Introduction
Hypertrophic scar (HS) formation is a common sequel
to injuries, burns and surgical operations. Up to 40–70% of all
surgical patients, and up to 91% of all burn patients are may be
affected by HS formation (1).
Hypertrophic scar has a detrimental effect on the patient's
physical as well as psychological health. The effects may include,
pain, itching, functional impairment, disfigurement, anxiety,
depression, and poor quality of life of the affected person
(2). Treatment of HS is typically
challenging; further research is required to devise appropriate
interventions.
The precise pathogenetic mechanism underlying HS
formation is not clearly understood1. However, it is known to
result from excessive post-traumatic regeneration of tissue,
abnormal extracellular matrix (ECM) deposition and collagen
remodeling (3). The
pathophysiological basis of HS formation involves interaction
between a diverse range of microcellular actors and processes.
These include abnormal activation, proliferation, synthesis, and
differentiation and secretions of connective tissue (such as
fibroblasts, myofibroblasts and keratinocytes), growth factors and
cytokines. This in turn leads to abnormal collagen synthesis and HS
formation via dysregulation of certain cellular signaling pathways,
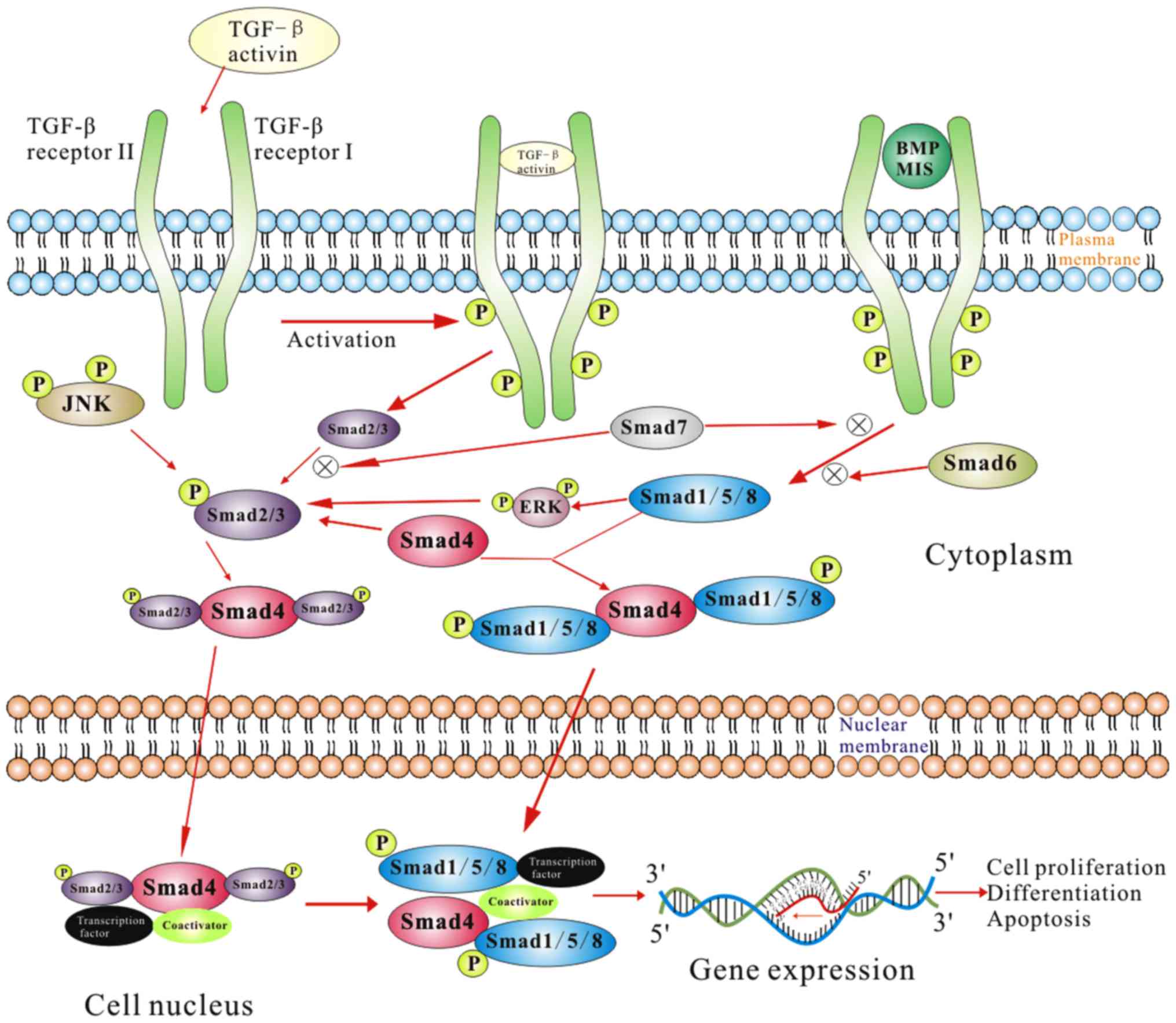
such as TGF-β1/Smad pathway (Fig.
1). In addition, other signaling pathways, such as
mitogen-activated protein kinase, extracellular signal-regulated
kinase and c-Jun N-terminal kinase pathway, have also been
implicated along with TGF-β signaling. However, the exact
mechanisms are not yet clear (4–7).
The goal of managing HS is symptom alleviation,
functional restoration and recurrence prevention. Currently
practiced therapeutic modalities for treatment of HS include,
topical and systemic medication (8),
surgery, physical treatment (e.g., laser and particle beams,
compression therapy and massage, etc (9–11),
silicone gel or sheeting (12),
radiotherapy (13), cryotherapy
(14), and mesenchymal or adipose
derived stem cell therapy (15). All
these therapeutic modalities have their inherent limitations and
advantages; there is no single universally accepted gold standard
therapy for HS (16). For example,
the disadvantages of intralesional injection treatment include
invasion, pain, pigmentation changes, ulceration, blister
formation, and necrosis (17).
