Introduction
Pulmonary arterial hypertension (PAH) is a
life-threatening disorder characterized by obstructive remodeling
of the pulmonary arteries, which may lead to right-sided heart
failure and mortality (1–2). PAH may be defined as a mean pulmonary
artery pressure (mPAP) of ≥20 mmHg at rest or >30 mmHg with
exercise, along with a pulmonary artery occlusion pressure of ≤15
mmHg (1–2). The estimated incidence of primary
pulmonary hypertension is 1–2/million in the global population
(1–2). There are currently three treatment
pathways, namely phosphodiesterase type 5 inhibitor or soluble
guanylate cyclase stimulator, prostacyclin class therapy and
endothelium receptor antagonist, which target the imbalances of
three substances; nitric oxide, prostacyclin and endothelin,
respectively (3). Despite the
efficacy of these pharmacological therapies in improving symptoms,
they do not prevent the progression of PAH or reduce the mortality
rate of patients (3). Therefore,
novel approaches that more effectively target PAH are required to
control the cellular components associated with pulmonary
remodeling. The chronic and continued proliferation of human
pulmonary artery smooth muscle cells (hPASMCs), in addition to
apoptotic resistance, leads to hypoxic pulmonary arterial
remodeling, which is considered to be a key mechanism for the
pathological development of PAH (4).
ATP-sensitive potassium (KATP) channels are located on
the cytoplasmic membrane and on subcellular membranes. Subcellular
membrane-associated KATP channels are divided into three
types: Sarcolemmal KATP, mitochondrial KATP
(mitoKATP) and nuclear KATP.
MitoKATP channels, which contribute to the control of
mitochondrial volume and energetic status (5–7), exhibit
high sensitivity to hypoxia (8).
Closed mitoKATP channels under normoxic conditions are
opened when cells are subjected to hypoxia, and depolarization of
the mitochondrial membrane potential (ΔΨm) is induced accordingly
(9). In addition, previous studies
indicate that the opening of mitoKATP channels leads to
the generation of reactive oxygen species (ROS) (5–7). ROS are
considered to be a double-edged sword, and their effect on cells as
either a survival or apoptotic signal is controlled by the dosage,
duration, type and site of ROS production (5–7).
Furthermore, opening of mitoKATP channels followed by
ΔΨm depolarization may contribute to increased expression of
hypoxia-inducible factor-1α (HIF-1α) and proliferation of PASMCs
(10). A previous study demonstrated
that mitoKATP channel opening participates in ROS
overproduction in hPASMCs through ΔΨm depolarization, which
indicates the involvement of mitoKATP in the chronic
proliferation and/or enhanced apoptotic resistance of hPASMCs
(11). However, the molecular
mechanism underlying the role of mitoKATP channels in
the abnormal proliferation or apoptotic resistance of hPASMCs
remains unknown.
The present study investigated whether
mitoKATP channels promote the aberrant prolonged
proliferation of hPASMCs by assessing ΔΨm and intracellular ROS.
The results indicated that mitoKATP channels contribute
to hypoxic pulmonary arterial remodeling via regulation of the
ROS/HIF/microRNA (miR)-210/iron-sulfur cluster protein (ISCU)
signaling pathway.
Materials and methods
Cell culture and treatment
hPASMCs were purchased from ScienCell Research
Laboratories (Carlsbad, CA, USA) and cultured in smooth muscle cell
medium (ScienCell Research Laboratories, Inc.) supplemented with 2%
fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), 100 U/ml penicillin, 100 µg/ml streptomycin and
1% smooth muscle cell growth supplement (all ScienCell Research
Laboratories) in an atmosphere containing 5% CO2 at
37°C. The culture medium was replaced every 2 to 3 days until cell
80% confluence was reached. hPASMCs at passages 4–10 were used in
the following experimental assays. Smooth muscle cells were
confirmed by immunohistochemistry using anti-α-actin monoclonal
antibody (dilution 1:300; sc-130616; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) incubated at 37°C for 2 h. The cells were
cultured at 37°C in hypoxic conditions (5% CO2 and 5%
O2) or normoxic conditions (1% CO2 and 20%
O2) as control for 6, 12 and 24 h respectively, prior to
the experiment. Subsequently, after treatment with
mitoKATP inhibitor 5-hydroxydecanoate (5-HD; 500
µmol/l), mitoKATP agonist diazoxide (100 µmol/l) or
HIF-1α inhibitor 3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole
(YC-1; 50 µmol/l; all from Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) or smooth muscle cell medium supplemented with 2% fetal
bovine serum as control for 1 h, immediately the cells were further
maintained at 37°C in a humidified atmosphere of 5% CO2
and 5% O2 for 6,12 and 24 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
mRNA and miRNA PCR experiments were performed in
triplicate. For mRNA expression assay, total RNA was extracted from
treated cells using TRIzol (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). cDNA was synthesized using a ReverTra Ace
qPCR RT kit (Toyobo Co., Ltd., Osaka, Japan). RNA concentration and
purity were assessed by UV spectrophotometry
(1.8<A260/A280<2.0). RNA integrity was assessed using
electrophoresis. Reverse transcription reaction was performed using
4 µg of total RNA and the First Strand cDNA Synthesis kit (Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. To generate standard curves, 1 µl of first-strand
cDNA was amplified using a Premix Ex Taq Version Kit (Takara Bio,
Inc., Otsu, Japan) according to the manufacturer's instructions and
quantification of PCR products was assessed to plot standard
curves. The qPCR reactions were performed using a SYBR Green PCR
Master mix (CWBIO; Beijing, China) in a CFX-96 system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) using the following primers:
HIF-1α, forward 5′-CTGATCATCTGACCAAAACTC-3′ and reverse
5′-GTTTCAACCCAGACATATCCAC-3′; and β-actin, forward
5′-ACTCTTCCAGCCTTCCTTCC-3′ and reverse 5′-CGTCATACTCCTGCTTGCTG-3′.
Real-time PCR was performed using the SYBR Premix Ex TaqTM II
(Takara Bio, Inc.) protocol on a Bio-Rad Connect real-time PCR
detection system with cycling conditions of 95°C for 3 min,
followed by 40 cycles of 95°C for 30 sec and 60°C for 30 sec.
