Introduction
Knee osteoarthritis (KOA) is a common type of joint
disease that affects the whole joint, including the articular
cartilage, synovial membrane, meniscus and subchondral bone
(1). It is characterized by
progressive articular cartilage degradation, subchondral bone
sclerosis, osteophyte formation and under-mineralization of the
trabecular structure (2,3). The precise underlying mechanism
responsible for KOA remains poorly understood but it is widely
accepted that biochemical and biomechanical factors serve important
roles in the pathogenesis of KOA (4–6).
Over the past two decades, studies investigating KOA
have focused on bodily fluid biomarkers, tissue biomarkers and
novel drug targets (7–12). Previous studies have demonstrated
that the receptor activator of nuclear factor-κB (RANK) signaling
pathway, transforming growth factor β1 (TGF-β1) pathway and nuclear
factor-κB pathway are all associated with KOA progression (13–15).
Additionally, Wnt inhibitory factor-1 (9), hypoxia-inducible factor-1α (10), osteopontin and Wnt5a (12) are associated with the severity of
KOA.
Cylindromatosis (CYLD) is a deubiquitinating enzyme
that has broad regulative effects on KOA, including its negative
regulation of the RANK (16) and
TGF-β1 signaling pathways (13,17).
Furthermore, CYLD is a crucial negative regulator of
osteoclastogenesis (16). Given the
roles of the aforementioned signaling pathways in articular
cartilage degradation and the subchondral bone remodeling
processes, it was hypothesized that the expression of CYLD in the
articular cartilage and subchondral bone may be associated with KOA
severity.
It has been reported that levels of CYLD mRNA in the
articular cartilage of patients with KOA are two-times higher than
those in the articular cartilage of healthy controls (18). However, limited data are available
regarding the expression of CYLD in other joint tissues and on the
association between CYLD expression in joint tissues and the
severity of KOA. Thus, the aim of the present study was to analyze
the expression patterns of CYLD in different sections of the knee
joint in patients with KOA to evaluate its potential association
with the severity of KOA.
Patients and methods
Patients
The protocol of the current study was approved by
the Ethics Committees of Shandong Provincial Hospital (Jinan,
China), the People's Hospital of Linzi (Linzi, China) and the
Central Hospital of Zibo Mining Group (Zibo, China). Human tibial
plateau (TP) samples were retrospectively collected from 129
patients with KOA that underwent primary total knee arthroplasty
due to KOA and 27 healthy controls who underwent primary amputation
due to severe lower-extremity trauma between January 2011 and
January 2016. All participants were enrolled from the
aforementioned three hospitals. Patients with KOA were diagnosed
according to the criteria of the American College of Rheumatology
(19). All patients and healthy
controls enrolled in the study had signed legally effective
informed consent forms. None of the enrolled subjects had a history
of bone tumors, conditions affecting bone remodeling, including
rheumatoid arthritis, osteoporosis, renal osteopathy or thyroid
disease, or use of drugs that affect bone metabolism. The Kellgren
Lawrence (KL) score was used to indicate the severity of KOA and
this was determined based on knee joint radiographs (20).
Histological analysis
TPs were harvested during surgery, washed with
normal saline to remove excess blood, wrapped with gauze and frozen
at −70°C. Samples were removed from storage 48 h prior to use and
thawed for 24 h at 4°C and 24 h at room temperature. For each TP, 9
samples were harvested from the medial, central and lateral
regions, respectively, at a depth of 1.0 cm (~0.3×0.3×1.0 cm). A
total of 3 samples were harvested from each region. Samples were
then fixed in 4% paraformaldehyde at room temperature for 24 h,
decalcified in 10% EDTA and dehydrated in graded ethanol. Following
dehydration, samples were embedded in paraffin, cut into 5-µm-thick
sections, placed on 3-aminopropyltriethoxy-silane coated slides and
stored at 4°C. Hematoxylin and eosin staining and safranin O
staining were performed following previously published protocols
(21).
Following histological staining, the severity of
articular cartilage damage was classified into four categories
based on the following modified Mankin system: Grade I (Mankin
score, 0–1); grade II (Mankin score, 2–5); grade III (Mankin score,
6–9); and grade IV (Mankin score, ≥10) (22).
