Introduction
The human body possesses ~640 skeletal muscles,
which account for ~40% of the whole body mass (1). These skeletal muscles are responsible
for body movement and fulfill the functions of locomotion,
respiration, swallowing and speech. Although they share the common
architectural traits of striated muscles, the skeletal muscles have
distinct embryonic origins (2).
Trunk and limb muscles derive from somites; the majority of head
muscles, such as masseter muscle and pharyngeal muscles, derive
from branchial arches; extraocular muscles derive from prechordal
mesoderm (3–5).
The prototype for skeletal muscle study is
somite-derived limb muscles, but these represent only a small
proportion of the whole skeletal muscle population (1). Investigations into head muscles have
revealed that they exhibit heterogeneity in comparison with their
limb counterparts. During embryonic myogenesis, T-box 1 and
pituitary homeobox 2 mediate the formation of head muscles, yet are
absent in limb muscle development (4,6). Head
muscles possess a visceral mesodermal origin and are closely
associated with heart development in myogenesis (7,8).
Mesoderm in the cardiopharyngeal field gives rise to both the
branchiomeric muscles and part of the heart (3). In addition, masseter muscle was
reported to regenerate less effectively compared with tibialis
anterior (TA) muscle (9). These
results point to the notion that head muscles and limb muscles are
markedly different skeletal muscle populations.
TA muscle has been studied extensively, particularly
its developmental origin, morphological characteristics and
augmentation potential (9–11). Levator veli palatini (LVP) muscle,
however, lacks investigation in spite of its critical role in
fulfilling velopharyngeal function and its core status in cleft
palate surgery (12–14). Plastic surgeons have long been
searching for strategies to augment LVP muscle, in order to
facilitate velopharyngeal closure and improve speech in patients
with cleft palates (15–17).
Strategies for muscle augmentation have been sought
for decades. Effective agents, such as insulin-like growth factor
(IGF)-1, angiotensin and Wnt7a, have previously been proposed to
increase myofiber diameter and enhance muscle function in atrophied
or normal muscle (10,18–20).
These studies were primarily performed in limb muscles like the TA
muscle. Considering the general differences between limb and head
muscle, combined with the knowledge gap for LVP muscle
augmentation, the present study was performed for two reasons: i)
To glean background information of LVP muscle, in comparison with
TA muscle; ii) to investigate whether the two muscles exhibited
different augmentation potential. In the present study, the
differences between TA and LVP muscle were characterized in terms
of muscle fiber composition, in situ stem cell population
and activation level of the Wnt signaling pathway under basal
conditions. Furthermore, it was investigated whether the two
muscles responded differently to growth factor stimulus.
Materials and methods
Animals
All experimental procedures on animals were approved
by the Institutional Animal Care and Use Committee at Sichuan
University (Chengdu, China). Adult male Sprague-Dawley rats (age,
10 weeks; weight, 280–300 g) were purchased from Chengdu Dashuo
Experimental Animal Center (Chengdu, China). The animals were
raised in a temperature- and humidity-controlled room (temperature,
21±2°C; relative humidity, 50±5%) on a 12-h light/dark schedule.
Food and water were freely accessible. A total of 18 animals were
used in the present study and were randomly allocated to the
following three groups: i) comparison between TA muscle and LVP
muscle under basal conditions (n=6); ii) intramuscular Wnt7a
administration of TA muscle (n=6); iii) intramuscular Wnt7a
administration of LVP muscle (n=6).
Intramuscular Wnt7a delivery
Recombinant human Wnt7a (R&D Systems, Inc.,
Minneapolis, MN, USA) was injected directly into the muscles. For
each TA muscle (n=6), 75 µl Wnt7a (100 µg/ml) was injected and 75
µl PBS was injected to the TA muscle in the contralateral leg as
control; for each LVP muscle (n=6), 25 µl Wnt7a (100 µg/ml) was
injected and 25 µl PBS was injected to the LVP muscle on the
contralateral side as control. Injection into LVP muscle was
carried out via an intraoral procedure. Rats were sacrificed 3
weeks after treatment and muscles were harvested for analyses.
Muscle harvest
TA muscle was cut from tendon to tendon on the tibia
anterior bone. LVP muscle was approached and isolated according to
Carvajal Monroy et al (21),
with small modifications. Briefly, a ventral incision extending
from the mandibular symphysis to the clavicle was made and the
subcutaneous tissue was separated to expose the salivary gland.
After removal of the salivary gland, the digastric and
sternocleidomastoid muscle was visible. The posterior belly of the
digastric muscle was dissected to its origin to expose the
stylohyoid muscle beneath it and the tympanic bulla. The stylohyoid
muscle was cut at its junction to the hyoid and pulled laterally to
visualize the LVP muscle with its tendon clearly attached to the
tympanic bulla. The LVP muscle was carefully dissected from its
origin in the tympanic bulla to its insertion in the soft
palate.