β-actin was used as an internal control. The relative mRNA
expression levels were determined using the 2−ΔΔCq
method and normalized to β-actin mRNA (12,13). For
the miR-210 expression assay, total cellular RNA was extracted with
TRIzol reagent. cDNA was synthesized by reverse transcription using
the miRNART-primer (RibBio Co., Ltd., Guangzhou, China). The qPCR
reactions were performed using a SYBR Green PCR Master mix using
miR-210 and U6 primer purchased from RibBio Co., Ltd. (sequences
not supplied), which served as normalization control. qPCR was
performed under the following steps: 95°C for 3 min, followed by 40
cycles of 95°C for 30 sec and 56°C for 30 sec. miR-210 expression
relative to U6 control was calculated using the 2−ΔΔCq
method (14).
Measurement of ΔΨm and intracellular
ROS
The ΔΨm was monitored using flow cytometry according
to a modified method (15). Briefly,
treated cells were detached by trypsinization and washed twice in
PBS. A total of 10 µg/ml rhodamine-123 (R-123, Sigma-Aldrich; Merck
KGaA) was then added and incubated for 30 min at 37°C in the dark.
The fluorescence intensity of cells was measured using a FACSAria
II cytometer (BD643182; BD Biosciences, San Jose, CA, USA).
Measurement of mitochondrial intracellular ROS was
performed as described previously (11). The fluorescent probe
2′,7′-dichlorofluorescin diacetate (DCFH-DA) was purchased from
Sigma-Aldrich; Merck KGaA.
RNA transfection
A total of 2×105 cells were plated into
6-well plates ins mooth muscle cell medium supplemented with 2%
fetal bovine serum in normoxic conditions (1% CO2 and
20% O2) at 37°C for 24 h. Cells were transfected with
miR-210 mimic (miR-210) or inhibitor (anti-210) (RibBio Co., Ltd.)
at a final concentration of 80 nmol/l, or three small interfering
RNAs (siRNA1, siRNA2 and siRNA3) against ISCU (GenePharma,
Shanghai, China) at a final concentration of 5 nmol/l using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
with serum-free smooth muscle cell medium, as described previously
(11). The follow sequences were
used: miR-210 mimic, forward 5′-CUGUGCGUGUGACAGCGGGUGA-3′ and
reverse 5′-UUGACACGCACACUGUCGCCGA-3′; and inhibitor, forward
5′-UCAGCCGCUGUCACACGCACAG-3′ and reverse
5′-CAGUACUUUUGUGUAGUACAA-3′. After 6 h, the culture medium was
replenished with fresh smooth muscle cell medium supplemented 2%
fetal bovine serum and cells were cultured for an additional 24 h
at 37°C, then subjected to hypoxia (5% CO2 and 5%
O2) and normoxia (1% CO2 and 20%
O2) at 37°C for 24 h. Control miR sequences and control
siRNA sequences were used (miR-con, anti-con, si-con) as negative
control. After miR-210 inhibitor and control transfection for 24 h,
cells were treated with 5-HD (500 µmol/l) or diazoxide (100 µmol/l)
and exposed to hypoxia for 48 h at 37°C.
Western blot analysis
Cells were lysed with lysis buffer (1% Triton X-100,
50 mmol/l Tris-HCl, pH 7.4, 25 mmol/l glycerophosphate, 150 mmol/l
NaCl, 2 mmol/l EDTA, 2 mmol/l ethylene glycol tetraacetic acid, 1
mmol/l phenylmethylsulfonyl fluoride, 10% glycerol and 1% protease
and phosphatase inhibitors) for 10 min on ice. The lysates were
collected and centrifuged at 15,000 × g for 15 min at 4°C. The
supernatant was recovered, and protein concentration was measured
using a protein assay kit (Pierce; Thermo Fisher Scientific, Inc.).
The proteins loaded (40 mg per lane) were subsequently separated by
12% SDS-PAGE and transferred to a polyvinylidene difluoride
membrane (PVDF; Thermo Fisher Scientific, Inc.), blocked at 37°C
for 2 h in 5% non-fat milk, and incubated with anti- proliferating
cell nuclear antigen (PCNA; sc-25280) or anti-ISCU antibody
(sc-271536) or β-actin antibody (sc-47778) or GAPDH (sc-25778) at
1:4,000 or 1:3,000 or 1:10,000 or 1:5,000, respectively (Santa Cruz
Biotechnology, Inc.) at 4°C overnight. After washing for 10 min in
1X Tris-buffered saline-Tween-20 solution three times, the target
protein was probed with the horseradish peroxidase-conjugated goat
anti-rabbit IgG antibody (sc-3841; at 1:6,000 dilution; Santa Cruz
Biotechnology, Inc.) at 37°C for 2 h. After washing for 10 min in
1X Tris-buffered saline-Tween-20 solution three times, the bound
antibody on the PVDF membrane was detected by chemiluminescence
with the ECL Detection Reagent kit (Pierce; Thermo Fisher
Scientific, Inc.) and the protein expression was analyzed using
Quantity One 4.6.2 (Bio-Rad Laboratories, Inc.) (16).
Statistical analysis
Statistical analysis was performed using one-way
analysis of variance and Duncan's test using Graphpad software
(version 5.0; GraphPad Software, Inc., La Jolla, CA, USA). The data
were expressed as the mean ± standard deviation in three
independent experiments. The statistical significance of
differences among groups was determined using an analysis of
variance or Student's t-test. P<0.05 was considered to represent
a statistically significant difference.
Results
MitoKATP increases ROS
generation in hPASMCs through the collapse of ΔΨm
Hypoxia-triggered mitoKATP channel
opening initiated by ATP depletion leads to the collapse of ΔΨm,
which has been implicated in the generation of ROS (17). Consistent with a previous report
(11), the present study observed
that R-123 fluorescence intensity was significantly increased in
hPASMCs subjected to hypoxia for 12 and 24 h (P<0.05 and
P<0.01, respectively), relative to normoxic control cells
(Fig. 1A), indicating that ΔΨm
depolarization had occurred. Furthermore, hPASMCs exhibited
significantly increased levels of ROS after 12 and 24 h of hypoxia
(P<0.05), as determined by DCFH-DA staining (Fig. 1B). To further investigate whether
mitoKATP channels were associated with ROS production in
hPASMCs, the levels of intracellular ROS were measured following
treatment with diazoxide or 5-HD, to activate or inhibit
mitoKATP, respectively. As predicted, opening of
mitoKATP significantly increased the level of
intracellular ROS, and closure of mitoKATP significantly
decreased the level of intracellular ROS under hypoxic conditions
(P<0.05; Fig. 1C). Furthermore,
under normoxic conditions, the opening of mitoKATP
significantly increased the level of intracellular ROS while
closure of mitoKATP significantly decreased the level of
intracellular ROS (P<0.05; Fig.
