Introduction
Clinically, severe drug eruption is typically
divided into toxic epidermal necrolysis (TEN), severe erythema
multiforme-type drug eruption (Steven-Johnson syndrome; SJS) and
exfoliative dermatitis (Erythrodermic drug eruption). Severe drug
eruption is a serious disease that is difficult to treat and has a
high mortality rate (1); for
example, the mortality rate of TEN is 30% (2). Corticosteroids are the preferred
therapeutic for severe drug eruption, however, the use of
corticosteroids to treat severe drug eruption is clinically
controversial (2). Engelhardt et
al (3) hypothesized that the use
of corticosteroids would not be able to shorten the course of drug
eruption. Instead, it would increase the risk of infection, sepsis
and gastrointestinal bleeding, prolonging hospital stays and
increasing mortality. A study by Guibal et al (4) demonstrated that although long-term
corticosteroid therapy may delay the progression of TEN, it was not
able to prevent the entire progression of the disease. Schneck
et al (5) revealed that the
use of corticosteroids was not beneficial in the reduction of SJS
and TEN-associated mortality in a large-scale retrospective study
in France and Germany.
Due to the side effect of corticosteroids, the use
of corticosteroids is restricted for patients with underlying
diseases, including diabetes, gastrointestinal bleeding,
peptic-ulcer, epilepsy, glaucoma, cataracts, osteoporosis,
tuberculosis or hypertension (6). In
the present study, the patient was admitted to hospital due to
upper gastrointestinal bleeding. Following anti-Helicobacter
pylori (HP) treatment, the patient presented with severe drug
eruption. Routine use of corticosteroids may have, in the present
case, increased the risk of aggravating the underlying disease.
Previous studies in which tumor necrosis factor (TNF)-α antagonists
have been successfully used to treat drug eruption have been
reported (2,7,8).
A TNF-α antagonist was subsequently used as
treatment and the patient was fully recovered after six injections.
Rash, pyrexia, peripheral blood leukocytes, eosinophils and the
evolution of the systematic damage were assessed and reported.
Case study
Patient
In May 2015, a 73-year-old female was admitted to
the gastroenterology clinic of The First People's Hospital of
Wujiang (Suzhou, China) due to abdominal pain that had persisted
for 1 week and melena for 5 days. Endoscopy indicated the presence
of a compound gastric ulcer with bleeding. Pathological diagnosis
demonstrated that the antral gastric mucosa exhibited chronic
active inflammation. Following treatment with amoxicillin,
clarithromycin, pantoprazole, and colloidal bismuth pectin
(quadruple anti-HP therapy), the patient's abdominal pain eased. On
day 4, an itchy erythema appeared around the patient's neck and the
patient was re-admitted to hospital.
The patient presented with no cough or expectoration
in the course of treatment. The patient had a history of
hypertension and was taking antihypertensive tablets daily to
control her blood pressure. No history of coronary heart disease,
trauma, blood transfusion or drug allergy was indicated. The
patient's stool was black and urine frequency, output and color
were normal.
Examination
Clinical examination demonstrated that the patient's
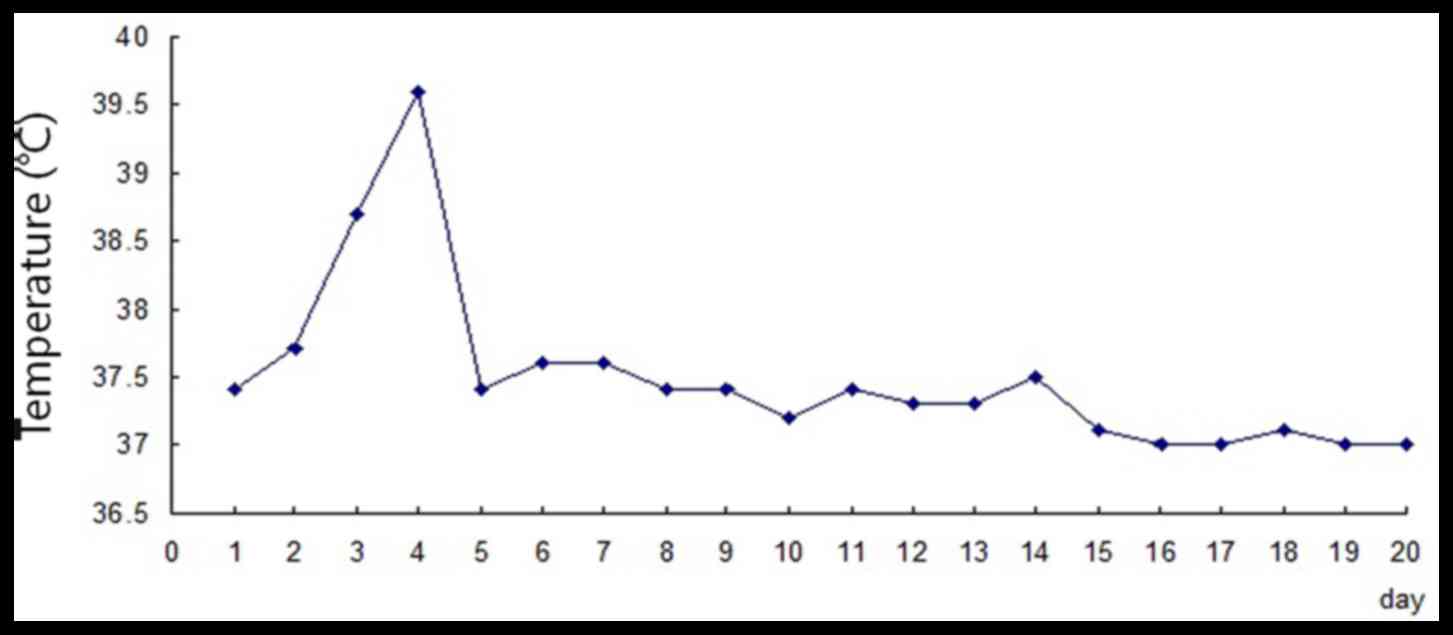
temperature was 37.4°C (Fig. 1),
pulse was 86 beats per minute, blood pressure was 144/79 mmHg,
respiratory rate was 18 beats per minute and SpO2 was
96%. The skin and mucous membranes across the entire body were not
cyanosed, icteric and pale. Superficial lymph nodes were
impalpable. Lung breath sounds were rough but rhonchi and moist
rale were not heard and the heartbeat was regular. Pathological
cardiac murmurs were not heard in the auscultation area of the
heart valves. The abdomen was soft, with upper abdominal tenderness
below the xiphoid process. Abdomen had no rebound tenderness or
muscle tension and abdominal mass was impalpable. The liver and
spleen were impalpable below the costal margin. Murphy's sign,
shifting dullness and renal percussive pain were all negative.