Immunofluorescence analysis
Muscle samples were attached to the chuck using
Tissue-Tek Optimal Cutting Temperature compound (Sakura Finetek
USA, Inc., Torrance, CA, USA) and frozen in isopentane cooled with
liquid nitrogen using Lawlor's method (22). Cryosections were made at 10 µm
thickness and fixed in 0°C acetone (100%) for 20 min. The sections
were dried at room temperature for 20 min and washed in 0.01 mol/l
phosphate-buffered saline (PBS). Sections were then blocked with
PBS containing 5% bovine serum albumin (Amresco, LLC, Solon, OH,
USA), and 5% donkey serum (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) for 1 h at 25°C, and
subsequently incubated overnight at 4°C with primary antibodies.
Following washing in PBS, sections were incubated for 1 h at 25°C
with Alexa Fluor 488-conjugated (A-21206; 1:500) and 568-conjugated
(A10037; 1:500) secondary antibodies (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). After several washes in PBS,
the nuclei were stained with DAPI. Images were captured with an
Olympus BX63 fluorescence microscope (Olympus Corporation, Tokyo,
Japan). The primary antibodies used were as follows: Rabbit
anti-laminin polyclonal antibody (L9393; 1:500; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany), mouse anti-Pax7 monoclonal
antibody (PAX7; 1:5; Developmental Studies Hybridoma Bank, Iowa
City, IA, USA), rabbit anti-Ki67 monoclonal antibody (ab1667;
1:500; Abcam, Cambridge, MA, USA), mouse anti-myosin heavy chain 1
(MyHC-1) monoclonal antibody (A4.840; 1:40; Developmental Studies
Hybridoma Bank), mouse anti-MyHC-2A monoclonal antibody (SC-71;
1:20; Developmental Studies Hybridoma Bank), mouse anti-MyHC-2X
monoclonal antibody (6H1; 1:5; Developmental Studies Hybridoma
Bank) and mouse anti-MyHC-2B monoclonal antibody (BF-F3; 1:5;
Developmental Studies Hybridoma Bank).
Western blot analysis
Muscle samples were minced and prepared in RIPA
lysis buffer (Beyotime Institute of Biotechnology, Haimen, China).
Tissues were incubated for 30 min on ice, followed by
centrifugation at 12,000 × g for 10 min at 4°C. Total protein
concentration in the supernatant was determined using a BCA protein
assay kit (Beijing Solarbio Science & Technology Co., Ltd.).
Protein extract (7 µl/lane) was loaded on 10% SDS-PAGE and
transferred onto polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA). After blocking with 5% bovine serum albumin in
0.5% TBS-Tween-20 at room temperature for 1 h, the membranes were
incubated with primary antibodies at 4°C overnight. Then, the
membranes were incubated with horseradish peroxidase-conjugated
secondary antibody for 1 h at 37°C. The protein bands were
visualized using an enhanced chemiluminescence system (G2014; Wuhan
Goodbio Technology Co., Ltd., Wuhan, China). Densitometry values
were normalized to the intensity of corresponding bands for GAPDH.
Quantitative analysis of western blotting was performed using
ImageJ version 1.50e (National Institutes of Health, Bethesda, MD,
USA). Primary antibodies were used as follows: Rabbit polyclonal
anti-Axin2 antibody (ab32197; 1:1,000; Abcam), sheep polyclonal
anti-Vangl2 antibody (AF4815; 1:1,000; R&D Systems, Inc.),
rabbit polyclonal anti-pS6 antibody (2211; 1:1,000; Cell Signaling
Technology, Inc., Danvers, MA, USA), rabbit polyclonal anti-pAkt
antibody (9271; 1:1,000, Cell Signaling Technology, Inc.) and
rabbit polyclonal anti-GAPDH antibody (ab9485; 1:1,000; Abcam).
Secondary antibodies were used as follows: Goat anti-rabbit IgG
(ab6721; 1:5,000; Abcam) and donkey anti-sheep IgG (ab97125;
1:5,000; Abcam).
Quantification and statistical
analyses
Myofiber size and overall cell density were
calculated using ImageJ 1.50e. Numbers of Pax7-positive nuclei,
Ki67-positive nuclei and the percentage of each specific fiber type
were manually counted with three fields of view selected for each
sample type. All data were analyzed using SPSS 19.0 (IBM Corp.,
Armonk, NY, USA) and results are presented as the mean ± standard
deviation. Student's t-test was used to evaluate statistical
differences. P<0.05 was considered to indicate a statistically
significant difference.