1C). These results suggest that mitoKATP channels
may be involved in the generation of ROS in hPASMCs under hypoxic
conditions, potentially through the collapse of ΔΨm.
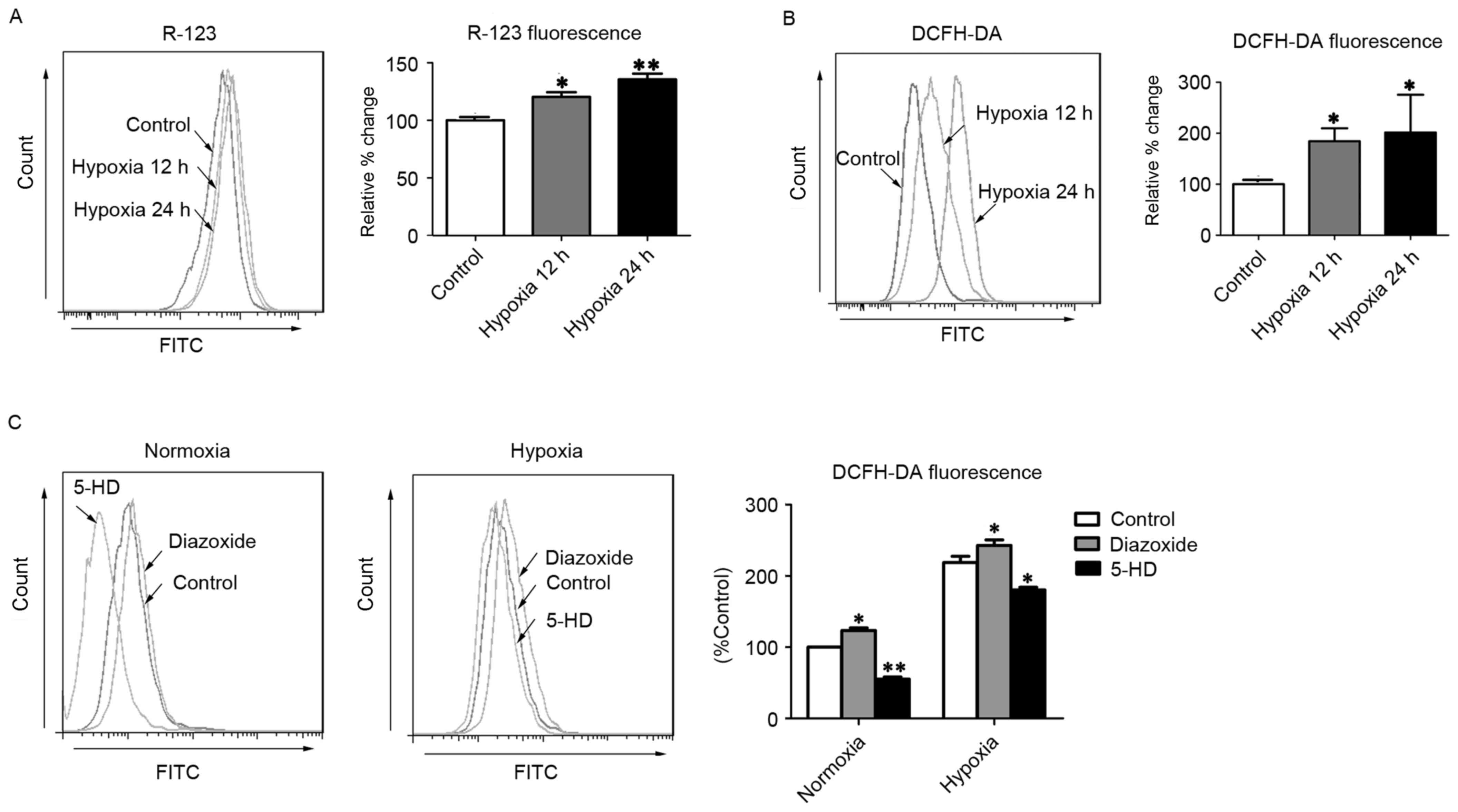 | Figure 1.MitoKATP channel opening
increases intercellular ROS in hPASMCs. (A) The ΔΨm was assessed
with R-123 fluorescence after cells were exposed to hypoxia for 12
or 24 h. (B) Levels of intracellular ROS were assessed by DCFH-DA
fluorescence after cells were exposed to hypoxia for 12 or 24 h.
(C) The intracellular ROS levels were assessed by DCFH-DA
fluorescence after cells were treated with 5-HD or diazoxide upon
normoxia or hypoxia. *P<0.05 and **P<0.01 vs. control. Data
are expressed as percentage of control (n=3), and were analyzed by
a Student's t-test. MitoKATP, mitochondrial
ATP-sensitive potassium channel; ROS, reactive oxygen species;
hPASMCs, human pulmonary artery smooth muscle cells; ΔΨm,
mitochondrial membrane potential; R-123, rhodamine-123; DCFH-DA,
2′,7′-dichlorofluorescin diacetate; 5-HD, 5-hydroxyde-canoate FITC,
fluorescein isothiocyanate. |
MitoKATP interacts with
HIF-1α in a positive feedback manner in hPASMCs
As HIF-1α is a critical oxygen sensor and master
transcriptional regulator of the hypoxic adaptive response, it has
been suggested that mitoKATP channels function in
combination with HIF-1α and its downstream targets, including
miR-210 and ISCU (12,18). To investigate whether
mitoKATP was associated with the expression of HIF-1α
and miR-210 in hPASMCs during hypoxia, the expression of HIF-1α
mRNA and miR-210 was measured using RT-qPCR. In hPASMCs subjected
to hypoxia, levels of HIF-1α mRNA were significantly increased at
12 and 24 h (P<0.01 and P<0.001, respectively), and miR-210
levels were significantly increased at 6 and 12 h (P<0.05;
Fig. 2A). In turn, expression of
miR-210 was significantly decreased following treatment with the
HIF-1α inhibitor YC-1 (P<0.01; Fig.
2B), and expression of HIF-1α was significantly increased
following treatment with the mitoKATP agonist diazoxide
(P<0.05; Fig. 2C). Furthermore,
the levels of HIF-1α mRNA and miR-210 were significantly decreased
following closure of mitoKATP with 5-HD (P<0.001 and
P<0.01, respectively; Fig. 2C).
To further investigate whether HIF-1α was involved in the
regulation of mitoKATP, ΔΨm and ROS levels were measured
in hPASMCs treated with YC-1. Significant decreases in the
fluorescence intensity of R-123 (P<0.05) and DCFH-DA (P<0.01)
were observed in hPASMCs upon inhibition of HIF-1α (Fig. 2D).