Bowel sounds occurred 5 times per min. Lower limbs had no edema.
Physiological reflex was normal and pathologic reflex was not
elicited.
Skin changes
Edematous erythema appeared on the head, face and
neck. The eyelids slightly swelled but the conjunctiva presented
with no congestion. The mouth and genitalia had no ulceration or
erosion.
Laboratory examination
Laboratory examinations were conducted and the
findings were as follows: Leukocyte count, 5.03×109
cells/l (normal, 4.00–10.0×109 cells/l); hemoglobin
(Hb), 105 g/l (normal, 110–150 g/l); neutrophil ratio, 74.7%
(normal, 50–70%); lymphocyte ratio, 14.3% (normal, 20~40%);
eosinophil ratio, 5.2% (normal, 0.5–5.0%); erythrocyte
sedimentation rate, 19 mm/h (normal, 0–20 mm/h); glycated
hemoglobin, 5.4% (normal, 4.0–6.0%); total glycated Hb, 6.4%
(normal, 3.9–7.3%); C-reactive protein, 2 mg/l (normal, 0.00–10.00
mg/l); ketone, 2+ (normal, 0–0.5 mmol/l); urine protein, negative;
D-dimer, 1.23 mg/l (normal, 0.00–0.55 mg/l) and total T3 0.92
nmol/l (normal, 1.34~2.73 nmol/l).
Gastroscopy (pre-admission)
The patient presented with a compound gastric ulcer
with bleeding. Pathological diagnosis demonstrated that the antral
gastric mucosa presented with chronic active inflammation.
Preliminary diagnosis was a compound gastric ulcer with bleeding
and drug eruption (slight) following treatment.
Admission course and treatment
procedure
The patient's rash further developed following
admittance to hospital. The rash on the face, neck, trunk and limbs
had worsened and the patient also had itchiness, fever and
increased peripheral blood leukocytes. Following intravenous
injection of 60 ml compound glycyrrhizin (20 ml contains 40 mg
glycyrrhizin, 400 mg aminoacetic acid and 20 mg cysteine
hydrochloride; Minophagen Pharmaceutical Co,. Ltd., Tokyo, Japan)
and administration of calamine lotion, the rash further increased
and integrated. The face became swollen, the conjunctiva exhibited
hyperemia and edema and the conjunctival secretion increased. On
day 3 following admission, the patient presented with a fever at
6:00 a.m. (body temperature, 38.7°C; Fig. 1). The rash was more swollen and
reddish, and had integrated further. Conjunctival secretion
markedly increased and the patient had difficulty opening their
eyes. Typical target-like erythematous papules appeared on the
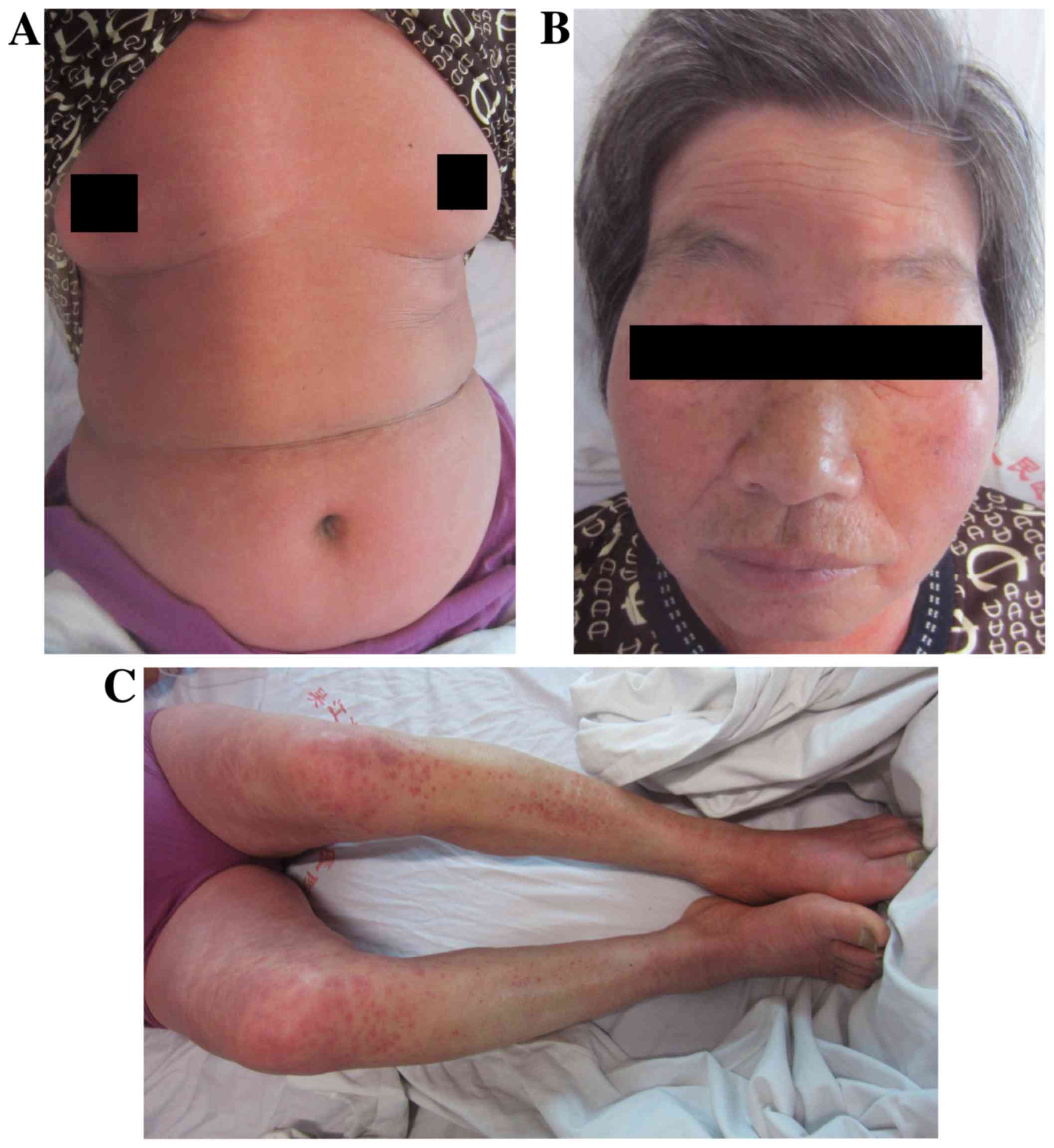
lower limbs. On day 4, the patient's condition aggravated further.