Results
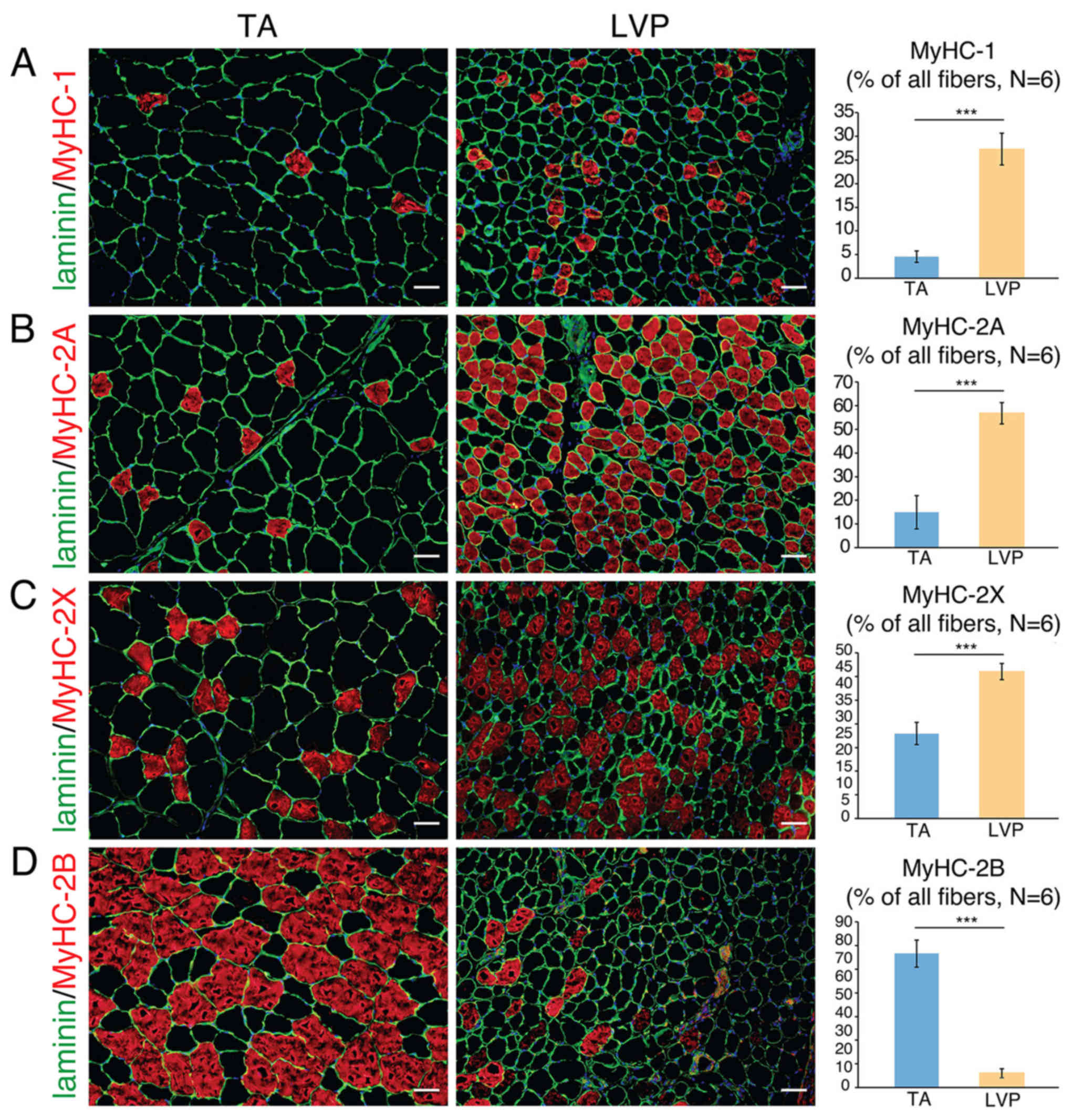
Fiber type composition of TA and LVP
muscle
Sections of muscle samples revealed that individual
fibers in TA muscle were larger compared with LVP muscle (data not
shown). With their corresponding markers immunostained, different
types of muscle fibers were identified and quantified in TA and LVP
muscle. The percentage of MyHC-1 fibers was significantly higher in
LVP muscle compared with TA muscle (~25 vs. ~5%; Fig. 1A). The percentage of MyHC-2A fibers
was significantly higher in LVP muscle compared with TA muscle (~60
vs. ~15%; Fig. 1B). The percentage
of MyHC-2X fibers was significantly higher in LVP muscle compared
with TA muscle (~40 vs. ~25%; Fig.
1C). By contrast, the percentage of MyHC-2B fibers was
significantly higher in TA muscle compared with LVP muscle (~75 vs.
~5%; Fig. 1D). These results
indicated that the vast majority of TA muscle is composed of type
2B fibers, while the majority of fibers in LVP muscle are types 2A
and 2X.