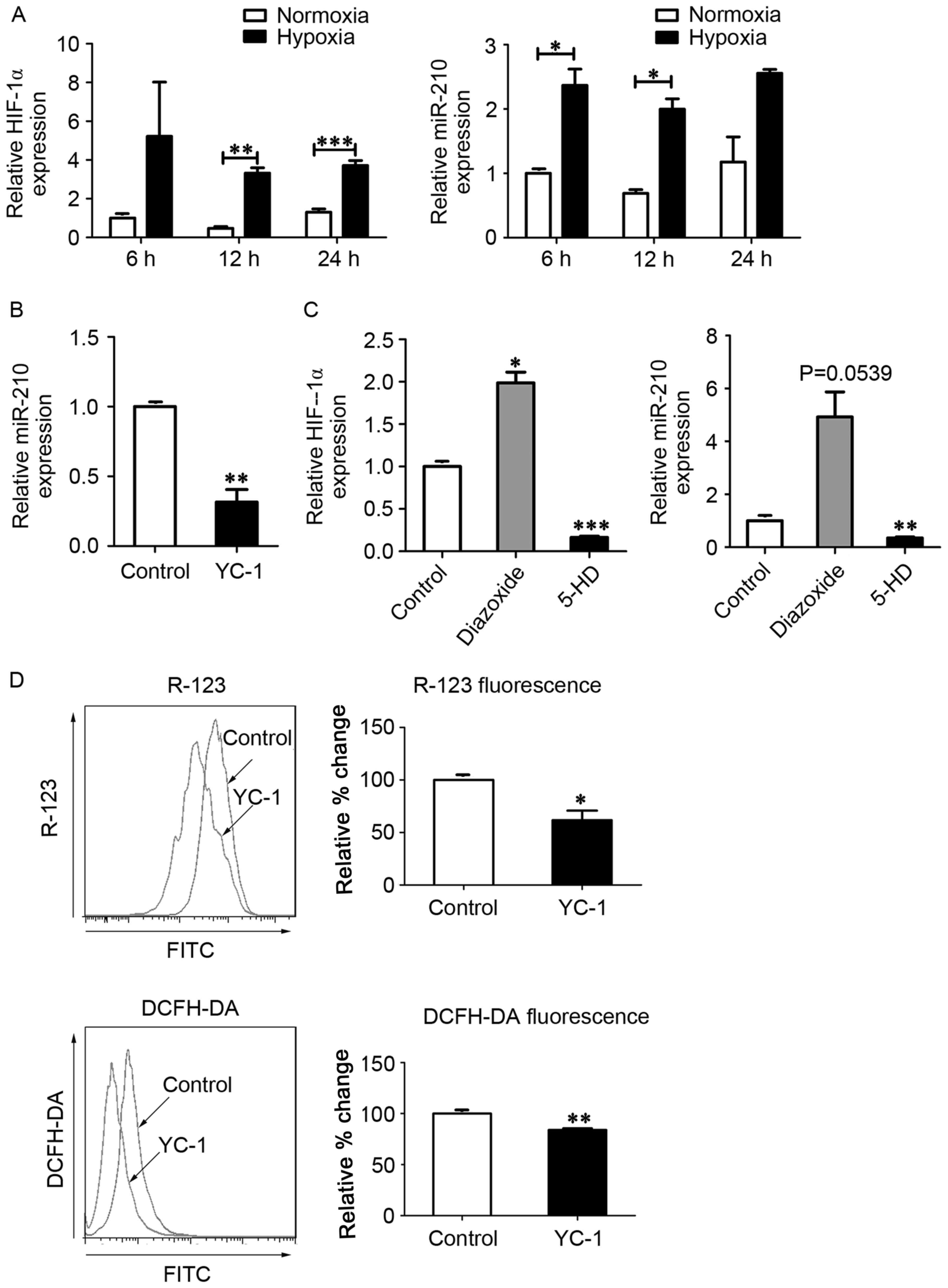 | Figure 2.MitoKATP interacts with
HIF-1α in a positive feedback manner. (A) Expression of HIF-1α and
miR-210 was assessed by qPCR after cells were exposed to normoxia
or hypoxia for 6, 12 and 24 h. Data are expressed as the mean ±
standard deviation (n=3). (B) hPASMCs were treated with YC-1 upon
hypoxia for 12 h, and the expression of miR-210 was measured by
qPCR. The value was relative to the control without YC-1, using U6
as a reference. (C) hPASMCs were treated with 5-HD or diazoxide in
a hypoxia state for 12 h, and the expression of HIF-1α and miR-210
was measured by qPCR. The value was relative to control (untreated)
cells, using β-actin as a reference. (D) hPASMCs were treated with
YC-1 upon hypoxia for 12 h. Left: Intracellular ROS content
analyzed by DCFH-DA fluorescence. Right: ΔΨm analyzed by R-123
fluorescence. Left and right: Results were expressed as a
percentage of control (untreated) cells and were presented as the
mean ± standard deviation (n=3). *P<0.05, **P<0.01 and
***P<0.001 vs. control. Data were analyzed by a Student's
t-test. MitoKATP, mitochondrial ATP-sensitive potassium
channel; HIF-1α, hypoxia-inducible factor-1α; miR, microRNA; qPCR,
quantitative polymerase chain reaction; hPASMCs, human pulmonary
artery smooth muscle cells; YC-1,
3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole; 5-HD,
5-hydroxyde-canoate; DCFH-DA, 2′,7′-dichlorofluorescin diacetate;
ΔΨm, mitochondrial membrane potential; R-123,
rhodamine-123. |
These data suggest that mitoKATP channel
opening increases the expression of HIF-1α and its downstream
target miR-210, leading to HIF-1α-mediated regulation of
mitoKATP through increased ROS levels in a positive
feedback manner in hPASMCs under hypoxic conditions.
MitoKATP participates in
ROS generation in hPASMCs under hypoxic conditions through
miR-210/ISCU
Previous studies have indicated that ISCU is a
downstream regulatory target of miR-210 (19,20).
ISCU, as an essential component of respiratory electron transfer
complexes, is involved in ROS generation in the mitochondria
(21). The present study blocked the
expression of miR-210 using an inhibitor of miR-210. Under normoxic
and hypoxic conditions, significant decreases in the fluorescence
intensity of R-123 (P<0.001 and P<0.01, respectively) and
DCFH-DA (P<0.01) were observed in hPASMCs upon treatment with
miR-210 inhibitor transfection (Fig. 3A
and B), indicating an involvement of miR-210 in the alteration
of ΔΨm and generation of ROS. The expression of ISCU was
subsequently assessed, and was demonstrated to be reduced or
increased following treatment with miR-210 mimic or miR-210
inhibitor under normoxic conditions, respectively (Fig. 3C). As shown in Fig. 3C, ISCU expression was reduced under
hypoxic conditions compared with normoxic conditions.
