Introduction
Both anterior and posterior cruciate ligaments are
intracapsular but extrasynovial structures and are nourished by the
middle genicular artery (1,2). Synovial vessels run obliquely and
longitudinally over the entire length of the anterior cruciate
ligament (ACL), beneath the synovial membrane. They arborize to
form a web-like network of periligamentous vessels that ensheath
the entire ligament (2).
Damage of cruciate ligaments may be accompanied by
disruption of the overlying synovial sleeve. In consequence,
fibroblasts forming ligaments may be exposed to the synovial fluid
(3) and, by the same token, more
accessible to biological stimulants introduced into the joint
cavity. When the fibroblasts from the ACL were exposed to the
synovial fluid, their plating efficiency was significantly reduced
suggesting that their ability to proliferate in a milieu of
synovial fluid is impaired (3).
Biological stimulants, however, such as platelet rich plasma (PRP)
preparations had beneficial influence on crucial ligaments healing
in animal experiments (4,5). Moreover, platelets together with plasma
proteins stimulated also collagen gene expression by cultured ACL
fibroblasts (6). In studies with
better defined growth factors, Lee et al (7) found that the stimulation of the cell
outgrowth in explants of rabbit anterior cruciate by basic
fibroblast growth factor (bFGF), insulin, transforming growth
factor-β 1 (TGFβ1), and platelet-derived growth factor-B (PDGF-B),
was much greater in the presence of all four growth factors than
the sum of the outgrowth with the individual factors. Stimulation
with TGFβ1 alone evoked strong proliferative response of cells from
explants of the ACL (8). TGFβ1
induced also dramatic elevation of metalloproteinase 2 (MMP2)
activities and the MMP2/tissue metalloproteinase inhibitors (TIMPs)
ratio in cells from ACL (9) and
significantly increased mRNA level of lysyl oxidase family members
(10) while tumor necrosis factor
(TNF) downregulated it (11).
Analysing both synovial fluid and growth factors
influence on the cruciate ligament fibroblasts (CLFs) it seems
advisable to include also factors produced by chondrocytes from
articular cartilage. McCutchen (12)
and others (13) formulated the
theory of ‘weeping’ lubrication in synovial joints. According to
their studies cartilage matrix contains a fluid phase, representing
~70% of its volume. During joint loading, ~10% of this liquid is
squeezed from the cartilage surface (which, in a molecular sense,
is porous) into the intra-articular cavity, and is responsible for
hydrostatic lubrication. Thus, it may be expected that cartilage
interstitial fluid (CIF) squeezed from cartilage during joint
loading contains cytokines produced by chondrocytes and affects
tissues of the joint. We have previously found that CIF released
from newborn rat cartilage contained bFGF, insulin-like growth
factor 1 (IGF1), TGFβ1, bone morphogenetic protein 7 (BMP7),
macrophage colony-stimulating factor (MCSF), granulocyte
colony-stimulating factor (GCSF) and leukemia inhibitory factor
(LIF). We also demonstrated that CIF stimulated a number of genes
in synovial membrane and dermal fibroblasts and these effects could
be partially imitated by CIF-like cocktail composed of factors
identified in CIF (14–16).
After crucial ligaments damage and tearing of
synovial tissue cover, their cells would be exposed to synovial
fluid, presumably containing factors not only produced by
synoviocytes but also released from articular cartilage. Thus, it
appeared interesting to establish influence of CIF on the cells
derived from the crucial ligaments, to see whether they react to
CIF stimulation similarly to dermal fibroblasts, or display
peculiarities which could be used in attempts to produce biological
constructs replacing damaged ligaments.
Materials and methods
Animals
Three-to five day-old inbred Lewis rats of both
sexes served as cartilage donors for CIF preparation. Crucial
ligaments were dissected from ten to twelve week-old male Lewis
rats. The animals were obtained from the Animal Unit of the Warsaw
Medical University. The study and the methods were approved by the
Animal Ethics Committee of the Warsaw Medical University (Warsaw,
Poland).
Preparation of CIF
CIF was prepared as described previously (14). Briefly, CIF was squeezed from the
articular-epiphyseal cartilage complexes dissected from the newborn
rats. After clearing from the surrounding tissues cartilages from 2
animals were put into 2 ml of PBS (Gibco BRL, Paisley, Scotland,
UK) and cut into small fragments which, together with PBS, were
transferred into a 50 ml Luer Lock syringe closed with the PTFE
Body Two-Way Valve from Hamilton (Sigma-Aldrich Chemie, Steinheim,
Germany). The air in the syringe was pressed with the plunger to
increase pressure up to three bars. Then the plunger was slowly
released. This procedure was repeated 20 times. The fluid was
separated from cartilage fragments by centrifugation, desalted on
PD-10 columns (Amersham Biosciences, Uppsala, Sweden) and
lyophilized. The lyophilisate was dissolved in RPMI medium (Gibco).
