Introduction
Aripiprazole (APZ) is a third-generation atypical
antipsychotic drug that is a partial agonist of the dopaminergic D2
receptor (D2R) and the serotonin 5-HT1A and 5-HT7 receptors. APZ is
used to treat schizophrenia (1–3) and acts
as a dopamine-serotonin system stabilizer in adjunct therapy for
major depressive disorder (3,4). Stroke
is a neurological disease that induces sustained damage in the
arteries in the brain and is often fatal. Studies have demonstrated
that >30% of stroke survivors experience depression, including
feelings of despair, anhedonia and anxiety (5,6).
APZ is widely used in combination with selective
serotonin reuptake inhibitors as a treatment of major depressive
disorder. Low-doses of APZ are also effective at treating patients
with post-stroke emotional disorders and impaired cognitive
function (3,7). Despite prospective clinical viewpoints,
the mechanisms underlying the curative efficacy of APZ in
post-stroke depression remain unclear. Previous studies have
demonstrated that APZ exhibits benefits in patients with
post-stroke depression, including the protection of primary lesions
and secondary extrafocal sites following ischemic stroke (8,9).
Additionally, APZ decreases striatal kainate-induced
lesion volumes in rodents by inducing a 5-HT1A-mediated protective
effect (10). Dopaminergic D2Rs
regulate inflammatory responses in the central nervous system and
ameliorate neurological dysfunction by reducing microglia
hyperactivity-related neuroinflammation (11). It has been demonstrated that dopamine
D2/D3 receptor agonists exhibit protective effects against
post-ischemic injury (12). However,
the neuroprotective effects of APZ have only been demonstrated in a
limited number of in vitro and in vivo studies
(8–10,13).
The present study was designed based on the
hypothesis that APZ inhibits the cell death and neuroinflammation
caused by ischemic assaults, thus exerting a neuroprotective
effect. To validate this hypothesis, the ability of APZ to induce
motor-function behaviors associated with equilibrium and rotation
asymmetry in a mouse model of middle cerebral artery occlusion
(MCAO) was evaluated. To further assess the neuroprotective effects
of APZ, histopathological analyses of brain sections were
performed. The chronological sequence of events is fundamental to
neuronal cell death and the neuroinflammatory response following
ischemic insult (14). Therefore, in
the current study, the subacute phase of ischemic stroke,
characterized by marked apoptosis and inflammation, was divided
into three periods according to the start of APZ treatment
following MCAO.
Materials and methods
Animals
A total of 30 male C57BL/6 mice aged 6 weeks old
(weight, 18–20 g) were purchased from DooYeol Biotech (Seoul,
Korea). Mice were housed at 22°C and 55±5% humidity under a 12-h
light-dark cycle and were fed a commercial diet. Mice had ad
libitum access to food and water. All experiments were approved by
the Pusan National University Animal Care and Use Committee in
accordance with the National Institutes of Health Guidelines
(approval no. PNU-2016-1149). After 1 week the mice were randomly
divided into 5 groups (n=6) as follows: A control group, a
MCAO+vehicle group and three MCAO+APZ treatment groups according to
the start of APZ treatment (1–5, 5–9 and 10–14 days following
MCAO). All mice were sacrificed at 24 days following MCAO.
MCAO model
Mice were anesthetized with isoflurane (Choongwae
Pharma Corp., Seoul, Korea) using a model VIP 3000 calibrated
vaporizer (Midmark Corporation, Orchard Park, OH, USA). Isoflurane
was induced at a concentration of 3% and maintained at a
concentration of 2% in 80% N2O and 20% O2.
Rectal temperatures were maintained at 36.5–37.5°C. Isoflurane
anesthesia was delivered using a facemask and a fibre optic probe
was fixed to the portion of skull that covered the middle cerebral
artery. Regional cerebral blood flow was then measured using the
PeriFlux Laser Doppler System 5000 (Perimed, Stockholm, Sweden) and
a left MCAO model was produced. A silicon-coated 7-0 monofilament
was advanced through the internal carotid artery to occlude the
middle cerebral artery for 30 min and subsequently withdrawn.
Reperfusion was confirmed using the Laser Doppler System. The
control group underwent isoflurane anesthesia, however no further
procedures were performed.
Drug administration
APZ was donated by Otsuka Pharmaceutical Co., Ltd.
(Tokyo, Japan). The drug was orally administered using a probe once
a day for 5 days. Treatment was initiated 1, 5 or 10 days following
MCAO, depending on the group mice were in. APZ was dissolved in
distilled water to obtain a concentration of 3 mg/kg. The vehicle
group were administrated the same volume of distilled water from 1
day following MCAO for 5 days.
