Introduction
With the improvement of living standards and the
increase in the number of obese people, it is estimated that the
number of diabetic patients in the world will reach 430 million by
2030 (1). Currently, China has the
second largest population in the world, and the number of diabetic
patients in China is expected to reach over 43.2 million by 2030
(2). Diabetic patients may suffer
from various vascular complications due to metabolic and endocrine
system disorders. This can eventually lead to diabetic retinopathy
(DR) and loss of vision in diabetic patients. Recent studies have
confirmed that inflammation plays a key role in the DR development
and progression (3).
Results obtained from several previous studies have
shown that long-term hyperglycemia can activate the advanced
glycation end products (AGEs) and receptor for advanced glycation
end products (RAGE) signal regulation pathway (4). AGEs are compounds formed by the
long-term hyperglycemia and protein glycosylation, which play
important roles in cell damage and chronic progression of diabetes
(5). AGEs, combined with RAGE, can
lead to intracellular oxidative stress, produce free radicals and
activate nuclear factor NF-κB. In addition, AGEs can also promote
the release of interleukin-1β (IL-1β), IL-6 and tumor necrosis
factor-α (TNF-α), leading to the inflammatory response. RAGE is the
key receptor protein of AGEs, and the abnormal expression of RAGE
gene or a change in RAGE gene activity change may affect the
development and progression of DR (6–8).
Studies have found that RAGE protein expression
levels in DR patients is abnormally high (9). DNA methylation is a common type of
epigenetic modification, and the gene expression can be lated when
CpG islands are methylated in some gene promoter regions (10). To our knowledge, no conclusive
research has been done on RAGE gene promoter methylation. In this
study, the methylation status of RAGE gene promoters in peripheral
blood mononuclear cells (PBMCs) was studied via
methylation-specific PCR (MSP). IL-1β, IL-6 and TNF-α levels in
serum were detected using enzyme-linked immunosorbent assay
(ELISA). RAGE gene promoter methylation was inhibited via methylase
inhibitor, 5′-aza-2′-deoxycytidine (5-aza-dC), and the effect of
methylation on IL-1β, IL-6 and TNF-α levels were studied. The
correlation between RAGE gene promoter methylation and diabetic
retinal inflammation was investigated.
Materials and methods
Materials and objects of study
5-aza-dC (Sigma, St. Louis, MO, USA); RPMI-1640
medium, fetal bovine serum (FBS) (HyClone Laboratories, UT, USA);
Ficoll Paque Plus (GE Healthcare, Bethesda, MD, USA); Epi-Tect
Bisulfite kits (Qiagen, Nordrhein-Westfalen, Germany); IL-1β, IL-6
and TNF-α ELISA kit (Beyotime Biotechnology Institute, Nantong,
China); DNA extraction kit (Tiangen, Beijing, China); TaqDNA
polymerase, dNTP mixture, DNA marker and primer synthesis (Takara,
Dalian, China).
A total of 80 patients diagnosed with type 2 DR in
Qilu Hospital, Shandong University from October, 2013 to October,
2015 were enrolled in this study. This included 44 males and 36
females aged 54.2±9.5 years. Diabetic patients were diagnosed
according to WHO standard in 1999, and all patients were definitely
diagnosed through the fundus fluorescein angiography and fundus
examination. There were 40 healthy subjects without diabetes, heart
disease, hypertension and other diseases in normal control group,
including 23 males and 17 females aged 55.3±10.7 years. The
participants had no specific heart, liver and lung diseases, and no
acute and chronic infectious diseases and malignant tumors. The
patients or their families signed the written informed consent, and
this study was approved by the Ethics Committee of Qilu
hospital.
Isolation and culture of PBMCs
Twenty microliters of venous blood was collected
from each patient, and PBMCs were isolated using Ficoll-Paque Plus
and cultured using the RPMI-1640 medium containing 10% autologous
serum under 5% CO2 at 37°C for 2 h. Suspended cells were
washed and adherent cells were collected, and the media were
replaced every 24 h, followed by digestive passage when cell fusion
was realized.