The rash extended to cover 80% of the body surface area and the
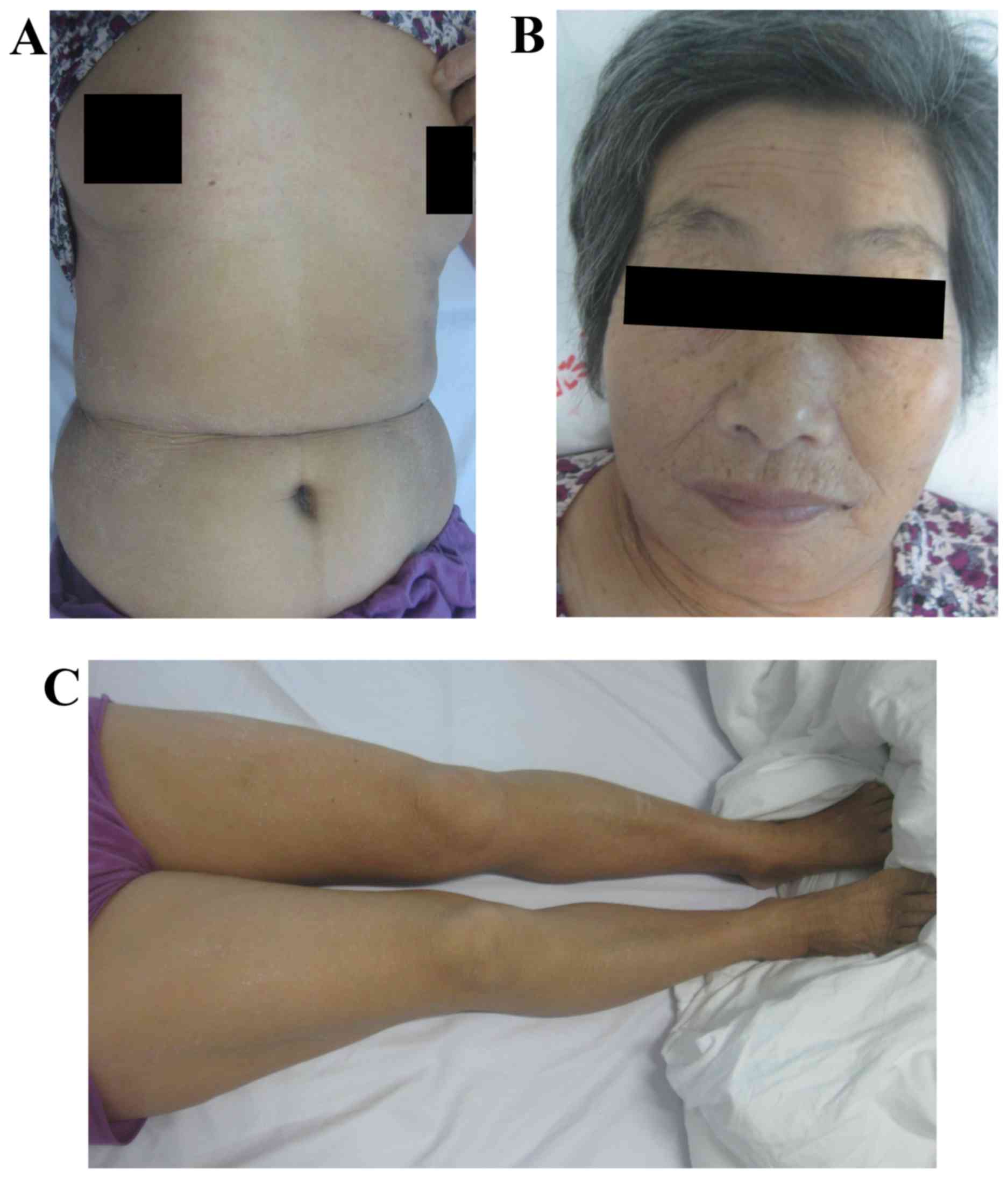
skin temperature increased (Fig.
2A-C) with the patient indicating that the itch was severe. Her
body temperature rose to 39.6°C (Fig.
1) at 6:00 p.m. Leukocyte and neutrophil counts markedly
increased as determined by laboratory examination: Leukocyte count,
24.70×109/l; neutrophil ratio, 93%; and eosinophil
ratio, 1.9%. A diagnosis of severe drug eruption (severe erythema
multiforme-type drug eruption) was given.