Behavioral experiments
To evaluate whether APZ had an effect on motor
function, cylinder, rotarod and wire suspension tests were
conducted in all groups once a week for 3 weeks following MCAO. The
cylinder test was performed to evaluate forelimb use and rotation
asymmetry (15). Each mouse was
individually placed on the floor of a plastic cylinder (diameter, 9
cm; height, 15 cm). The number of times that mice used their front
paws to touch the cylinder was recorded and repeated 20 times. The
motor coordination and equilibrium of mice were measured using a
rotarod apparatus (Panlab S.L.U, Barcelona, Spain). All mice were
pre-trained with two trials per day for two days on a rotarod
apparatus at a fixed speed (20 rpm). Mice were then placed on the
rotating rod, with a cut-off time of 3 min (16). The experiment comprised of five
trials per day, once a week over a 3-week period. The wire
suspension test was performed to measure muscular strength and
neuromuscular endurance of mice following MCAO (17). The grip capabilities of the mice were
evaluated using a sustained horizontal bar. The time that mice
spent hanging on the bar was recorded and the mean of five trials
from each mouse was analyzed.
Determination of infarct volume
To measure ischemic damage, mice were fully
anesthetized using 500 mg/kg chloral hydrate (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) and received an intraperitoneal perfusion
of saline followed by 4% paraformaldehyde in PBS. Brains were
removed, post-fixed in the same fixative for 24 h at 4°C and
immersed in 30% sucrose solution for 72 h at 4°C for
cryoprotection. Brain infarct sizes or atrophies were estimated by
staining frozen sections of 30-µm thickness with 0.1% cresyl violet
at room temperature for 2 min (Sigma-Aldrich; Merck KGaA) and
mounting slides in mounting medium (cat. no. H-5000; Vector
Laboratories, Inc., Burlingame, CA, USA). To measure infarct area,
the contralateral and ipsilateral segmentum sizes of each section
(including the striatum, corpus callosum, cortex and midbrain)
images were captured at magnification ×10 using a Stemi 305 light
microscope (Carl Zeiss AG, Oberkochen, Germany) and quantified
using i-solution full image analysis software (version 10.1; Image
and Microscope Technology, Hackettstown, NJ, USA).
Immunohistochemistry
The 30-µm-thick brain sections were frozen at −25°C
and then incubated in blocking buffer [1X PBS/5% normal goat serum
(cat. no. s-1000; Vector Laboratories Inc.)/0.3% Triton X-100] for
1 h at room temperature. Sections were incubated with the following
primary antibodies for 48 h in PBS at 4°C: Neuronal nuclei (NeuN;
cat. no. MAB377; 1:500; EMD Millipore, Billerica, MA, USA),
tyrosine hydroxylase (TH; cat. no. AB152; 1:500; EMD Millipore),
dopamine D2R (cat. no. AB5084p; 1:100; EMD Millipore),
Ca2+/calmodulin-dependent protein kinase IIδ (CaMKIIδ;
cat. no. ab181052; 1:100; Abcam, Cambridge, UK), ionized calcium
binding adaptor molecule 1 (Iba1; cat. no. 019-19741; 1:500; Wako
Pure Chemical Industries, Ltd., Osaka, Japan) and cluster of
differentiation 68 (CD68; cat. no. MCA1957; 1:500; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Slides were then washed
with PBS and sections were incubated with the
fluorescein-conjugated goat-anti-rabbit (cat. no. A11008; 1:500) or
Texas red-conjugated goat-anti-mouse (cat. no. A11005; 1:500),
goat-anti-rat (cat. no. A11007; 1:500) and DAPI (cat. no. H3570;
1:10,000) (all Thermo Fisher Scientific, Inc., Waltham, MA, USA)
for 2 h at room temperature in the dark and then washed with PBS
three times. Subsequently, slides were mounted in mounting medium
(Vector Laboratories, Inc.) and captured at magnifications ×25 and
×400 using a fluorescence microscope (Carl Zeiss Imager M1; Carl
Zeiss AG, Oberkochen, Germany) and confocal microscope (Carl Zeiss
observer Z1; Carl Zeiss AG).
Measurement of apoptotic cells
Apoptotic cells were identified using staining, with
a terminal deoxynucleotidyl transferase-mediated dUTP nick end
labeling (TUNEL) assay and TH. The TUNEL assay was performed using
a TUNEL assay kit (DeadEnd™ Fluorometric TUNEL System;
Promega Corporation, Madison, WI, USA) following the manufacturer's
protocol. Slides were mounted in mounting medium (Vector
Laboratories, Inc.). The number of TUNEL/TH-positive cells were
counted. Quantitative blind analysis was performed by counting the
number of apoptotic cells using a fluorescence microscope. Data are
presented as the mean number of apoptotic cells from all brain
tissue samples as counted in one field of the striatum at
magnification ×200.
Data analyses
All data are expressed as mean ± standard error of
the mean and analyzed using the Sigma statistical program version
11.2 (Systat Software Inc., San Jose, CA, USA). Data were analyzed
using one-way analysis of variance for repeated measures and
Tukey's post hoc test of least significant difference. P<0.05
was considered to indicate statistically significance.
