Introduction
With long time of work and study, cervical
spondylosis is becoming a common disease in modern society.
However, the specific pathogenesis has remained to be fully
clarified and may result from multiple factors. Cervical
instability is considered the main etiological basis of cervical
spondylosis. It has been confirmed that destroying the stability of
dynamic and static forces of rats may promote cervical disc
degeneration (1), which is in line
with a previous study by our group (unpublished data). At the same
time, the mechanisms associated with changes of inflammatory
cytokines in disc degeneration have remained elusive. Mern et
al (2) linked vertebral disc
degeneration with intradiscal cytokine imbalance, which leads to
decreased disc cell density. Wuertz and Haglund (3) suggested that the presence of
interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α
exacerbates disc degeneration. Shamji et al (4) and Akyol et al (5) reported that disc samples of patients
with cervical spondylosis contained higher levels of IL-2, IL-4,
IL-10, IL-12 and IL-17 compared with those in healthy control
subjects. However, earlier studies have mainly focused on IL-1β and
TNF-α, which are considered the first inflammatory cytokines in
intervertebral discs (6,7), while more recent studies have assessed
IL-6, IL-8 and other cytokines (8–12). As
the majority of cervical spondylosis patients are administered
drugs, physiotherapy and other conservative treatments, this may
interfere with the detection of cytokines to a certain extent.
Furthermore, previous studies have used post-operative human
cervical nucleus pulposus as samples, while domestic and
international studies assessing inflammatory cytokines in the serum
appear to be lacking. Finally, the nucleus pulposus is easily
contaminated with blood and tissue fluid, which may affect the
outcome during the sampling. Therefore, the present study used a
rat model of cervical spondylosis by disturbing the cervical
equilibrium of dynamic and static forces in order to induce
cervical degeneration (13). By
detecting the levels of IL-1β, IL-6, IL-10, IL-12, transforming
growth factor (TGF)-β and TNF-α in the serum of experimental and
control rats, the association between changes of inflammatory
cytokines and cervical degeneration were investigated. The present
study provided an experimental basis and reference for clinical
prevention and treatment of cervical spondylosis.
Materials and methods
Animals
A total of 60 adult and healthy male Sprague Dawley
(SD) rats (age, 6 weeks; weight, 220–250 g; Shanghai Laboratory
Animal Center, Shanghai, China) were randomized into test (n=45)
and control (n=15) groups, which were subdivided into one-, three-
and six months post-operation groups. The test group included 10,
15, 20 rats at the corresponding post-operative stages and the
control group contained five rats at each time-point. The present
study was performed in strict accordance with the recommendations
in the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health. The animal protocol was reviewed and
approved by the Institutional Animal Care and Use Committee (IACUC)
of Nanjing Medical University (Nanjing, China).
Model establishment
Rats in the test group that had been solid-food
fasted for 12 h prior to surgery were anesthetized by
intraperitoneal injection with 10% chloral hydrate (300 mg/kg;
Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) and fixed on
the operating table in the prone position with a 50-ml falcon tube
under the neck. Subsequent to shaving the nape of the neck and
disinfection, a 2–2.5 cm longitudinal incision was made at the
midline to perforate the skin and subcutaneous tissue. Every muscle
layer was fully separated. Superficial muscles, platysma muscle,
trapezius and rhomboideus, and deeper muscles, splenius cervicis
muscle, longissimus capitis et atlantis, longissimus cervicis,
hiocostalis cervicis and semispinalis capitis were transected
successively, and 1.5 cm of those muscles were resected to avoid
coalescence. At last, supraspinous ligament and interspinous
ligaments from C2 to C7 were cut off prior to suturing skin layers
successively, without removing the sutures, which came off
naturally. The preparation prior to surgery and anesthesia for rats
in the pseudo-surgery (control) group was the same as that in the
test group. The skin incision of rats in the control group was
sutured without resecting or cutting any muscle or ligament. Each
rat was fed a standard diet and had access to food and water ad
libitum in individual cages under normal conditions at 23–25°C
with a relative humidity of 40–70% and a 12-h light/dark cycle with
intramuscular injection of 50,000 units penicillin sodium to
prevent infection following surgery.
Index assessment
At one, three or six months post-surgery, the serum
of rats in the control and experimental groups was obtained
following intraperitoneal anesthesia with 10% chloral hydrate from
the intraperitoneal venous blood by centrifugation at 630 × g for
15 min at 4°C, and preserved at −80°C after. Serum IL-1β, IL-6,
IL-10, TGF-β1 and TNF-α levels were measured using a standard
quantitative sandwich ELISA (MultiSciences Biotech Co., Ltd.,
Hangzhou, Zhejiang, China), and serum IL-12 levels were measured
using a standard quantitative sandwich ELISA (Nanjing Jiancheng
Bioengineering Institute, Nanjing China) with a 1.42 pg/ml (IL-1β),
2.57 pg/ml (IL-6), 0.48 pg/ml (IL-10), 3 pg/ml (IL-12), 7.14 pg/ml
(TNF-α), 31.25 pg/ml (TGF-β1) detection limit of sensitivity. The
minimum detectable dose was determined by adding two standard
deviations to the mean optical density value of ten zero standard
replicates and calculating the corresponding concentration. Serum
samples were diluted 1:5 or 1:10 in PBS. Concentrations were
reported as pg/ml. All analyses and calibrations were performed in
duplicate. Optical densities were determined using an absorbance
microplate reader (Elx808™; Bio-Tek Instruments, Winooski, VT, USA)
at 450 nm. GraphPad Prism Data Analysis software 6 (GraphPad
Software, Inc., La Jolla, CA, USA). was used to analyze all
materials and depict the standard curve.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Statistical analyses were performed with GraphPad Prism
6 (GraphPad Software, Inc.). F-test was used to assess the equality
of variances. If variances were equal, a Students t-test was
employed to compare mean values among groups. The Rank-sum test was
performed if data were not normally distributed or variances were
not equal. P<0.05 was determined to indicate statistically
significant difference.
