Introduction
Stroke is a severe health issue worldwide. Available
treatments are limited and it is currently the second leading cause
of death (1). Prompt thrombolytic
therapy is critical for improving the clinical prognosis of
patients with acute ischemic stroke (AIS). The collateral
circulation is a major factor affecting the effect of thrombolytic
therapy and good collateral circulation significantly influences
the prognosis of patients, thereby preventing and delaying
permanent neurological damage (2).
The collateral circulation maintains the blood supply to the
infarction area prior to recanalization after AIS (3). It also prevents the expansion of the
infarct size, provides a better clinical prognosis, increases the
rate of recanalization and potentially prolongs the time window
prior to the requirement of endovascular treatment (ET) (4–7). In
addition, good collateral circulation reduces the risk of
hemorrhagic transformation (HT) and mortality after thrombolysis
therapy (8).
A previous systematic review by Leng et al
(9) analyzed the available evidence
on the correlations between collateral circulation and the outcomes
in patients with AIS following intravenous thrombolysis therapy
(IVT). However, it did not include studies in which endovascular
treatment was used. In the present study, the relevant literature
published until November 2016 was systematically reviewed and a
meta-analysis was performed to evaluate the association between
collateral circulation determined prior to thrombolytic treatment
and outcomes.
Materials and methods
Reporting and definitions
The relevant studies were reported according to the
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
and the Meta-Analysis of Observational Studies in Epidemiology
(10,11). The definition of good or poor
collateral circulation status prior to treatment was in accordance
with that in the primary studies; for studies classifying the
collateral status in >2 categories, it was categorized as good
and poor in the present analysis by adopting the dichotomization
methods from other primary studies using the same imaging modality
to gauge the collateral status. The outcomes, including good
recanalization/reperfusion, HT, final infarct size, mortality and
the favorable functional outcome at 3–6 months, were defined in the
studies.
Information sources
Potentially eligible studies as full-text articles,
published from January 2000 to November 2016, were identified
through a search of the Cochrane Library, Ovid, Embase, Medline and
PubMed databases. The search was restricted to articles published
in English language. In brief, the search terms included ‘stroke’,
‘collateral’, ‘thrombolysis’, ‘thromboly’ and ‘endovascular’. A
manual search was also performed by checking the references of
pertinent review articles and relevant original research
articles.
Study selection and eligibility
criteria
The criteria for the included studies were as
follows: i) Case-control, cohort or randomized controlled trial
(RCT) studies on patients (>18 years of age) with acute ischemic
stroke; ii) collateral circulation status was evaluated prior to
the initiation of thrombolysis treatment; iii) The correlation
between pre-treatment collateral circulation status and outcome in
patients with AIS was described. Animal studies, non-RCTs and
duplicate reports were excluded.
Data collection and parameters
All titles and abstracts were initially screened by
one investigator to identify potentially relevant studies for
inclusion. Relevant studies were retrieved in full text and
reassessed by two researchers to determine the eligibility for
inclusion with regard to publication characteristics, study
populations, patient demographics, onset-to-treatment time, mode of
thrombolysis therapy, methods to assess the collateral circulation
status, methods to define successful reperfusion and/or
recanalization and definition of HT.
Statistical analysis
Cochrane RevMan (version 5.3; The Cochrane
Institute, London, UK) was used for analyzing the data. The impact
of good vs. poor pre-treatment collateral circulation on the
outcomes was evaluated by a fixed-effects model if the
heterogeneity was low or the random-effects model with the results
expressed as the risk ratio (RR) and 95% confidence interval (CI).
For the clinical or imaging outcomes, subgroup analyses were
performed with stratification by different sample sizes, prescribed
durations of thrombolysis treatment, median baseline National
Institutes of Health Stroke Scale (NIHSS), treatment type and mean
(or median) age. To assess the publication bias, a visual
inspection of the funnel plot was applied in the analysis of any
assessed variable.
Inter-study and -subgroup heterogeneities were
evaluated by χ2 and І2 statistics (P<0.10
and І2 >50% was considered to indicate significant
heterogeneity). A sensitivity analysis was also performed by
removing individual trials from the meta-analysis.