Results
Effect of APZ on motor-function
behaviors
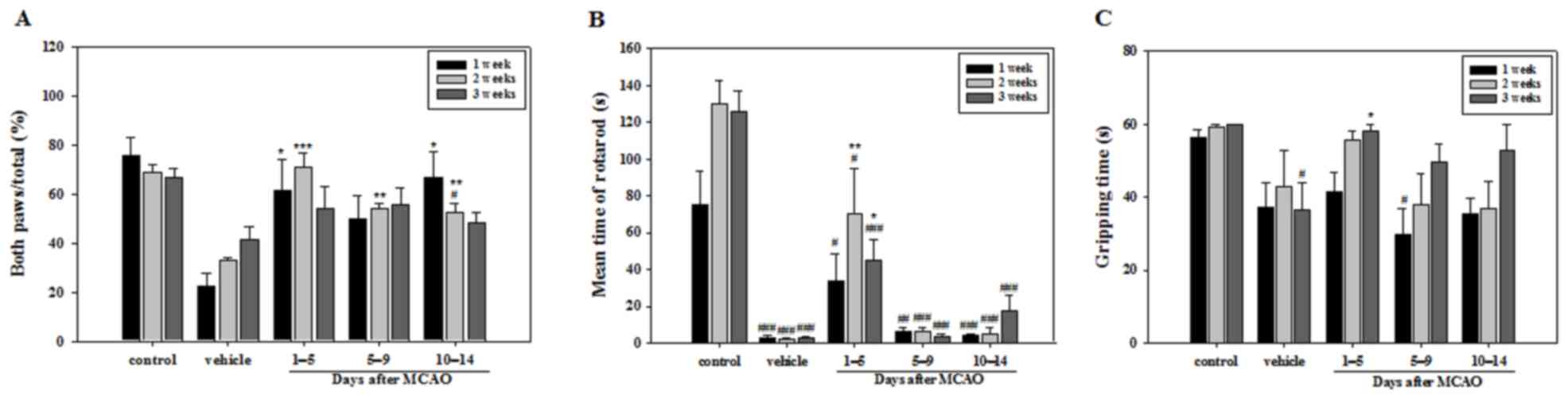
Various symptoms, including loss of balance and arm
weakness or numbness, were observed during behavioral experiments
conducted following MCAO. The number of times a mouse touched the
cylinder with both paws during the cylinder test was significantly
higher in all APZ-treated groups compared with the vehicle group at
2 weeks. However, no significant differences were observed between
any of the treatment groups and the vehicle group at 3 weeks. At
week 1 only the groups treated with APZ between 1–5 and 10–14 days
following MCAO demonstrated a significant difference compared with
the vehicle group (Fig. 1A). Mice
treated with APZ 1–5 days following MCAO attained a significantly
higher time in the rotarod test compared with the vehicle group at
2 and 3 weeks, indicating that immediate APZ administration