The patient was hospitalized due to upper
gastrointestinal bleeding and a peptic ulcer. Following anti-HP
treatment, a severe drug eruption occurred. Therefore, the routine
use of corticosteroids posed an increased risk to this patient. The
patient was assessed for viral hepatitis, cancer and tuberculosis
through hepatitis series, tumor series and X-ray testing, all of
which were negative. Written informed consent was provided by the
patient, prior to the subcutaneous injection of the recombinant
human TNF receptor II-antibody fusion protein (etanercept) with a
25 mg/needle (CITIC National Health Pharmaceutical Co., Ltd.,
Shanghai, China) every three days with the initial dose being 50
mg/day and the maintenance dose as 25 mg/day. Following six
injections with the protein, the patient's symptoms were
improved.
As severe drug eruption lacks acknowledged
evaluation criteria, the present study referred to an
eruption-severity scoring method, outlined by Chen et al
(8), that utilizes a drug eruption
area and severity index (DASI). According to this, the evolution of
the rash was recorded and quantified. The method was as follows: i)
Evaluating rash area (A). Head/neck made up 10%, trunk made up 30%,
upper limbs made up 20%, and lower limbs made up 40%. If no rash
was observed, the area was assigned a score of 0. The present study
assigned points as follows: 1, rash area <10%; 2, 10–29%; 3,
30–49%; 4, 50–69%; 5, 70–89%; and 6, 90–100%. Overall rating scores
were between 0 and 24 points. Upper limbs included axillas and
hands. The trunk included the groin and middle axillary axial area.
Lower limbs included hips and feet. ii) Evaluating the rash
severity. The following features were evaluated: Erythema (E),
infiltration or edema (I), erosion or vesicle (Ev), and
desquamation (D). Each feature was evaluated using a 0–3 rating: 0,
none; 1, slight condition; 2, moderate condition; and 3, severe
condition. D was scored in reverse. iii) Evaluating mucosal damage.
The mucosa included the eyes, nose, mouth, genitalia, anus and
rectum. Involvement of the eyes and oral mucosa scored 2 points and
the rest scored 1 point. Severe eye mucosal damage may cause
blindness and severe oral mucosal damage affects eating and
therefore, the involvement of two mucosal areas is very important.
Severity of mucosal damage was scored using a 4-point system, as
follows: 0 point, none; 1 point, <5 blisters; 2 points, >5
blisters and (or) mild erosion; and 3 points, severe erosion. DASI
total scores were calculated as follows: DASI total scores = (E
head + I head + Ev head + D head) × A head × 0.1 + (E upper limbs +
I upper limbs + Ev upper limbs + D upper limbs) × A upper limbs ×
0.2 + (E trunk + I trunk + Ev trunk + D trunk) × A trunk × 0.3 + (E
lower limbs + I lower limbs + Ev lower limbs + D lower limbs) × A
lower limbs × 0.4 + Mucosal area score × Mucosal damage severity
score. Total scores were between 0 and 93 points, according to the
formula.
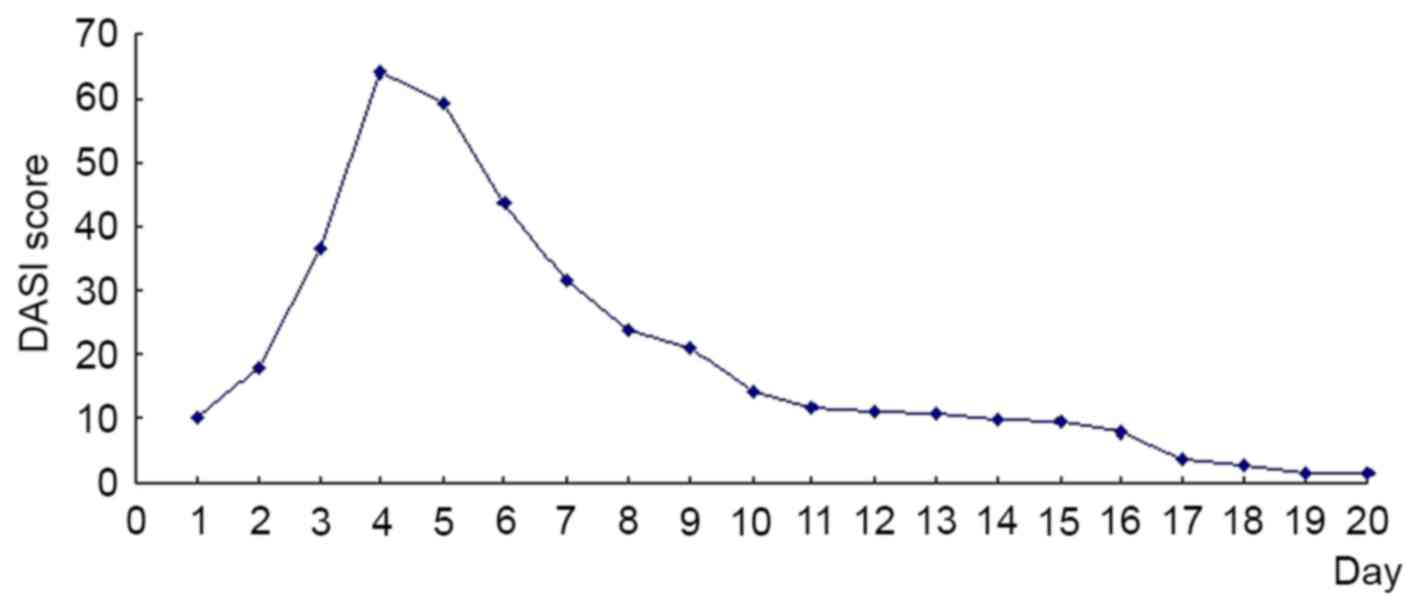
The patient's initial DASI score on admission was
9.9 points (Fig. 3). The score prior
to injection was 64 points (Fig. 3);
meanwhile the rash area covered 80% of the body surface area.