Results
General observation
Forty-five SD rats in the test group underwent
surgery without any perioperative mortality and were characterized
by head bobbing, twisting and shaking that disappeared within
approximately one week. At seven days after surgery, five rats had
died (Table I) and were dissected,
revealing cervical infection and intestinal tympaniteses. No
further mortality was observed at one, three and six months
post-operation.
 | Table I.Survival rate of animals in the
present study. |
Table I.
Survival rate of animals in the
present study.
|
| Control group | Experimental
group |
|---|
|
|
|
|
|---|
| Time (months) | Total (n) | Survived, n
(%) | Died (n) | Total (n) | Survived, n
(%) | Died (n) |
|---|
| 1 | 5 | 5 (100) | 0 | 10 | 9 (90) | 1 |
| 3 | 5 | 5 (100) | 0 | 15 | 13 (87) | 2 |
| 6 | 5 | 5 (100) | 0 | 20 | 18 (90) | 2 |
Changes in inflammatory cytokine
levels
The serum inflammatory cytokine levels in the two
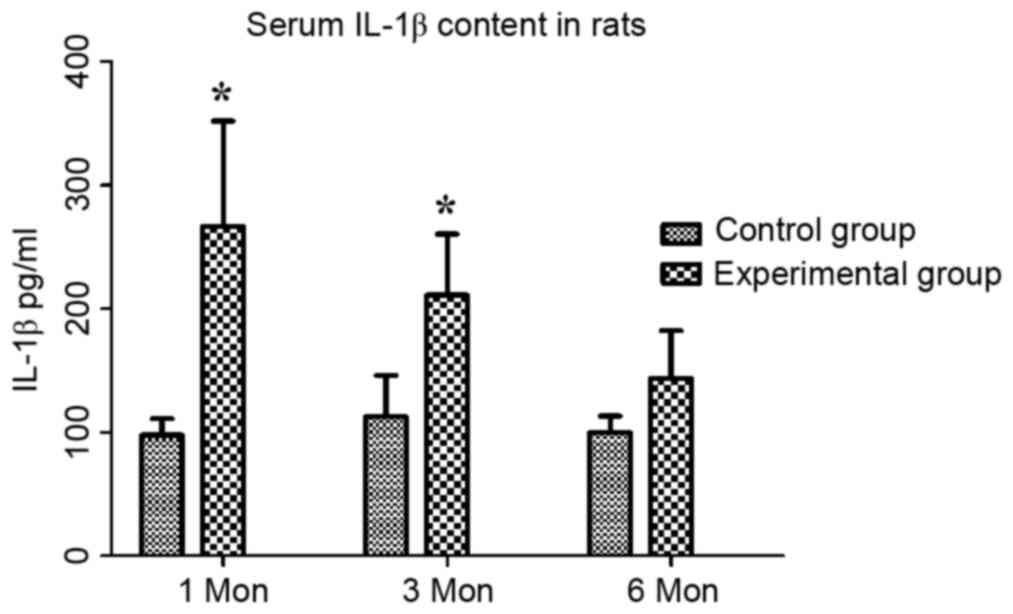
groups at different time-points are shown in Table II. The content of IL-1β in the
control group at one, three and six months post-operation was
97.76±13.33, 112.69±33.35 and 99.78±13.44 pg/ml, respectively,
while that in the experimental group was 266.69±85.33, 211.22±49.23
and 143.71±38.58 pg/ml, respectively. Compared with that in the
control group, the serum IL-1β content in the experimental group
was significantly increased at one and three months (P<0.05;
Fig. 1).
 | Table II.Serum inflammatory cytokine content
in rats (pg/ml). |
Table II.
Serum inflammatory cytokine content
in rats (pg/ml).
|
|
| Group |
|---|
|
|
|
|
|---|
| Cytokine | Time (months) | Control group | Experimental
group |
|---|
| IL-1β | 1 |
97.76±13.33 |
266.69±85.33a |
|
| 3 |
112.69±33.35 |
211.22±49.23a |
|
| 6 |
99.78±13.44 |
143.71±38.58 |
| IL-6 | 1 |
261.05±14.69 |
269.99±13.87 |
|
| 3 |
257.84±7.87 |
262.61±12.20 |
|
| 6 |
254.80±21.13 |
266.40±16.59 |
| IL-10 | 1 |
59.81±3.68 |
94.65±9.37b |
|
| 3 |
64.60±0.96 |
111.73±10.42b |
|
| 6 |
59.82±13.11 |
69.93±9.69 |
| IL-12 | 1 |
315.74±34.32 |
333.73±37.25 |
|
| 3 |
268.21±77.31 |
375.56±40.70a |
|
| 6 |
322.83±34.77 |
339.37±20.56 |
| TNF-α | 1 |
79.55±7.64 |
147.15±26.12c |
|
| 3 |
74.54±2.23 |
144.39±29.45c |
|
| 6 |
79.60±11.76 |
101.59±16.19 |
| TGF-β1 | 1 |
4,214.21±258.59 |
4,789.72±633.27 |
|
| 3 |
4,894.47±327.32 |
4,044.79±361.26c |
|
| 6 |
3,608.41±387.11 |
3,333.52±251.41 |
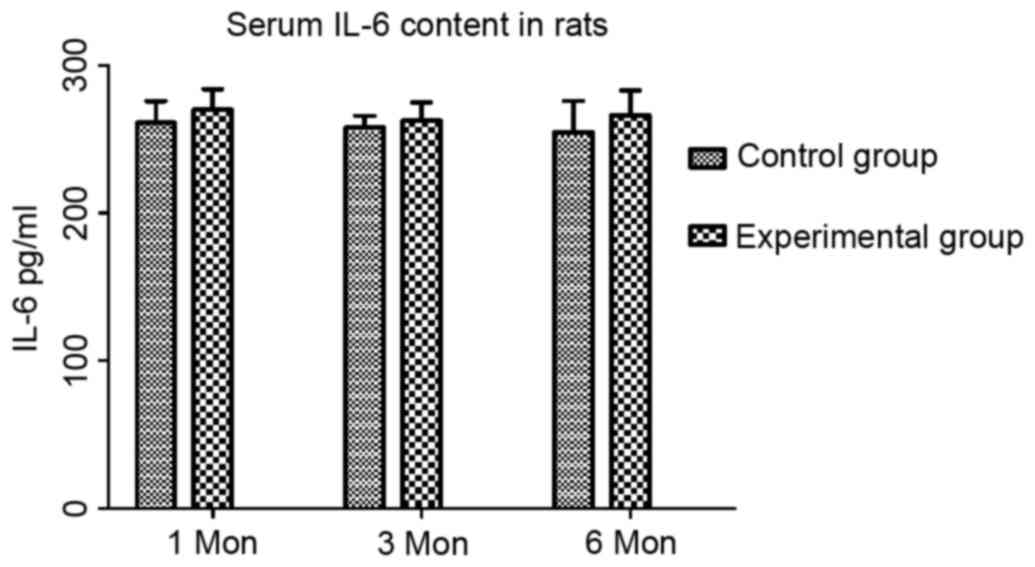
The content of IL-6 in the control group at one,
three and six months post-operation was 261.05±14.69, 257.84±7.87
and 254.80±21.13 pg/ml, respectively, while that in the
experimental group was 269.99±13.87, 262.61±12.20 and 266.40±16.59
pg/ml, respectively. Compared with that in the control group, the
IL-6 content in the experimental group showed no significant change
at any of the time-points (Fig.
