N and Fazan R Jr:
Antihypertensive action of amiodarone in spontaneously hypertensive
rats. Hypertension. 38:597–601. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang LP, Jiang YC, Yu XF, Xu HL, Li M,
Zhao XZ and Sui DY: Ginsenoside Rg3 improves cardiac function after
myocardial ischemia/reperfusion via attenuating apoptosis and
inflammation. Evid Based Complement Alternat Med. 2016:69678532016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Williams GH, Braley LM and Menachery A:
Decreased adrenal responsiveness to angiotensin II: A defect
present in spontaneously hypertensive rats. A possible model of
human essential hypertension. J Clin Invest. 69:31–37. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Duprez DA: Role of the
renin-angiotensin-aldosterone system in vascular remodeling and
inflammation: A clinical review. J Hypertens. 24:983–991. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhong J, Basu R, Guo D, Chow FL, Byrns S,
Schuster M, Loibner H, Wang XH, Penninger JM, Kassiri Z and Oudit
GY: Angiotensin-converting enzyme 2 suppresses pathological
hypertrophy, myocardial fibrosis, and cardiac dysfunction.
Circulation. 122:717–728. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li M, Jiang Y, Jing W, Sun B, Miao C and
Ren L: Quercetin provides greater cardioprotective effect than its
glycoside derivative rutin on isoproterenol-induced cardiac
fibrosis in the rat. Can J Physiol Pharmacol. 91:951–959. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zong WN, Yang XH, Chen XM, Huang HJ, Zheng
HJ, Qin XY, Yong YH, Cao K, Huang J and Lu XZ: Regulation of
angiotensin-(1–7) and angiotensin II type 1 receptor by telmisartan
and losartan in adriamycin-induced rat heart failure. Acta
Pharmacol Sin. 32:1345–1350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Singal PK, Li T, Kumar D, Danelisen I and
Iliskovic N: Adriamycin-induced heart failure: Mechanism and
modulation. Mol Cell Biochem. 207:77–86. 2000. View Article : Google Scholar : PubMed/NCBI
|