According to SCORNTEN scoring method (9), this case's score was 2 points and
predicted mortality was 12.1%. Following the first injection of
etanercept, the fever was under control within 1 h. The rash was
under control within 24 h and no new rashes appeared. The score was
59.4 points (Fig. 3). The rash
further improved over the next 72 h. The rash color turned dark and
the itchiness and conjunctival secretion markedly reduced. Slight
scaling appeared on the face and some normal skin was revealed. The
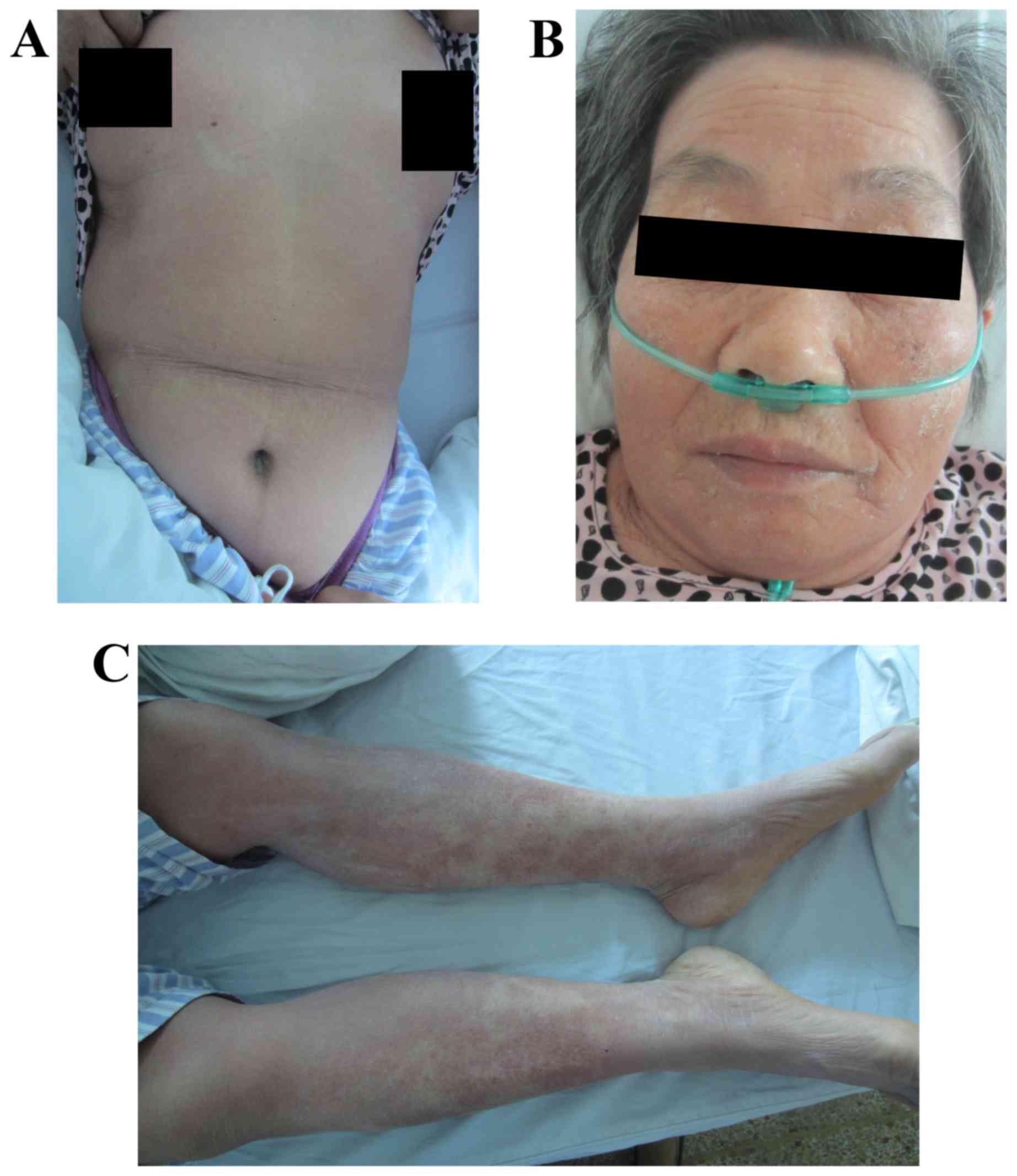
rash covered 45% of the whole body surface area (Fig. 4A-C) and scored 23.9 points (Fig. 3). The patient did not feel itchy and
clear desquamation began to appear on the head, face and trunk
following 96 h. Conjunctival erosion was almost recovered and the
score was 20.9 points (Fig. 3). Two
herpes simplex sores appeared separately on the right lip and the
back of the right shoulder-back on day 5 following the injection.
The patient was treated with penciclovir cream, and recovered 1
week later. The rash on the patient's limbs, in particular the
lower limbs, faded markedly by day 7 following injection and the
target-like erythema disappeared. The trunk and limbs markedly
began to scale, the face and neck returned to normal and the score
was 10.9 points (Fig. 3). The
majority of the patient's skin returned to normal and the primary
rash was shedding on the lower limbs on day 14 (Fig. 5A-C), and the score was 1.7 points
(Fig. 3). On day 16, the patient was
discharged and the score was 0.4 points (Fig. 3). Following admission, the water and
electrolyte balance was maintained at 2,000–2,500 ml daily fluid
infusion to allow adequate urine output. The patient was also
instructed drink milk and was treated with an intravenous drip of
compound glycyrrhizin (80 ml daily). The patient was asked to
gargle with chlorhexidine solution and protect her eyes with
chlortetracycline eye ointment. During the treatment, the patient
presented with no nausea, vomiting, diarrhea, hematochezia, cough
or abdominal pain.