Immunohistochemistry
Immunohistochemical staining was performed to assess
the expression of CYLD in TP samples using Histostain-SP kits
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
(23). Fixed paraffin-embedded
samples were heated at 60°C for 30 min, deparaffinized in xylene
(10 min × 2), rehydrated in alcohol (100% alcohol for 5 min × 2,
95% alcohol for 5 min × 2, and 85, 75 and 50% alcohol for 5 min
each), and washed with distilled water and PBS for 5 min each. The
samples were then treated successively with 3%
H2O2 in methanol at room temperature for 10
min and 20% goat serum (both Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) at room temperature for 30 min to block endogenous
peroxidase activity and nonspecific antibody binding. Subsequently,
sections were incubated with diluted rabbit polyclonal anti-CYLD
antibody (1:100; cat. no. ab137524; Abcam, Cambridge, UK) at 37°C
for 2 h, then with goat anti-rabbit immunoglobulin G (1:1,000; cat.
no. A0545; Sigma-Aldrich; Merck KGaA) at 37°C for 30 min. Finally,
samples were stained with diaminobenzidine tetrachloride at room
temperature for 8 min and counterstained with hematoxylin at room
temperature for 1 min. Sections prepared using PBS instead of
primary antibody were used as negative controls. All the sections
were examined by a blinded independent pathologist using a BX51
microscope (Olympus Corporation, Tokyo, Japan) at a magnification
of ×100.
CYLD levels were expressed as normalized optical
density (OD) values and were determined using a MetaMorph/DPIO/BX41
morphology image analysis system (Olympus Corporation). PBS was
used for OD normalization and the experiment was repeated in
triplicate. The variation coefficients of CYLD expression in the
articular cartilage and subchondral bone were <2%.
Statistical analyses
Data were analyzed using SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA). Normally distributed measurement data were
expressed as the mean ± standard deviation. Data were compared
using one-way analysis of variance with a Tukey's honest
significant difference post hoc test or t-tests. Skewed measurement
data were expressed as the median and interquartile range and
compared using Mann-Whitney U-tests. Numerical data were expressed
as percentages and differences between groups were compared using
the Pearson's χ2 test. Associations between CYLD
expression in TP samples and the severity of KOA were analyzed
using Spearman's correlation analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
A total of 156 participants were enrolled in the
present study. Baseline features of the patients that may have been
associated with the severity of articular cartilage degeneration
are listed in Table I. No
significant differences were identified in the age, sex or body
mass index between healthy controls and patients with KOA
(P>0.05). KL and Mankin scores of the patients with KOA from
three hospitals are listed in Table
II. Disease severity did not differ significantly among the
patients from the different hospitals (P>0.05).
 | Table I.Baseline features of patients. |
Table I.
Baseline features of patients.
| Parameter | n | Age [years, M
(QR)] | Sex (n, %
female) | BMI
(kg/m2, x¯±s) |
|---|
| Control group | 27 | 61.50
(49.00–71.00) | 12 (44.4) | 25.38±3.55 |
| KOA group | 129 | 63.00
(49.00–71.00) | 63 (48.8) | 26.89±3.58 |
|
χ2/t/z | NA | −0.203 | 0.173 | 1.975 |
| P-value | NA |
0.839 | 0.678 | 0.048 |
 | Table II.Baseline features of patients with
knee osteoarthritis from the three different hospitals. |
Table II.
Baseline features of patients with
knee osteoarthritis from the three different hospitals.
|
|
|
|
|
| KL scores (n,
%) | Mankin scores (n,
%) |
|---|
|
|
|
|
|
|
|
|
|---|
| Hospital names | n | Age (years) | Sex (n, %
female) | BMI
(kg/m2) | III | IV | II | III | IV |
|---|
| SDH | 59 | 65.00
(47.00–72.00) | 30 (50.8) | 27.14
(21.93–33.72) | 13 (22.03) | 46 (77.97) | 8 (13.56) | 18 (30.51) | 33 (55.93) |
| LZH | 42 | 60.00
(48.00–71.00) | 19 (45.2) | 25.64
(20.86–32.16) | 10 (23.81) | 32 (76.19) | 7 (16.67) | 18 (42.86) | 17 (40.47) |
| ZBH | 28 | 64.00
(49.00–71.00) | 14 (50.0) | 28.24
(23.03–34.32) | 7 (25.00) | 21 (75.00) | 6 (21.43) | 9 (32.14) | 13 (46.43) |
|
χ2/z | NA | −1.310 | 0.328 | −1.642 |
0.104 |
|
3.120 |
|
| P-value | NA | 0.190 | 0.849 | 0.102 |
0.949 |
|
0.538 |
|
Expression of CYLD in different TP
regions
CYLD expression in the TP samples was detected by
immunohistochemistry and was determined using normalized OD values.