Detection of RAGE gene promoter
methylation in PBMCs via MSP
Total DNA was extracted using DNA extraction kit,
and the DNA content and purity (qualified if A260/A280 >1.8)
were detected using ultraviolet spectrophotometer (U-3010; Hitachi,
Tokyo, Japan). Total DNA extracted received bisulfite modification
according to instructions provided by EpiTect Bisulfite kits, and
methylation-specific PCR and unmethylated-specific PCR were
performed for modified DNA. PCR reaction was 2 µl 10X PCR buffer
(20 µl in total), 20 pmol primers (Fig.
1, sequence), dNTP (final concentration of 0.2 mmol/l),
MgCl2 (final concentration of 1.0 mmol/l), 1 unit Hot
Start Taq enzyme and 100 ng modified DNA template. The specific
conditions for PCR amplification were as follows: 95°C for 5 min,
degeneration at 95°C for 45 sec, annealing at 67°C for 45 sec,
extension at 72°C for 60 sec, a total of 30 cycles, and reaction
termination after extension at 72°C for 10 min. PCR product was
transferred to 2% agarose gel electrophoresis, ethidium bromide
staining, photography and result analysis using gel-imaging system
(UVP, LLC, Upland, CA, USA) (Table
I).
 | Table I.RAGE gene promoter methylated and
unmethylated primer sequence. |
Table I.
RAGE gene promoter methylated and
unmethylated primer sequence.
| Primer | Primer sequences | Product size
(bp) |
|---|
| RAGE-MF |
5-TTTTAGAATTTTTTTTAGAATTCGA-3 | 181 |
| RAGE-MR |
5-CTTACACTTCAACACCAATAACTCG-3 |
|
| RAGE-UF |
5-TTTTAGAATTTTTTTTAGAATTTGA-3 | 186 |
| RAGE-UR |
5-TACACTTCAACACCAATAACTCACC-3 |
|
Detection of IL-1β, IL-6 and TNF-α
contents in serum via ELISA
Three milliliters fasting venous blood was drawn
from DR patients and those in normal control group in the early
morning, and centrifuged after water bath at 37°C for 10 min. The
serum samples were collected and stored in the refrigerator at
−20°C, and the IL-1β, IL-6 and TNF-α contents in serum were
detected according to instructions of ELISA kit.
Detection of effect of 5-aza-dC on
RAGE gene promoter methylation in PBMCs via MSP
PBMCs of DR patients with positive RAGE gene
promoter methylation were cultured according to standard method,
and cells were divided into non-treatment group (NT) and 5-aza-dC
treatment group. Cells in NT group were cultured using the normal
culture method, while cells in 5-aza-dC treatment group were
treated with 5-aza-dC with the final concentration of 5 µmol/l.
After 24 h, cells were digested by trypsin and collected, and the
total DNA was extracted using the DNA extraction kit. The RAGE gene
promoter methylation in each group was then detected.
Detection of effect of 5-aza-dC on
IL-1β, IL-6 and TNF-α in culture supernatant of PBMCs via
ELISA
PBMCs in logarithmic growth phase were collected and
added into each well (2×105 cells/ml). After the culture
of PBMCs suspension in the 6-well plate for 24 h, the cells were
treated according to the method in 1.5. After 24 h, 50 µl culture
supernatant was drawn and the contents of IL-1β, IL-6 and TNF-α in
culture supernatant were detected according to instructions of
ELISA kit.
Statistical analysis
SPSS 17.0 (IBM Corp., Armonk, NY, USA) was used for
data processing in this study. Measurement data were presented as
mean ± standard deviation. LSD test was performed for multiple
comparisons test. p<0.05 indicates a statistically significant
difference.