CIF from 10–20 rats was pooled to obtain more uniform material and
the protein content was determined using BCA protein assay reagent
(Pierce, Rockford, IL, USA). The total amount of protein in CIF
squeezed from cartilage obtained from one animal varied from 0.87
to 1.1 mg. Working solution of CIF was standardized to contain 1
mg/ml of protein in RPMI supplemented with 2% of FCS and 1% of
Antibiotic/Antimycotic Solution (all from Gibco).
CIF-like cocktail preparation
CIF-like cocktail contained commercial cytokines
dissolved in RPMI medium with 0.1% of bovine serum albumin (BSA)
and 2% of FCS (all from Gibco). Cytokines were used in
concentration identical to that found in CIF: 25 pg/ml G-CSF, 60
pg/ml M-CSF, 25 pg/ml LIF, 80 pg/ml BMP7, 2.5 ng/ml bFGF
(PromoKine; PromoCell GmbH, Heidelberg, Germany), 0.5 ng/ml TGFβ1
(Sigma-Aldrich Chemie) and 2 ng/ml IGF1 (R&D Systems, MN,
USA).
Dissection of crucial ligaments
Knee joints were cut off together with fragments of
femur and tibia from 10–12 week old rats. Joint cavity was opened
and the synovial membrane was excised together with patella,
patellar ligament and joint capsule. Then all tissues covering the
joint together with remnants of joint capsule were removed. The
further preparation was done under dissecting microscope. Anterior
and posterior crucial ligaments, together with menisci were
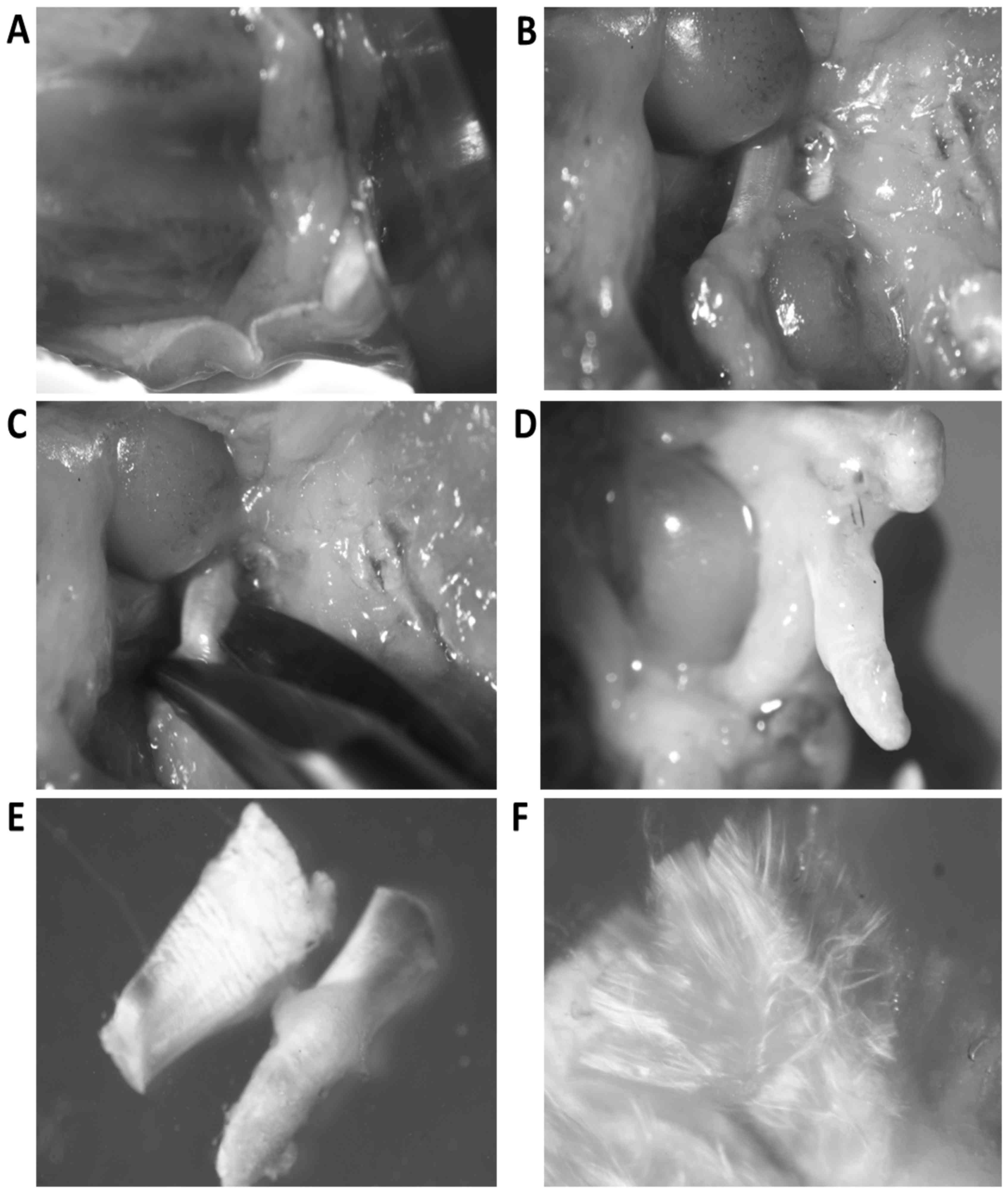
separated from the tibia (Fig. 1A and
B). The femur was grasped with sharp forceps and both crucial
ligaments were cut off from the menisci and afterwards from the
condyles of the femur (Fig.