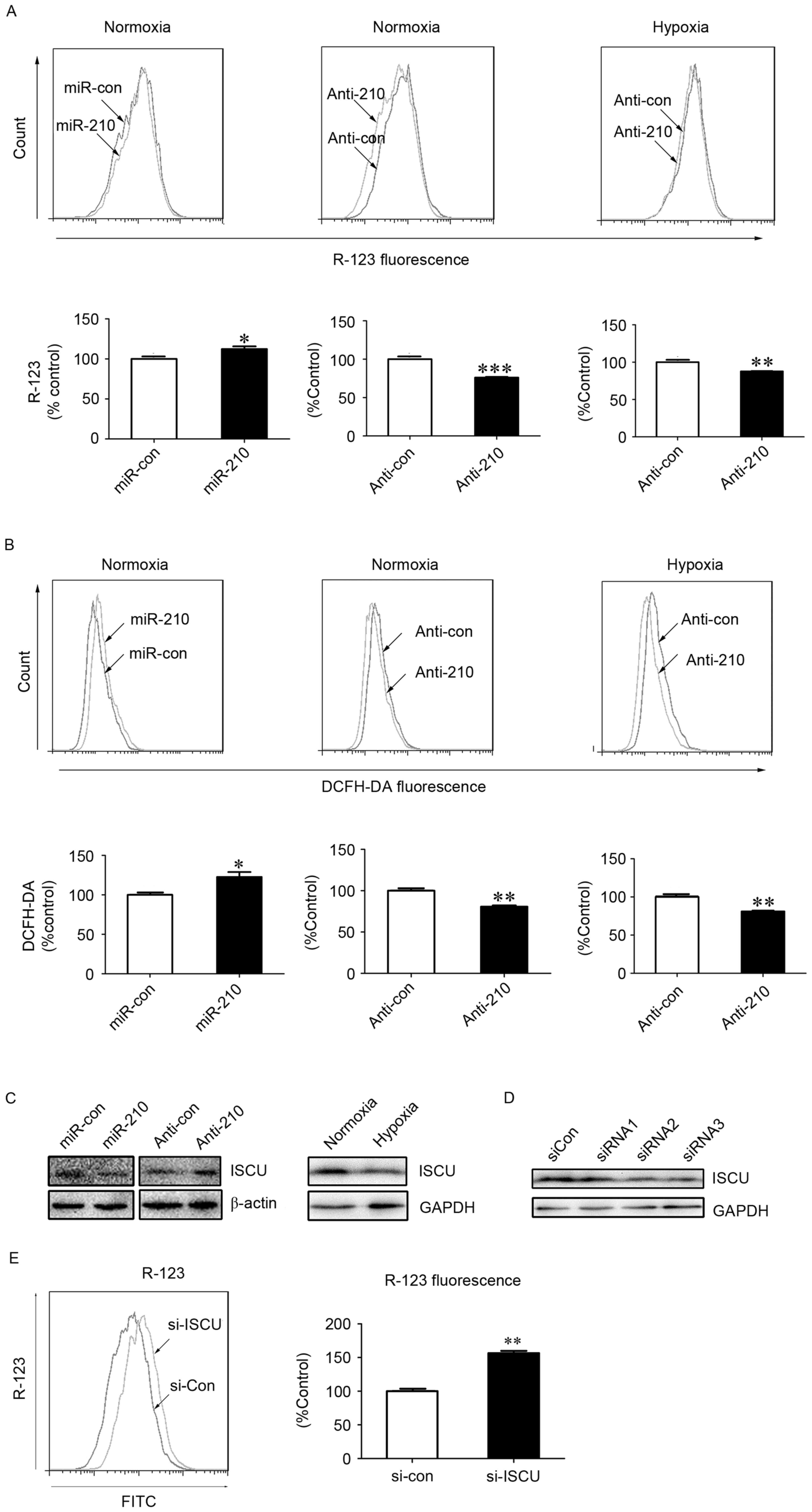 | Figure 3.MitoKATP participates in
ROS generation in hPASMCs under hypoxic conditions via the
miR-210/ISCU pathway. The (A) ΔΨm and (B) intracellular ROS levels
were measured in hPASMCs transfected with miR-210 mimic or
inhibitor for 24 h, prior to normoxia or hypoxia for an additional
24 h. Data are presented as a percentage of control (n=3), and
analyzed by a Student's t-test. (C) Expression of ISCU was assessed
using western blot analysis upon hypoxia or normoxia, or cells were
transfected with miR-210 mimic or inhibitor upon normoxia for 48 h.
The β-actin or GAPDH acted as loading control. (D) hPASMCs were
transfected with 5 nM of siRNAs against ISCU or si-Con for 48 h.
The silencing efficiencies of ISCU (si-ISCU) were measured by
western blot analysis. GAPDH acted as loading control. (E) ΔΨm in
hPASMCs were transfected with si-Con or si-ISCU upon normoxia for
24 h. The ΔΨm was analyzed by R-123 fluorescence. Data is expressed
as a percentage of the control (n=3), and were analyzed by a
Student's t-test. *P<0.05, **P<0.01 and ***P<0.001 vs.
control. MitoKATP, mitochondrial ATP-sensitive potassium
channel; ROS, reactive oxygen species; hPASMCs, human pulmonary
artery smooth muscle cells; miR, microRNA; si-Con, scramble
control; si, small interfering; ISCU, iron-sulfur cluster protein;
ΔΨm, mitochondrial membrane potential; YC-1,
3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole; DCFH-DA,
2′,7′-dichlorofluorescin diacetate; R-123, Rhodamine-123; GAPDH,
glyceraldehyde 3-phsphate dehydrogenase; con, control; si, small
interfering RNA; FITC, fluorescein isothiocyanate. |
Furthermore, in order to determine the role of ISCU
in the activity of mitoKATP, specific siRNA against ISCU
or scrambled control siRNA was transfected into hPASMCs. Western
blot analysis indicated that two of the three siRNAs specifically
reduced the expression of ISCU (Fig.
3D). In addition, a significant increase in the fluorescence
intensity of R-123 was observed in hPASMCs upon treatment with ISCU
siRNA (P<0.01; Fig. 3E), which
suggests a role of ISCU in the collapse of ΔΨm, the fluorescence
intensity of R-123 was lower in hypoxic environments. Loss of ISCU
may impair the function of the electron transfer chain, leading to
increased generation of ROS and decreased production of ATP.