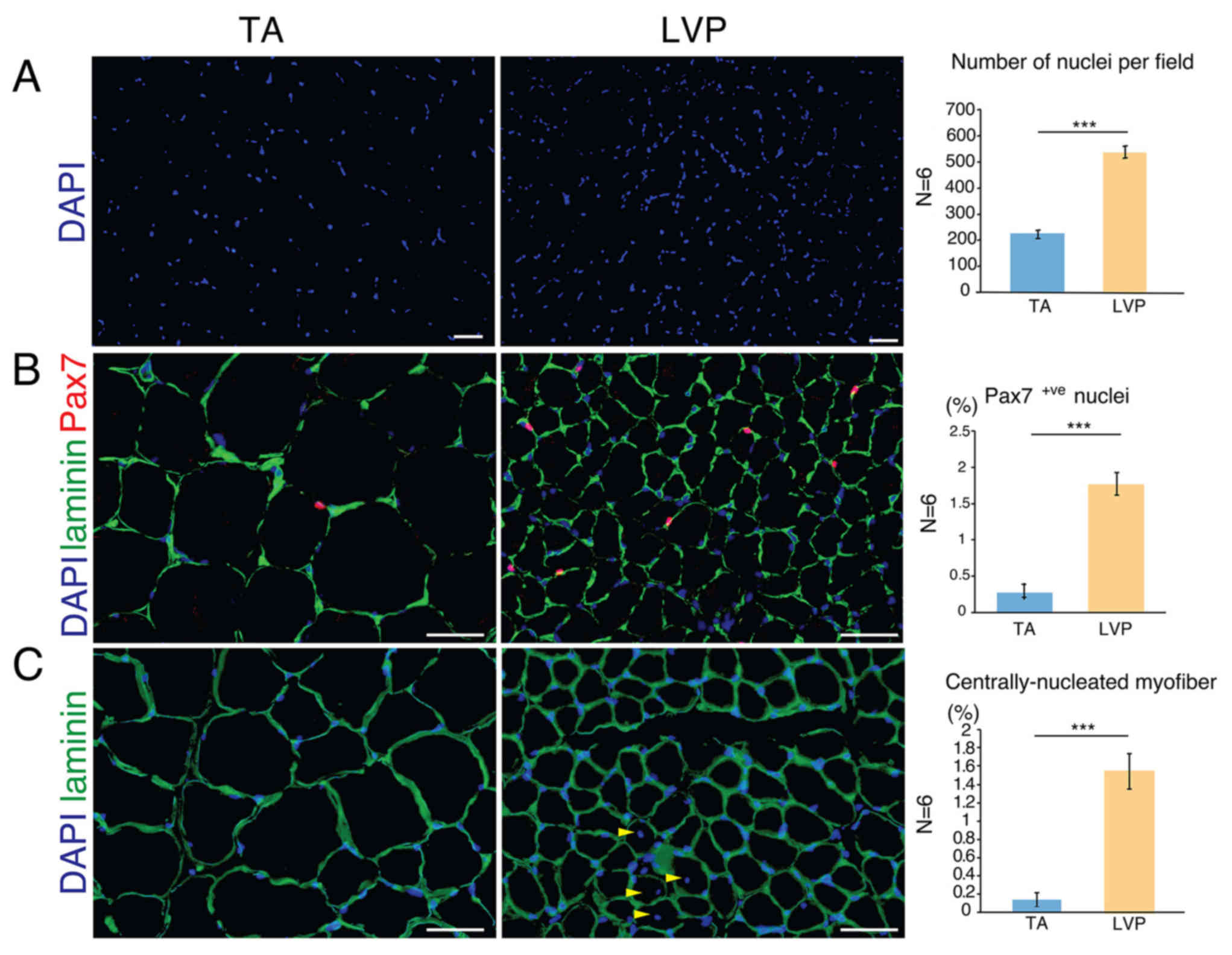
Satellite cells and
centrally-nucleated myofibers in TA and LVP muscle
The number of nuclei per area was significantly
higher in LVP muscle compared with TA muscle (Fig. 2A). The number of Pax7-positive
nuclei, an indicator of satellite cells, was significantly higher
in LVP muscle compared with TA muscle (~2.2% vs. ~0.3%; Fig. 2B). Satellite cells are resident stem
cells in skeletal muscle tissue, wedged in the interspace between
sarcolemma and basal lamina (23).
When activated, satellite cells proliferate and differentiate
either to merge into pre-existing fibers or form new fibers,
distinguishing the involved myofibers as centrally-nucleated
myofibers (24). In TA muscle,
centrally-nucleated myofibers were only scarcely identified (~0.1%
of total fibers), but they were observed significantly more
frequently in LVP muscle (~1.5% of total fibers; Fig. 2C). These data suggested that LVP
muscle possesses a broader stem cell pool and more active myonuclei
turnover compared with TA muscle. Given that Wnt signaling is
closely associated with satellite cell activity (25), further investigation was performed in
the Wnt pathway.