As presented in Table III, CYLD
expression in the articular cartilage of patients with KOA was
significantly higher than that of the healthy controls
(t=8.66, P<0.001). By contrast, CYLD expression in the
subchondral bone of patients with KOA was significantly lower than
in the healthy controls (t=−15.004, P<0.001).
 | Table III.Expression of CYLD in the TP of
patients with KOA and healthy controls. |
Table III.
Expression of CYLD in the TP of
patients with KOA and healthy controls.
| CYLD levels
(%) | Articular
cartilage | Subchondral
bone | t | P-value |
|---|
| Control group | 6.53±2.01 | 11.46±2.34 | 8.295 | <0.001 |
| KOA group | 28.69±13.23 | 3.50±2.54 | 21.235 | <0.001 |
| t | 8.66 | −15.004 | NA | NA |
| P-value | <0.001 | <0.001 | NA | NA |
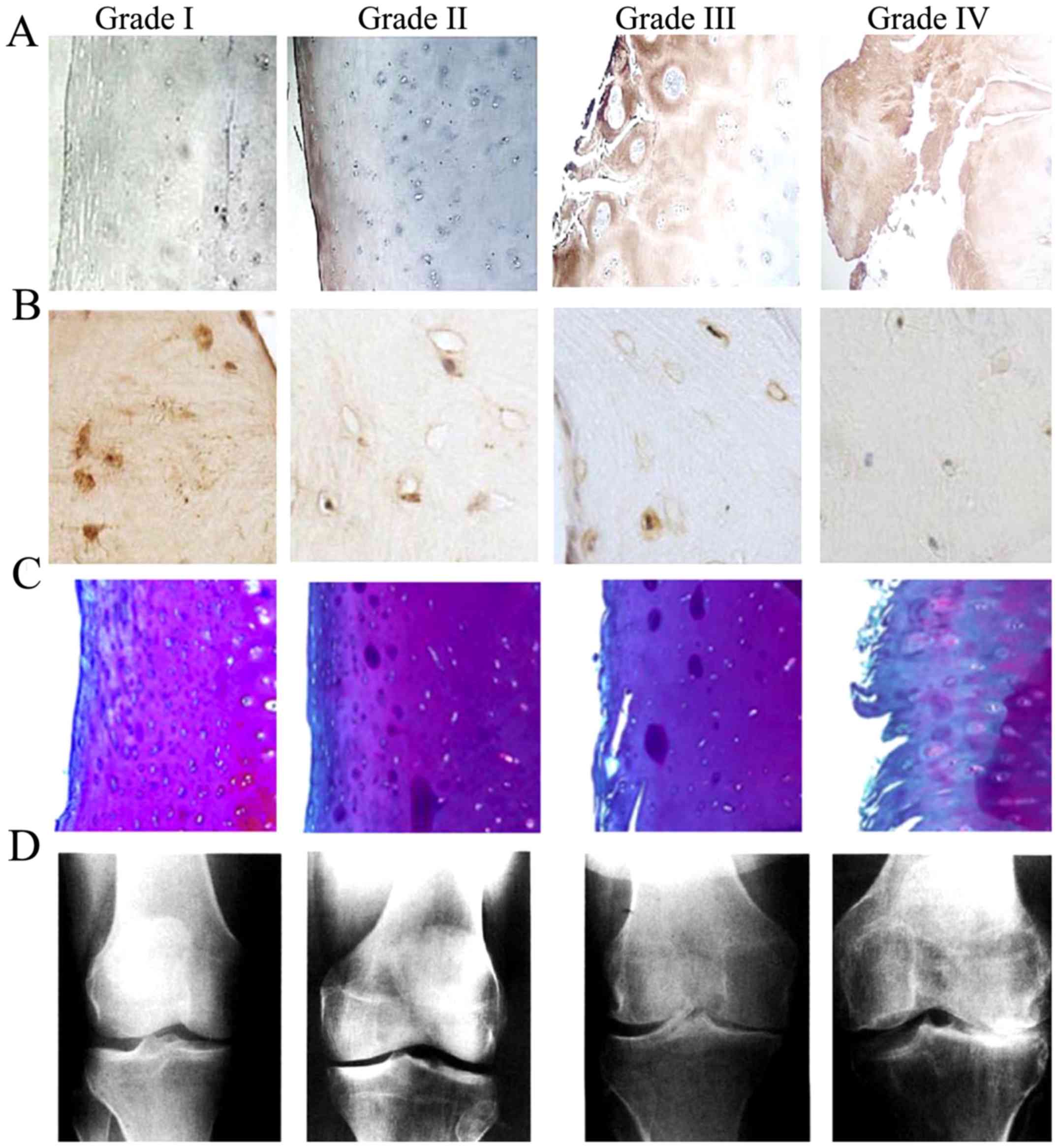
Representative immunohistochemical staining images
and radiographs of the TP samples are presented in Fig. 1. Immunohistochemistry detected CYLD
expression in the cell nuclei and cytoplasm; regions with positive
CYLD immunostaining were indicated by dark brown granular staining
(Fig. 1A and B). Safranin O stained
sections revealed that the degree of cartilage destruction was
positively associated with the severity of KOA (Fig. 1C). Radiographs of the KOA patients
identified clear narrowing of the joint space, which was also
positively associated with the severity of KOA (Fig. 1D). As shown in Fig. 1A compared with Fig. 1C and D, elevated CYLD expression in
the articular cartilage of patients with KOA was concomitant with
the severity of KOA. The opposite pattern of CYLD expression was
observed in the subchondral bone of patients with KOA, which was
demonstrated in Fig. 1B compared
with Fig. 1C and D.