Compression therapy for HS is liable to induce severe discomfort,
limitation in movement because of pressure effect, ulceration,
blister formation, itching and rashes (18). Hypopigmentation and the potential for
carcinogenesis are some of the concerns associated with the use of
cryotherapy (19) and radiotherapy
(20), respectively. Therefore,
developing a non-invasive, safe, efficacious and cost-effective
treatment modality for HS will be of immense benefit.
Extracorporeal shock wave therapy (ESWT) is a type
of pulsed acoustic wave resulting from dramatic changes in
pressure. ESWT was originally used for urinary lithotripsy
(21). Of late, its application has
been extended to other areas including treatment of fracture
nonunion, musculoskeletal disorders and soft tissue wounds
(22–24). Radial ESWT, also referred as
unfocused ESWT, is generally divided into two categories based on
the energy flux density (EFD) applied, i.e., higher EFD rESWT and
lower EFD rESWT. However, there is a lack of consensus on the
validity of this classification. Speed et al (25) recommended a threshold level of 0.12
mJ/mm2 for differentiating between higher EFD and lower
EFD rESWT, while Goertz et al suggested a threshold level of
0.15 mJ/mm2 (26). Radial
ESWT is known to alleviate tissue ischemia, heal burn wounds and
diabetic foot ulcers, improve uptake of skin grafts, and has the
advantage of being non-invasive, safe and convenient to use
(27,28). Radial ESWT is known to promote
angiogenesis, inhibit local inflammation, promote recruitment of
mesenchymal stem cells and endothelial progenitor cells at the
injured site, stimulate cellular proliferation, regeneration, and
decrease bacterial colonization (29).
Despite its advantages, the use of rESWT in the
treatment of HS has not been adequately investigated. Fioramonti
et al (30) documented
satisfactory results, in terms of texture and color improvement,
from use of ESWT in 16 patients with post-burn scars. Cho et
al (31) report that ESWT
significantly reduced scar pain in burn patients after wound
recovery. Similarly, Saggini et al (32) evaluated the efficacy of unfocused
shock wave treatment on retracting scars of the hands. They
observed significant early improvement in modified Vancouver Scar
Scale after treatment. Comparable results were obtained with
respect to pain and range of movements. However, few studies were
able to elucidate the underlying mechanism of action. Histologic
changes in connective tissue appearance, scar vascularization and
density of fXIIIa and CD34-positive cells were found following ESWT
treatment (32).
In the present study, we investigated whether rESWT
can serve as an effective modality for improving characteristics of
hypertrophic scar by interfering with the TGF-β1/Smad signaling
pathway.
Materials and methods
Ethical approval
The study protocol was approved by the Institutional
Ethics Committee at The First hospital of Jilin University.
Establishment of rabbit ear HS
model
Twenty five adult white rabbits (male: female ratio,
13: 12, weight: 2.0–2.3 kg each) were obtained from the Laboratory
Animal Center of Jilin University. The rabbits were acclimatized
and housed separately at 22–25°C with 60–70% humidity, in a 12-h
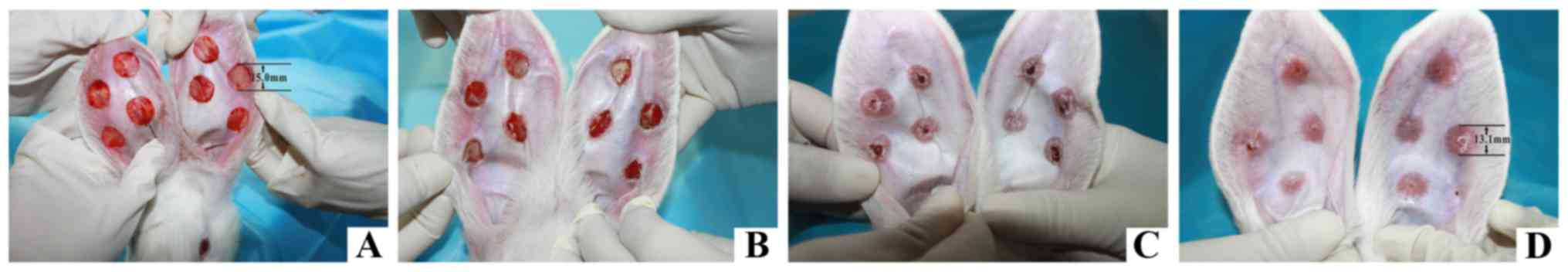
light/dark cycle for 48 h. The HS model was established as
described by Morris et al (33). In brief, the rabbits were
anesthetized with intravenous ketamine (30 mg/kg) injection into
auricular vein. Four full thickness circular wounds (15 mm
diameter) were inflicted on the ventral surface of each ear with a
scalpel, avoiding damage to the visible vessels. The separation
between each wound was >15 mm. The epidermis, dermis, and
perichondrium were carefully stripped. The full-thickness wounds
with exposed cartilage were covered with erythromycin eye ointment.
Post anesthesia, the rabbits were placed in their previous cages.
Secretions and exudates were gently removed on the following day.
No topical wound care was provided and the wounds were left to heal
spontaneously. Hypertrophic scars were formed three weeks after the
surgery and histopathological examination was performed (Fig. 2).