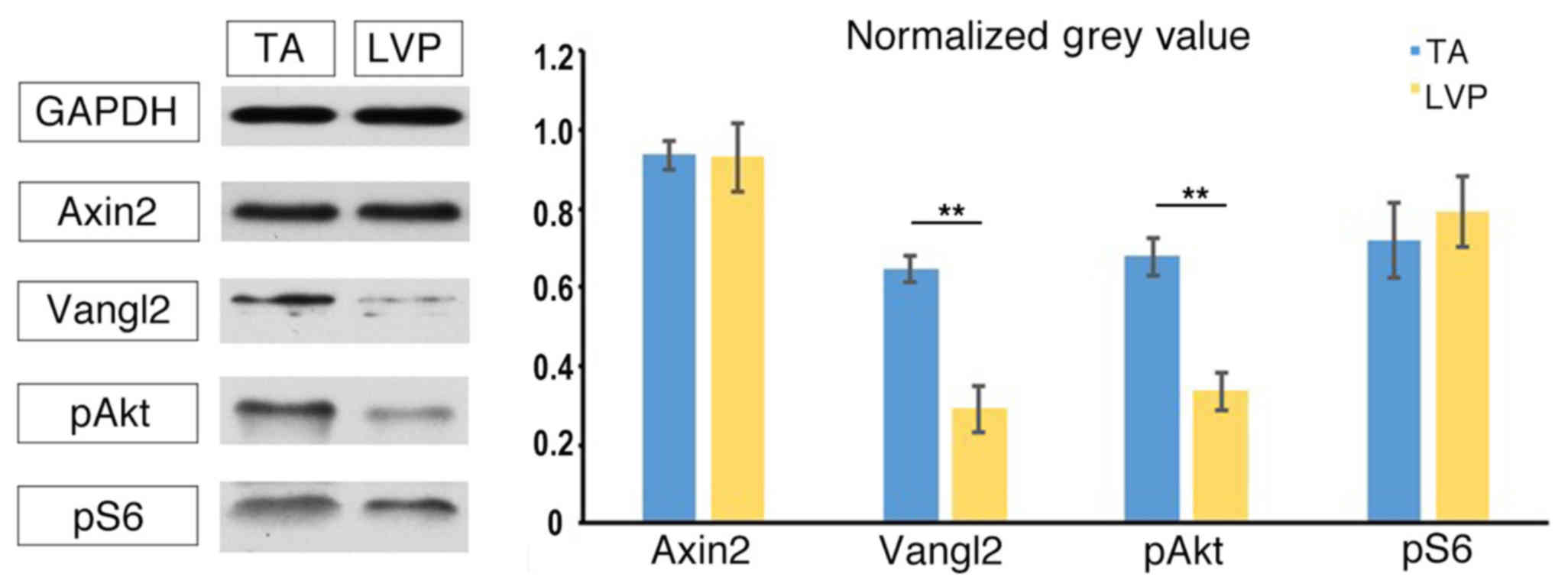
Wnt signaling hubs in TA muscle and
LVP muscle
In canonical Wnt pathways, Axin2 is a direct target
gene of Wnt and its expression is a reliable marker for the
activation of Wnt signaling (26).
For non-canonical Wnt pathways, the expression of Vangl2 reflects
the activation of Wnt/planar cell polarity pathway (27), while pAkt and pS6 are indicators of
Wnt/Akt/mechanistic target of rapamycin pathway activation
(10). No significant differences
were identified in the expression of Axin2 between TA muscle and
LVP muscle (Fig. 3). The expression
level of Vangl2 was observed to be significantly higher in TA
muscle compared with LVP muscle (Fig.
3). Similarly, pAkt was expressed at a significantly higher
level in TA muscle compared with LVP muscle (Fig. 3). No significant differences were
identified in the expression of pS6 between TA muscle and LVP
muscle (Fig. 3). Based on these
differences in the baseline level of Wnt hubs, it was investigated
whether LVP and TA muscle responded differently to Wnt ligand
stimulus.
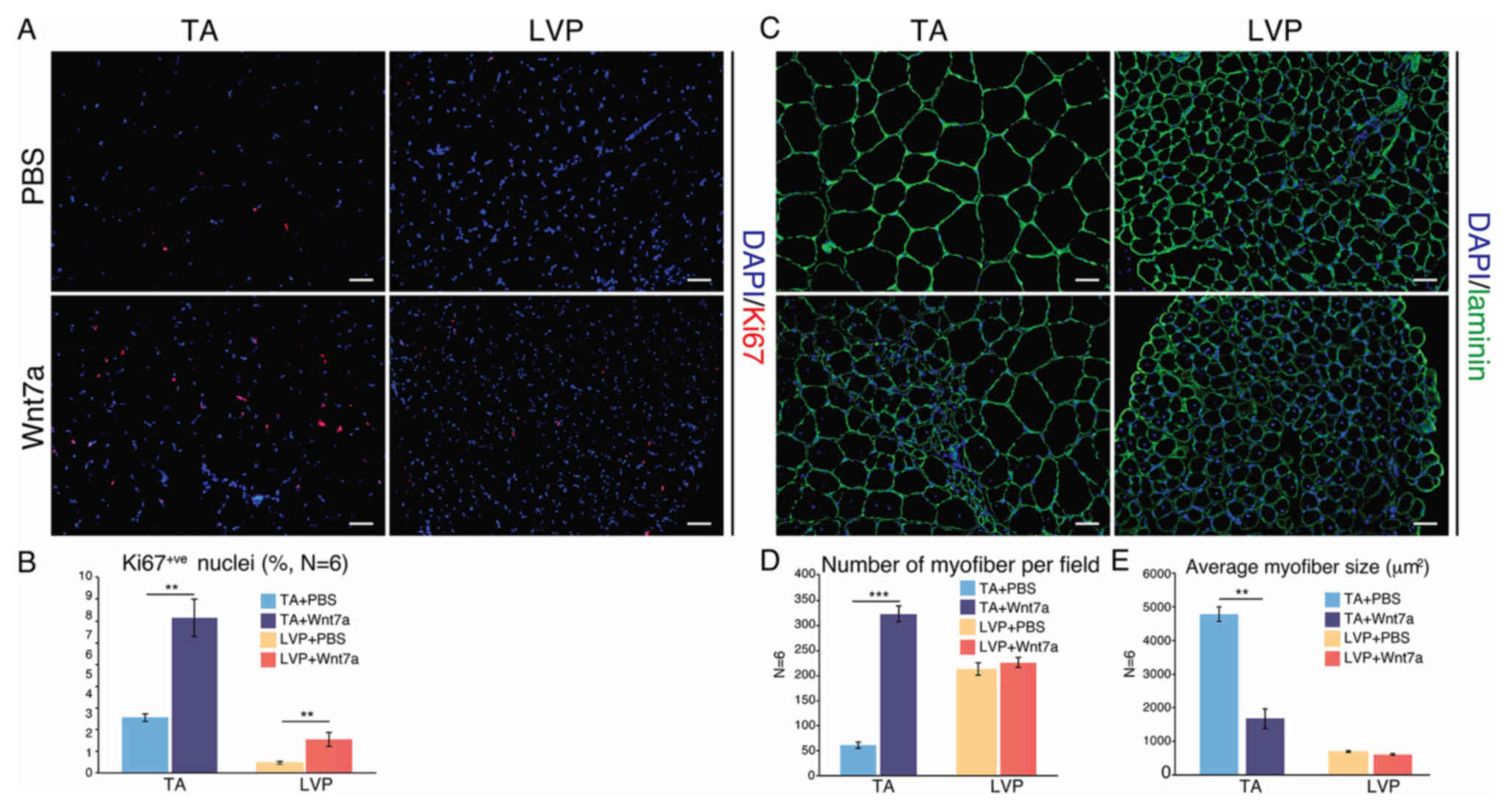
TA and LVP muscles respond differently
to exogenous Wnt7a stimulus
Wnt7a has been demonstrated to be effective in
expanding TA muscles (10,28), but to the best of our knowledge, has
not been tested in LVP muscle before. At 3 weeks after a single
injection of recombinant Wnt7a protein, TA and LVP muscles were
harvested for comparison. After Wnt7a administration, the
percentage of Ki67-positive nuclei, which indicated proliferative
cells, increased significantly in TA and LVP muscle (Fig. 4A and B). The two muscle types
exhibited a marked increase in centrally-nucleated myofibers after
Wnt7a administration (Fig. 4C).
Notably, in TA muscle, a significant increase in myofibers was
observed after Wnt7a injection, leading to a five-fold increase in
the number of myofibers and a significant decrease in the average
myofiber size (Fig. 4C and D). In
LVP muscle, however, the number and the average size of the
myofibers stayed constant after Wnt7a administration (Fig. 4C and E).
Discussion
From the perspective of embryonic origin, TA and LVP
muscle fall into different categories: TA muscle derives from
somites (2), while LVP muscle
derives from the fourth and sixth branchial arches (13). Apart from their differences in
embryonic origin, the current study has characterized further
heterogeneities between the two muscle types.
Until now, the predominant model of fiber types has
been based on four major MyHC isoforms: MyHC-1, −2A, −2X and −2B
(29). From type 1 to type 2B
fibers, the shortening velocity gradually increases and resistance
to fatigue decreases (29). The
composition of different fiber types is closely associated with the