Results
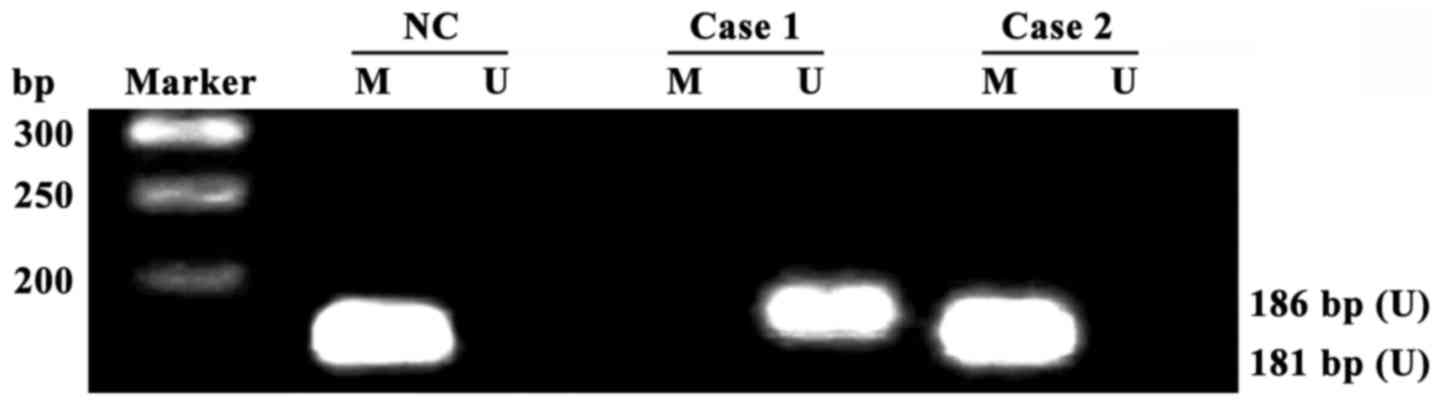
RAGE gene promoter methylation in
PBMCs
All RAGE gene promoters in PBMCs of 40 healthy
adults were methylated, and the detection rate of RAGE gene
promoter methylation in PBMCs of DR patients was 32.50% (26/80)
(Fig. 1). The methylation rate of
RAGE gene promoters had a significant difference in PBMCs between
DR patients and healthy adults (p<0.01).
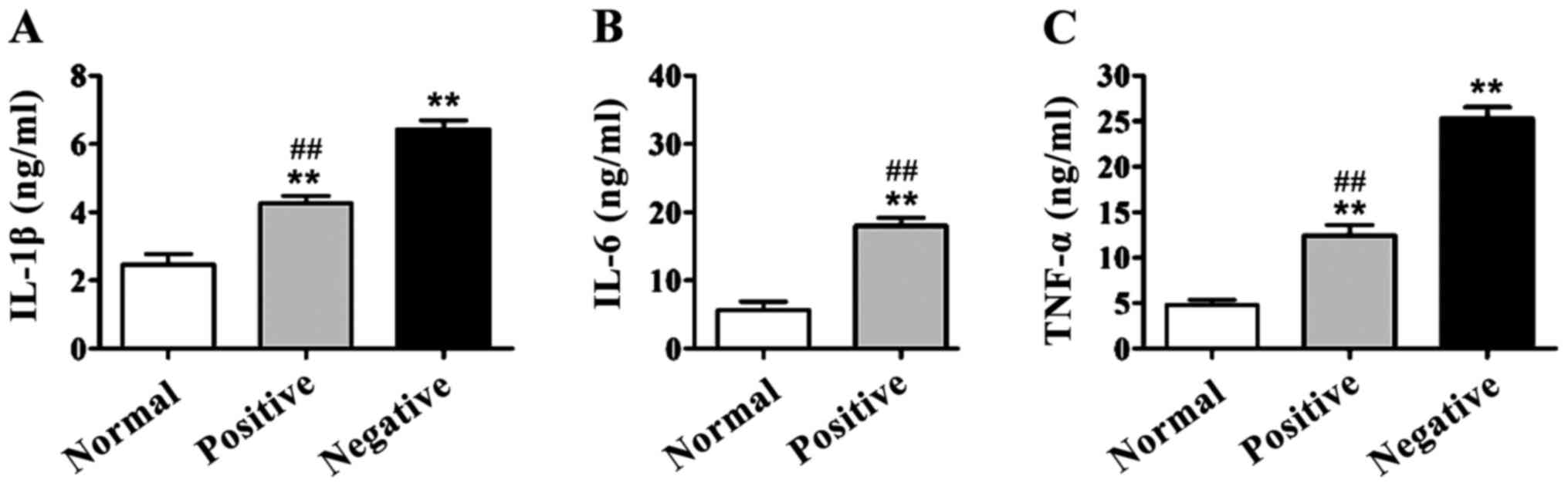
IL-1β, IL-6 and TNF-α levels in
serum
In DR group, the serum levels of IL-1β, IL-6 and
TNF-α of patients with and without RAGE gene promoter methylation
were higher than those in the normal control group (p<0.01).