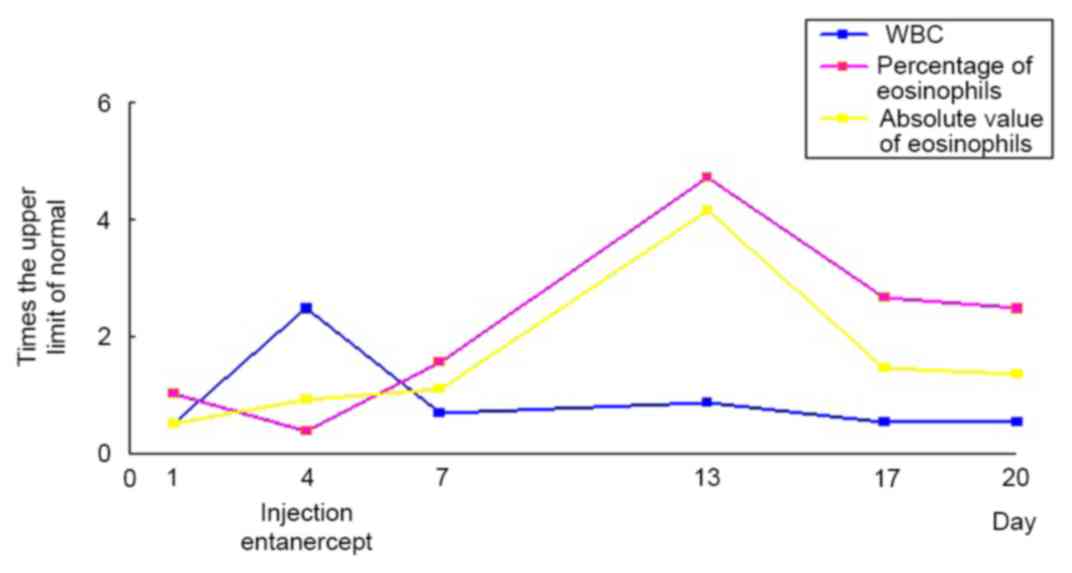
Laboratory examination on the first day of admission
indicated that the leukocyte count was 5.03×109 cells/l
(normal, 4.00–10.0×109 cells/l; Fig. 6) and the percentage of eosinophils
was 5.2% (normal, 0.5–5.0%; Fig. 6).
Prior to etanercept injection, the leukocyte count rose to
24.70×109/l (Fig. 6) and
the percentage of neutrophils increased to 93.0%. The percentage of
eosinophils was 1.9% (Fig. 6) and
biochemical indicators were normal, with the exception of lactate
dehydrogenase (LDH) being slightly increased at 369 U/l (normal,
109–245 U/l), and total protein slightly dropped to 56.7 g/l
(normal, 60.0–85.0 g/l).
The peripheral blood eosinophil count continued to
rise and the percentage peaked at 23.6% (Fig. 6) following the third injection of
etanercept. When the patient was discharged, the percentage of
eosinophils remained higher than normal and reached up to 12.4%
(Fig. 6). The patient's hepatic and
renal function remained normal during hospitalization. On day 3
following the injection of etanercept, LDH was slightly increased
at 271 U/l (normal, 109–245 U/l), serum total protein was lowest at
49.6 g/l (normal, 60.0–85.0 g/l) and albumin was lowest at 30.6 g/l
(normal, 35.0–55.0 g/l).
Albumin was not administered but the patient was
instructed to drink more milk. The level of albumin returned to
normal when reviewed. On day 9 following injection, the level of
triglycerides rose to 2.35 mmol/l (normal, 0.33–1.70 mmol/l) and
returned to normal when the patient was discharged. Written
informed consent was obtained from the patient for publication of
the images and medical data in this report.
Discussion
The patient in the current study presented with
generalized edematous erythema, lymphadenectasis, fever, severe
itch, conjunctival erosion and increased levels of leukocytes and
eosinophils in the peripheral blood following antibiotic treatment.
Parts of the rash were target-like and therefore, the diagnosis was
severe erythema multiforme-type drug eruption (Stevens-Johnson
syndrome, SJS). According to the probability of drug eruption,
amoxicillin allergy was the most probable cause.
Severe erythema multiforme-type drug eruption is a
serious adverse drug reaction and it has a risk of mortality.
According to previous studies (10,11), the
average mortality rate for SJS is 1–5%, and the risk of mortality
is higher in elderly patients. The most common drugs that cause
allergic reactions are penicillin, sulfonamides and nonsteroidal
anti-inflammatory drugs (12,13).
TNF-α is a cytokine secreted by macrophages, lymphocytes and other
immune cells that are activated by endotoxins. TNF-α is the
strongest anti-tumor and anti-inflammation cytokine identified,
thus far. TNF-α has a role in the occurrence and development of a
number of different diseases and illnesses, including cancer,
infection, fever, endotoxin shock, autoimmune diseases and
transplant rejection (14–18). The more severe the trauma is, the
higher the level of TNF-α is (19,20).
Epidermal necrosis in SJS and TEN is considered to
be caused by cytotoxic T cells. Perforin, which is released with
TNF-α and other cytokines, may induce keratinocyte necrosis. Paquet
et al (21) demonstrated that
there is a high expression of TNF-α in the rashes of patients with
TEN and hypothesized that TNF-α mediates apoptosis. Carr et
al (22) revealed that TNF-α was
detected in the rashes of exanthematous drug eruption of patients
with HIV infection and the positive rate was ~45%. Nassif et
al (23) indicated marked TNF-α
expression in the blister fluid of patients with TEN. Paul et
al (24) observed that the
rashes of 5 patients with TEN and SJS-TEN overlap and demonstrated
that all the rashes exhibited full-thickness necrosis of epidermal
keratinocytes. This was hypothesized to be due to extensive
apoptosis following the release of TNF-α, which may enhance this
toxicity. This suggests that the inhibition of apoptosis may be an
important means of treating TEN.