post-MCAO increases motor coordination and balance performance
(Fig. 1B). Gripping time in the wire
suspension test was also significantly increased at 3 weeks in the
group treated with APZ between 1–5 days following MCAO (Fig. 1C). These results suggest that
treatment with APZ reverses motor dysfunction and that APZ
treatment is most effective when administered immediately following
stroke induction.
Effect of APZ on atrophic changes and
dopaminergic neuronal injury in the brain
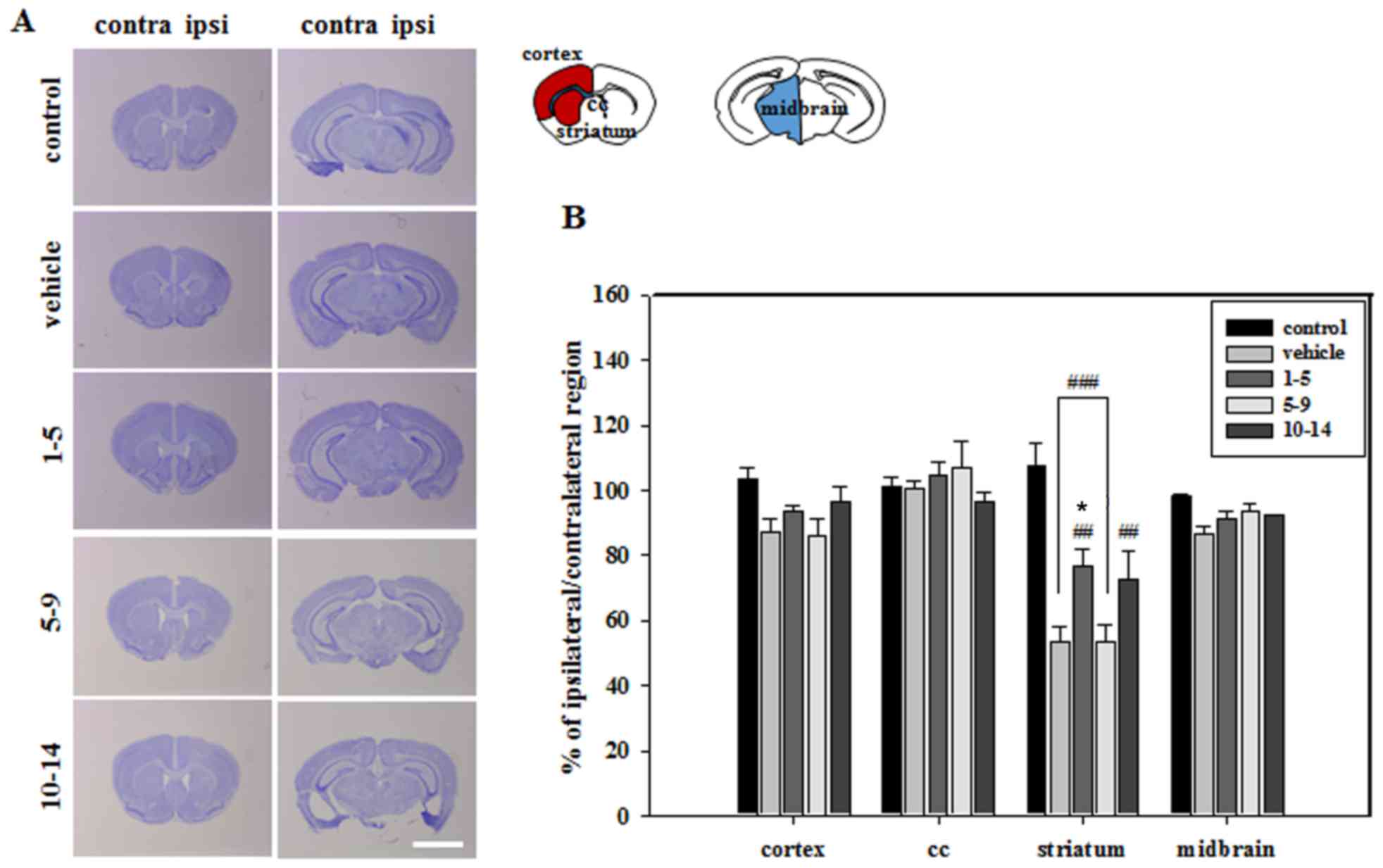
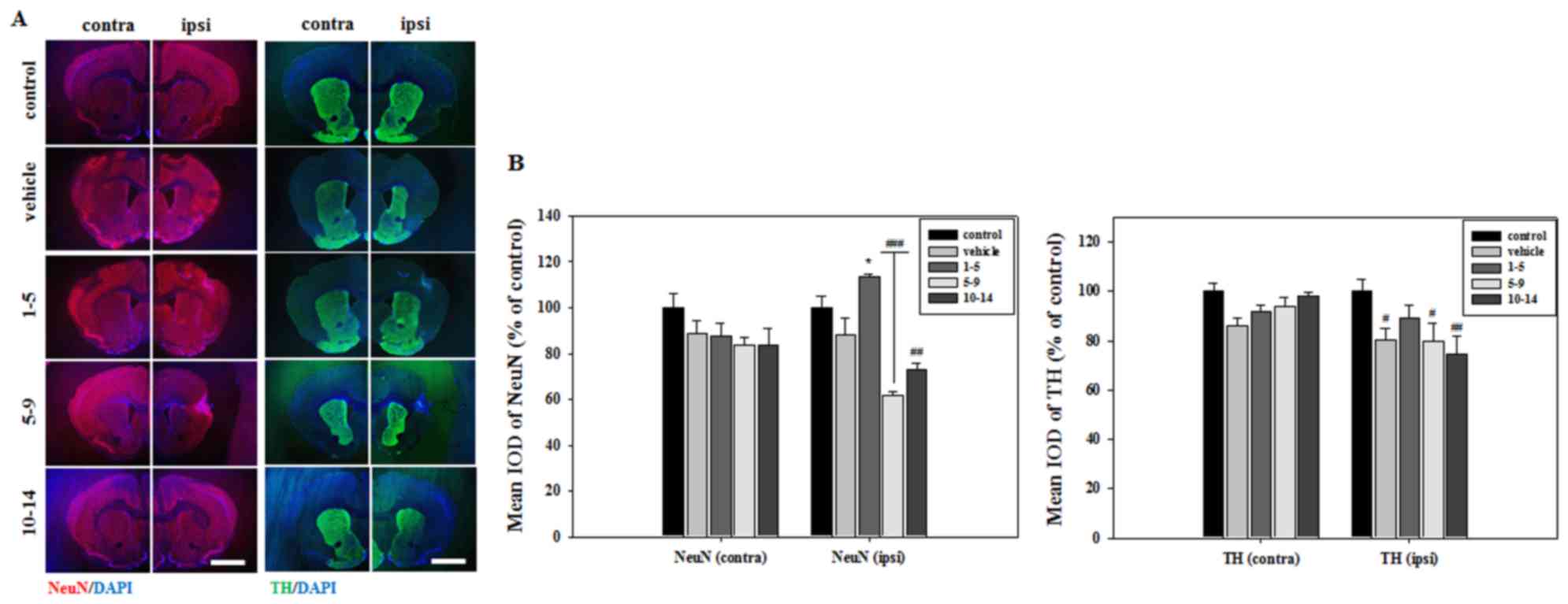
Histological analysis of brain sections revealed
that severe atrophic changes in the vehicle group only occurred in
the striatum, which was the primary lesion site of MCAO (Fig. 2). These atrophic changes were
countered by treating mice with APZ 1–5 days following MCAO
(Fig. 2). Brain sections were then
stained with NeuN to verify neuronal cell survival in the striatum.