2).
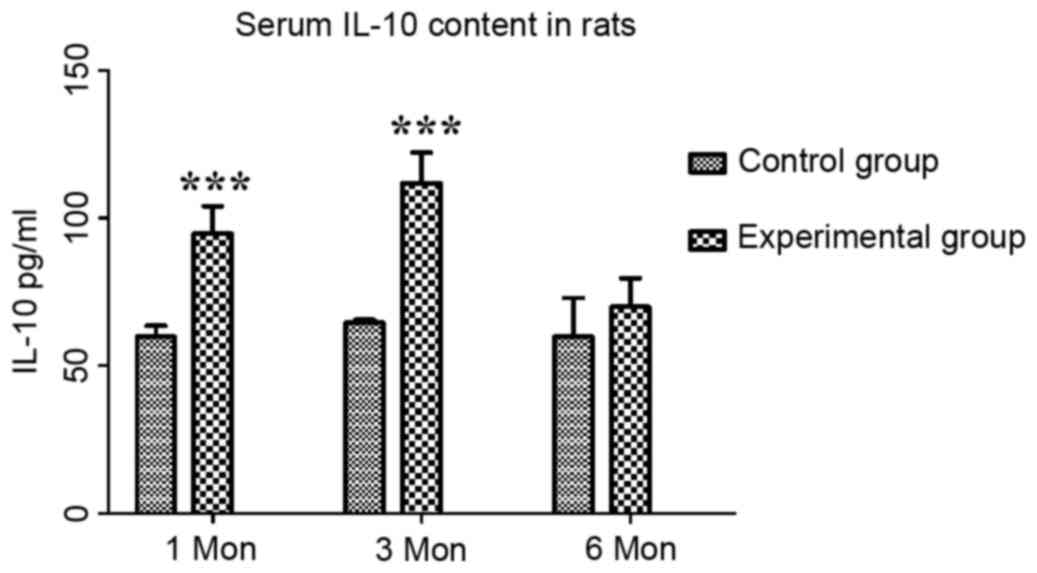
The content of IL-10 in the control group at one,
three and six months post-operation was 59.81±3.68, 64.60±0.96 and
59.82±13.11 pg/ml, respectively, while that in the experimental
group was 94.65±9.37, 111.73±10.42 and 69.93±9.69 pg/ml,
respectively. Compared with those in the control group, the serum
levels of IL-10 in the experimental group were significantly
increased at one and three months (P<0.001; Fig. 3).
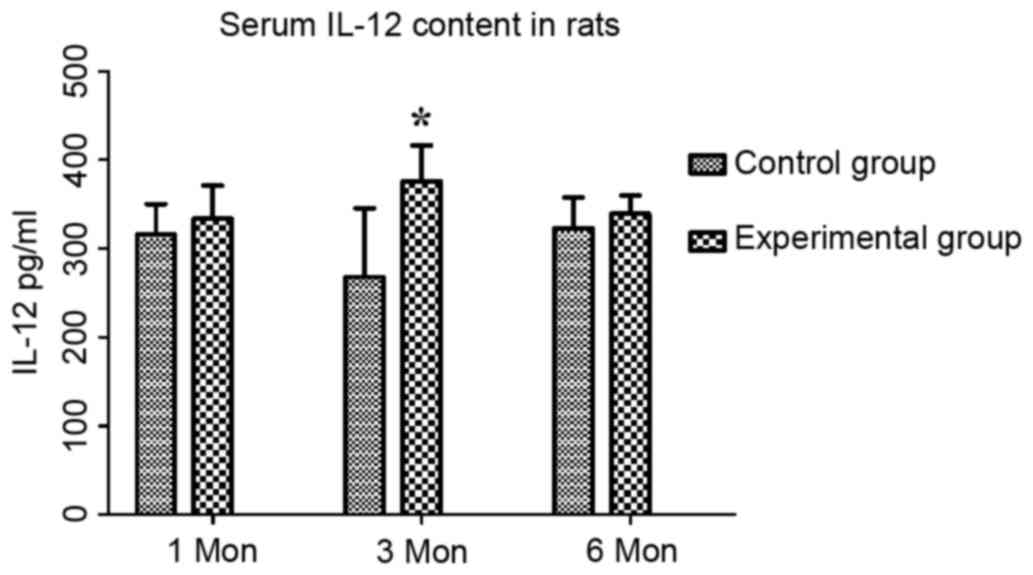
The content of IL-12 in the control group at one,
three and six months post-surgery was 315.74±34.32, 268.21±77.31
and 322.83±34.77 pg/ml, respectively, while that in the
experimental group was 333.73±37.25, 375.56±40.70 and 339.37±20.56
pg/ml, respectively. Compared with the control group, the serum
levels of IL-12 in the experimental group were significantly
increased at three month (P<0.05; Fig. 4).