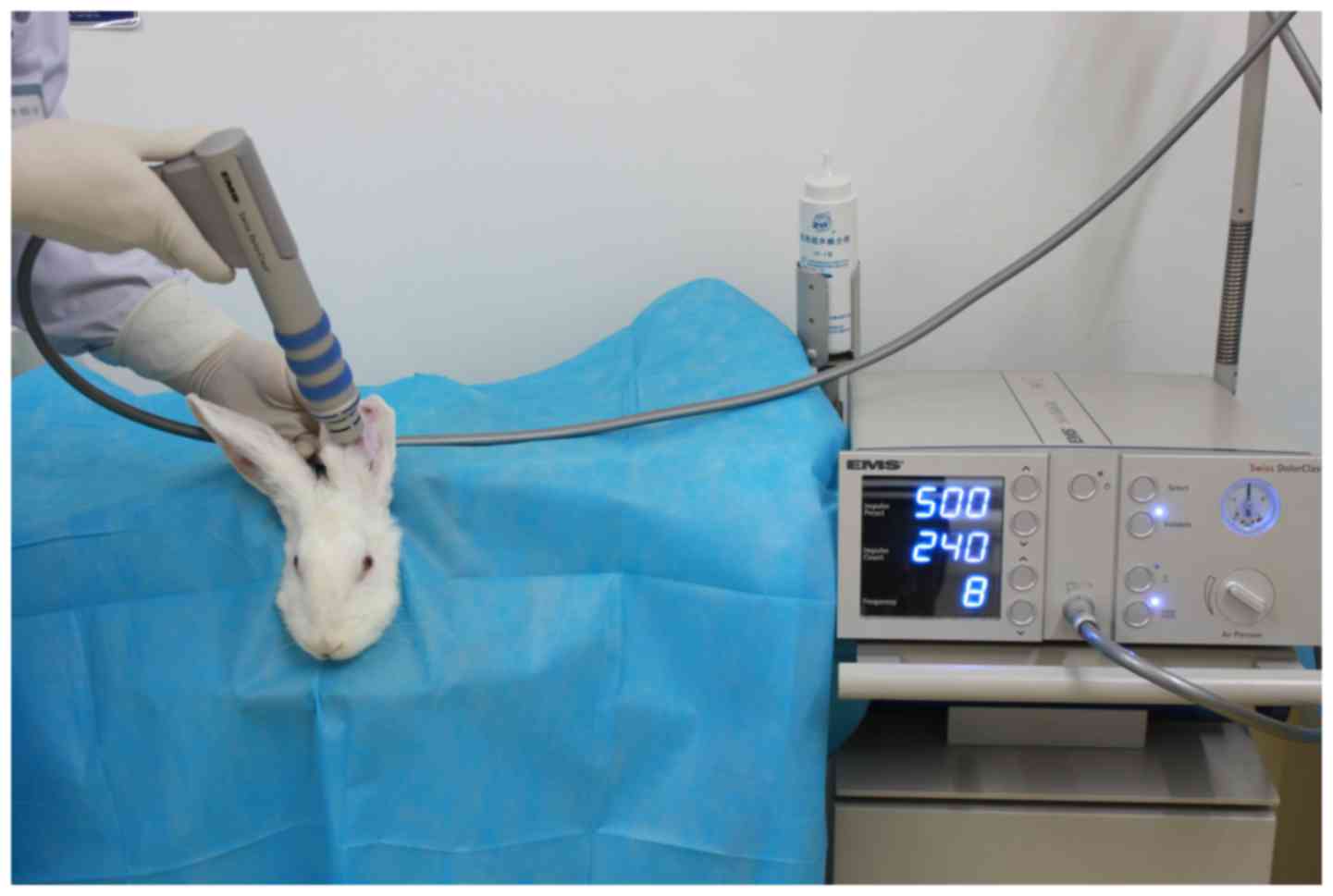
ESWT treatment protocol
Three weeks after HS model establishment, twenty
four rabbits (one was already used for HS testing) were randomly
allocated to three groups: rESWT with lower EFD of 0.1
mJ/mm2 (L-ESWT), rESWT with higher EFD of 0.18
mJ/mm2 (H-ESWT) and sham ESWT treatment group (S-ESWT).
The Swiss DolorClast® Classic (EMS Electro Medical
Systems, Nyon, Switzerland) convex 15-mm shock wave applicator was
gently placed on the HS surface which was covered with a liberal
amount of medical ultrasound coupling agent as a conductive gel. In
the L-ESWT and H-ESWT groups, a single shock wave treatment with
500 impulses at a frequency of 8 Hz was administered once a week
for four weeks (0.1 mJ/mm2 for L-ESWT and 0.18
mJ/mm2 for H-ESWT, respectively) (Fig. 3). S-ESWT group also received
identical treatment; however, no shock wave impulse was
administered. Rabbits appeared to tolerate the rESWT treatment well
without anesthetic cover.
Samples and data collection
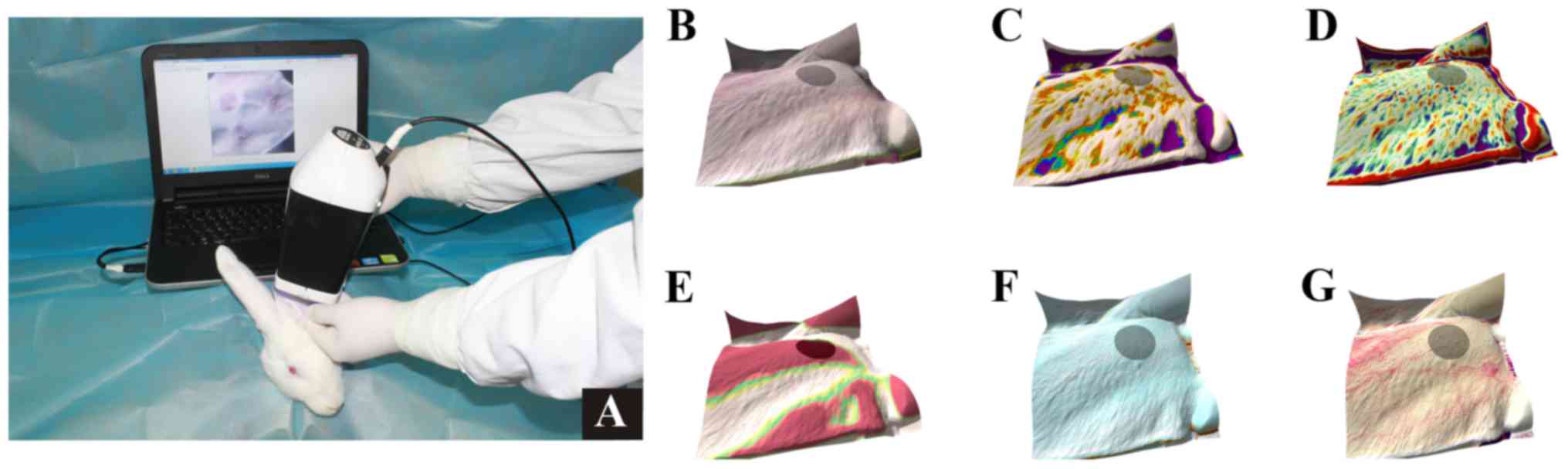
The characteristics of HS were assessed using Antera
3D® (v2.0; Miravex Limited, Dublin 2, Dublin, Ireland),
with the camera held in tight apposition to the wound and the push
button on the camera clicked to obtain a higher resolution image.