TNF-α is a pro-inflammatory cytokine. The
pathogenesis of TNF-α may involve severe eruption due to the
following aspects: i) As the drug is absorbed into the body, it
activates drug-specific T cells that secrete TNF-α, stimulate
keratinocytes and endothelial cells and mediate the immune
responses of skin and mucosa; ii) TNF-α mediates type IVa
hypersensitivity; and iii) in TEN, TNF-α has a role in
FasL-mediated keratinocyte apoptosis by activating inducible nitric
oxide synthase (25).
TNF antagonists may block the biological activity of
TNF by specific binding, which may function to control the
inflammation, leading to recovery from the disease. Previously,
there have been a number of cases of single use TNF factor
antagonists to successfully treat severe drug eruption, globally.
Hunger et al (2) used single
infliximab (5 mg/kg) to treat a 69-year-old female patient with
TEN, and successfully identified the point when the recovery time
was significantly shorter than the gamma globulin. Kreft et
al (7) used infliximab to
successfully treat a case of celecoxib-induced TEN. Chen et
al (8) injected etanercept every
other day for 16 days and successfully treated a patient with Dress
accompanied diabetes.
In the present study, the patient recovered and was
discharged on day 16, following subcutaneous injections of 25 mg
(initial dose, 50 mg) etanercept every 3 days for 6 times. The
total amount of etanercept treatment included seven injections. It
is evident that TNF-α antagonist is able to target and inhibit the
early inflammatory mediator TNF-α in a timely and effective manner,
terminate the inflammatory cascade and then block the pathogenesis
of drug eruption. Therefore, TNF-α antagonist is effective in the
treatment of severe drug eruption.
Prior to treatment by injection, the total level of
leucocytes and neutrophils markedly increased. Considering that the
patient presented with no clear evidence of infection, it was
speculated that this was associated with increased interleukin
(IL)-8 in the drug eruption pathogenesis. IL-8 has the effect of
stimulating neutrophil chemotaxis and releasing lysosomes.
Therefore, no antibiotics were used during treatment. TNF-α is able
to induce IL-8. In this case, the patient's leukocytes and
neutrophils quickly returned to normal following treatment with the
TNF-α antagonist. In the follow-up examinations, the level of
leukocytes and neutrophils remained within the normal range. This
may be because the TNF-α antagonist inhibits TNF-α, reduce IL-8 and
then inhibit the chemotaxis of neutrophils.
The peripheral blood eosinophil continued to rise
following the injection of TNF-α antagonist in the present case and
reached its peak on day 9 following the injection. From then, it
declined slowly, but remained significantly higher than normal when
the patient was discharged (Fig. 6).
This observation is the same as Chen et al's (8). This suggests that TNF antagonist is a
safe, fast and effective therapeutic for severe drug eruption, but
it may not prevent the rise of peripheral blood eosinophils, as
they are not affected by the treatment.
In addition, in the process of using TNF-α
antagonist, the patient's gastrointestinal symptoms were
continuously improved and melena did not recur. It indicated that
TNF-α antagonist does not affect the prognosis of underlying
diseases such as upper gastrointestinal bleeding and peptic-ulcer
in the present case.
In gastroenterology, anti-HP treatment typically
uses penicillin antibiotics. Penicillin is associated with a higher
probability of leading to drug allergies or even severe drug
eruption (26,27). As the patients using anti-HP therapy
typically also have upper gastrointestinal bleeding, the
contraindication is to use glucocorticoids. It is therefore,
difficult to treat drug eruption in these cases. The patient of the
current study was treated with a TNF-α antagonist, which achieved
promising results.
In conclusion, the present case of drug eruption was
healed within 16 days of injection treatments and this treatment
did not affect the underlying diseases, including gastrointestinal
ulcer bleeding. Therefore, TNF-α antagonist may be a good treatment
in similar situations. TNF-α antagonist opens new perspectives for
the treatment of severe drug eruption. Due to our treatment being
administered to a single case, well-conducted studies of the
efficacy and safety of such treatment are urgently needed.
References
|
1
|
Kardaun SH and Jonkman MF: Dexamethasone
pulse therapy for Stevens-Johnson syndrome/toxic epidermal
necrolysis. Acta Derm Venereol. 87:144–148. 2007. View Article : Google Scholar
|
|
2
|
Hunger RE, Hunziker T, Buettiker U,
Braathen LR and Yawalkar N: Rapid resolution of toxic epidermal
necrolysis with anti-TNF-alpha treatment. J Allergy Clin Immunol.
116:923–924. 2005. View Article : Google Scholar
|
|
3
|
Engelhardt SL, Schurr MJ and Helgerson RB:
Toxic epidermal necrolysis: An analysis of referral patterns and
steroid usage. J Burn Care Rehabil. 18:520–524. 1997. View Article : Google Scholar
|
|
4
|
Guibal F, Bastuji-Garin S, Chosidow O,
Saiag P, Revuz J and Roujeau JC: Characteristics of toxic epidermal
necrolysis in patients undergoing long-term glucocorticoid therapy.
Arch Dermatol. 131:669–672. 1995. View Article : Google Scholar
|
|
5
|
Schneck J, Fagot JP, Sekula P, Sassolas B,
Roujeau JC and Mockenhaupt M: Effects of treatments on the
mortality of Stevens-Johnson syndrome and toxic epidermal
necrolysis: A retrospective study on patients included in the
prospective EuroSCAR study. J Am Acad Dermatol. 58:33–40. 2008.
View Article : Google Scholar
|
|
6
|
Moghadam-Kia S and Werth VP: Prevention
and treatment of systemic glucocorticoid side effects. Int J
Dermatol. 49:239–248. 2010. View Article : Google Scholar
|
|
7
|
Kreft B, Wohlrab J, Bramsiepe I, Eismann
R, Winkler M and Marsch WC: Etoricoxib-induced toxic epidermal
necrolysis: Successful treatment with infliximab. J Dermatol.