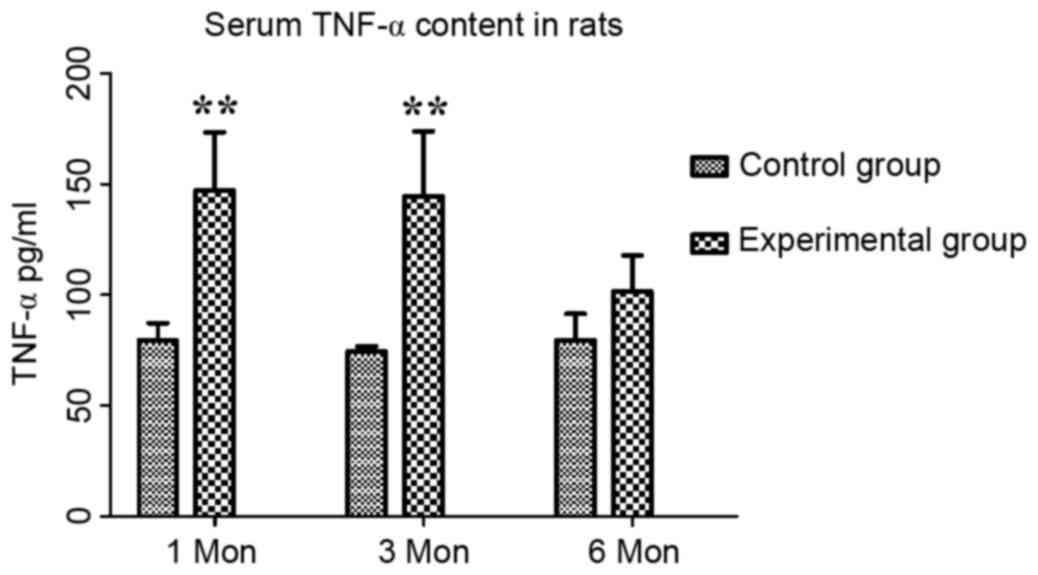
The content of TNF-α in the control group at one,
three and six months post-operation was 79.55±7.64, 74.54±2.23 and
79.60±11.76 pg/ml, respectively, while that in the experimental
group was 147.15±26.12, 144.39±29.45 and 101.59±16.19 pg/ml,
respectively. Compared with those in the control group, the serum
levels of TNF-α in the experimental group were significantly
increased at one and three months (P<0.01; Fig. 5).
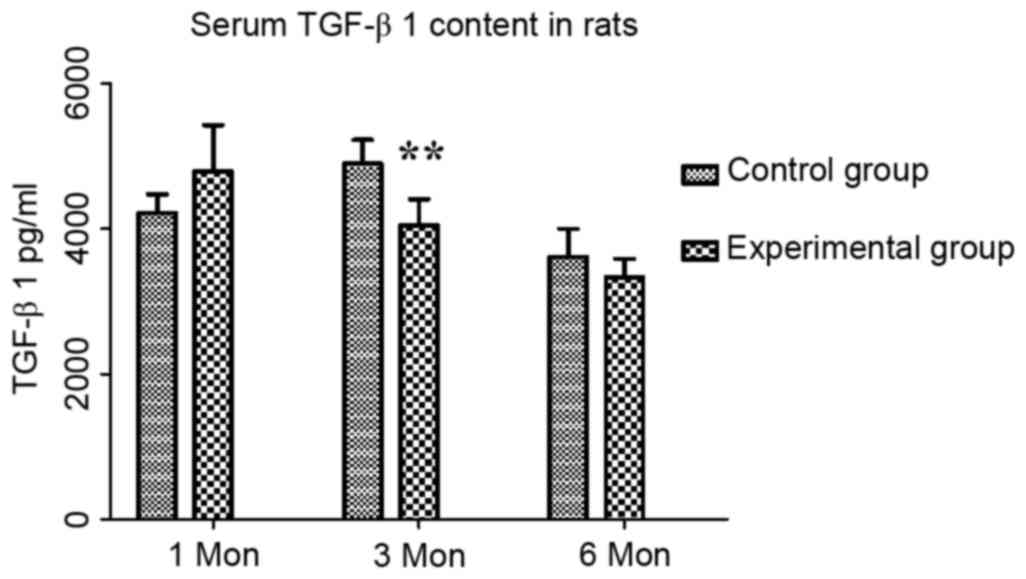
The content of TGF-β1 in the control group at one,
three and six months post-operation were 4,214.21±258.59,
4,894.47±327.32 and 3,608.41±387.11 pg/ml, respectively, while
those in the experimental group were 4,789.72±633.27,
4,044.79±361.26 and 3,333.52±251.41 pg/ml, respectively. Compared
with the control group, there was an increasing trend in TGF-β1
content at one month, which may have been a compensatory increase,
followed by a significant decrease at three months (P<0.01;
Fig. 6).