1C-E).
Isolation and culture of ligament
fibroblasts
Bundles of ligament collagen fibers were separated
with thin needles to expose fibroblasts located between bundles
(Fig. 1F). Ligaments were incubated
in enzymic solution containing 0.25% collagenase (Type I), 0.05%
DNase, 17.5 µM N-a-p tosyl-l-lysine chloromethylketone (TLCK) and
1% antibiotic-antimycotic solution (all from Sigma-Aldrich Chemie)
in RPMI medium, (Gibco). Ligaments were delicately shaken for 40
min in humified atmosphere of 5% CO2 in air at 37°C. The
digested material was filtered through a 40-µm mesh nylon filter.
The liberated cells were rinsed 3 times with the RPMI medium
(Gibco) supplemented with 10% FCS and 1% Antibiotic/Antimycotic
solution (all from Gibco) and seeded into 25 cm2 flasks
(Corning Inc., Corning, NY, USA) in 5 ml of medium. After the cells
reached subconfluency, they were detached with 0.25% trypsin-EDTA
(Sigma-Aldrich Chemie), suspended in CIF, CIF-like cocktail or
control medium (RPMI with 0.1% of BSA, 1% of Antibiotic/Antimycotic
solution and 2% of FCS) and seeded into flat-bottomed 24-well
plates (Corning) at the density 5×104 per well. The
cells were incubated in humified atmosphere of 5% CO2 in
air at 37°C for 24 h. After incubation the total RNA from cultured
cells was isolated and the expression of genes encoding: Hyaluronan
synthases (HAS1 and HAS2), extracellular matrix proteins (collagen
type I, versican, aggrecan and lubricin), matrix metalloproteinases
(MMP2 and MMP3), tissue inhibitors of metalloproteinases (TIMP1,
TIMP2 and TIMP3), and cytokines (TGFβ1, TNF, IL1β and IL6) was
examined.
Some fibroblasts in control or experimental medium
were also seeded onto 12 mm glass slides placed in 24 well plates
and, after 24 h culture, were fixed and stained with
hematoxylin/eosin.
Total RNA isolation
RNA was isolated with NucleoSpin®RNA II
kit (Macherey-Nagel, Duren, Germany), according to manufacturer's
protocol. The quantity and quality of the isolated total RNA was
evaluated spectrophotometrically using ND-2000-Spectrophotometer
NanoDrop 2000 with software for analysis of nucleic acids (Thermo
Fisher Scientific, Wilmington, Delaware, USA).
Reverse transcription
Reverse transcription was performed using the High
Capacity cDNA Reverse Transcription kit (Applied Biosystems,
Cheshire, UK), according to the manufacturer's protocol in
Eppendorf Mastercycler gradient (10 min at 25°C, 120 min at 37°C
and 5 sec. at 85°C). Briefly, 2 µl of 10X RT buffer, 0.8 µl of 25×
dNTP Mix, 2 µl of 10× Random Primers, 1 µl of Multiscribe Reverse
Transcriptase, 4.2 µl of nuclease-free water and 10 µl of mRNA (0.5
µg) per one reaction. cDNA samples were stored at −20°C.
Real-time PCR
Real-time PCR was performed in the ABI PRISM 7500
(Applied Biosystems) using 96-well optical plates. Each sample was
run in triplicate and was supplied with an endogenous control (Rat
GAPDH endogenous control Rn01775763_g1). For gene expression
analysis, the proper TaqMan expression assays was used.
(Rn00597231_m1 for HAS1, Rn00565774_m1 for HAS2, Rn01526721_m1 for
collagen type I, Rn01493763_m1 for versican, Rn00573424_m1 for
aggrecan, Rn01490812_m1 for lubricin, Rn01538167 for MMP2,
Rn00591740_m1 for MMP3, Rn 00587558_m1 for TIMP1, Rn00573232_m1 for
TIMP2, Rn00441826_m1 for TIMP3, Rn00572010_m1 for TGFβ1,
Rn99999017_m1 for TNF, Rn00580432_m1 for IL1β and Rn01410330_m1 for
IL6). All probes were stained with FAM (Applied Biosystems).