The image was saved and the characteristics were measured in terms
of the presence of wrinkles, texture, volume of elevation
(mm3), area (mm2), diameter (mm), melanin and
hemoglobin of scar using the mode selection menu (2 mm filter was
used for wrinkles, texture and elevations) (Fig. 4). Findings were assessed with Antera
3D® on days 1, 4, 7, 10, 14, 21, 28 and 35 of initial
rESWT treatment.
A separate set of fresh tissue samples were
harvested for determining gene and protein expression under
intravenous anesthesia, as described above, on days 1, 4, 7, 10,
14, 21, 28 and 35 of the initial rESWT treatment. One wound per
rabbit and time point for taking samples. Prior to their
processing, all specimens were snap-frozen in liquid nitrogen.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Hypertrophic scar tissues were homogenized using an
electronic high-speed homogenizer. Total RNA was obtained using
TRIzol® Reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA) according to the manufacturer's instructions. The total
RNA concentration in each HS sample was determined with Epoch™
Multi-Volum Microplate Spectrophotometer System (BioTek
Instruments, Inc., Winooski, VT, USA). After reverse transcription
with M-MLV Reverse Transcriptase (Promega Corp., Madison, WI, USA)
using Oligo (dT)15 Primer (Promega) for 75 min (37°C for 5 min,
42°C for 60 min, and 95°C for 10 min), 2 µl first strand cDNA
template was amplified by polymerase chain reaction (PCR) with 25
µl GoTaq® Green Master Mix (Promega), 19 µl Nuclease
Free Water (Promega), and 2 µl each of primer. The specimens were
denatured first at 94°C for 30s, followed by annealing for 30s at
58°C for TGF-β1; 50°C for 30s for smad2; 50°C for 35s for smad3;
and 65°C for 30s for GAPDH, and extension at 72°C for 40s (25
cycles for TGF-β1, 30 cycles for smad2, smad3 and GAPDH). Reverse
transcriptase (RT)-PCR-amplified products were analyzed by 1.5%
agarose gel electrophoresis containing ethidium bromide. Results
were photographed using the Tanon 2500 Gel Imaging System (Tanon
Science & Technology Co., Ltd., Shanghai, China). Band
intensities were semi-quantified using Tanon Gel Image System 1D
software (v4.1.2), and subsequently normalized by dividing the band
gray value of the target gene by the intensity of its corresponding
GAPDH. Primer sequences were synthesized by BGI Tech Solutions Co.,
Ltd., (Shenzhen, China) as follows: TGF-β1 forward primer
5′-CGGCAGCTGTACATTGACTT-3′ and reverse primer
5′-AGCGCACGATCATGTTGGAC-3′ (size 271 basepairs), smad2 forward
primer 5′-ATGCCACGGTAGAAATGAC-3′ and reverse primer
5′-TTGAGCAACGCACTGAAGG-3′ (size 429 basepairs), smad3 forward
primer 5′-ACCACGCAGAACGTGAACAC-3′ and reverse primer
5′-GCTGGTTTTCCTTGGGTACC-3′ (size 419 basepairs), GAPDH forward
primer 5′-GCGCCTGGTCACCAGGGCTGCTT-3′ and reverse primer
5′-TGCCGAAGTGGTCGTGGATGACCT-3′ (size 464 basepairs).
Enzyme-linked immunosorbent assay
(ELISA)
The concentration of rabbit TGF-β1, Smad2, Smad3,
and Smad7 proteins in the HS tissue were determined using
commercially available ELISA kits according to the manufacturer's
instructions (R&D Systems, Inc., Minneapolis, MN, USA).
Statistical analysis
Data from Antera 3D® were expressed as
mean ± standard deviation (SD); intergroup differences were
assessed by paired t-test using SPSS software ver 19.0 (IBM Corp.,
NY, USA). Findings of RT-PCR and ELISA were expressed as mean ±
standard error of the mean (SEM); intergroup differences were
assessed using Analysis Of Variance (ANOVA). A P-value <0.05 was
considered indicative of a statistically significant
difference.
Results
Antera 3D® evaluation of
HS
Prior to rESWT treatment, there were no
statistically significant inter-group differences with respect to
scar characteristics in the three study groups (data not provided).
Until two weeks of rESWT treatment, no significant intergroup
differences in terms of wrinkles and hemoglobin were observed
between L-ESWT and S-ESWT groups (P=0.042 and P=0.040,
respectively), between L-ESWT and H-ESWT (P=0.700 and P=0.176,
respectively), or between H-ESWT and S-ESWT (P=0.080 and P=0.226,
repectively). However, three weeks later, wrinkles and hemoglobin
in both L-ESWT and H-ESWT groups were significantly less than that
in observed in the S-ESWT group (L-ESWT and S-ESWT, P=0.043 and
P=0.043, respectively; H-ESWT and S-ESWT, P=0.037 and P=0.025,
respectively). However, no significant difference was observed
between L-ESWT and H-ESWT in this respect. Similar findings were
observed till the fourth week (L-ESWT and S-ESWT, P=0.042 and
P=0.013, respectively; H-ESWT and S-ESWT, P=0.026 and P=0.044,
respectively). Four weeks later, significant difference in the
appearance of texture was observed between L-ESWT and S-ESWT
(P=0.014) groups. However, no such difference was observed between
L-ESWT and H-ESWT, or between H-ESWT and S-ESWT groups. No
significant differences were observed between the three study
groups with respect to diameter, area, elevation of volume and
melanin of HS.