Results
Study selection and
characteristics
The selection of the studies identified through the
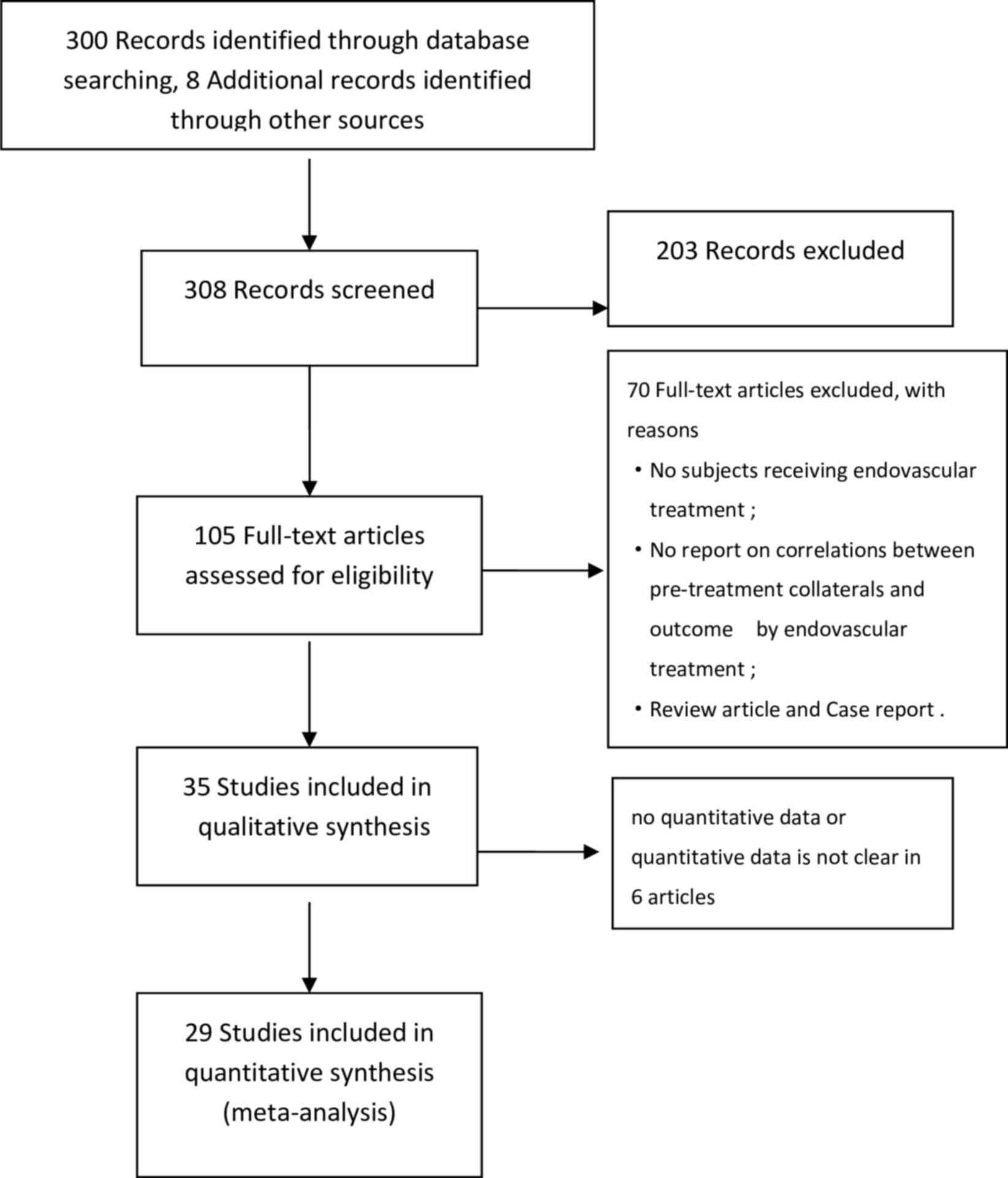
literature search was schematically represented in a flowchart
illustrated in Fig. 1. Of the 308
records retrieved from several databases and manual searches, 29
primary studies comprising 4,053 subjects were included in the
systematic review, of which 12 were retrospective studies, 17 were
prospective studies, 7 were multicenter studies and 22 were
single-center studies. A total of 9 studies described the treatment
with IVT alone, and in the other 20 studies, patients received IVT
with subsequent ET or ET only (Table
I) (7,8,12–38). All
of the studies used tissue plasminogen activator for the
thrombolysis treatment, while that by Christoforidis et al
(7) used tissue plasminogen
activator, urokinase and pro-urokinase. The duration of
thrombolysis treatment was 3–6 h in 12 studies and >6 h in 5
studies, extending up to 12 h. However, the time window was not
prescribed in 6 of the studies. The assessment of the pre-treatment
collateral circulation status was performed using different imaging
methods, which were computed tomography (CT) angiography in 22
studies (7,8,12,14,15,17,18,22–35,37),
while others included digital subtraction angiography (13,36),
fluid-attenuated inversion recovery imaging (19,21), and
CT or magnetic resonance perfusion imaging (16,20,38)
(Table I).