Reactions was run in 25 µl of volume with TaqMan Universal Master
Mix, appropriate primer set, MGB probe and 50 ng of cDNA template.
Universal thermal conditions, 10 min at 95°C, 40 cycles of 15 sec
at 95°C and 1 min at 60°C, were used. Data analysis was done with
sequence detection software version 1.2 (Applied Biosystems).
Statistical analysis
Data were analyzed by the Mann-Whitney U test
(Statistica12 software; StatSoft Polska, Krakow, Poland). P<0.05
was considered to indicate a statistically significant difference
(17).
Results
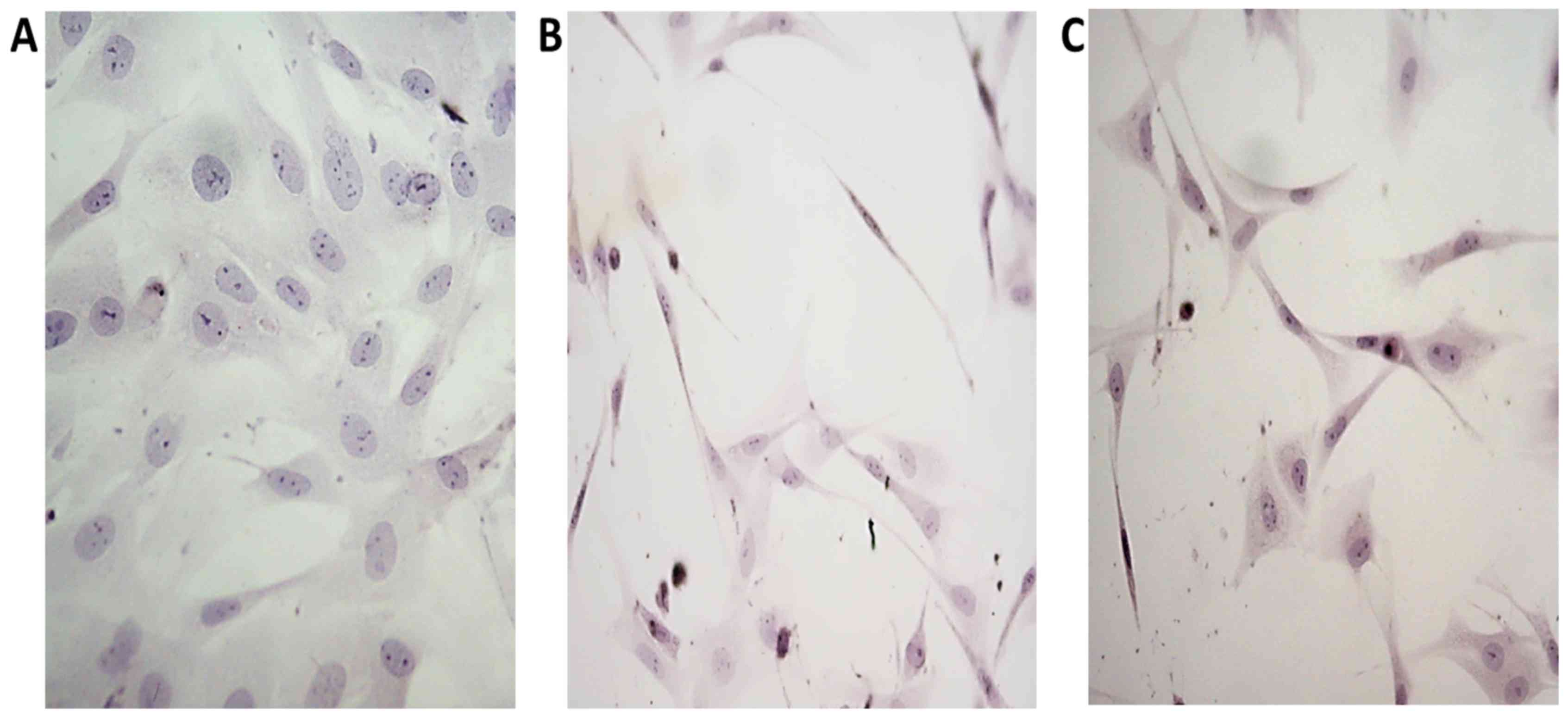
Ligament fibroblasts cultured in the control medium
had multiform appearance and possess scanty, usually short
processes, characteristic for typical fibroblasts in culture.
(Fig. 2A). In CIF treated cultures
numerous fibroblasts assumed spindle-like form, with long, thin
processes or were triangular with slim, very long extensions.
(Fig. 2B). The influence of CIF-like
cocktail on the morphology of cultured fibroblasts was similar to
the influence of CIF (Fig. 2C).