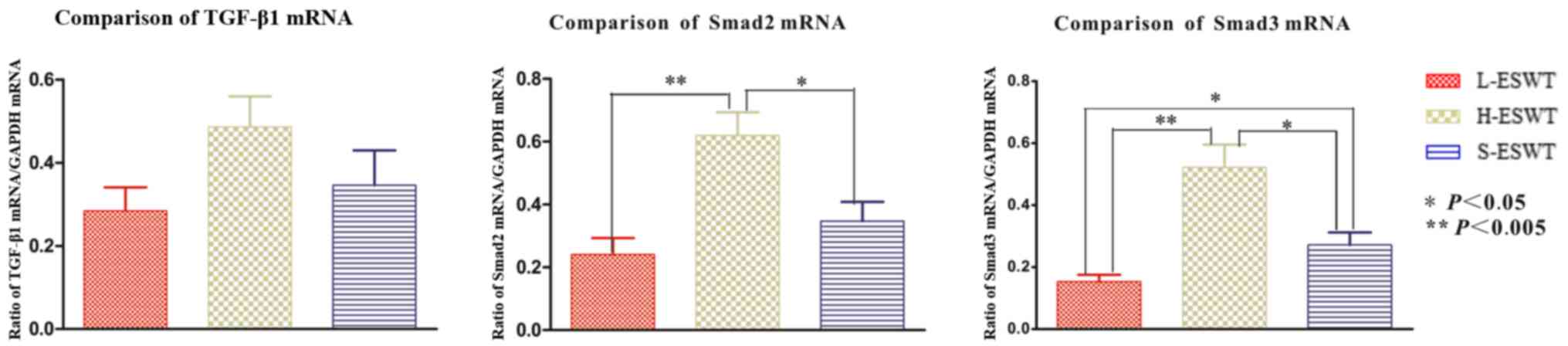
Gene expression of TGF-β1/Smad
pathway
The effect of L-ESWT and H-ESWT on TGF-β1/smad mRNA
expression was assessed by RT-PCR, against that associated with use
of GAPDH as an internal standard control. No significant difference
in TGF-β1 mRNA expression was found between the three groups. The
smad2 mRNA levels were significantly higher in H-ESWT as compared
to that in the L-ESWT and S-ESWT groups (P=0.001 and P=0.024,
respectively). No difference in smad2 expression was observed
between the L-ESWT and S-ESWT (P=0.476). The level of smad3 were
found to be significantly decreased in L-ESWT as compared to that
in H-ESWT and S-ESWT groups (P=0.000 and P=0.047, respectively).
The smad3 mRNA level in H-ESWT was significantly higher than that
in the S-ESWT group (P=0.019) (Fig.
5).
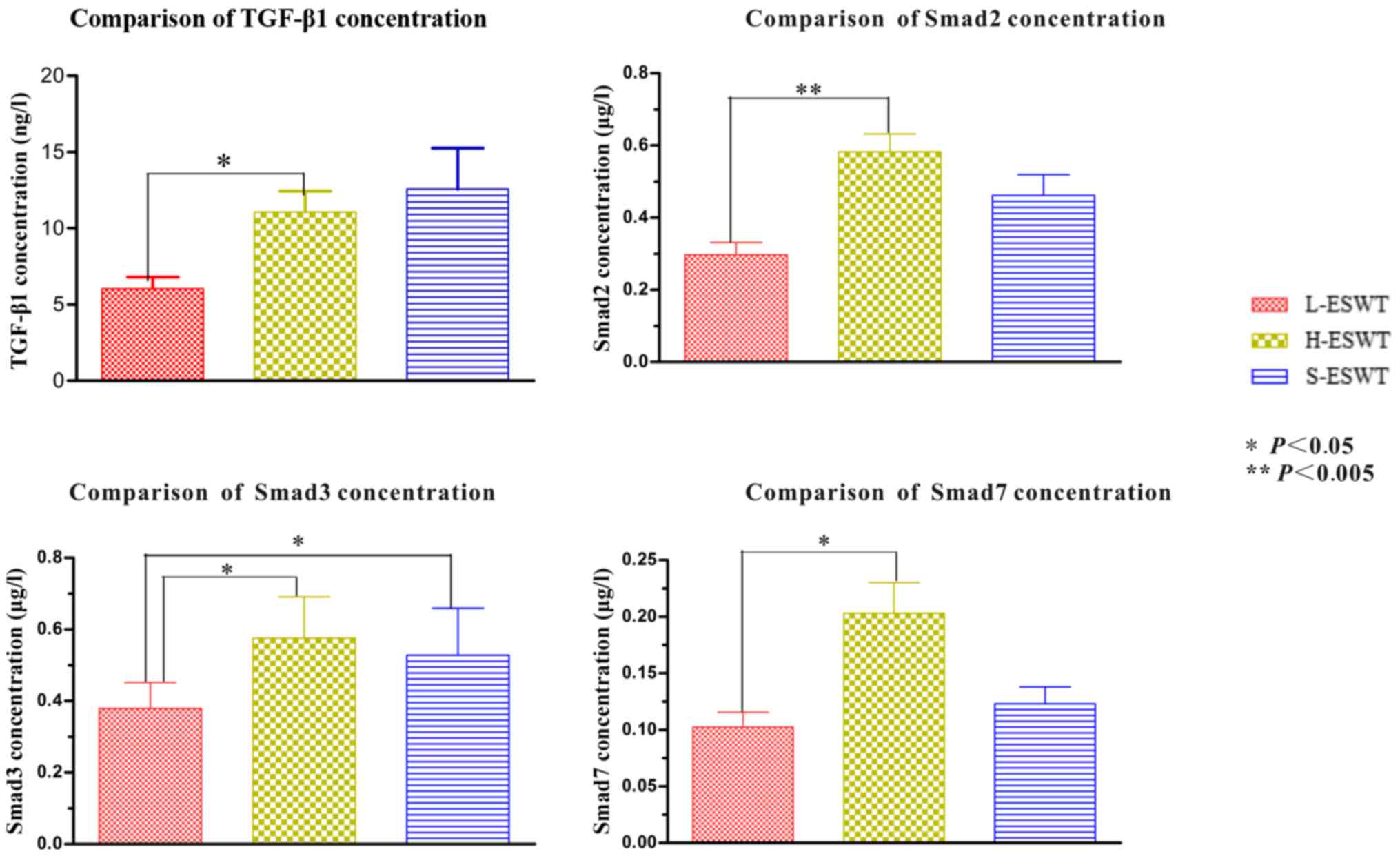
Protein determination of TGF-β1/Smad
pathway
No significant differences in the concentrations of
TGF-β1, smad2 and smad7 protein were observed in both L-ESWT and
H-ESWT groups when compared to those in the S-ESWT group. The smad3
protein levels in the L-ESWT were significantly lower than those in
the H-ESWT and S-ESWT groups (P=0.012 and P=0.048, respectively).