The mean integrated optical density (IOD) of NeuN expression was
significantly increased in the striatum of mice treated with APZ
1–5 days following MCAO (Fig. 3).
APZ treatment also increased the number of TH-positive dopaminergic
cells in the striatum of the same group, although this increase was
not significant (Fig. 3). These
results indicate that APZ may have a neuroprotective effect on
dopaminergic neuronal cells, protecting them from damage caused by
ischemic assaults.
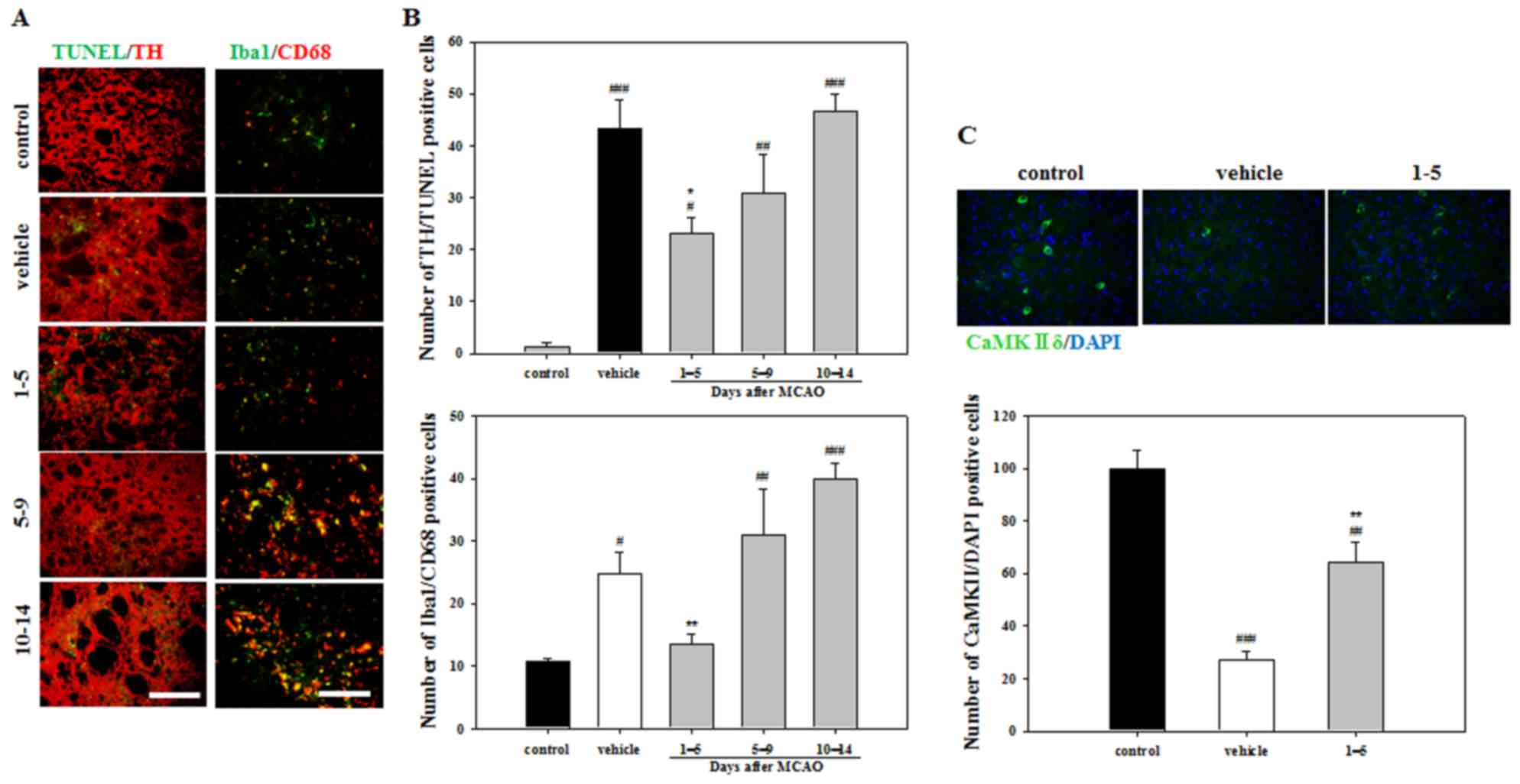
Effect of APZ on cell death and
activation of microglia
To evaluate apoptosis, TUNEL and TH double staining
was performed. Staining indicated that the vehicle group exhibited
a significantly increased number of apoptotic cells compared with
the control group (Fig. 4A and B).