IL-1β, IL-6 and TNF-α levels in patients with positive RAGE gene
promoter were significantly lower than those of patients with
negative RAGE gene promoter methylation, and there were significant
differences (p<0.01) (Fig.
2).
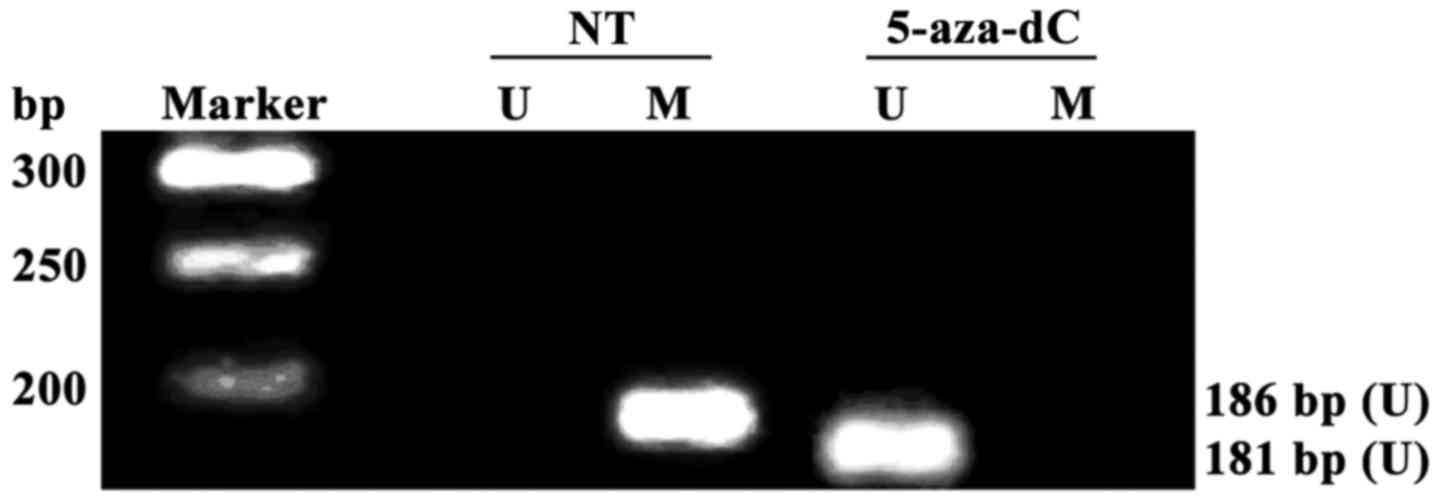
Effect of 5-aza-dC on RAGE gene
promoter methylation in PBMCs of patients
All RAGE gene promoters were methylated in
non-treatment group (NT), and no RAGE gene promoter methylation was
detected in 5-aza-dC treatment group, suggesting that the RAGE gene
promoter methylation in PBMCs of DR patients with positive RAGE
gene promoter methylation can be completely inhibited after
intervention with 5-aza-dC for 24 h (Fig. 3A).