Decreased levels of ATP may then trigger opening of
mitoKATP channels and a collapse of ΔΨm.
These data suggest that mitoKATP channels
indirectly participate in ROS generation in hPASMCs under hypoxic
conditions via the miR-210/ISCU pathway. This may supplement the
potential direct involvement of mitoKATP in ROS
generation, mediated by channel opening and ΔΨm collapse.
MitoKATP promotes the
proliferation of hPASMCs through miR-210 signaling
As hPASMCs grow relatively slowly, the proliferation
of hPASMCs was evaluated by measuring the expression of PCNA, as an
established marker of cell proliferation that is synthesized in the
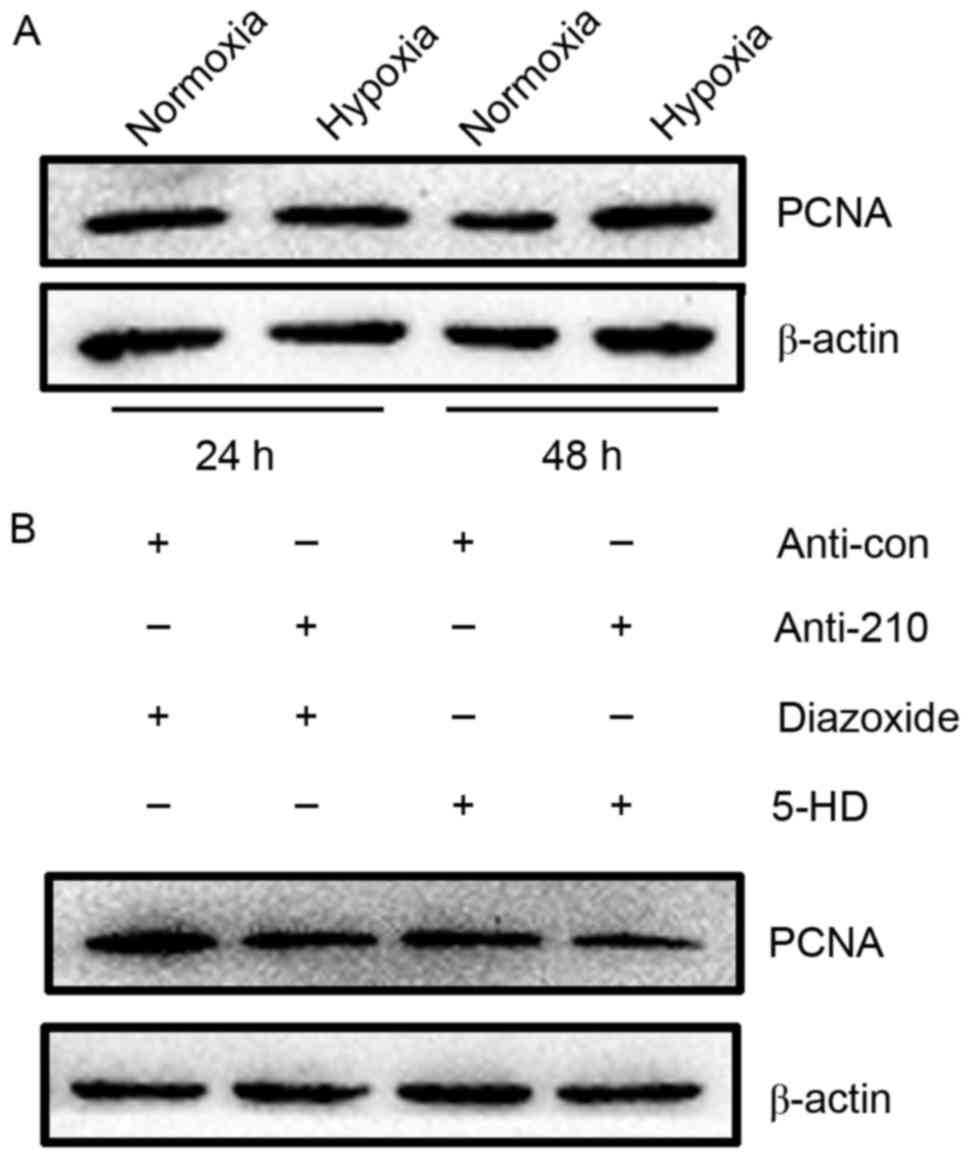
early G1 and S phases of the cell cycle (22). Results of western blot analysis
showed that PCNA expression was markedly increased in hPASMCs
subjected to hypoxia for 48 h, relative to normoxic controls
(Fig. 4A). The proliferation of
hPASMCs upon activation or inhibition of mitoKATP and
inhibition of miR-210 was subsequently assessed. It was observed
that activation of mitoKATP increased the expression of
PCNA, while inhibition of mitoKATP and miR-210 decreased
PCNA expression (Fig. 4B). These
results indicate that mitoKATP promotes the
proliferation of hPASMCs under hypoxic conditions through the
miR-210 signaling pathway.
Discussion
The proliferation and survival of hPASMCs are
essential in hypoxic pulmonary arterial remodeling, which typically
leads to PAH (4). KATP
channels were initially identified in the inner membrane of liver
mitochondria (23). Previous results
suggest that the opening of mitoKATP channels, which
induces the collapse of ΔΨm, contributes to the proliferation and
survival of different cell types, including cardiac myocytes
(24), renal epithelial cells
(25), cerebellar granule neurons
(26) and skin cells (27). A previous study also demonstrated
that hypoxic proliferation of hPASMCs was associated with
mitoKATP channel opening (11). However, the molecular mechanism
underlying the promotive effect of mitoKATP channel
opening on hPASMC proliferation in PAH remains unclear.
ROS has been implicated as a trigger that causes
aberrant proliferation or apoptotic resistance in hPASMCs under
hypoxic conditions (28). It has
been reported that a collapse of ΔΨm, resulting from
mitoKATP channel opening, leads to mitochondrial
respiratory chain disorders associated with an increase in complex
III-dependent ROS and a decrease in complex I-dependent ROS
(21). Thus, complex III is
considered to be a major ROS-releasing site during phases of
decreased membrane potential mediated by mitoKATP
channel opening (28).