muscle function. Specifically, TA muscle is more suitable for fast
and powerful actions such as jumping and kicking, while LVP muscle
is streamlined for slower contractions with longer durations in
elevating and stretching the soft palate (30,31).
Consequently, it was identified in the present study that TA muscle
is largely composed of MyHC-2B myofibers, while LVP muscle
primarily consists of MyHC-2A and MyHC-2X fibers. Hybrid fibers
co-expressing different MyHC isoforms are common (29), since boundaries between different
fiber types are not clear. This could explain why the sum of all
four MyHC isoforms in the muscles was >100%. Apart from gleaning
baseline information of fiber type composition in the two specific
muscles, the data presented here could also be relevant to clinical
practice. Fiber type composition can have profound impacts on the
specific muscle's susceptibility to disease. For example, type 2
fibers are preferentially affected in Duchenne muscular dystrophy
(DMD), and inducing type 1 fibers can ameliorate DMD (32).
Satellite cells are well-recognized resident stem
cells within skeletal muscle tissue (23). It was revealed in the present study
that LVP muscle maintains a higher satellite cell content compared
with TA muscle. During muscle repair or regeneration, satellite
cells undergo asymmetric division and differentiate into myogenic
progenitors, either fusing into the existing myofibers or becoming
de novo myofibers (33). In
this process, the involved myofibers are distinguished as
centrally-nucleated myofibers. It has long been speculated that
satellite cells remain quiescent in their sublaminar niche until
required for skeletal muscle repair (23,25,33).
However, recent studies performed by Randolph et al
(34) and Keefe et al
(35) have demonstrated that
satellite cells contribute nuclei to myofibers in adult muscles in
sedentary mice, challenging this conventional view. Similarly, in
the present study, satellite cell activity in 2-month-old rat
skeletal muscle was detected under basal conditions, as evidenced
by the presence of centrally-nucleated myofibers. It was also
identified that the percentage of centrally-nucleated myofibers was
significantly higher in LVP muscle compared with TA muscle. With
regards to the core components in Wnt signaling, protein analyses
revealed that Vangl2 and pAkt are expressed at a higher level in TA
muscle compared with LVP muscle. These molecular differences
indicated different Wnt pathway activation levels under basal
conditions in TA muscle and LVP muscle. However, the mechanisms
underlying the broader stem cell pool and more active myonuclei
turnover in LVP are yet to be elucidated.