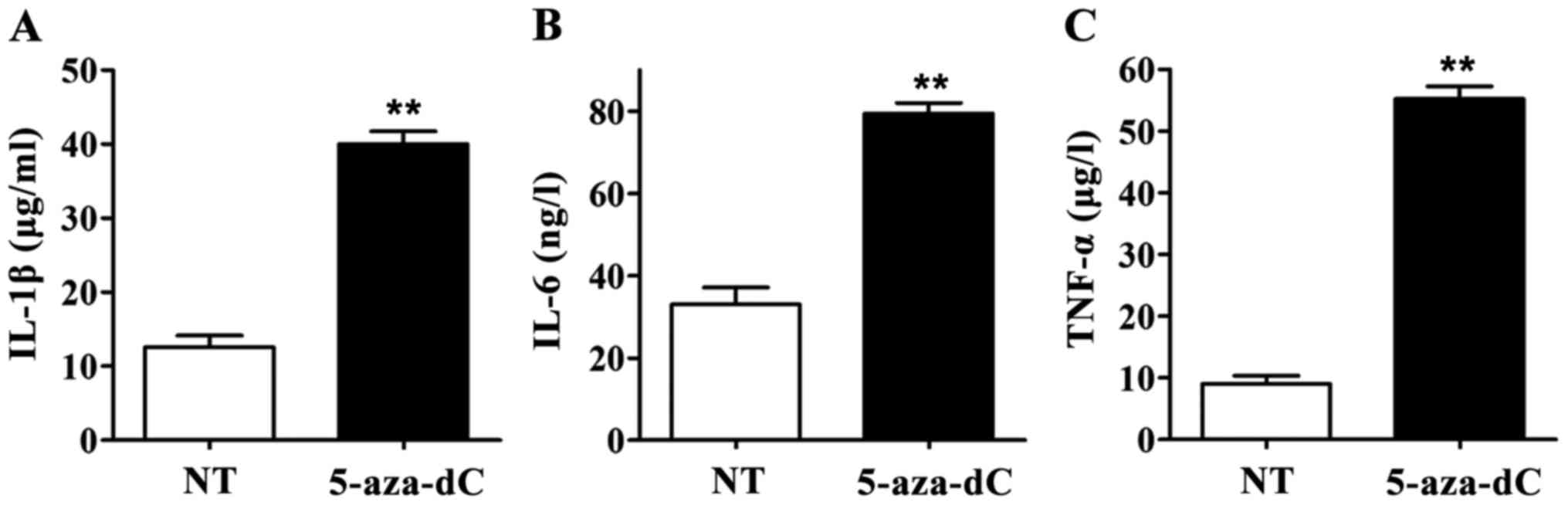
Effect of 5-aza-dC on IL-1β, IL-6 and
TNF-α levels in culture supernatant of PBMCs
When the RAGE gene promoter methylation in PBMCs was
inhibited by 5-aza-Dc, IL-1β, IL-6 and TNF-α levels in culture
supernatant in the 5-aza-dC group were significantly higher than
those in the NT group. All differences were statistically
significant (p<0.01) (Fig. 4),
suggesting that inhibiting the RAGE gene promoter methylation in
PBMCs can significantly increase the levels of IL-1β, IL-6 and
TNF-α in culture supernatant of PBMCs.
Discussion
Diabetic retinopathy is a serious complication in
patients suffering from diabetes, which can cause serious lesions
in retinal capillaries, and eventually may lead to blindness
(11). RAGE is a transmembrane
receptor with multiple ligands (12). RAGE, combined with its ligand AGEs,
can activate a series of signaling pathways, which may lead to
synthesis and release of different cytokines and growth factors. It
can cause vascular endothelial injury, abnormal cellular matrix
proliferation and other pathological changes, ultimately
participating in the occurrence and development of diabetic
complications (13). Epidemiological
studies have confirmed that the activation of RAGE signaling
pathways plays an important role in the inflammatory response of
human body and vascular disease in diabetic patients (14).