However, it is established that HIF-1α is a critical
regulator of the hypoxic adaptive response during hypoxia stress
(22,23). Under normoxic conditions, HIF-1α is
hydroxylated by HIF prolyl-hydroxylases, which enables its
recognition and ubiquitination by von Hippel-Lindau E3 ubiquitin
ligase, with ubiquitin serving as a signal for proteosomal
degradation (24,25). As HIF prolyl-hydroxylase utilizes
oxygen as a co-substrate, it is inhibited in response to hypoxia,
which allows HIF-1α to function. This negative regulation mediated
by HIF prolyl-hydroxylase is an additional mechanism of HIF-1α
regulation linked to ROS (11,28).
Increases in mitochondrial ROS induced by hypoxia has been
demonstrated to contribute to HIF-1α stabilization under hypoxic
conditions (29–31). As HIF-1α is a major transcriptional
regulator of the cellular and developmental response to hypoxia
(32,33), it is possible that crosstalk occurs
between HIF-1α and mitoKATP during hPASMC proliferation
under hypoxic conditions. In accordance with this assumption, the
present data indicated that mitoKATP channel opening
participates in the chronic proliferation of hPASMCs in
collaboration with HIF-1α under hypoxic conditions.
miR-210, which is upregulated by HIF-1α, is
considered to be among the most hypoxia-sensitive miRs and a
microregulator of the hypoxia pathway (34). As a downstream target of miR-210
(12,33,35),
ISCU is responsible for the assembly of iron sulfur clusters [Fe-S]
in mitochondrial respiratory complexes (complexes I, II and III)
(21,36). Dysfunction of ISCU leads to an
impaired electron transport chain, which results in two
consequences: i) ROS generation or ii) a decreased H+
gradient leading to suppressed production of ATP. Subsequently,
mitoKATP channels are opened in response to reduced
levels of ATP (11,28). Previous data suggest that
HIF-1/miR-210/ISCU may act as a critical signaling axis in the
regulation of mitochondrial metabolism during hypoxia (19,36–38).
Consistent with these findings, results of the present study
suggested that mitoKATP promotes the proliferation of
hPASMCs via upregulation of the ROS/HIF/miR-210/ISCU signaling
pathway.
The present study demonstrated that
mitoKATP channels were involved in the proliferation of
hPASMCs under hypoxic conditions. An increase in ROS during hypoxia
leads to a downregulation in HIF prolyl-hydroxylase, enabling it to
function as a regulator of the hypoxic response. Due to dysfunction
of respiratory chain complexes in the inner mitochondrial membrane,
ROS levels may be generated by two positive feedback loops:
ROS/HIF/miR-210/ISCU and ATP/mitoKATP/ ΔΨm. Aberrancies
in the levels of ROS ultimately lead to the proliferation of
hPASMCs under hypoxic conditions. The potential association between
mitoKATP and the chronic proliferation of hPASMCs
suggests that targeting of mitoKATP may be a useful
strategy for the treatment of hypoxia-associated pulmonary
diseases, including PAH.
In conclusion, HIF-1α functions in the hypoxia
adaptive response following reduced inhibition by
prolyl-hydroxylase, and serves its role due to increased generation
of ROS. Elevated levels of ROS, which have been associated with
dysfunction of respiratory chain complexes, may be further
upregulated by two positive feedback loops: ROS/HIF/miR-210/ISCU
and ATP/mitoKATP/ΔΨm.
Acknowledgements
The present study was supported by the Wuhan Health
and Family Planning Commission Project (grant nos. WX14B09 and
WX13B07).
Glossary
Abbreviations
Abbreviations:
|
hPASMCs
|
human pulmonary artery smooth muscle
cells
|
|
mitoKATP
|
mitochondrial ATP-sensitive potassium
channel
|
|
HIF
|
hypoxia-inducible factor
|
|
ROS
|
reactive oxygen species
|
|
PAH
|
pulmonary arterial hypertension
|
|
ΔΨm
|
mitochondrial membrane potential
|
|
miR
|
microRNA
|
|
5-HD
|
5-hydroxydecanoate
|
|
R-123
|
rhodamine-123
|
|
ISCU
|
iron-sulfur cluster protein
|
|
DCFH-DA
|
2′,7′-dichlorofluorescin diacetate
|
|
YC-1
|
3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole
|
|
siRNA
|
small interfering RNA
|
References
|
1
|
Blok IM, van Riel ACMJ, van Dijk APJ,
Mulder BJM and Bouma BJ: From bosentan to macitentan for pulmonary
arterial hypertension and adult congenital heart disease: Further
improvement? Int J Cardiol. 227:51–52. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ryan JJ and Archer SL: Emerging concepts
in the molecular basis of pulmonary arterial hypertension (PAH):
Part I: Metabolic plasticity and mitochondrial dynamics in the
pulmonary circulation and right ventricle in pulmonary arterial
hypertension. Circulation. 131:1691–1702. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Frumkin LR: The pharmacological treatment
of pulmonary arterial hypertension. Pharmacol Rev. 64:583–620.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ibe JC, Zhou Q, Chen T, Tang H, Yuan JX,
Raj JU and Zhou G: Adenosine monophosphate-activated protein kinase
is required for pulmonary artery smooth muscle cell survival and
the development of hypoxic pulmonary hypertension. Am J Respir Cell
Mol Biol. 49:609–618. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vanden Hoek TL, Becker LB, Shao Z, Li C
and Schumacker PT: Reactive oxygen species released from
mitochondria during brief hypoxia induce preconditioning in
cardiomyocytes. J Biol Chem. 273:18092–18098. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pain T, Yang XM, Critz SD, Yue Y, Nakano
A, Liu GS, Heusch G, Cohen MV and Downey JM: Opening of
mitochondrial K(ATP) channels triggers the preconditioned state by
generating free radicals. Circ Res. 87:460–466. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han J, Kim N, Park J, Seog DH, Joo H and
Kim E: Opening of mitochondrial ATP-sensitive potassium channels
evokes oxygen radical generation in rabbit heart slices. J Biochem.