In the present study, it was further examined
whether TA and LVP muscle responded differently to exogenous
myogenic growth factor. Wnt7a was selected because it was less
likely to induce resistance to growth hormone or cause
hypoglycemia, when compared with IGF-1 (10). Previous studies by von Maltzahn et
al (10,28) have indicated the key role of
recombinant Wnt7a protein in augmenting mouse limb muscle, leading
to significant myofiber hypertrophy and enhanced muscle function.
Notably, there were two primary findings in the present study.
First, Wnt7a induced hyperplasia rather than hypertrophy in TA
muscle. This discrepancy may be attributed to the different
postnatal growth patterns in muscle fibers between mice and rats.
Typically, the number of myofibers remains constant after birth in
mice, while the number and size of myofibers continue to increase
for 10 weeks in rats (20,36). Secondly, TA and LVP muscle reflected
different augmentation potential after Wnt7a delivery. In TA
muscle, the number of myofibers increased significantly, a large
proportion of which were observed to be centrally-nucleated
myofibers. However, LVP muscle did not exhibit an increase in
myofiber number, despite markedly upregulating the number of
centrally-located myofibers. The two muscle types exhibited
augmentation potential in response to Wnt7a stimulus, but had
different responses. The reason for these differences requires
further investigation.
LVP muscle was selected for investigation in the
current study primarily due to its clinical significance. LVP
muscle elevates the soft palate and serves a critical function in
velopharyngeal closure. Reduced LVP muscle volume and compromised
LVP muscle function in patients with cleft palate results in
affected speech (12). Effective
augmentation of LVP muscle via biological therapeutics may
revolutionize the management of cleft palate.
The present study has certain limitations. The
newly-formed myofibers with small diameters observed in TA muscle 3
weeks after Wnt7a administration were immature. To probe the
augmentation effect, a longer observation time is required in order
to assess the development of these new fibers. In addition, the
notable discrepancies between the responses of TA and LVP muscle to
Wnt7a administration highlights the requirement for more in-depth
work in order to reveal the underlying mechanisms.
In conclusion, LVP muscle is distinct from TA muscle
in its myofiber composition, in-situ stem cell population,
Wnt signaling hub expression and augmentation potential. Therefore,
therapeutics that are effective in TA muscle may not be effective
in LVP muscle, and further studies are required in order to
elucidate the differences between the muscle types.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China to Dr Jingtao Li (grant no.
81500829) and Professor Bing Shi (grant no. 81470729).
Glossary
Abbreviations
Abbreviations:
|
LVP
|
levator veli palatini
|
|
TA
|
tibia anterior
|
References
|
1
|
Randolph ME and Pavlath GK: A muscle stem
cell for every muscle: Variability of satellite cell biology among
different muscle groups. Front Aging Neurosci. 7:1902015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sambasivan R, Kuratani S and Tajbakhsh S:
An eye on the head: The development and evolution of craniofacial
muscles. Development. 138:2401–2415. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Diogo R, Kelly RG, Christiaen L, Levine M,
Ziermann JM, Molnar JL, Noden DM and Tzahor E: A new heart for a
new head in vertebrate cardiopharyngeal evolution. Nature.
520:466–473. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kelly RG: Core issues in craniofacial
myogenesis. Exp Cell Res. 316:3034–3041. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Michailovici I, Eigler T and Tzahor E:
Craniofacial muscle development. Curr Top Dev Biol. 115:3–30. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shih HP, Gross MK and Kioussi C: Cranial
muscle defects of Pitx2 mutants result from specification defects
in the first branchial arch. Proc Natl Acad Sci USA. 104:pp.
5907–5912. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grifone R and Kelly RG: Heartening news
for head muscle development. Trends Genet. 23:365–369. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tzahor E and Evans SM: Pharyngeal mesoderm
development during embryogenesis: Implications for both heart and
head myogenesis. Cardiovasc Res. 91:196–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pavlath GK, Thaloor D, Rando TA, Cheong M,
English AW and Zheng B: Heterogeneity among muscle precursor cells
in adult skeletal muscles with differing regenerative capacities.
Dev Dyn. 212:495–508. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
von Maltzahn J, Bentzinger CF and Rudnicki
MA: Wnt7a-Fzd7 signalling directly activates the Akt/mTOR anabolic
growth pathway in skeletal muscle. Nat Cell Biol. 14:186–191. 2012.
View Article : Google Scholar
|
|
11
|
Stuelsatz P, Shearer A, Li Y, Muir LA,
Ieronimakis N, Shen QW, Kirillova I and Yablonka-Reuveni Z:
Extraocular muscle satellite cells are high performance myo-engines
retaining efficient regenerative capacity in dystrophin deficiency.