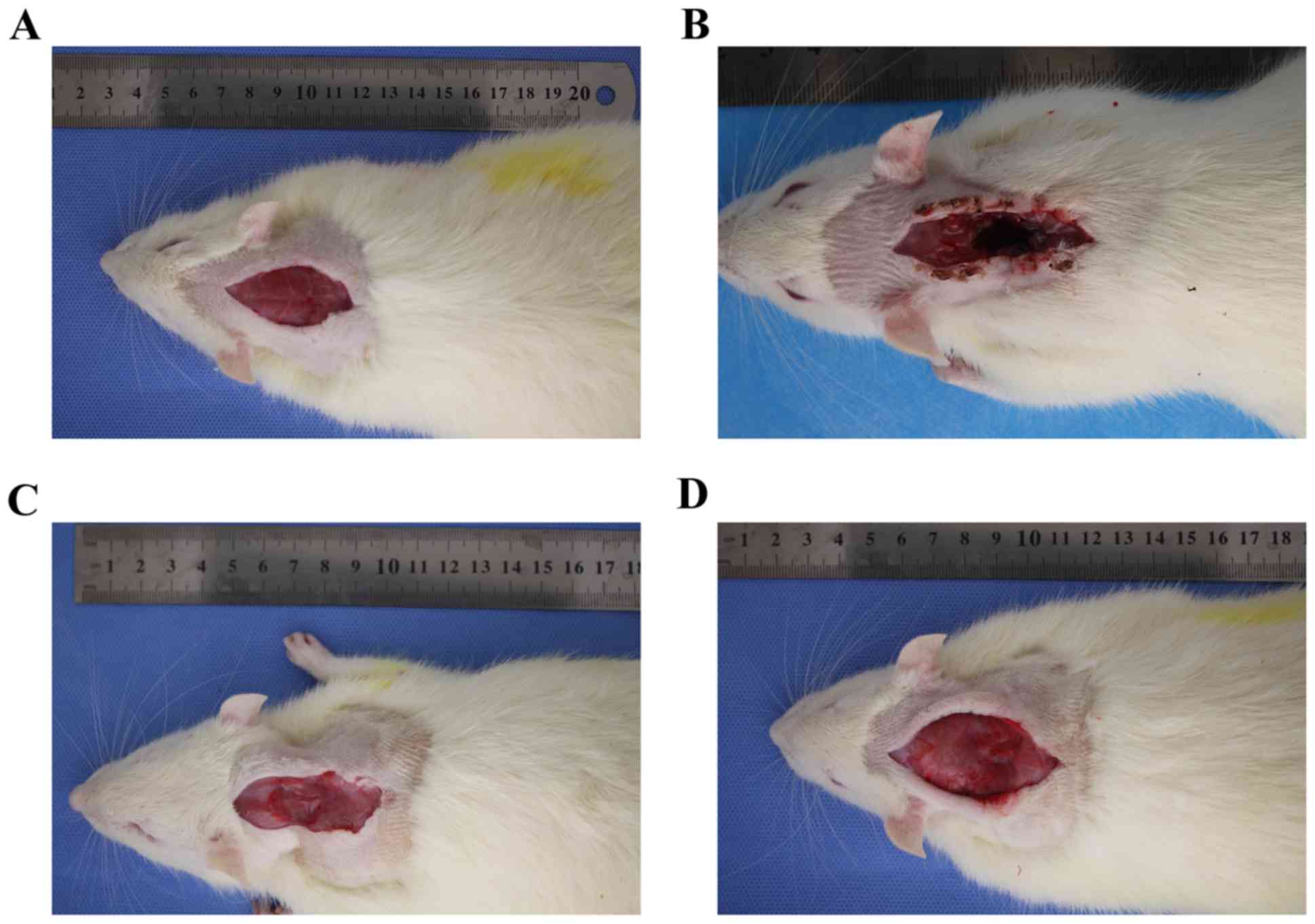
Following investigation of post-operative healing in
the two groups it was demonstrated that part of the cervical back
muscle in the experimental group healed and exhibited scarring over
time, especially at 6 months post-surgery (Fig. 7). In the control group, the incision
healed well and the cervical back muscle was well arranged under
the subcutaneous tissue.
Discussion
The present study successfully established a rat
model of cervical spondylosis by creating a non-equilibrium of
dynamic and static forces. X-ray analysis (unpublished data) of the
cervical spine of experimental group rats revealed degenerative
manifestations, including disappearance of the cervical
physiological curvature, reduction of the intervertebral foramen in
size and narrowing of the disc space. The animal model of cervical
degeneration provided a reliable sample for future studies on the
association between serum inflammatory cytokines and cervical
degeneration.
Cytokines are glycoproteins produced by a variety of
cells and are secreted into the extracellular space to participate
in the immune response and inflammatory regulation. As a research
hot spot, IL-1β is thought to be the most important cytokine, with
a strong pro-inflammatory activity by stimulating the production of
multiple pro-inflammatory mediators such as cytokines, chemokines
and matrix metalloproteinases (14–16). In
addition, IL-1β promotes oxidative stress and accelerates the
degradation of extracellular matrix by inducing cell senescence
apoptosis, thereby accelerating disc degeneration (17). In the present study, the serum IL-1β
content was elevated in parallel with the progression of cervical
degeneration at one and three months, suggesting that IL-1β is an
important inflammatory mediator with a pivotal role in cervical
degeneration. Degeneration of the cervical vertebrae accompanied by
a high level of local IL-1β and IL-1β mediated a strong
inflammatory response, and local inflammation accelerates the
oxidation of intervertebral disc cells and degradation of
extracellular matrix, which facilitates disc degeneration. In
another study by Smith et al (18), co-treatment with IL-1β and IL-1
receptor antagonist almost completely reversed degenerative changes
of bovine nucleus pulposus cells induced by IL-1β. This conclusion
inspired us to treat cervical disc degeneration by eliminating
local inflammatory factors and cytokines to restore intra-disc
homeostasis, e.g. by intra-disc injection of cytokine receptor
antagonists. Sainoh et al (19) demonstrated that intradiscal
tocilizumab injection exerted a short-term analgesic effect in
patients with discogenic low back pain, however further research is
required to determine the long-term effects.