131:721–727. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Teramoto N: Physiological roles of
ATP-sensitive K+ channels in smooth muscle. J Physiol. 572:617–624.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Samavati L, Monick MM, Sanlioglu S,
Buettner GR, Oberley LW and Hunninghake GW: Mitochondrial K(ATP)
channel openers activate the ERK kinase by an oxidant-dependent
mechanism. Am J Physiol Cell Physiol. 283:C273–C281. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao JP, Guo Z, Zhou ZG, Chen J, Hu HL,
Wang T and Zhang ZX: Effect of opening of mitochondrial
ATP-sensitive K(+) channels on the expression of hypoxia inducible
factor-1alpha and cell proliferation in pulmonary arterial smooth
muscle cells of rats. Sheng Li Xue Bao. 59:157–162. 2007.(In
Chinese). PubMed/NCBI
|
|
11
|
Hu HL, Zhang ZX, Chen CS, Cai C, Zhao JP
and Wang X: Effects of mitochondrial potassium channel and membrane
potential on hypoxic human pulmonary artery smooth muscle cells. Am
J Respir Cell Mol Biol. 42:661–666. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Liu Y, Chen J, Tang MJ, Zhang SL,
Wei LN, Li CH and Wei DB: Restriction-ligation-free (RLF) cloning:
A high-throughput cloning method by in vivo homologous
recombination of two PCR products. Genet Mol Res. 14:12306–12315.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jin Z, Sun T, Xia X, Wei Q, Song Y, Han Q,
Chen Q, Hu J and Zhang J: Optimized expression, purification of
herpes B virus gD protein in Escherichia coli, and production of
its monoclonal antibodies. Jundishapur J Microbiol. 9:e321832016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu YY, Chen TS, Qu JL, Pan WL, Sun L and
Wei XB: Dihydroartemisinin (DHA) induces caspase-3-dependent
apoptosis in human lung adenocarcinoma ASTC-a-1 cells. J Biomed
Sci. 16:162009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang J, Jin Z, Sun T, Jiang Y, Han Q,
Song Y, Chen Q and Xia X: Prokaryotic expression, purification and
polyclonal antibody production of a truncated recombinant rabies
virus L protein. Iranian J Biotechnol. 13:18–24. 2015. View Article : Google Scholar
|
|
17
|
Suski JM, Lebiedzinska M, Bonora M, Pinton
P, Duszynski J and Wieckowski MR: Relation between mitochondrial
membrane potential and ROS formation. Methods Mol Biol.
810:183–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y and Wei L, Wei D, Li X, Xu L and
Wei L: Testis-specific lactate dehydrogenase (LDH-C4) in skeletal
muscle enhances a pika's sprint-running capacity in hypoxic
environment. Int J Environ Res Public Health. 12:9218–9236. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Favaro E, Ramachandran A, McCormick R, Gee
H, Blancher C, Crosby M, Devlin C, Blick C, Buffa F, Li JL, et al:
MicroRNA-210 regulates mitochondrial free radical response to
hypoxia and krebs cycle in cancer cells by targeting iron sulfur
cluster protein ISCU. PLoS One. 5:e103452010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee DC, Romero R, Kim JS, Tarca AL,
Montenegro D, Pineles BL, Kim E, Lee J, Kim SY, Draghici S, et al:
miR-210 targets iron-sulfur cluster scaffold homologue in human
trophoblast cell lines: Siderosis of interstitial trophoblasts as a
novel pathology of preterm preeclampsia and
small-for-gestational-age pregnancies. Am J Pathol. 179:590–602.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rouault TA and Tong WH: Iron-sulfur
cluster biogenesis and human disease. Trends Genet. 24:398–407.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang H, Sun K, Ding J, Xu H, Zhu L, Zhang
K, Li X and Sun W: Harmine induces apoptosis and inhibits tumor
cell proliferation, migration and invasion through down-regulation
of cyclooxygenase-2 expression in gastric cancer. Phytomedicine.
21:348–355. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Noma A: ATP-regulated K+ channels in
cardiac muscle. Nature. 305:147–148. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Costa AD, Quinlan CL, Andrukhiv A, West
IC, Jabůrek M and Garlid KD: The direct physiological effects of
mitoK(ATP) opening on heart mitochondria. Am J Physiol Heart Circ
Physiol. 290:H406–H415. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nilakantan V, Liang H, Mortensen J, Taylor
E and Johnson CP: Variable effects of the mitoK(ATP) channel
modulators diazoxide and 5-HD in ATP-depleted renal epithelial
cells. Mol Cell Biochem. 335:211–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Teshima Y, Akao M, Li RA, Chong TH,
Baumgartner WA, Johnston MV and Marbán E: Mitochondrial
ATP-sensitive potassium channel activation protects cerebellar
granule neurons from apoptosis induced by oxidative stress. Stroke.
34:1796–1802. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cao C, Healey S, Amaral A, Lee-Couture A,
Wan S, Kouttab N, Chu W and Wan Y: ATP-sensitive potassium channel:
A novel target for protection against UV-induced human skin cell
damage. J Cell Physiol. 212:252–263. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Malinska D, Mirandola SR and Kunz WS:
Mitochondrial potassium channels and reactive oxygen species. FEBS
Lett. 584:2043–2048. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brunelle JK, Bell EL, Quesada NM,
Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC and Chandel NS:
Oxygen sensing requires mitochondrial ROS but not oxidative
phosphorylation. Cell Metab. 1:409–414. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guzy RD, Hoyos B, Robin E, Chen H, Liu L,
Mansfield KD, Simon MC, Hammerling U and Schumacker PT:
Mitochondrial complex III is required for hypoxia-induced ROS
production and cellular oxygen sensing. Cell Metab. 1:401–408.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Simon MC: Mitochondrial reactive oxygen
species are required for hypoxic HIF alpha stabilization. Adv Exp
Med Biol. 588:165–170. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Semenza GL: Hydroxylation of HIF-1: Oxygen
sensing at the molecular level. Physiology (Bethesda). 19:176–182.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Majmundar AJ, Wong WJ and Simon MC:
Hypoxia-inducible factors and the response to hypoxic stress. Mol
Cell. 40:294–309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chavez A, Miranda LF, Pichiule P and
Chavez JC: Mitochondria and hypoxia-induced gene expression
mediated by hypoxia-inducible factors. Ann N Y Acad Sci.
1147:312–320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Maxwell PH, Wiesener MS, Chang GW,
Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER and
Ratcliffe PJ: The tumour suppressor protein VHL targets
hypoxia-inducible factors for oxygen-dependent proteolysis. Nature.
399:271–275. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chan SY, Zhang YY, Hemann C, Mahoney CE,
Zweier JL and Loscalzo J: MicroRNA-210 controls mitochondrial
metabolism during hypoxia by repressing the iron-sulfur cluster
assembly proteins ISCU1/2. Cell Metab. 10:273–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang X, Le QT and Giaccia AJ:
MiR-210-micromanager of the hypoxia pathway. Trends Mol Med.
16:230–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen Z, Li Y, Zhang H, Huang P and Luthra
R: Hypoxia-regulated microRNA-210 modulates mitochondrial function
and decreases ISCU and COX10 expression. Oncogene. 29:4362–4368.
2010. View Article : Google Scholar : PubMed/NCBI
|