Prior studies showed that inflammatory symptoms can
de seen in diabetic patients, and IL-1β, IL-6, TNF-α expressions
may abnormally increase in these patients (15). Results obtained from other studies
revealed that hyperglycemia can promote the secretion of IL-1β,
IL-6 and IL-1. It was also discovered that IL-6 can promote the B
lymphocyte differentiation, and the production of the
antibody-activated T lymphocytes, it may also promote the death of
pancreatic islet B cells (16). At
the same time, IL-1 and IL-6 may promote the proliferation of
smooth muscle cells and can increase the endothelial permeability,
and cause vascular injury (17).
TNF-α mediates inflammatory responses, and is an important index of
inflammation and tissue damage, which can damage the blood-retinal
barrier and increase the permeability of retinal vessels (18). Studies have found that TNF-α
increases the adhesion of neutrophils and retinal endothelial cells
through increasing the activity of glycosylase (19).
In this study, MSP was used to detect the
methylation status of RAGE gene promoters in PBMCs. The results
showed that the methylation rate of RAGE gene promoters in PBMCs of
DR patients was 32.50%, and all RAGE gene promoters in PBMCs in
healthy adults were methylated. The results of ELISA showed that
IL-1β, IL-6 and TNF-α levels in serum of patients with positive
RAGE gene promoter methylation were significantly lower than those
of patients with negative RAGE gene promoter methylation. In order
to confirm the effect of RAGE promoter methylation on diabetic
retinal inflammation, 5-aza-dC was used to inhibit the RAGE gene
promoter methylation in PBMCs. Consequently, the effect of
inhibiting RAGE gene promoter methylation on IL-1β, IL-6 and TNF-α
levels in PBMC was investigated. Results showed that inhibiting
RAGE gene promoter methylation could significantly increase IL-1β,
IL-6 and TNF-α levels in the supernatant, indicating that RAGE gene
promoter methylation can inhibit diabetic retinal inflammation.
Yamamoto et al studied the role of RAGE in
diabetic nephropathy using transgenic technology, and they
discovered that the RAGE gene knockout can slow down the occurrence
of diabetic nephropathy (20). In
addition, in other studies it was revealed that lesions may occur
in retinas when RAGE protein is overexpressed in mice with
transgenic diabetes. Also, it was shown that the retinal
inflammation symptoms were significantly improved when the RAGE
overexpression in diabetic mice was inhibited (21,22). In
this study, it was demonstrated that RAGE gene promoter methylation
could inhibit diabetic retinal inflammation.
We conclud that RAGE gene promoter methylation
reduce diabetic retinal inflammation. Also, DNA methylation is a
way to regulate gene expression, therefore inducing RAGE gene
promoter methylation may become a new method for reducing the
inflammation of DR patients.
References
|
1
|
Wild S, Roglic G, Green A, Sicree R and
King H: Global prevalence of diabetes: Estimates for the year 2000
and projections for 2030. Diabetes Care. 27:1047–1053. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mehuys E, De Bolle L, Van Bortel L,
Annemans L, Van Tongelen I, Remon JP and Giri M: Medication use and
disease management of type 2 diabetes in Belgium. Pharm World Sci.
30:51–56. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Studholme S: Diabetic retinopathy. J
Perioper Pract. 18:205–210. 2008.PubMed/NCBI
|
|
4
|
Lee K, Ahn JM, Kim EK and Kim TI:
Comparison of optical quality parameters and ocular aberrations
after wavefront-guided laser in-situ keratomileusis versus
wavefront-guided laser epithelial keratomileusis for myopia.
Graefes Arch Clin Exp Ophthalmol. 251:2163–2169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yan SF, D'Agati V, Schmidt AM and Ramasamy
R: Receptor for advanced glycation endproducts (RAGE): A formidable
force in the pathogenesis of the cardiovascular complications of
diabetes and aging. Curr Mol Med. 7:699–710. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bierhaus A, Hofmann MA, Ziegler R and
Nawroth PP: AGEs and their interaction with AGE-receptors in
vascular disease and diabetes mellitus. I. The AGE concept.