A previous study suggested that the level of TNF-α
is closely associated with cervical pain of patients with cervical
spondylosis. TNF-α facilitates neurovascular ingrowth via
increasing the production of nerve growth factor and vascular
endothelial growth factor. It also facilitates matrix degradation
and upregulates substance P, suggesting it may sensitize nerves to
painful responses or facilitate structural disruption (20,21). Lai
et al (22) confirmed that
compared with the changed cervical structure or partial
neurovascular hyperplasia, the pain caused by cervical spondylosis
was more closely linked with local inflammation. When comparing
nucleus pulposus material from disk herniation vs. painful
degenerative disk disease, higher levels of TNF-α were detected in
the painful degenerative disk disease group (23,24). In
the present study, elevated serum TNF-α content and cervical
degeneration showed a positive association at the first and third
months, suggesting that the pain caused by cervical degeneration
was chronically sustained and intensified over the first three
months, accompanied by degeneration of the cervical vertebrae.
IL-12 is mainly produced by dendritic cells and
macrophages, with a wide range of immune and pro-inflammatory
effects by promoting the secretion of interferon-γ and has an
enhanced role in other pro-inflammatory reactions (25,26).
T-lymphocytes are major inflammatory cells in cervical disc tissue,
its cell subsets, particularly the imbalance of T-helper type 1 vs.
type 2 cell subsets and cytokines may be associated with cervical
degeneration, and IL-12 has an important role in T-cell mediated
autoimmune diseases (27). Akyol
et al (28) found that the
expression of IL-12 significantly increased in cervical disc tissue
of cervical spondylosis patients compared with that in the control
group. The present study assessed serum samples and found that the
amount of IL-12 in the experimental group was significantly
increased at 3 months post-operation compared with that in the
control group. However, the increasing trend of IL-12 in serum
samples was not as remarkable as that in disc samples. It is
therefore speculated that IL-12 is mainly involved in the immune
response in disc tissue but less in serum.
IL-10 is a homodimeric anti-inflammatory 36-kDa
cytokine produced by monocytes and lymphocytes. Anti-inflammatory
IL-10 is probably increased in intervertebral disk disease as a
result of the tight coupling of the pro-inflammatory arm of the
local disk disease process with an anti-inflammatory regulatory arm
of the response, which is necessary to prevent excessive
stimulation and tissue destruction (29). A previous study found more
IL-10-positive cells in the degenerated discs than in the control
discs (30). Similarly, the present
study revealed that the expression of IL-10 was gradually
increased, which is consistent with the above conclusions. IL-10 is
known to have a major role in the anti-inflammatory response and is
raised as a feedback effect. Li et al (31) observed that either TGF-β or IL-10
alone suppressed the expression of inflammatory cytokines.
Furthermore, their combined use produced a higher level of
inhibition of TNF-α and IL-1β than either TGF-β or IL-10 alone. It
was therefore speculated that IL-10 may be involved in an
anti-degeneration mechanism in the cervical degeneration
process.
It is similar to the cytokine IL-10, Maltman et
al (32) found that TGF-β guides
abnormal bone remodeling, and has a vital role in maintaining
articular cartilage and subchondral bone homeostasis. TGF-β has
been reported to increase proteoglycan production to adjust the
proliferation of type II collagen intervertebral disk cells and
reduce matrix degradation to thereby regulate the metabolism of
intervertebral disc cells (33,34).
While certain studies found that proteoglycan production in nucleus
pulposus cells was increased through gene therapy with TGF-β, it is
inferred that TGF-β is a protective cytokine in the intervertebral
disc (35–37). In the present study, an increasing
trend of TGF-β1 at 1 month post-operation was identified, while it
had significantly decreased at 3 months. This may be the body's
protective response to cervical degeneration. As TGF-β decreased
earlier than other cytokines, it may be speculated that the early
stage of cervical degeneration may be accompanied with the increase
of TGF-β1. TGF-β1 may then activate the body's anti-cervical
degeneration mechanism by increasing the production and reducing
the degradation of proteoglycan matrix, guiding bone and cartilage
remodeling to restore the stability of the local cervical spine.
The specific mechanisms still require to be further
investigated.
Kishimoto et al (38) proposed that IL-6 is a multifunctional
cytokine involved in cell proliferation and differentiation,
maintaining immune homeostasis, macrophage function and other key
functions. It has been confirmed that IL-6 can be used as a
pro-inflammatory cytokine, which may cause secondary injury
(39,40). Sainoh et al (41) achieved a pain reduction by intra-disc
injection of IL-6 inhibitor. Miyagi et al (42) found that the expression of IL-6 in a
rat model of intervertebral disc injury increased steadily from the
first to the fourth day compared with that in the control group.
Sainoh et al (41) found that
IL-6 and IL-6 receptor levels reached a maximum on the first day
post-disc injury and then gradually decreased in injured
intervertebral discs over time compared to those in the disks of
non-injured mice. Conversely, in the present study, no significant
changes in the serum levels of IL-6 were found in the test and
control groups at any time-point. The possible reasons may be as
follows: First, the sample detection in the above studies was
usually within a few days after model establishment, but was at
least one month in the present study. Differences in species
between studies may be another factor affecting the results of the
experiments. Finally, the present study assessed serum samples,
while the above studies assessed cytokine levels in the
intervertebral disc, which may have influenced the outcome. Xiang
et al (43) reported that
inflammatory cytokines in degenerative disc tissue were
significantly higher than those in serum, which confirms this
conjecture.
Of note, the present study found that in the
experimental group, the levels of IL-1β, IL-6, IL-10, IL-12, TGF-β
and TNF-α at six months had obviously decreased compared with those
at one and three months. This may be associated with the partial
healing of neck muscles and scarring, which may in part lead to the
recovery of cervical vertebral stability. The recovery of cervical
vertebral stability retards the process of cervical degeneration,
resulting in the decrease of inflammatory cytokines. It is
therefore inferred that the changes of inflammatory cytokines were
associated with cervical vertebral stability, but not with cervical
degeneration. The initiating factors of cervical spondylosis
therefore remain to be elucidated.