Association between CYLD expression
and KL score
KL scores of all patients with KOA were >III,
which was in accordance for what is expected in patients requiring
total knee replacement. The potential association between CYLD
expression in the TP samples and KL score was analyzed by
Spearman's correlation analysis. The results are presented in
Table IV and indicate that the
expression of CYLD in the articular cartilage was positively
correlated with the KL score (r=0.837, P<0.001), and that
CYLD expression in the subchondral bone was negatively correlated
with the KL score (r=−0.802, P<0.001).
 | Table IV.Association between CYLD expression
in the TP and KL scores. |
Table IV.
Association between CYLD expression
in the TP and KL scores.
| CYLD levels
(%) | I–II | III | IV | r | P-value |
|---|
| n | 27 | 30 | 99 | NA | NA |
| Articular
cartilage |
6.53±2.01 | 14.22±4.17 | 33.08±11.83 |
0.837 | <0.001 |
| Subchondral
bone | 11.46±2.34 |
6.77±2.22 | 2.51±1.64 | −0.802 | <0.001 |
Associations between CYLD expression
and Mankin score
Articular cartilage sections were classified using
modified Mankin scores. As presented in Table V, the articular cartilage sections
classified as grades I, II, III and IV exhibited CYLD expression of
6.53±2.01, 14.23±4.66, 21.13±5.13 and 38.91±10.82%, respectively.
CYLD expression in the corresponding graded subchondral bone
sections of TP samples were 11.46±2.34, 7.81±1.66, 3.97±1.41 and
1.73±1.17%, respectively.
 | Table V.Association between CYLD expression
in the TP and Mankin scores. |
Table V.
Association between CYLD expression
in the TP and Mankin scores.
| CYLD levels
(%) | I | II | III | IV | r | P-value |
|---|
| n | 27 | 21 | 45 | 63 | NA | NA |
| Articular
cartilage |
6.53±2.01 | 14.23±4.66 | 21.13±5.13 | 38.91±10.82 |
0.925 | <0.001 |
| Subchondral
bone | 11.46±2.34 |
7.81±1.66 |
3.97±1.41 | 1.73±1.17 | −0.844 | <0.001 |
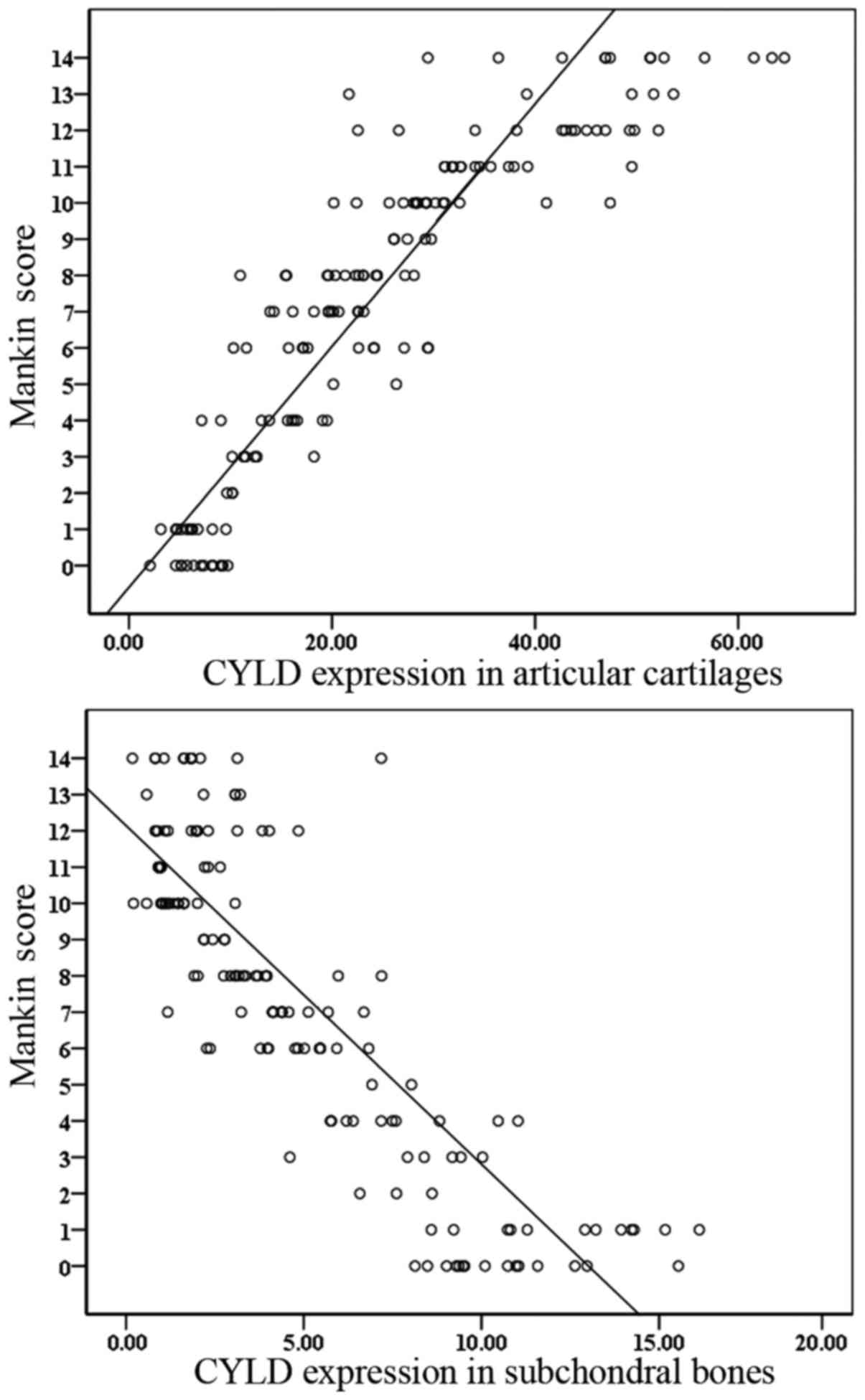
Spearman's correlation analysis was conducted to
assess the association between CYLD expression and Mankin scores.
The results indicated that CYLD expression in the articular
cartilage and subchondral bone was significantly correlated with
the Mankin score (r=0.925 and r=−0.844, all
P<0.001). Scatter diagrams were used to plot the correlation
between CYLD expression and Mankin score for the TP samples