No significant difference was observed between H-ESWT and S-ESWT
groups with respect to smad3 concentration (P=0.289) (Fig. 6).
Discussion
Treatment of HS is typically challenging due in part
to the limited understanding of its pathogenesis. TGF-β1 is known
to stimulate synthesis of collagen and proteoglycan, which may
promote HS formation via its influence on the metabolism of ECM
(34). Fibroblasts isolated from HS
tissue have been shown to have an increased expression of TGF-β1
and TGF-βR as compared to those from normal skin and are thought to
play a vital role in HS formation, including the resultant
increased synthesis of tissue inhibitor of matrix
metalloproteinases, and which in turn decreases the activity of
matrix metalloproteinases (35),
resulting in excessive collagen synthesis and deposition (36). Further, TGF-β1 was also shown to
induce alpha-smooth muscle actin expression in myofibroblasts,
conferring an increased ability for collagen synthesis and
resistance to apoptotic inductors (37,38).
There are various receptors to which TGF-β can bind to, the most
important being two ligand-dependent complexes of heterodimeric
transmembrane serine/threonine kinases, i.e., TGF-β receptor I
(TGF-βRI) and TGF-β receptor II (TGF-βRII).
Among the various downstream signaling pathways
mediated by TGF-β1, the Smad pathway is thought to be of vital
importance (6). Smad proteins
respond to activated TGF-β receptor complex and play an important
role in the TGF-β signal transduction from cell surface receptors
to the nucleus. Smad family consists of at least eight subtypes of
smad proteins, and these subtypes participate in various signal
transduction pathways of various TGF-β superfamily members.
Classically, smad proteins have been classified into
three categories, namely, the receptor-regulated smads (R-Smads),
common mediator smad (Co-Smad) and inhibitory smads (I-Smads)
(39). R-Smads are amenable to
activation after binding with TGF-βRI to form a complex. R-Smads
are classified into two categories depending on the activation by
activin or TGF-β (including smad2 and smad3). In addition, smads
are activated by bone morphogenetic proteins (including smadl,
smad5 and smad8 (40–42), too.
Co-Smad consists of smad4 protein, which is involved
in nearly all TGF-β superfamily signal transduction pathways.
I-Smads (including smad6 and smad7) are considered to be the
negative feedback regulators, with the ability to bind to activated
TGF-βRI and block TGF-β signal pathway (38). Smad6 mainly inhibits the bone
morphogenetic protein pathway, while smad7 mainly blocks the TGF-β
signal pathway (43).
In normal tissues, TGF-β isoforms exist in a
precursor form, and combine with latent TGF-β1 binding proteins to
form a complex (44). Intracellular
signaling of TGF-β1 is initiated after latent TGF-β1 binding
proteins is dissociated from this complex (6). The activated TGF-β1 is released and
binds to TGF-βRII, which in turn activates the TGF-βRI.
Activin-receptor-like kinase 5, one of the isoforms of TGF-βRIs
phosphorylates and activates smad2 and samd3; combine with smad4
and translocate into the nucleus to function as transcription
factors, or participate in transcriptional control of certain
genes.
The TGF-β1/Smad pathway in HS tissue has been shown
to manifest certain typical characteristics. These include,
increased expression and phosphorylation of samd2 and/or samd3, but
not smad7, during HS formation (39). Similar findings implicating TGF-β1,
smad2 and smad3 in HS formation have been reported by Chen et
al (45). Therefore, blockage of
the fibrotic processes mediated by TGF-β1/smad signaling is a
potential therapeutic target for prevention and treatment of HS
(46–48). In this study, we treated HS in rabbit
ear model with different EFD of ESWT and found no significant
difference in the expression of TGF-β1, smad2 and smad7. However,
L-ESWT appeared to significantly inhibit smad3 expression.
Previous studies have shown that a reduction in
smad3 expression may contribute to the reduction of collagen I
synthesis and enhance wound healing which makes it a potential
molecular target for treatment of HS (45,49,50). In
view of the important role of smad3 in the TGF-β1/smad signaling
pathway, we speculate that this may be one of the mechanisms for
the inhibitory effect of L-ESWT on HS formation. A comprehensive
pre- and post-treatment of HS tissue could be invaluable in
devising future therapeutic interventions.
Several instruments are currently available for
assessment of HS, such as, patient and observer scar assessment
scales, Vancouver scar scale and visual analog scale. However,
these tools do not allow for an objective assessment of improvement
of HS. Instead, a variety of techniques and instruments were used
before and after treatment to detect the improvement of scar, for
example, in a randomized controlled trial, Cho et al
(10) documented the characteristics
of scar in terms of thickness, melanin, erythema, transepidermal
water loss, sebum, and elasticity with the help of different
equipments such as ultrasonography, Mexameter®,
Tewameter®, Sebumeter®, and
Cutometer®, respectively. Although this method affords a
comprehensive and accurate assessment of scar improvement, the
requirement for multiple equipment and devices with varying
operating requirements is a limitation.
Antera 3D® allows for acquisition of 3D
images and 3D data which provides the basis for an objective
quantification of the treatment efficacy and monitors changes over
time. In the present study, we assessed the changes in wrinkles,
texture, diameter, area, elevation of volume, melanin and
hemoglobin of HS tissue in real-time using Antera 3D®.
This allowed for a comprehensive and objective assessment. Our
study indicates that both L-ESWT and H-ESWT may improve the
wrinkles and hemoglobin of HS. Only L-ESWT treatment was associated
with a significant improvement in the texture of HS. However, there
was no apparent influence of rESWT on the scar diameter, area and
melanin content. This may be attributed to the lack of efficacy of
ESWT treatment, or to the relatively short period of study. Future
studies with a longer time frame are required to confirm our
findings.