 | Table I.Summary of the characteristics of the
primary studies. |
Table I.
Summary of the characteristics of the
primary studies.
|
| Collateral
circulation grading |
|
|
|---|
|
|
|
|
|
|---|
|
| Dichotomized
collateral status |
|
|
|---|
|
|
|
|
|
|---|
| First author | Country | Year | Sample size
(n) | Thrombolysis
type | Mean age
(years) | Median NIHSS at
baseline | Duration of IVT
(h) | Mean interval from
onset to treatment (min) | Imaging
modality | Grading method | Good | Poor | Study
qualitya | (Refs.) |
|---|
| García-Tornel | Canada | 2016 | 108 | IVT, ET | 70 | 17 | 8 | 215 | CTA | University of
Calgary collateral circulation scale (0–5) | 4–5 | 0–1 | A | (12) |
| Angermaier | Germany | 2011 | 25 | ET | 67 | 14 | 6 | 244 | CTA | LCC graded on a
4-point scale (0–3) | 2–3 | 0–1 | A | (13) |
| Bang | USA/Korea | 2011 | 222 | ET | 65 | 16 | UNK | UNK | DSA | ASITN/SIR
collateral circulation graded on a 5-point scale (0–4) | 2-4; | 0–1 | A | (8) |
| Berkhemer | Netherlands | 2016 | 231 | ET | UNK | UNK | 6 | UNK | CTA | LCC graded on a
4-point scale (0–3) | 2–3 | 0–1 | A | (14) |
| Brunner | Germany | 2014 | 246 | IVT | 74 | 14 | 3 or 4.5 | 160 | CTA | LCC graded on a
4-point scale (0–3) | 2–3 | 0–1 | A | (15) |
| Calleja | Spain | 2013 | 54 | IVT | 73 | 10 | 4.5 or >4.5
based on imaging | 237 | CTP | LCC graded on a
4-point scale (0–3) | 2–3 | 0–1 | A | (16) |
| Christoforidis | USA | 2008 | 104 | ET | 68 | 16 | 12 | 285 | DSA | LCC graded on a
5-point scale (1–5) | 1–2 | 3–5 | A | (7) |
| Fanou | Canada | 2015 | 395 | IVT, ET | 72 | 14 | 4.5 | 147 | CTA | LCC graded on a
4-point scale (0–3) | 2–3 | 0–1 | A | (17) |
| Gerber | Germany | 2016 | 93 | IVT, ET | 69 | 17 | UNK | 252 | CTA | LCC graded on a
4-point scale (0–3) | 2–3 | 0–1 | A | (18) |
| Kufner | Germany | 2015 | 62 | IVT | 71 | 11 | 4.5 | UNK | FLAIR MRI | Number of sections
of FLAIR with hyperintense vessels | ≤4 | >4 | A | (19) |
| Lee | Korea | 2000 | 17 | IVT | 63 | 15 | 3 or 7 | UNK | CTP | Percentage of
severe Vdeficit in MCA territory | ≤erc | 33–50% | A | (20) |
| Lee | USA | 2009 | 52 | IVT | 69 | 8 | 3 | UNK | FLAIR MRI | Distal hyperintense
vessels (none/subtle/prominent) |
Prominentb | Noneb | A | (21) |
| Lima | USA | 2010 | 196 | IVT, ET | 69 | 13 | UNK | UNK | CTA | LCC graded on a
5-point scale (1–5) | UNK | UNK | A | (22) |
| Menon | North America,