However, significantly fewer TUNEL/TH-positive cells were detected
in the mice treated with APZ 1–5 days following MCAO compared with
the vehicle group (Fig. 4B). To
better understand the activation of microglia in damaged striatum,
Iba1 and CD68 double staining was performed. The number of
activated microglia exhibiting Iba1/CD68 double-positive expression
were decreased in mice treated with APZ 1–5 days following MCAO
compared with the vehicle group (Fig. 4A
and C). To investigate the neuroprotective effects of the
dopamine receptor, brain sections were stained using the CaMKIIδ
antibody, which regulates Ca2+-mediated neuronal
activities in the brain. The number of CaMKIIδ-positive cells in
the striatum was decreased following MCAO, but increased following
APZ treatment administered 1–5 days following MCAO (Fig. 4C). These results indicate that APZ
may reduce the dopaminergic neuronal cell death and microglial
activation caused by ischemic assaults in the striatum, while
enhancing CaMKIIδ expression.
Discussion
The present study evaluated the neuroprotective
effects of APZ, an atypical antipsychotic drug, in a mouse model of
ischemic stroke. The results indicated that APZ induces the
functional recovery of neurological deficit caused by ischemia and
reduces the atrophic changes in the striatum of the brain.
Additionally, APZ treatment reduced dopaminergic neuronal cell
injury and neuroinflammation in the striatum, while enhancing
CaMKIIδ expression, indicating that APZ enhances
neuroprotection.
Atypical antipsychotics are associated with a lower
risk of all-cause mortality and extrapyramidal symptoms. However,
certain atypical antipsychotics induce a higher risk of stroke
compared with conventional antipsychotics (18). In previous studies, APZ treatment has
been associated with a lower risk of cardiovascular morbidity and
mortality (19), while inducing
positive effects following multiple episodes of schizophrenia
(4,20,21).
Therefore, the present study hypothesized that APZ treatment
following ischemic assaults may enhance functional recovery via
neuroprotection.
APZ exhibits antidepressant effects, which makes it
particularly useful for treating complex post-stroke emotional
disorders (7,8). APZ has also been demonstrated to
recover dopaminergic neuronal cells that serve beneficial roles in
protecting against neurodegeneration (8). Additionally, certain antipsychotics
including APZ, may slow the neurodegenerative changes that occur in
patients with schizophrenia for whom such treatment may be useful
(13). Thus, APZ may enhance
functional recovery following stroke by protecting neuronal
cells.
The present study identified the effect of APZ on
behavioral function. Motor function test results were improved
following treatment with APZ, particularly when administered 1–5
days following MCAO. When stroke occurs, it causes brain atrophy
and the loss of brain cells (22).
The degradation of motor function and asymmetry may occur due to
the atrophic changes that occur in various regions of the brain.
Therefore, the atrophic changes occurring in the brain cortex,
corpus callosum, striatum and midbrain were analyzed in the present
study. Severe atrophic changes in the striatum, the primary lesion
site of MCAO, were alleviated with APZ treatment administered 1–5
days following MCAO. This result was similar to that of a previous
study, which demonstrated that APZ decreases striatal
kainate-induced lesion volumes in rodents (10).
APZ exerts antioxidant effects, meaning that it is
highly effective at preventing the cell death that occurs as a
result of oxidative stress (23). It
has also been demonstated that APZ treatment enhances neurite
extension and inhibits cell death in cultured dopaminergic neurons
(24). Another dopamine D2/D3
receptor agonist, pramipexole, exhibits protective effects against
post-ischemic damage (12). Dopamine
is an important neurotransmitter that maintains and controls
attention and body movement (11).
Therefore, APZ treatment may preserve the survival of dopaminergic
neurons. In the present study, the survival of dopaminergic neurons
in the striatum was analyzed. Many neuronal cells in the striatum
demonstrated NeuN and TH immunoreactions in APZ treated mice
compared with vehicle-treated mice, suggesting that APZ exerts a
strong protective effect on dopaminergic neuronal cells. However,
the results of D2R IOD did not vary with dopamine levels and its
variation was very small (data not shown).