Dev Biol. 397:31–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fisher DM and Sommerlad BC: Cleft lip,
cleft palate, and velopharyngeal insufficiency. Plast Reconstr
Surg. 128:342e–360e. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cohen SR, Chen L, Trotman CA and Burdi AR:
Soft-palate myogenesis: A developmental field paradigm. Cleft
Palate Craniofac J. 30:441–446. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koch KH, Grzonka MA and Koch J: The
pathology of the velopharyngeal musculature in cleft palates. Ann
Anat. 181:123–136. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Monroy PL Carvajal, Grefte S,
Kuijpers-Jagtman AM, Wagener FA and Von den Hoff JW: Strategies to
improve regeneration of the soft palate muscles after cleft palate
repair. Tissue Eng Part B Rev. 18:468–477. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iwata J, Suzuki A, Yokota T, Ho TV,
Pelikan R, Urata M, Sanchez-Lara PA and Chai Y: TGFβ regulates
epithelial-mesenchymal interactions through WNT signaling activity
to control muscle development in the soft palate. Development.
141:909–917. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Crockett DJ and Goudy SL: Update on
surgery for velopharyngeal dysfunction. Curr Opin Otolaryngol Head
Neck Surg. 22:267–275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chakravarthy MV, Bradley SD and Frank WB:
IGF-1 restores satellite cell proliferation potential in
immobilized old skeletal muscle. J Appl Physiol (1985).
89:1365–1379. 2000.PubMed/NCBI
|
|
19
|
Márquez-Miranda V, Abrigo J, Rivera JC,
Araya-Durán I, Aravena J, Simon F, Pacheco N, González-Nilo FD and
Cabello-Verrugio C: The complex of PAMAM-OH dendrimer with
Angiotensin (1–7) prevented the disuse-induced skeletal muscle
atrophy in mice. Int J Nanomedicine. 12:1985–1999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
White RB, Bierinx AS, Gnocchi VF and
Zammit PS: Dynamics of muscle fibre growth during postnatal mouse
development. BMC Dev Biol. 10:212010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Monroy PL Carvajal, Grefte S,
Kuijpers-Jagtman AM, Helmich MP, Ulrich DJ, Von den Hoff JW and
Wagener FA: A rat model for muscle regeneration in the soft palate.
PLoS One. 8:e591932013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meng H, Janssen PM, Grange RW, Yang L,
Beggs AH, Swanson LC, Cossette SA, Frase A, Childers MK, Granzier
H, et al: Tissue triage and freezing for models of skeletal muscle
disease. J Vis Exp. 2014. View
Article : Google Scholar
|
|
23
|
Chang NC and Rudnicki MA: Satellite cells:
The architects of skeletal muscle. Curr Top Dev Biol. 107:161–181.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cadot B, Gache V and Gomes ER: Moving and
positioning the nucleus in skeletal muscle-one step at a time.
Nucleus. 6:373–381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dumont NA, Bentzinger CF, Sincennes MC and
Rudnicki MA: Satellite cells and skeletal muscle regeneration.
Compr Physiol. 5:1027–1059. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huraskin D, Eiber N, Reichel M, Zidek LM,
Kravic B, Bernkopf D, von Maltzahn J, Behrens J and
Hashemolhosseini S: Wnt/β-catenin signaling via Axin2 is required
for myogenesis and, together with YAP/Taz and Tead1, active in
IIa/IIx muscle fibers. Development. 143:3128–3142. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Le Grand F, Jones AE, Seale V, Scimè A and
Rudnicki MA: Wnt7a activates the planar cell polarity pathway to
drive the symmetric expansion of satellite stem cells. Cell Stem
Cell. 4:535–547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
von Maltzahn J, Renaud JM, Parise G and
Rudnicki MA: Wnt7a treatment ameliorates muscular dystrophy. Proc
Natl Acad Sci USA. 109:pp. 20614–20619. 2012; View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schiaffino S and Reggiani C: Fiber types
in mammalian skeletal muscles. Physiol Rev. 91:1447–1531. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Perry JL, Kuehn DP and Sutton BP:
Morphology of the levator veli palatini muscle using magnetic
resonance imaging. Cleft Palate Craniofac J. 50:64–75. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lindman R, Paulin G and Stål PS:
Morphological Characterization of the levator veli palatini muscle
in children born with cleft palates. Cleft Palate Craniofac J.
38:438–448. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Talbot J and Maves L: Skeletal muscle
fiber type: Using insights from muscle developmental biology to
dissect targets for susceptibility and resistance to muscle
disease. Wiley Interdiscip Rev Dev Biol. 5:518–534. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yin H, Price F and Rudnicki MA: Satellite
cells and the muscle stem cell niche. Physiol Rev. 93:23–67. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Randolph ME, Phillips BL, Choo HJ, Vest
KE, Vera Y and Pavlath GK: Pharyngeal satellite cells undergo
myogenesis under basal conditions and are required for pharyngeal
muscle maintenance. Stem Cells. 33:3581–3595. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Keefe AC, Lawson JA, Flygare SD, Fox ZD,
Colasanto MP, Mathew SJ, Yandell M and Kardon G: Muscle stem cells
contribute to myofibres in sedentary adult mice. Nat Commun.
6:70872015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tamaki T, Akatsuka A, Yorshimura S, Roy RR
and Edgerton VR: New fiber formation in the interstitial spacs of
rat skeletal muscle during postnatal growth. J Histochem Cytochem.
50:1097–1111. 2002. View Article : Google Scholar : PubMed/NCBI
|