37:904–906. 2010. View Article : Google Scholar
|
|
8
|
Chen LL, Lu DD, Shi X, Sun XD, Xie LX,
Chen XJ, Ding L, Jin WJ and Wang YF: Drug rash with eosinophilia
and systemic symptoms controlled by tumor necrosis factor-α
antagonist: A clinical observation. Zhonghua Pi Fu Ke Za Zhi.
46:621–625. 2013.(In Chinese).
|
|
9
|
Bastuji-Garin S, Fouchard N, Bertocchi M,
Roujeau JC, Revuz J and Wolkenstein P: SCORTEN: A
severity-of-illness score for toxic epidermal necrolysis. J Invest
Dermatol. 115:149–153. 2000. View Article : Google Scholar
|
|
10
|
Harr T and French LE: Stevens-Johnson
syndrome and toxic epidermal necrolysis. Chem Immunol Allergy.
97:149–166. 2012. View Article : Google Scholar
|
|
11
|
Miliszewski MA, Kirchhof MG, Sikora S,
Papp A and Dutz JP: Stevens-Johnson syndrome and toxic epidermal
necrolysis: An analysis of triggers and implications for improving
prevention. Am J Med. 129:1221–1225. 2016. View Article : Google Scholar
|
|
12
|
Lalosevic J, Nikolic M, Gajic-Veljic M,
Skiljevic D and Medenica L: Stevens-Johnson syndrome and toxic
epidermal necrolysis: A 20-year single-center experience. Int J
Dermatol. 54:978–984. 2015. View Article : Google Scholar
|
|
13
|
Greenberger PA: Drug allergy. J Allergy
Clin Immunol. 117 2 Suppl Mini-Primer:S464–S470. 2006. View Article : Google Scholar
|
|
14
|
Waters JP, Pober JS and Bradley JR: Tumour
necrosis factor in infectious disease. J Pathol. 230:132–147. 2013.
View Article : Google Scholar
|
|
15
|
Stefferl A, Hopkins SJ, Rothwell NJ and
Luheshi GN: The role of TNF-alpha in fever: Opposing actions of
human and murine TNF-alpha and interactions with IL-beta in the
rat. Br J Pharmacol. 118:1919–1924. 1996. View Article : Google Scholar
|
|
16
|
Nakayama M, Niki Y, Kawasaki T, Takeda Y,
Horiuchi K, Sasaki A, Okada Y, Umezawa K, Ikegami H, Toyama Y and
Miyamoto T: Enhanced susceptibility to lipopolysaccharide-induced
arthritis and endotoxin shock in interleukin-32 alpha transgenic
mice through induction of tumor necrosis factor alpha. Arthritis
Res Ther. 14:R1202012. View
Article : Google Scholar
|
|
17
|
Doss GP, Agoramoorthy G and Chakraborty C:
TNF/TNFR: Drug target for autoimmune diseases and immune-mediated
inflammatory diseases. Front Biosci (Landmark Ed). 19:1028–1040.
2014. View Article : Google Scholar
|
|
18
|
Azzawi M, Hasleton PS and Hutchinson IV:
TNF-alpha in acute cardiac transplant rejection. Cytokines Cell Mol
Ther. 5:41–49. 1999.
|
|
19
|
Esposito E and Cuzzocrea S: TNF-alpha as a
therapeutic target in inflammatory diseases, ischemia-reperfusion
injury and trauma. Curr Med Chem. 16:3152–3167. 2009. View Article : Google Scholar
|
|
20
|
He M, Dong LM and Hou XB: Time-related
expression of IL-6, IL-8 and TNF-alpha following explosive injury
to rabbit's chest. Fa Yi Xue Za Zhi. 30:85–87. 2014.(In
Chinese).
|
|
21
|
Paquet P, Nikkels A, Arrese JE,
Vanderkelen A and Piérard GE: Macrophages and tumor necrosis factor
alpha in toxic epidermal necrolysis. Arch Dermatol. 130:605–608.
1994. View Article : Google Scholar
|
|
22
|
Carr A, Vasak E, Munro V, Penny R and
Cooper DA: Immunohistological assessment of cutaneous drug
hypersensitivity in patients with HIV infection. Clin Exp Immunol.
97:260–265. 1994. View Article : Google Scholar
|
|
23
|
Nassif A, Bensussan A, Dorothée G,
Mami-Chouaib F, Bachot N, Bagot M, Boumsell L and Roujeau JC: Drug
specific cytotoxic T-cells in the skin lesions of a patient with
toxic epidermal necrolysis. J Invest Dermatol. 118:728–733. 2002.
View Article : Google Scholar
|
|
24
|
Paul C, Wolkenstein P, Adle H, Wechsler J,
Garchon HJ, Revuz J and Roujeau JC: Apoptosis as a mechanism of
keratinocyte death in toxic epidermal necrolysis. Br J Dermatol.
134:710–714. 1996. View Article : Google Scholar
|
|
25
|
Viard-Leveugle I, Gaide O, Jankovic D,
Feldmeyer L, Kerl K, Pickard C, Roques S, Friedmann PS, Contassot E
and French LE: TNF-α and IFN-γ are potential inducers of
Fas-mediated keratinocyte apoptosis through activation of inducible
nitric oxide synthase in toxic epidermal necrolysis. J Invest
Dermatol. 133:489–498. 2013. View Article : Google Scholar
|
|
26
|
Lin YF, Yang CH, Sindy H, Lin JY, Hui
Rosaline CY, Tsai YC, Wu TS, Huang CT, Kao KC, Hu HC, et al: Severe
cutaneous adverse reactions related to systemic antibiotics. Clin
Infect Dis. 58:1377–1385. 2014. View Article : Google Scholar
|
|
27
|
Strom BL, Carson JL, Halpern AC, Schinnar
R, Snyder ES, Shaw M, Tilson HH, Joseph M, Dai WS, Chen D, et al: A
population-based study of Stevens-Johnson syndrome. Incidence and
antecedent drug exposures. Arch Dermatol. 127:831–838. 1991.
View Article : Google Scholar
|