Abundant apoptotic cells were detected in the
pre-infarction area of mice following ischemic stroke. However,
stroke-induced apoptosis was reduced during APZ treatment
administered 1–5 days following MCAO. The chronic stimulation of
dopamine D2R by APZ activates CaMKIIδ3, which regulates the
transcription of the neurotrophin brain-derived neurotrophic factor
(BDNF) by activating various nuclear proteins, including cyclic
adenosine 3′,5′-monophosphate response element-binding protein
(24). CaMKIIδ staining in
APZ-treated groups revealed that the number of
CaMKIIδ-immunopositive cells were increased compared with the
vehicle-treated group, indicating that the increase in BDNF
expression induced by CaMKIIδ is involved in the neuroprotective
effect of APZ.
Dopamine D2R agonists, including quinpirole and
ropinirole, regulate the inflammatory response by alleviating
microglia hyperactivity-induced neuroinflammation, thus attenuating
brain injury following intracerebral hemorrhage (11,25). It
has been demonstrated that DRD2−/− mice exhibit
pronounced microglial activation as part of the inflammatory
response that occurs in Parkinson's disease (26). Cerebral ischemia induces the
expression of dopamine D2R on activated resident microglia in the
brain, which is thought to modulate microglia function during
neuroinflammation (27). APZ induces
anti-inflammatory effects that occur as following the inhibition of
microglial activation (28).
Therefore, CD68 and Iba1 double-staining was performed in the
present study to evaluate the neuroinflammatory response following
treatment with APZ. APZ treatment reduced Iba1/CD68 double-positive
cell numbers, indicating that microglial activation was inhibited
following stroke.
In conclusion, the present study demonstrated that
treatment of ischemic mice with APZ ameliorated various behavioral
characteristics of motor dysfunction by inhibiting dopaminergic
neuronal cell injury and neuroinflammation. This neuroprotective
effect may occur via dopamine D2R stimulation, which may in turn,
activate CaMKII. Further studies are required to confirm this
hypothesis; other potential mechanisms of APZ action, which may
involve agonist and antagonistic activity at serotonin receptors
were not assessed. However, the results of the present study
provide evidence of APZ-mediated neuroprotection and a novel
therapeutic insight into the overall pathogenesis of ischemic
stroke.
Acknowledgements
The present study was supported by the National
Research Foundation of Korea and funded by the Korean government
(grant no. 2014R1A5A2009936).
References
|
1
|
Shapiro DA, Renock S, Arrington E, Chiodo
LA, Liu LX, Sibley DR, Roth BL and Mailman R: Aripiprazole, a novel
atypical antipsychotic drug with a unique and robust pharmacology.
Neuropsychopharmacology. 28:1400–1411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Greenaway M and Elbe D: Focus on
aripiprazole: A review of its use in child and adolescent
psychiatry. J Can Acad Child Adolesc Psychiatry. 18:250–260.
2009.PubMed/NCBI
|
|
3
|
Russo E, Citraro R, Davoli A, Gallelli L,
Di Paola ED and De Sarro G: Ameliorating effects of aripiprazole on
cognitive functions and depressive-like behavior in a genetic rat
model of absence epilepsy and mild-depression comorbidity.
Neuropharmacology. 64:371–379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burda K, Czubak A, Kus K, Nowakowska E,
Ratajczak P and Zin J: Influence of aripiprazole on the
antidepressant, anxiolytic and cognitive functions of rats.
Pharmacol Rep. 63:898–907. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kronenberg G, Gertz K, Heinz A and Endres
M: Of mice and men: Modelling post-stroke depression
experimentally. Br J Pharmacol. 171:4673–4689. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Loubinoux I, Kronenberg G, Endres M,
Schumann-Bard P, Freret T, Filipkowski RK, Kaczmarek L and
Popa-Wagner A: Post-stroke depression: Mechanisms, translation and
therapy. J Cell Mol Med. 16:1961–1969. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shimoda K and Kimura M: Two cases of
emotional disorder after middle cerebral artery infarction showing
distinct responses to antidepressant treatment. Neuropsychiatr Dis
Treat. 10:965–970. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim YR, Kim HN, Pak ME, Ahn SM, Hong KH,
Shin HK and Choi BT: Studies on the animal model of post-stroke
depression and application of antipsychotic aripiprazole. Behav
Brain Res. 287:294–303. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim YR, Kim HN, Hong KW, Shin HK and Choi
BT: Antidepressant effects of aripiprazole augmentation for
cilostazol-treated mice exposed to chronic mild stress after
ischemic stroke. Int J Mol Sci. 18:pii: E3552017. View Article : Google Scholar
|
|
10
|
Cosi C, Waget A, Rollet K, Tesori V and
Newman-Tancredi A: Clozapine, ziprasidone and aripiprazole but not
haloperidol protect against kainic acid-induced lesion of the
striatum in mice, in vivo: Role of 5-HT1A receptor activation.