(Fig. 2) and demonstrated that an
increased Mankin score is correlated with increased CYLD expression
in the articular cartilage and reduced CYLD expression in the
subchondral bone.
Discussion
The present study may represent the first attempt to
systematically evaluate CYLD expression in the TP tissue of
patients with KOA and determine its association with the severity
of KOA. KL and Mankin scores were used to grade the severity of
knee OA and the expression of CYLD in the articular cartilage and
subchondral bone was determined by immunohistochemistry. Notably,
it was determined that CYLD expression was significantly increased
in the articular cartilage but significantly reduced in the
subchondral bone of patients with KOA. Although there are very few
published studies investigating the expression of CYLD in TP
tissue, the results of certain reports are partially consistent
with those of the current study. Song et al (18) reported that CYLD expression in the
articular cartilage of patients with KOA was significantly higher
than in healthy controls.
Progressive articular cartilage degradation and
subchondral bone sclerosis are typical pathological changes that
occur in KOA. Despite extensive investigations into the sequence of
these pathological changes, a generally accepted mechanism has yet
to be established (24–28). However, an increasing number of
studies have demonstrated that there is molecular crosstalk between
the articular cartilage and subchondral bone (28–31). In
the present study, it was identified that the expression of CYLD in
the articular cartilage was positively correlated with the KL
(r=0.837, P<0.001) and Mankin scores (r=0.925,
P<0.001), whereas CYLD expression in the subchondral bone was
negatively correlated with KL (r=−0.802, P<0.001) and
Mankin scores (r=−0.844, P<0.001). These results indicate
that CYLD expression may be a potential biomarker for the diagnosis
of KOA, as well for monitoring the severity of KOA. Changes in the
expression of CYLD may be an early event involved in the
pathological processes of articular cartilage degradation and
subchondral bone remodeling abnormalities. Additionally, CYLD may
serve a crucial role in the molecular crosstalk that occurs between
the articular cartilage and subchondral bone in KOA.