The present study preliminarily assessed the
association between serum inflammatory cytokines and cervical
degeneration. However, there is a limitation as the addition of
another control group, in which comparable muscles in different
parts of mice were cut, is required. The changes in cytokines may
have been due to muscle ligation rather than spine-associated
processes. However, the expression of associated genes and signal
transduction pathways in the process of cervical degeneration has
remained indeterminate and will be the focus of further study.
Acknowledgements
The present study was supported by the Project of
Invigorating Health Care Through Science, Technology and Education
(Jiangsu Provincial Medical Youth Talent), Changzhou City High
Level Health Personnel Training Project (grant no. 2016CZBJ029),
Changzhou International Scientific and Technological Cooperation
Project (grant no. CZ20170021), Jiangsu Postdoctoral Research
supported project (grant no. 1701001A).
References
|
1
|
Miyamoto S, Yonenobu K and Ono K:
Experimental cervical spondylosis in the mouse. Spine (Phila Pa
1976). 16(10 Suppl): S495–S500. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mern DS, Beierfuss A, Thome C and Hegewald
AN: Enhancing human nucleus pulposus cells for biological treatment
approaches of degenerative intervertebral disc diseases: A
systematic review. J Tissue Eng Regenerative Med. 8:925–936. 2014.
View Article : Google Scholar
|
|
3
|
Wuertz K and Haglund L: Inflammatory
mediators in intervertebral disk degeneration and discogenic pain.
Global Spine J. 3:175–184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shamji MF, Setton LA, Jarvis W, So S, Chen
J, Jing L, Bullock R, Isaacs RE, Brown C and Richardson WJ:
Proinflammatory cytokine expression profile in degenerated and
herniated human intervertebral disc tissues. Arthritis Rheum.
62:1974–1982. 2010.PubMed/NCBI
|
|
5
|
Akyol S, Eraslan BS, Etyemez H, Tanriverdi
T and Hanci M: Cataboliccytokine expressions in patients with
degenerative disc disease. Turk Neurosurg. 20:492–499.
2010.PubMed/NCBI
|
|
6
|
Le Maitre CL, Freemont AJ and Hoyland JA:
The role of interleukin-1 in the pathogenesis of human
intervertebral disc degeneration. Arthritis Res Ther. 7:R732–R745.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Le Maitre CL, Hoyland JA and Freemont AJ:
Catabolic cytokine expression in degenerate and herniated human
intervertebral discs: IL-1beta and TNFalpha expression profile.
Arthritis Res Ther. 9:R772007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Risbud MV and Shapiro IM: Role of
cytokines in intervertebral disc degeneration: Pain and disc
content. Nat Rev Rheumatol. 10:44–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sutovsky J, Benco M, Sutovska M, Kocmalova
M, Pappova L, Miklusica J, Frano A and Kurca E: Cytokine and
chemokine profile changes in patients with lower segment lumbar
degenerative spondylolisthesis. Int J Surg. 43:163–170. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Zhao Y, Li J, Wang S, Liu Y, Nie
L and Cheng L: Interleukin-9 Promotes TNF-α and PGE2 release in
human degenerated intervertebral disc tissues. Spine (Phila Pa
1976). 41:1631–1640. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schroeder GD, Markova DZ, Koerner JD, Rihn
JA, Hilibrand AS, Vaccaro AR, Anderson DG and Kepler CK: Are Modic
changes associated with intervertebral disc cytokine profiles?
Spine J. 17:129–134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuelling FA, Foley KT, Liu JJ, Liebenberg
E, Sin AH, Matsukawa A and Lotz JC: The anabolic effect of
plasma-mediated ablation on the intervertebral disc: Stimulation of
proteoglycan and interleukin-8 production. Spine J. 14:2479–2487.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang YJ, Shi Q, Lu WW, Cheung KC, Darowish
M, Li TF, Dong YF, Zhou CJ, Zhou Q, Hu ZJ, et al: Cervical
intervertebral disc degeneration induced by unbalanced dynamic and
static forces: A novel in vivo rat model. Spine (Phila Pa 1976).
31:1532–1538. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dinarello CA: Immunological and
inflammatory functions of the interleukin-1 family. Annu Rev
Immunol. 27:1–550. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dinarello CA: Interleukin-1 in the
pathogenesis and treatment of inflammatory diseases. Blood.
117:3720–3732. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rosenzweig JM, Lei J and Burd I:
Interleukin-1 receptor blockade in perinatal brain injury. Front
Pediatr. 2:1082014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang W, Yu XH, Wang C, He WS, Zhang SJ,
Yan YG, Zhang J, Xiang YX and Wang WJ: Interleukin-1β in
intervertebral disk degeneration. Clinica Chimica Acta. 450:1–272.
2015. View Article : Google Scholar
|
|
18
|
Smith LJ, Chiaro JA, Nerurkar NL, Cortes
DH, Horava SD, Hebela NM, Mauck RL, Dodge GR and Elliott DM:
Nucleus pulposus cells synthesize a functional extracellular matrix
and respond to inflammatory cytokine challenge following long-term
agarose culture. Eur Cell Mater. 22:291–301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sainoh T, Orita S, Miyagi M, Inoue G,
Kamoda H, Ishikawa T, Yamauchi K, Suzuki M, Sakuma Y, Kubota G, et
al: Single intradiscal administration of the tumor necrosis
factor-alpha inhibitor, etanercept, for patients with discogenic
low back pain. Pain Med. 17:40–45. 2016.PubMed/NCBI
|
|
20
|
Séguin CA, Pilliar RM, Roughley PJ and
Kandel RA: Tumor necrosis factor-alpha modulates matrix production
and catabolism in nucleus pulposus tissue. Spine (Phila Pa 1976).
30:1940–1948. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Purmessur D, Freemont AJ and Hoyland JA:
Expression and regulation of neurotrophins in the nondegenerate and
degenerate human intervertebral disc. Arthritis Res Ther.