Brain Res. 1043:32–41. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Chen Y, Wu J, Manaenko A, Yang P,
Tang J, Fu W and Zhang JH: Activation of dopamine D2 receptor
suppresses neuroinflammation through αB-crystalline by inhibition
of NF-κB nuclear translocation in experimental ICH mice model.
Stroke. 46:2637–2646. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hall ED, Andrus PK, Oostveen JA, Althaus
JS and VonVoigtlander PF: Neuroprotective effects of the dopamine
D2/D3 agonist pramipexole against postischemic or
methamphetamine-induced degeneration of nigrostriatal neurons.
Brain Res. 742:80–88. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koprivica V, Regardie K, Wolff C, Fernalld
R, Murphy JJ, Kambayashi J, Kikuchi T and Jordan S: Aripiprazole
protects cortical neurons from glutamate toxicity. Eur J Pharmacol.
651:73–76. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zaleska MM, Mercado ML, Chavez J,
Feuerstein GZ, Pangalos MN and Wood A: The development of stroke
therapeutics: Promising mechanisms and translational challenges.
Neuropharmacology. 56:329–341. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hua Y, Schallert T, Keep RF, Wu J, Hoff JT
and Xi G: Behavioral tests after intracerebral hemorrhage in the
rat. Stroke. 33:2478–2484. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patil SP, Jain PD, Sancheti JS, Ghumatkar
PJ, Tambe R and Sathaye S: Neuroprotective and neurotrophic effects
of Apigenin and Luteolin in MPTP induced parkinsonism in mice.
Neuropharmacology. 86:192–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Taleb O, Bouzobra F, Tekin-Pala H, Meyer
L, Mensah-Nyagan AG and Patte-Mensah C: Behavioral and
electromyographic assessment of oxaliplatin-induced motor
dysfunctions: Evidence for a therapeutic effect of
allopregnanolone. Behav Brain Res. 320:440–449. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Farlow MR and Shamliyan TA: Benefits and
harms of atypical antipsychotics for agitation in adults with
dementia. Eur Neuropsychopharmacol. 27:217–231. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kasteng F, Eriksson J, Sennfält K and
Lindgren P: Metabolic effects and cost-effectiveness of
aripiprazole versus olanzapine in schizophrenia and bipolar
disorder. Acta Psychiatr Scand. 124:214–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Citrome L, Kalsekar I, Baker RA and Hebden
T: A review of real-world data on the effects of aripiprazole on
weight and metabolic outcomes in adults. Curr Med Res Opin.
30:1629–1641. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khanna P, Suo T, Komossa K, Ma H,
Rummel-Kluge C, El-Sayeh HG, Leucht S and Xia J: Aripiprazole
versus other atypical antipsychotics for schizophrenia. Cochrane
Database Syst Rev: CD006569. 2014. View Article : Google Scholar
|
|
22
|
Zhu H, Zhang Y, Shi Z, Lu D, Li T, Ding Y,
Ruan Y and Xu A: The neuroprotection of liraglutide against
ischaemia-induced apoptosis through the activation of the PI3K/AKT
and MAPK pathways. Sci Rep. 6:268592016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park SW, Lee CH, Lee JG, Kim LW, Shin BS,
Lee BJ and Kim YH: Protective effects of atypical antipsychotic
drugs against MPP(+)-induced oxidative stress in PC12 cells.
Neurosci Res. 69:283–290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shioda N, Sawai M, Ishizuka Y, Shirao T
and Fukunaga K: Nuclear translocation of
calcium/calmodulin-dependent protein kinase IIδ3 promoted by
protein phosphatase-1 enhances brain-derived neurotrophic factor in
dopaminergic neurons. J Biol Chem. 290:21663–21675. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Farber K, Pannasch U and Kettenmann H:
Dopamine and noradrenaline control distinct functions in rodent
microglial cells. Mol Cell Neurosci. 29:128–138. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shao W, Zhang SZ, Tang M, Zhang XH, Zhou
Z, Yin YQ, Zhou QB, Huang YY, Liu YJ, Wawrousek E, et al:
Suppression of neuroinflammation by astrocytic dopamine D2
receptors via αB-crystallin. Nature. 494:90–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huck JH, Freyer D, Böttcher C, Mladinov M,
Muselmann-Genschow C, Thielke M, Gladow N, Bloomquist D,
Mergenthaler P and Priller J: De novo expression of dopamine D2
receptors on microglia after stroke. J Cereb Blood Flow Metab.
35:1804–1811. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kato T, Mizoguchi Y, Monji A, Horikawa H,
Suzuki SO, Seki Y, Iwaki T, Hashioka S and Kanba S: Inhibitory
effects of aripiprazole on interferon-gamma-induced microglial
activation via intracellular Ca2+ regulation in vitro. J Neurochem.
106:815–825. 2008. View Article : Google Scholar : PubMed/NCBI
|