Several signaling pathways have been implicated in
the molecular crosstalk between articular cartilage and subchondral
bone, including the TGF-β and Wnt signaling pathways (28–31).
CYLD may negatively regulate these signaling pathways during the
pathological processes of KOA (16,17).
This may explain why CYLD expression is increased in the articular
cartilage but decreased in the subchondral bone of patients with
KOA and may explain its correlation with the severity of KOA.
TGF-β also exhibits inverse expression trends in
KOA; its expression is decreased in the articular cartilage and
increased in the subchondral bone (29,32,33).
Furthermore, inhibition of TGF-β expression in the articular
cartilage or upregulation of TGF-β expression in the subchondral
bone aggravates the degeneration of articular cartilage (32). CYLD negatively regulates TGF-β
expression by deubiquitinating protein kinase B (17). Elevated Wnt signaling may also induce
bone sclerosis (34) and this may be
associated with the reduced deubiquitinating activity of CYLD
(35). Additionally, decreased CYLD
expression in the subchondral bone may induce subchondral bone
remodeling abnormalities via negative regulation of the RANK
signaling pathway (16).
Collectively, the aforementioned findings support the hypothesis
that CYLD exhibits regulatory activity during the processes of
articular cartilage degradation and subchondral bone remodeling in
KOA. However it remains unknown whether the articular cartilage and
subchondral bone influence each other via CYLD expression. Further
studies are required to elucidate the precise mechanisms of CYLD in
KOA, particularly regarding its potential effects on osteoblasts,
osteoclasts and chondrocytes.
The sample size of the present study was larger than
that of previous studies (9,12), and in the present study, TP samples
were collected from subjects admitted to three different public
hospitals, including one 2A hospital, one 3B hospital and one 3A
hospital. Hospitals in China are classified into 9 grades according
to the size of the hospital, medical technology, medical equipment,
management and medical quality; they are as follows: 1A, 1B, 1C,
2A, 2B, 2C, 3A, 3B and 3C. Hospitals grades as 1A, 1B and 1C are
township hospitals, which provide preventive care and minimal
health care. Hospitals grades as 2A, 2B, 2C, 3A, 3B and 3C are
affiliated with large and medium-sized cities, and responsible for
providing specialist health services. Most Chinese patients with
mild and moderate-severe diseases tend to choose large hospitals,
including 2A, 3A, 3B and 3C hospitals, for specialist treatment
(36). This means that samples
included in the present study were more likely to be representative
of all patients with KOA. Nevertheless, the present study still had
a number of limitations. The current study was retrospective; thus,
the collection of blood or synovial fluid samples from patients was
not possible. Given that biomarkers included in the bodily fluid
are more favorable for diagnosis (37), further studies are required to
investigate the associations between CYLD levels in bodily fluids
and the severity of KOA, which may assist the early diagnosis and
estimations of prognosis in patients. Furthermore, KL and Mankin
scores are artificial classification systems used for grading the
severity of KOA. The KL score is considered to be imprecise and
indefinite (37,38) and neither of these classification
systems fully reflect the severity of subchondral bone remodeling
abnormalities. Therefore, more detailed studies are necessary to
investigate the associations between CYLD levels in TP tissues, and
the activities of osteoblasts, osteoclasts and chondrocytes.
In conclusion, despite these limitations, the
present study demonstrated that CYLD levels in the articular
cartilage and subchondral bone of patients with KOA were associated
with the severity of KOA. Thus, CYLD may be a potential diagnostic
and predictive biomarker for KOA.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 30973040) and the
Natural Science Foundation of Shandong Province (grant no.
2012ZRB127AO).