In crucial ligament fibroblasts (CLFs) CIF
stimulated the expression of genes encoding HAS1, HAS2, aggrecan,
lubricin, MMP3, TIMP3 and TGFβ1. Expression of collagen type I,
versican, MMP2, TIMP2, TNF and IL1β was inhibited. CIF-like
cocktail stimulated expression of HAS1, HAS2, collagen type I,
versican, aggrecan, lubricin, TIMP1, TGFβ1, IL1β and IL6 and
inhibited of MMP3 and TNF expression. Both agents exerted the
similar effect on the expression of genes encoding HAS2, aggrecan,
lubricin, TGFβ1 and TNF (Fig.
3).
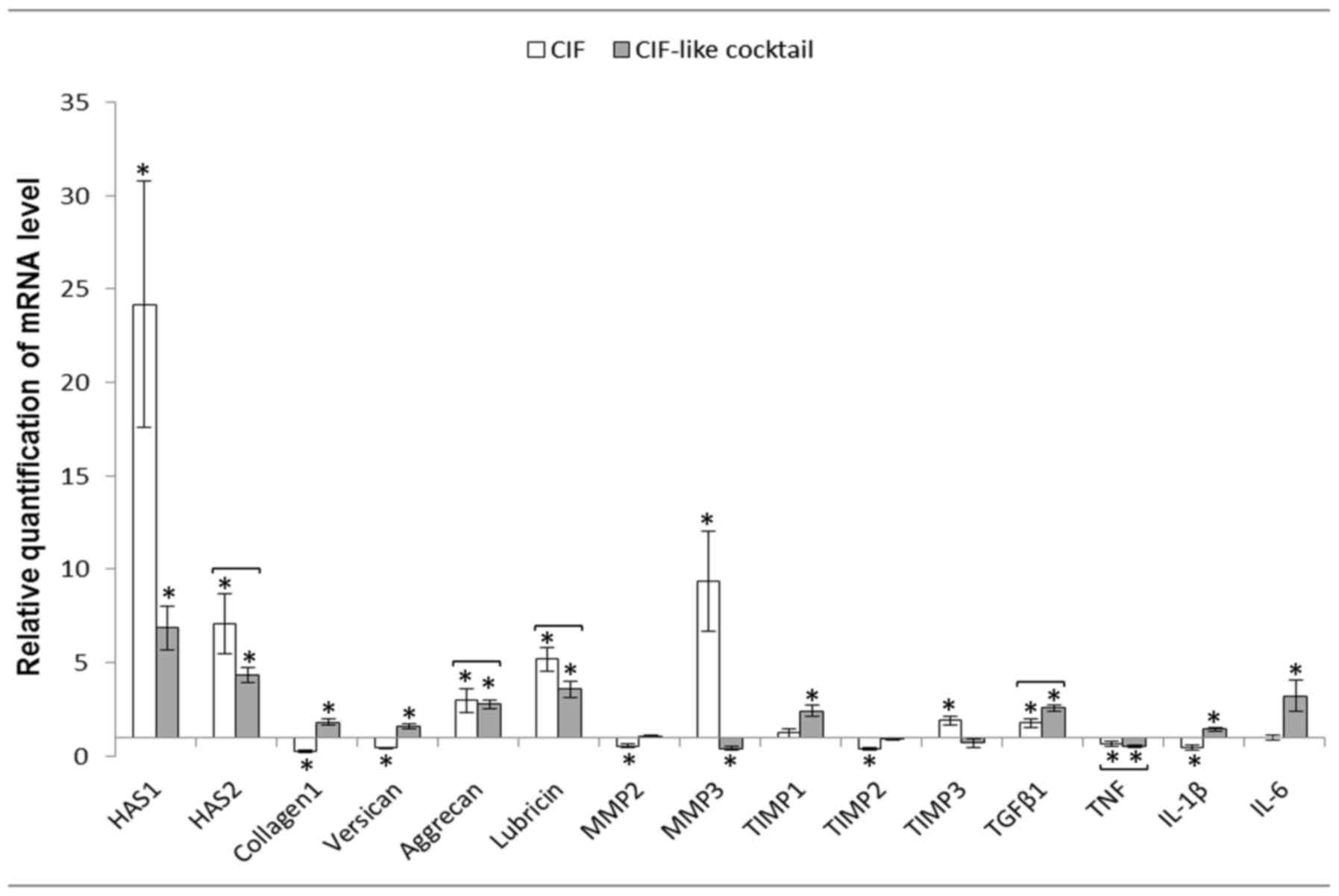 | Figure 3.Mean ± standard error of HASs, matrix
proteins, MMPs, TIMPs and cytokine mRNA levels in the cruciate
ligament fibroblasts after 24 h of incubation with CIF or CIF-like
cocktail (n=12). Relative expression was calculated against the
reference gene, GAPDH. Analysis was conducted as a relative
quantification study using fibroblasts cultured in control medium
(RPMI medium containing 2% fetal calf serum) gene expression as a
calibrator (value=1). *P<0.05 between control and experimental
groups. Statistically insignificant differences between the
influence of CIF and CIF-like cocktail are joined by brackets,
because the lack of differences suggests that CIF-like cocktail
acts similarly to CIF. CIF, cartilage interstitial fluid; HAS,
hyaluronan synthases; MMP, matrix metalloproteinase; TIMP, tissue
inhibitors of metalloproteinases; TGF, transforming growth factor;
TNF, tumor necrosis factor; Il, interleukin. |
Discussion
In the previous work (15) the influence of CIF and CIF-like
cocktail on the gene expression in dermal fibroblasts was studied.