Cardiovasc Res. 37:586–600. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singh R, Barden A, Mori T and Beilin L:
Advanced glycation end-products: A review. Diabetologia.
44:129–146. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Adamis AP: Is diabetic retinopathy an
inflammatory disease? Br J Ophthalmol. 86:363–365. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seiler T, Kaemmerer M, Mierdel P and
Krinke HE: Ocular optical aberrations after photorefractive
keratectomy for myopia and myopic astigmatism. Arch Ophthalmol.
118:17–21. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jubb AM, Bell SM and Quirke P: Methylation
and colorectal cancer. J Pathol. 195:111–134. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lam DW and LeRoith D: The worldwide
diabetes epidemic. Curr Opin Endocrinol Diabetes Obes. 19:93–96.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schmidt AM, Vianna M, Gerlach M, Brett J,
Ryan J, Kao J, Esposito C, Hegarty H, Hurley W, Clauss M, et al:
Isolation and characterization of two binding proteins for advanced
glycosylation end products from bovine lung which are present on
the endothelial cell surface. J Biol Chem. 267:14987–14997.
1992.PubMed/NCBI
|
|
13
|
Logsdon CD, Fuentes MK, Huang EH and
Arumugam T: RAGE and RAGE ligands in cancer. Curr Mol Med.
7:777–789. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan SF, Ramasamy R and Schmidt AM:
Mechanisms of disease: Advanced glycation end-products and their
receptor in inflammation and diabetes complications. Nat Clin Pract
Endocrinol Metab. 4:285–293. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carey AL, Lamont B, Andrikopoulos S,
Koukoulas I, Proietto J and Febbraio MA: Interleukin-6 gene
expression is increased in insulin-resistant rat skeletal muscle
following insulin stimulation. Biochem Biophys Res Commun.
302:837–840. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aljada A, Garg R, Ghanim H, Mohanty P,
Hamouda W, Assian E and Dandona P: Nuclear factor-kappaB
suppressive and inhibitor-kappaB stimulatory effects of
troglitazone in obese patients with type 2 diabetes: Evidence of an
antiinflammatory action? J Clin Endocrinol Metab. 86:3250–3256.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
André-Schmutz I, Hindelang C, Benoist C
and Mathis D: Cellular and molecular changes accompanying the
progression from insulitis to diabetes. Eur J Immunol. 29:245–255.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Krylova IN, Sablin EP, Moore J, Xu RX,
Waitt GM, MacKay JA, Juzumiene D, Bynum JM, Madauss K, Montana V,
et al: Structural analyses reveal phosphatidyl inositols as ligands
for the NR5 orphan receptors SF-1 and LRH-1. Cell. 120:343–355.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ben-Mahmud BM, Chan WH, Abdulahad RM,
Datti A, Orlacchio A, Kohner EM and Chibber R: Clinical validation
of a link between TNF-α and the glycosylation enzyme core 2
GlcNAc-T and the relationship of this link to diabetic retinopathy.
Diabetologia. 49:2185–2191. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamamoto Y, Kato I, Doi T, Yonekura H,
Ohashi S, Takeuchi M, Watanabe T, Yamagishi S, Sakurai S, Takasawa
S, et al: Development and prevention of advanced diabetic
nephropathy in RAGE-overexpressing mice. J Clin Invest.
108:261–268. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamagishi S, Nakamura K, Matsui T, Noda Y
and Imaizumi T: Receptor for advanced glycation end products
(RAGE): A novel therapeutic target for diabetic vascular
complication. Curr Pharm Des. 14:487–495. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reiniger N, Lau K, McCalla D, Eby B, Cheng
B, Lu Y, Qu W, Quadri N, Ananthakrishnan R, Furmansky M, et al:
Deletion of the receptor for advanced glycation end products
reduces glomerulosclerosis and preserves renal function in the
diabetic OVE26 mouse. Diabetes. 59:2043–2054. 2010. View Article : Google Scholar : PubMed/NCBI
|