Glossary
Abbreviations
Abbreviations:
|
CYLD
|
cylindromatosis
|
|
KOA
|
knee osteoarthritis
|
|
TP
|
tibial plateau
|
|
KL
|
Kellgren Lawrence
|
|
RANK
|
receptor activator of nuclear
factor-κB
|
|
TGF-β1
|
transforming growth factor β1
|
|
TP
|
tibial plateau
|
References
|
1
|
Heidari B: Knee osteoarthritis prevalence,
risk factors, pathogenesis and features: Part I. Caspian J Intern
Med. 2:205–212. 2011.PubMed/NCBI
|
|
2
|
Man GS and Mologhianu G: Osteoarthritis
pathogenesis-a complex process that involves the entire joint. J
Med Life. 7:37–41. 2014.PubMed/NCBI
|
|
3
|
Hayami T: Osteoarthritis of the knee joint
as a cause of musculoskeletal ambulation disability symptom complex
(MADS). Clin Calcium. 18:1574–1580. 2008.(In Japanese). PubMed/NCBI
|
|
4
|
Guilak F: Biomechanical factors in
osteoarthritis. Best Pract Res Clin Rheumatol. 25:815–823. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Powell A, Teichtahl AJ, Wluka AE and
Cicuttini FM: Obesity: A preventable risk factor for large joint
osteoarthritis which may act through biomechanical factors. Br J
Sports Med. 39:4–5. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fukui N, Ikeda Y, Ohnuki T, Tanaka N,
Hikita A, Mitomi H, Mori T, Juji T, Katsuragawa Y, Yamamoto S, et
al: Regional differences in chondrocyte metabolism in
osteoarthritis: A detailed analysis by laser capture
microdissection. Arthritis Rheum. 58:154–163. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hunter DJ, Nevitt M, Losina E and Kraus V:
Biomarkers for osteoarthritis: Current position and steps towards
further validation. Best Pract Res Clin Rheumatol. 28:61–71. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Attur M, Krasnokutsky-Samuels S, Samuels J
and Abramson SB: Prognostic biomarkers in osteoarthritis. Curr Opin
Rheumatol. 25:136–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao SG, Zeng C, Liu JJ, Tian J, Cheng C,
Zhang FJ, Xiong YL, Pan D, Xiao YB and Lei GH: Association between
Wnt inhibitory factor-1 expression levels in articular cartilage
and the disease severity of patients with osteoarthritis of the
knee. Exp Ther Med. 11:1405–1409. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qing L, Lei P, Liu H, Xie J, Wang L, Wen T
and Hu Y: Expression of hypoxia-inducible factor-1α in synovial
fluid and articular cartilage is associated with disease severity
in knee osteoarthritis. Exp Ther Med. 13:63–68. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Al-Jarallah KF, Shehab D, Al-Awadhi A,
Nahar I, Haider MZ and Moussa MA: Are 25(OH)D levels related to the
severity of knee osteoarthritis and function? Med Princ Pract.
21:74–78. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Y, Xiao W, Sun M, Deng Z, Zeng C, Li H,
Yang T, Li L, Luo W and Lei G: The expression of osteopontin and
Wnt5a in articular cartilage of patients with knee osteoarthritis
and its correlation with disease severity. Biomed Res Int.
2016:95610582016.PubMed/NCBI
|
|
13
|
Fang J, Xu L, Li Y and Zhao Z: Roles of
TGF-beta 1 signaling in the development of osteoarthritis. Histol
Histopathol. 31:1161–1167. 2016.PubMed/NCBI
|
|
14
|
Rigoglou S and Papavassiliou AG: The NF-κB
signalling pathway in osteoarthritis. Int J Biochem Cell Biol.
45:2580–2584. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zeng GQ, Chen AB, Li W, Song JH and Gao
CY: High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis.
Genet Mol Res. 14:14811–14822. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin W, Chang M, Paul EM, Babu G, Lee AJ,
Reiley W, Wright A, Zhang M, You J and Sun SC: Deubiquitinating
enzyme CYLD negatively regulates RANK signaling and
osteoclastogenesis in mice. J Clin Invest. 118:1858–1866. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lim JH, Jono H, Komatsu K, Woo CH, Lee J,
Miyata M, Matsuno T, Xu X, Huang Y, Zhang W, et al: CYLD negatively
regulates transforming growth factor-β-signalling via
deubiquitinating Akt. Nat Commun. 3:7712012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song J, Jin EH, Kim D, Kim KY, Chun CH and
Jin EJ: MicroRNA-222 regulates MMP-13 via targeting HDAC-4 during
osteoarthritis pathogenesis. BBA Clin. 3:79–89. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aletaha D, Neogi T, Silman AJ, Funovits J,
Felson DT, Bingham CO III, Birnbaum NS, Burmester GR, Bykerk VP,
Cohen MD, et al: 2010 rheumatoid arthritis classification criteria:
An American College of Rheumatology/European League Against
Rheumatism collaborative initiative. Arthritis Rheum. 62:2569–2581.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Edwards MH, Parsons C, Bruyère O, Dop
Petit F, Chapurlat R, Roemer FW, Guermazi A, Zaim S, Genant H,
Reginster JY, et al: High kellgren-lawrence grade and bone marrow
lesions predict worsening rates of radiographic joint space
narrowing; the SEKOIA study. J Rheumatol. 43:657–665. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jaiprakash A, Prasadam I, Feng JQ, Liu Y,
Crawford R and Xiao Y: Phenotypic characterization of
osteoarthritic osteocytes from the sclerotic zones: A possible
pathological role in subchondral bone sclerosis. Int J Biol Sci.