In the present work we examined the influence of CIF and CIF-like
cocktail on fibroblasts isolated from crucial ligaments.
Morphological changes of ligament fibroblasts under the influence
of CIF or CIF-like cocktail were similar to those occurring in
dermal fibroblasts and probably were caused by the reorganization
of the cytoskeleton (Fig. 2B and C).
The effects exerted by CIF and CIF-like cocktail on gene expression
in dermal and CLFs demonstrated several differences (Tables I and II).
 | Table I.Influence of CIF on cruciate ligament
and dermal fibroblasts. |
Table I.
Influence of CIF on cruciate ligament
and dermal fibroblasts.
| Gene | Cruciate ligament
fibroblasts | Dermal
fibroblastsa |
|---|
| The same effect (no
statistically significant differences) |
|
|
| HAS2 | 7.10±1.60 | 6.92±1.06 |
|
Collagen1 | 0.27±0.05 | 0.36±0.06 |
| MMP2 | 0.53±0.11 | 0.53±0.05 |
|
TIMP1 | 1.23±0.22 | 1.10±0.15 |
|
TIMP2 | 0.40±0.05 | 0.39±0.07 |
|
TGFβ1 | 1.78±0.24 | 2.32±0.36 |
|
IL-1β | 0.45±0.13 | 0.63±0.09 |
| Similar effect
(statistically significant differences) |
|
|
| HAS1 | 24.17±6.58 | 1.56±0.09 |
|
Versican | 0.43±0.05 | 0.67±0.05 |
|
Lubricin | 5.2±0.64 | 1.52±0.33 |
| MMP3 | 9.37±2.65 | 276.91±53.35 |
| The opposite
effect |
|
|
|
Aggrecan | 2.97±0.62 | 0.12±0.05 |
|
TIMP3 | 1.92±0.24 | 0.09±0.02 |
| TNF | 0.66±0.15 | 2.32±0.63 |
| IL-6 | 1.0±0.16 | 1.84±0.35 |
 | Table II.Influence of CIF-like cocktail on
cruciate ligament and dermal fibroblasts. |
Table II.
Influence of CIF-like cocktail on
cruciate ligament and dermal fibroblasts.
| Gene | Cruciate ligament
fibroblasts | Dermal
fibroblastsa |
|---|
| The same effect (no
statistically significant differences) |
|
|
|
Collagen1 | 1.82±0.19 | 1.40±0.10 |
| MMP2 | 1.03±0.07 | 1.14±0.04 |
|
TIMP2 | 0.92±0.07 | 1.09±0.06 |
|
TIMP3 | 0.70±0.22 | 1.15±0.09 |
|
IL-1β | 1.43±0.09 | 1.24±0.35 |
| Similar effect
(statistically significant differences) |
|
|
|
HAS1 | 6.85±1.19 | 1.36±0.19 |
|
HAS2 | 4.33±0.40 | 1.60±0.16 |
|
TIMP1 | 2.43±0.31 | 1.25±0.12 |
|
TGFβ1 | 2.57±0.17 | 1.52±0.14 |
| The opposite
effect |
|
|
|
Versican | 1.60±0.15 | 1.08±0.07 |
|
Aggrecan | 2.77±0.26 | 1.08±0.19 |
|
Lubricin | 3.58±0.45 | 0.95±0.16 |
|
MMP3 | 0.42±0.10 | 0.96±0.10 |
|
TNF | 0.52±0.09 | 2.16±0.35 |
|
IL-6 | 3.23±0.81 | 1.17±0.09 |
In case of HAS1 expression, CIF and CIF-like
cocktail influence was markedly stronger on CLFs than on dermal
fibroblasts (DFs). Similarly, HAS2 expression under the influence
CIF-like cocktail was stronger in CLFs than in DFs, however, CIF
stimulation of HAS2 was within the same range (Tables I and II). Hyaluronic acid present in synovial
fluid is synthesized in synovial membrane by fibroblast-like
synoviocytes, which express hyaluronan synthases (18). The same authors (18) demonstrated that TGFβ1 is the potent
stimulus for HAS1 transcription. Thus, TGFβ1 which is present both
in CIF and CIF-like cocktail could be responsible for raise in HAS1
expression. In short, lasting 4 h only stimulation of the synovial
membrane, expression of HAS1 was, however, not stimulated by TGFβ1
or IGF1 acting alone, but both factors applied together exerted
distinct stimulatory effect suggestive of certain synergism
(14,16). Since platelet-rich plasma (PRP), used
for the treatment of joint ailments, contains, among others, TGFβ1,
IGF1 and bFGF (19), the same
factors which are present in CIF, administration of PRP could also
stimulate HAS1 expression in crucial ligaments.