In addition to the relatively short-term period we
have investigated in the present study, the other limitation of the
study was the retrieval of 8 separate tissue biopsy samples under
anesthesia over the course of 35 days post-treatment, which may
have impacted on the gene and protein expression results.
Radial ESWT is a non-invasive, safe and well
tolerated treatment modality for HS. In this study, L-ESWT
treatment was associated with a significant improvement in
wrinkles, hemoglobin and texture of HS. Downregulation of smad3 may
be one of the mechanism of action of L-ESWT. Future studies are
required to determine other potential mechanisms in order to
further develop and improve efficacy of L-ESWT-based therapy in the
clinical management of HS.
References
|
1
|
Gauglitz GG, Korting HC, Pavicic T,
Ruzicka T and Jeschke MG: Hypertrophic scarring and keloids:
Pathomechanisms and current and emerging treatment strategies. Mol
Med. 17:113–125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Niessen FB, Spauwen PH, Schalkwijk J and
Kon M: On the nature of hypertrophic scars and keloids: A review.
Plast Reconstr Surg. 104:1435–1458. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hardy MA: The biology of scar formation.
Phys Ther. 69:1014–1024. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hayashida T, Decaestecker M and Schnaper
HW: Cross-talk between ERK MAP kinase and Smad signaling pathways
enhances TGF-beta-dependent responses in human mesangial cells.
FASEB J. 17:1576–1578. 2003.PubMed/NCBI
|
|
5
|
Asano Y, Ihn H, Yamane K, Jinnin M, Mimura
Y and Tamaki K: Phosphatidylinositol 3-kinase is involved in
alpha2(I) collagen gene expression in normal and scleroderma
fibroblasts. J Immunol. 172:7123–7135. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu Z, Ding J, Shankowsky HA and Tredget
EE: The molecular mechanism of hypertrophic scar. J Cell Commun
Signal. 7:239–252. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Klass BR, Grobbelaar AO and Rolfe KJ:
Transforming growth factor beta1 signalling, wound healing and
repair: A multifunctional cytokine with clinical implications for
wound repair, a delicate balance. Postgrad Med J. 85:9–14. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Trisliana Perdanasari A, Torresetti M,
Grassetti L, Nicoli F, Zhang YX, Dashti T, Di Benedetto G and
Lazzeri D: Intralesional injection treatment of hypertrophic scars
and keloids: A systematic review regarding outcomes. Burns Trauma.
3:142015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Steinstraesser L, Flak E, Witte B, Ring A,
Tilkorn D, Hauser J, Langer S, Steinau HU and Al-Benna S: Pressure
garment therapy alone and in combination with silicone for the
prevention of hypertrophic scarring: Randomized controlled trial
with intraindividual comparison. Plast Reconstr Surg.
128:306e–313e. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cho YS, Jeon JH, Hong A, Yang HT, Yim H,
Cho YS, Kim DH, Hur J, Kim JH, Chun W, et al: The effect of burn
rehabilitation massage therapy on hypertrophic scar after burn: A
randomized controlled trial. Burns. 40:1513–1520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Azzam OA, Bassiouny DA, El-Hawary MS, El
Maadawi ZM, Sobhi RM and El-Mesidy MS: Treatment of hypertrophic
scars and keloids by fractional carbon dioxide laser: A clinical,
histological, and immunohistochemical study. Laser Med Sci.
31:9–18. 2016. View Article : Google Scholar
|
|
12
|
O'Brien L and Jones DJ: Silicone gel
sheeting for preventing and treating hypertrophic and keloid scars.
Cochrane Database Syst Rev. 9:CD0038262013.
|
|
13
|
Ogawa R, Miyashita T, Hyakusoku H, Akaishi
S, Kuribayashi S and Tateno A: Postoperative radiation protocol for
keloids and hypertrophic scars: Statistical analysis of 370 sites
followed for over 18 months. Ann Plast Surg. 59:688–691. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zouboulis CC: Cryosurgery in dermatology.
Hautarzt. 66:834–848. 2015.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Q, Liu LN, Yong Q, Deng JC and Cao
WG: Intralesional injection of adipose-derived stem cells reduces
hypertrophic scarring in a rabbit ear model. Stem Cell Res Ther.
6:1452015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Williams CC and De Groote S: Clinical
inquiry: What treatment is best for hypertrophic scars and keloids?
J Fam Pract. 60:757–758. 2011.PubMed/NCBI
|
|
17
|
Ledon JA, Savas J, Franca K, Chacon A and
Nouri K: Intralesional treatment for keloids and hypertrophic
scars: A review. Dermatol Surg. 39:1745–1757. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Macintyre L and Baird M: Pressure garments
for use in the treatment of hypertrophic scars-a review of the
problems associated with their use. Burns. 32:10–15. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mustoe TA, Cooter RD, Gold MH, Hobbs FD,
Ramelet AA, Shakespeare PG, Stella M, Téot L, Wood FM and Ziegler
UE; International Advisory Panel on Scar Management, :
International clinical recommendations on scar management. Plast
Reconstr Surg. 110:560–571. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meaume S, Le Pillouer-Prost A, Richert B,
Roseeuw D and Vadoud J: Management of scars: Updated practical
guidelines and use of silicones. Eur J Dermatol. 24:435–443.
2014.PubMed/NCBI
|
|
21
|
Chaussy C, Brendel W and Schmiedt E:
Extracorporeally induced destruction of kidney stones by shock
waves. Lancet. 2:1265–1268. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rassweiler J, Rassweiler MC, Frede T and
Alken P: Extracorporeal shock wave lithotripsy: An opinion on its
future. Indian J Urol. 30:73–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Valchanou VD and Michailov P: High energy
shock waves in the treatment of delayed and nonunion of fractures.
Int Orthop. 15:181–184. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maffulli G, Hemmings S and Maffulli N:
Assessment of the effectiveness of extracorporeal shock wave
therapy (ESWT) for soft tissue injuries (ASSERT): An online
database protocol. Transl Med UniSa. 10:46–51. 2014.PubMed/NCBI
|
|
25
|
Speed C: A systematic review of shockwave
therapies in soft tissue conditions: Focusing on the evidence. Br J
Sports Med. 48:1538–1542. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goertz O, Lauer H, Hirsch T, Ring A,
Lehnhardt M, Langer S, Steinau HU and Hauser J: Extracorporeal
shock waves improve angiogenesis after full thickness burn. Burns.