8:406–417. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wei F, Zhou J, Wei X, Zhang J, Fleming BC,
Terek R, Pei M, Chen Q, Liu T and Wei L: Activation of Indian
hedgehog promotes chondrocyte hypertrophy and upregulation of
MMP-13 in human osteoarthritic cartilage. Osteoarthritis Cartilage.
20:755–763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Welte S, Urbanik T, Elßner C, Kautz N,
Koehler BC, Waldburger N, Bermejo JL, Pinna F, Weiss KH, Schemmer
P, et al: Nuclear expression of the deubiquitinase CYLD is
associated with improved survival in human hepatocellular
carcinoma. PLoS One. 9:e1105912014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Burr DB and Gallant MA: Bone remodelling
in osteoarthritis. Nat Rev Rheumatol. 8:665–673. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Anderson-MacKenzie JM, Quasnichka HL,
Starr RL, Lewis EJ, Billingham ME and Bailey AJ: Fundamental
subchondral bone changes in spontaneous knee osteoarthritis. Int J
Biochem Cell Biol. 37:224–236. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hayami T, Pickarski M, Zhuo Y, Wesolowski
GA, Rodan GA and Duong LT: Characterization of articular cartilage
and subchondral bone changes in the rat anterior cruciate ligament
transection and meniscectomized models of osteoarthritis. Bone.
38:234–243. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Day JS, Ding M, van der Linden JC, Hvid I,
Sumner DR and Weinans H: A decreased subchondral trabecular bone
tissue elastic modulus is associated with pre-arthritic cartilage
damage. J Orthop Res. 19:914–918. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang LZ, Zheng HA, Jiang Y, Tu YH, Jiang
PH and Yang AL: Mechanical and biologic link between cartilage and
subchondral bone in osteoarthritis. Arthritis Care Res (Hoboken).
64:960–967. 2012.PubMed/NCBI
|
|
29
|
Sharma AR, Jagga S, Lee SS and Nam JS:
Interplay between cartilage and subchondral bone contributing to
pathogenesis of osteoarthritis. Int J Mol Sci. 14:19805–19830.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yuan XL, Meng HY, Wang YC, Peng J, Guo QY,
Wang AY and Lu SB: Bone-cartilage interface crosstalk in
osteoarthritis: Potential pathways and future therapeutic
strategies. Osteoarthritis Cartilage. 22:1077–1089. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Findlay DM and Kuliwaba JS: Bone-cartilage
crosstalk: A conversation for understanding osteoarthritis. Bone
Res. 4:160282016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shen J, Li S and Chen D: TGF-β signaling
and the development of osteoarthritis. Bone Res. 2:pii: 14002.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Van der Kraan PM: Age-related alterations
in TGF beta signaling as a causal factor of cartilage degeneration
in osteoarthritis. Biomed Mater Eng. 24 1 Suppl:S75–S80. 2014.
|
|
34
|
Jenkins ZA, van Kogelenberg M, Morgan T,
Jeffs A, Fukuzawa R, Pearl E, Thaller C, Hing AV, Porteous ME,
Garcia-Miñaur S, et al: Germline mutations in WTX cause a
sclerosing skeletal dysplasia but do not predispose to
tumorigenesis. Nat Genet. 41:95–100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tauriello DV, Haegebarth A, Kuper I,
Edelmann MJ, Henraat M, Canninga-van Dijk MR, Kessler BM, Clevers H
and Maurice MM: Loss of the tumor suppressor CYLD enhances
Wnt/beta-catenin signaling through K63-linked ubiquitination of
Dvl. Mol Cell. 37:607–619. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li X, Huang J and Zhang H: An analysis and
of hospital preparedness capacity for public health emergency in
four regions of China: Beijing, Shandong Guangxi and Hainan. BMC
Public Health. 8:3192008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guermazi A, Hayashi D, Roemer F, Felson
DT, Wang K, Lynch J, Amin S, Torner J, Lewis CE and Nevitt MC:
Severe radiographic knee osteoarthritis-does Kellgren and Lawrence
grade 4 represent end stage disease?-the MOST study. Osteoarthritis
Cartilage. 23:1499–1505. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schiphof D, Boers M and Bierma-Zeinstra
SM: Differences in descriptions of Kellgren and Lawrence grades of
knee osteoarthritis. Ann Rheum Dis. 67:1034–1036. 2008. View Article : Google Scholar : PubMed/NCBI
|