CIF inhibited gene expression of versican and
collagen type I both on CLF and DF while CIF like cocktail
stimulated it in CLFs (Tables I and
II). Cells from human cruciate
ligaments expanded in culture and treated with TGFβ1 and bFGF
increased expression and production of collagenous and
noncollagenous extracellular matrix proteins (6) similarly as CIF-like cocktail in which
both these factors were present. Thus the marked difference between
CIF and CIF-like cocktail suggests that the CIF contains some, as
yet unidentified, inhibitory factor(s). A decrease in versican
content detected in ruptured ACLs of patients in comparison to
normal controls (20) could also
depend on the influence of the same or similar factor(s) which
gained access to the torn ligaments.
Expression of lubricin was stimulated by both agents
only in the CLFs (Tables I and
II). Similar stimulation was also
evident in the rat synovial membrane (14). In torn ACLs, lubricin was generally
found as a discrete layer covering the torn surface. Lubricin was
also found on the native surfaces of intact ACLs and on torn edges
of ACLs. Thus, it may interfere with the integrative healing
process needed for ACL repair (21).
Both CIF and CIF-like cocktail stimulated aggrecan
expression in CLFs but not in DFs (Tables I and II). Presence of aggrecan in
fibrocartilaginous ligaments was also reported previously (22).
In synovial fluid samples of patients with crucial
ligaments injury the average concentrations of MMP-3 was highly
elevated in comparison with normal controls. The concentration of
MMP-3 remained high, independent of the duration since the injury
(23). In our experiments
stimulation of MMP3 by CIF was evident in the CLFs, although was
many times stronger in DFs. CIF-like cocktail inhibited its
expression in CLFs and had no influence in DFs (Tables I and II). Reactions of crucial ligaments may
considerably differ from those of tendons, since downregulated
expression of MMP3 mRNA have been found in injured or ruptured
Achilles and supraspinatus tendons as compared to non-injured
tendon groups (24).
The expression of TGFβ1 was stimulated by CIF and
CIF-like cocktail in DFs and CLFs (Tables I and II) and its secretion was also detected in
human tendon fibroblasts (25).
Many articles refer to the fibroblast gene
expression of the ligaments in damaged or pathological joints
(20,21,23,24). Our
study shows the influence of factors present in normal joint fluid
on the gene expression in ACL fibroblasts. Therefore, our results
may be the basis for evaluation the changes occurring in the
pathological states of synovial joints.
It is evident from the presented data that CIF
contains both inhibitory and stimulatory factors and some of them
are not included in CIF-like cocktail. Melas et al (26) found that cultured chondrocytes may
release up to 55 cytokines and other active agents either
spontaneously or after stimulation by various ligands and list of
growth factors produced by chondrocyte may still not be complete.
Thus, in view of the powerful influence exerted by CIF both on the
synovial membrane (14) and CLFs as
evident in this work, further studies on its composition are
needed, particularly that improved CIF like-cocktail could find
application in treatment of various joint or tendon ailments.
Acknowledgements
The present study was supported by the National
Science Centre of Poland (grant no. DEC-2012/05/B/NZ4/02646).
Glossary
Abbreviation
Abbreviations:
|
CIF
|
cartilage interstitial fluid
|
References
|
1
|
de Carvalho RT, Ramos LA, Novaretti JV,
Ribeiro LM, Szeles PR, Ingham SJ and Abdalla RJ: Relationship
between the middle genicular artery and the posterior structures of
the knee: A cadaveric study. Orthop J Sports Med.
4:23259671166735792016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Duthon VB, Barea C, Abrassart S, Fasel JH,
Fritschy DJ and Ménétrey J: Anatomy of the anterior cruciate
ligament. Knee Surg Sports Traumatol Arthrosc. 14:204–213. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Andrish J and Holmes R: Effects of
synovial fluid on fibroblasts in tissue culture. Clin Orthop Relat
Res. 138:279–283. 1979.
|
|
4
|
Murray MM, Spindler KP, Abreu E, Muller
JA, Nedder A, Kelly M, Frino J, Zurakowski D, Valenza M, Snyder BD
and Connolly SA: Collagen-platelet rich plasma hydrogel enhances
primary repair of the porcine anterior cruciate ligament. J Orthop
Res. 25:81–91. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Murray MM and Fleming BC: Use of a
bioactive scaffold to stimulate anterior cruciate ligament healing
also minimizes posttraumatic osteoarthritis after surgery. Am J
Sports Med. 41:1762–1770. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng M, Wang H, Yoshida R and Murray MM:
Platelets and plasma proteins are both required to stimulate
collagen gene expression by anterior cruciate ligament cells in
three-dimensional culture. Tissue Eng Part A. 16:1479–1489. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee J, Green MH and Amiel DJ: Synergistic
effect of growth factors on cell outgrowth from explants of rabbit
anterior cruciate and medial collateral ligaments. J Orthop Res.