10:R992008. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lai A, Moon A, Purmessur D, Skovrlj B,
Laudier DM, Winkelstein BA, Cho SK, Hecht AC and Iatridis JC:
Annular puncture with tumor necrosis factor-alpha injection
enhances painful behavior with disc degeneration in vivo. Spine J.
16:420–431. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee S, Moon CS, Sul D, Lee J, Bae M, Hong
Y, Lee M, Choi S, Derby R, Kim BJ, et al: Comparison of growth
factor and cytokine expression in patients with degenerated disc
disease and herniated nucleus pulposus. Clin Biochem. 42:1504–1511.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Burke JG, Watson RW, McCormack D, Dowling
FE, Walsh MG and Fitzpatrick JM: Intervertebral discs which cause
low back pain secrete high levels of proinflammatory mediators. J
Bone Joint Surg Br. 84:196–201. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Starbeck-Miller GR, Xue HH and Harty JT:
IL-12 and type I interferon prolong the division of activated CD8 T
cells by maintaining high-affinity IL-2 signaling in vivo. J Exp
Med. 211:1–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Garcia K, Sun Z, Mattson E, Li L, Smyth K
and Xiao Z: IL-12 is required for mTOR regulation of memory CTLs
during viral infection. Genes Immun. 15:413–423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abdi K and Singh NJ: Singh Making many
from few: IL-12p40 as a model for the combinatorial assembly of
heterodimeric cytokines. Cytokine. 76:53–57. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Akyol S, Eraslan BS, Etyemez H, Tanriverdi
T and Hanci M: Catabolic cytokine expressions in patients with
degenerative disc disease. Turk Neurosurg. 20:492–499.
2010.PubMed/NCBI
|
|
29
|
Lin WP, Lin JH, Chen XW, Wu CY, Zhang LQ
and Lai JM: Interleukin-10 promoter polymorphisms associated with
susceptibility to lumbar disc degeneration in a Chinese cohort.
Genetics Mol Res. 10:1719–1727. 2011. View Article : Google Scholar
|
|
30
|
Holm S, Mackiewicz Z, Holm AK, Konttinen
YT, Kouri VP, Indahl A and Salo J: Pro-inflammatory, pleiotropic,
and anti-inflammatory TNF-alpha, IL-6, and IL-10 in experimental
porcine intervertebral disk degeneration. Vet Pathol. 46:1292–1300.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li W, Liu T, Wu L, Chen C, Jia Z, Bai X
and Ruan D: Blocking the function of inflammatory cytokines and
mediators by using IL-10 and TGF-β: A potential biological
immunotherapy for intervertebral disc degeneration in a beagle
model. Int J Mol Sci. 15:17270–17283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maltman J, Pragnell IB and Graham GJ:
Specificity and reciprocity in the interactions between TGF-beta
and macrophage inflammatory protein-1 alpha. J Immunol.
156:1566–1571. 1996.PubMed/NCBI
|
|
33
|
Zhen G and Cao X: Targeting TGFβ signaling
in subchondral bone and articular cartilage homeostasis. Trends
Pharmacol Sci. 35:227–236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nishida K, Kang JD, Gilbertson LG, Moon
SH, Suh JK, Vogt MT, Robbins PD and Evans CH: Modulation of the
biologic activity of the rabbit intervertebral disc by gene
therapy: An in vivo study of adenovirus-mediated transfer of the
human transforming growth factor beta 1 encoding gene. Spine (Phila
Pa 1976). 24:2419–2425. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Illien-Jünger S, Lu Y, Purmessur D, Mayer
JE, Walter BA, Roughley PJ, Qureshi SA, Hecht AC and Iatridis JC:
Detrimental effects of discectomy on intervertebral disc biology
can be decelerated by growth factor treatment during surgery - a
large animal organ culture mode. Spine J. 14:2724–2732. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cho H, Lee S, Park SH, Huang J, Hasty KA
and Kim SJ: Synergistic effect of combined growth factors in
porcine intervertebral disc degeneration. Connect Tissue Res.
54:181–186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Abbott RD, Purmessur D, Monsey RD,
Brigstock DR, Laudier DM and Iatridis JC: Degenerative grade
affects the responses of human nucleus pulposus cells to link-N,
CTGF, and TGFβ3. J Spinal Disord Tech. 26:E86–E94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kishimoto T, Akira S, Narazaki M and Taga
T: Interleukin-6 family of cytokines and gp130. Blood.
86:1243–1254. 1995.PubMed/NCBI
|
|
39
|
Gruol DL: IL-6 regulation of synaptic
function in the CNS. Neuropharmacology. 96:42–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gruol DL, Puro A, Hao C, Blakely P,
Janneke E and Vo K: Neuroadaptive changes in cerebellar neurons
induced by chronic exposure to IL-6. J Neuroimmunol. 239:28–36.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sainoh T, Orita S, Miyagi M, Sakuma Y,
Yamauchi K, Suzuki M, Kubota G, Oikawa Y, Inage K, Sato J, et al:
Interleukin-6 and interleukin-6 receptor expression, localization
and involvement in pain-sensing neuron activation in a mouse
intervertebral disc injury mode. J Orthop Res. 33:1508–1514. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Miyagi M, Ishikawa T, Orita S, Eguchi Y,
Kamoda H, Arai G, Suzuki M, Inoue G, Aoki Y, Toyone T, et al: Disk
injury in rats produces persistent increases in pain-related
neuropeptides in dorsal root ganglia and spinal cord glia but only
transient increases in inflammatory mediators: Pathomechanism of
chronic diskogenic low back pain. Spine (PhilaPa 1976).
36:2260–2266. 2011. View Article : Google Scholar
|
|
43
|
Xiang YJ, Shen XH, Shen Z, et al: Local
inflammatory cytokine changes in degenerative vertebal discs in
patients with cervical spondylotic myelopathy. Acad J Sec Mil Univ.
24:788–790. 2003.
|