38:1010–1018. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dymarek R, Halski T, Ptaszkowski K,
Slupska L, Rosinczuk J and Taradaj J: Extracorporeal shock wave
therapy as an adjunct wound treatment: A systematic review of the
literature. Ostomy Wound Manage. 60:26–39. 2014.PubMed/NCBI
|
|
28
|
Zhao JC, Xian CJ, Yu JA and Shi K:
Advancement in the research of effect of extracorporeal shock wave
therapy on wound angiogenesis. Chin J Injury Repair Wound Healing.
9:80–84. 2014.(In Chinese).
|
|
29
|
Zhao J, Xue Y, Yu J, Shi K, Xian C and
Zhou X: Advances in the research of mechanism of enhancement of
wound healing with extracorporeal shock wave therapy. Zhonghua Shao
Shang Za Zhi. 31:315–317. 2015.(In Chinese). PubMed/NCBI
|
|
30
|
Fioramonti P, Cigna E, Onesti MG, Fino P,
Fallico N and Scuderi N: Extracorporeal shock wave therapy for the
management of burn scars. Dermatol Surg. 38:778–782. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cho YS, Joo SY, Cui H, Cho SR, Yim H and
Seo CH: Effect of extracorporeal shock wave therapy on scar pain in
burn patients: A prospective, randomized, single-blind,
placebo-controlled study. Medicine (Baltimore). 95:e45752016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Saggini R, Saggini A, Spagnoli AM, Dodaj
I, Cigna E, Maruccia M, Soda G, Bellomo RG and Scuderi N:
Extracorporeal shock wave therapy: An emerging treatment modality
for retracting scars of the hands. Ultrasound Med Biol. 42:185–195.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Morris DE, Wu L, Zhao LL, Bolton L, Roth
SI, Ladin DA and Mustoe TA: Acute and chronic animal models for
excessive dermal scarring: Quantitative studies. Plast Reconstr
Surg. 100:674–681. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Köse O and Waseem A: Keloids and
hypertrophic scars: Are they two different sides of the same coin?
Dermatol Surg. 34:336–346. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Scott PG, Dodd CM, Tredget EE, Ghahary A
and Rahemtulla F: Immunohistochemical localization of the
proteoglycans decorin, biglycan and versican and transforming
growth factor-beta in human post-burn hypertrophic and mature
scars. Histopathology. 26:423–431. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shah M, Foreman DM and Ferguson MW:
Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition
of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci.
108:985–1002. 1995.PubMed/NCBI
|
|
37
|
Desmoulière A, Geinoz A, Gabbiani F and
Gabbiani G: Transforming growth factor-beta 1 induces alpha-smooth
muscle actin expression in granulation tissue myofibroblasts and in
quiescent and growing cultured fibroblasts. J Cell Biol.
122:103–111. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Moulin V, Larochelle S, Langlois C,
Thibault I, Lopez-Vallé CA and Roy M: Normal skin wound and
hypertrophic scar myofibroblasts have differential responses to
apoptotic inductors. J Cell Physiol. 198:350–358. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kopp J, Preis E, Said H, Hafemann B,
Wickert L, Gressner AM, Pallua N and Dooley S: Abrogation of
transforming growth factor-beta signaling by SMAD7 inhibits
collagen gel contraction of human dermal fibroblasts. J Biol Chem.
280:21570–21576. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu J, Lamouille S and Derynck R:
TGF-beta-induced epithelial to mesenchymal transition. Cell Res.
19:156–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schmierer B and Hill CS: TGFbeta-SMAD
signal transduction: Molecular specificity and functional
flexibility. Nat Rev Mol Cell Biol. 8:970–982. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Massagué J: How cells read TGF-beta
signals. Nat Rev Mol Cell Biol. 1:169–178. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Arno AI, Gauglitz GG, Barret JP and
Jeschke MG: New molecular medicine-based scar management
strategies. Burns. 40:539–551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Annes JP, Munger JS and Rifkin DB: Making
sense of latent TGFbeta activation. J Cell Sci. 116:217–224. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen W, Fu X, Sun T, Sun X, Zhao Z and
Sheng Z: Change of gene expression of transforming growth
factor-beta1, Smad 2 and Smad 3 in hypertrophic scars skins.
Zhonghua Wai Ke Za Zhi. 40:17–19. 2002.(In Chinese). PubMed/NCBI
|
|
46
|
Wu C, Jiang J, Boye A, Jiang Y and Yang Y:
Compound Astragalus and Salvia miltiorrhiza extract suppresses
rabbits' hypertrophic scar by modulating the TGF-β/Smad signal.
Dermatology. 229:363–368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bai X, He T, Liu J, Wang Y, Fan L, Tao K,
Shi J, Tang C, Su L and Hu D: Loureirin B inhibits fibroblast
proliferation and extracellular matrix deposition in hypertrophic
scar via TGF-β/Smad pathway. Exp Dermatol. 24:355–360. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang X, Chu J, Wen CJ, Fu SB, Qian YL, Wo
Y, Wang C and Wang DR: Functional characterization of TRAP1-like
protein involved in modulating fibrotic processes mediated by
TGF-β/Smad signaling in hypertrophic scar fibroblasts. Exp Cell
Res. 332:202–211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Schultze-Mosgau S, Blaese MA, Grabenbauer
G, Wehrhan F, Kopp J, Amann K, Rodemann HP and Rödel F: Smad-3 and
Smad-7 expression following anti-transforming growth factor beta 1
(TGFbeta1)-treatment in irradiated rat tissue. Radiother Oncol.
70:249–259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ashcroft GS, Yang X, Glick AB, Weinstein
M, Letterio JL, Mizel DE, Anzano M, Greenwell-Wild T, Wahl SM, Deng
C and Roberts AB: Mice lacking Smad3 show accelerated wound healing
and an impaired local inflammatory response. Nat Cell Biol.
1:260–266. 1999. View
Article : Google Scholar : PubMed/NCBI
|