13:435–441. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Spindler KP, Imro AK, Mayes CE and
Davidson JM: Patellar tendon and anterior cruciate ligament have
different mitogenic responses to platelet-derived growth factor and
transforming growth factor beta. J Orthop Res. 14:542–546. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Tang Z, Xue R, Singh GK, Lv Y, Shi
K, Cai K, Deng L and Yang L: TGF-β1 promoted MMP-2 mediated wound
healing of anterior cruciate ligament fibroblasts through NF-κB.
Connect Tissue Res. 52:218–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie J, Jiang J, Zhang Y, Xu C, Yin L, Wang
C, Chen PC and Sung KL: Up-regulation expressions of lysyl oxidase
family in anterior cruciate ligament and medial collateral ligament
fibroblasts induced by transforming growth factor-beta 1. Int
Orthop. 36:207–213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie J, Jiang J, Huang W, Zhang Y, Xu C,
Wang C, Yin L, Chen PC and Sung KL: TNF-α induced down-regulation
of lysyl oxidase family in anterior cruciate ligament and medial
collateral ligament fibroblasts. Knee. 21:47–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McCutchen CW: Sponge-hydrostatic and
weeping bearings. Nature. 184:1284–1285. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Caligaris M and Ateshian GA: Effects of
sustained interstitial fluid pressurization under migrating contact
area and boundary lubrication by synovial fluid, on cartilage
friction. Osteoarthritis Cartilage. 16:1220–1227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hyc A, Moskalewski S and Osiecka-Iwan A:
Influence of cartilage interstitial fluid on the mRNA levels of
matrix proteins, cytokines, metalloproteases and their inhibitors
in synovial membrane. Int J Mol Med. 38:937–942. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hyc A, Moskalewski S, Stankevic D and
Osiecka-Iwan A: Influence of cartilage interstitial fluid on gene
expression in dermal fibroblasts. Folia Biologica (Kraków).
65:27–32. 2017.
|
|
16
|
Osiecka-Iwan A, Moskalewski S and Hyc A:
Influence of IGF1, TGFb1, bFGF and G-CSF/M-CSF on the mRNA levels
of selected matrix proteins, cytokines, metalloproteinase 3 and
TIMP1 in rat synovial membrane cells. Folia Histochem Cytobiol.
54:159–165. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stuhlmeier KM and Pollaschek C:
Differential effect of transforming growth factor beta (TGF-beta)
on the genes encoding hyaluronan synthases and utilization of the
p38 MAPK pathway in TGF-beta-induced hyaluronan synthase 1
activation. J Biol Chem. 279:8753–8760. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kabiri A, Esfandiari E, Esmaeili A,
Hashemibeni B, Pourazar A and Mardani M: Platelet-rich plasma
application in chondrogenesis. Adv Biomed Res. 3:1382014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Young K, Samiric T, Feller J and Cook J:
Extracellular matrix content of ruptured anterior cruciate ligament
tissue. Knee. 18:242–246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang D, Cheriyan T, Martin SD, Gomoll AH,
Schmid TM and Spector M: Lubricin distribution in the torn human
anterior cruciate ligament and meniscus. J Orthop Res.
29:1916–1922. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Milz S, Aktas T, Putz R and Benjamin M:
Expression of extracellular matrix molecules typical of articular
cartilage in the human scapholunate interosseous ligament. J Anat.
208:671–679. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Higuchi H, Shirakura K, Kimura M, Terauchi
M, Shinozaki T, Watanabe H and Takagishi K: Changes in biochemical
parameters after anterior cruciate ligament injury. Int Orthop.
30:43–47. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ireland D, Harrall R, Curry V, Holloway G,
Hackney R, Hazleman B and Riley G: Multiple changes in gene
expression in chronic human Achilles tendinopathy. Matrix Biol.
20:159–169. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Skutek M, van Griensven M, Zeichen J,
Brauer N and Bosch U: Cyclic mechanical stretching modulates
secretion pattern of growth factors in human tendon fibroblasts.
Eur J Appl Physiol. 86:48–52. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Melas IN, Chairakaki AD, Chatzopoulou EI,
Messinis DE, Katopodi T, Pliaka V, Samara S, Mitsos A, Dailiana Z,
Kollia P and Alexopoulos LG: Modeling of signaling pathways in
chondrocytes based on phosphoproteomic and cytokine release data.
Osteoarthritis Cartilage. 22:509–518. 2014. View Article : Google Scholar : PubMed/NCBI
|