Australia, Europe | 2015 | 185 | IVT, ET | UNK | UNK | 3 | UNK | CTA | LCC in ACA-MCA and
PCA-MCA regions (0–10) | 6–10 | 0–5 | A | (23) |
| Marks | USA | 2014 | 60 | IVT, ET | 64 | 19 | 12 | 360 | MRI | Collateral Flow
Grading System with a 4-point scale (0–4) | 3–4 | 0–2 | A | (24) |
| Miteff | Australia | 2009 | 92 | IVT, ET | 74 | 17 | 6 | UNK | CTA | LCC graded into 3
categories (good, moderate, poor) | Good | Moderate, poor | A | (25) |
| Nambiar | Canada | 2013 | 84 | IVT, ET | 65 | 14 | 3 for IVT | UNK | CTA | rLMC score based on
ASPECTS | rLMC score
(11–20) | rLMC score
(0–10) | A | (26) |
| Ramaiah | Australia | 2013 | 87 | IVT, ET | 66 | 18 | UNK | 329 | CTA | LCC graded on a
4-point scale (0–3) | 3 | 0–2 | A | (27) |
| Saarinen | Finland | 2014 | 105 | IVT | 69 | 13 | 3 | 132 | CTA | LCC graded on a
5-point scale (0–4) | 2–4 | 0–1 | A | (28) |
| Sallustio | Italy | 2016 | 135 | IVT, ET | 69 | UNK | UNK | UNK | CTA | LCC graded on a
4-point scale (0–3) | 2–3 | 0–1 | A | (29) |
| Mangiafico | Italy | 2014 | 103 | ET | 61 | 20 | 3–6 | 270 | CTA | Careggi collateral
score 6 categories (0–5) | 2–5 | 0–1 | A | (30) |
| van Seeters | Netherlands | 2016 | 484 | IVT, ET | 66 | 13 | 9 | 361 | CTA | Collateral filling
of Vterritory of the affected MCA or MCA branch territory | ≥ran | <50% | A | (31) |
| Sheth | USA | 2016 | 117 | IVT, ET | UNK | UNK | UNK | UNK | DSA | ASITN/SIR
collateral circulation graded on a 5-point scale (0–4) | 3–4 | 0–2 | A | (32) |
| Shin | Korea | 2014 | 43 | ET | UNK | UNK | 6 | UNK | CTA | Graded on CTA and
delayed contrast CECT axial MIP imaging on a 4-point scale | 2–3 | 0–1 | A | (33) |
| Souza | USA | 2012 | 197 | IVT, ET | 67 | 15 | 9 | UNK | CTA | LCC graded on a
4-point scale (0–3) | 1–3 | 0 | A | (34) |
| Sung | Korea | 2014 | 30 | IVT, ET | 61 | 16 | 8 | 324 | DSA | LCC graded on a
5-point scale (0–4) | UNK | UNK | A | (35) |
| Yeo | Singapore | 2015 | 200 | IVT | 63 | 19 | UNK | 155 | CTA | LCC by four
different scores (i.e., the Miteff system, scores 1–3) | 2–3 | 1 | A | (36) |
| Zhang | China | 2016 | 80 | IVT | 68 | 13 | 6 | 195 | CTA | rLMC score (rLMC-P
and rLMC-M) | rLMC-P >11
rLMC-M>16 | rLMC-P ≤11 rLMC-M
≤MM | A | (37) |
| Zhang | China | 2016 | 66 | IVT | UNK | UNK | 6 | UNK | PWI | ATD was defined as
the velocity of collateral flow | ATD <2.3
sec | ATD ≥TD sec | A | (38) |
Favorable functional outcome
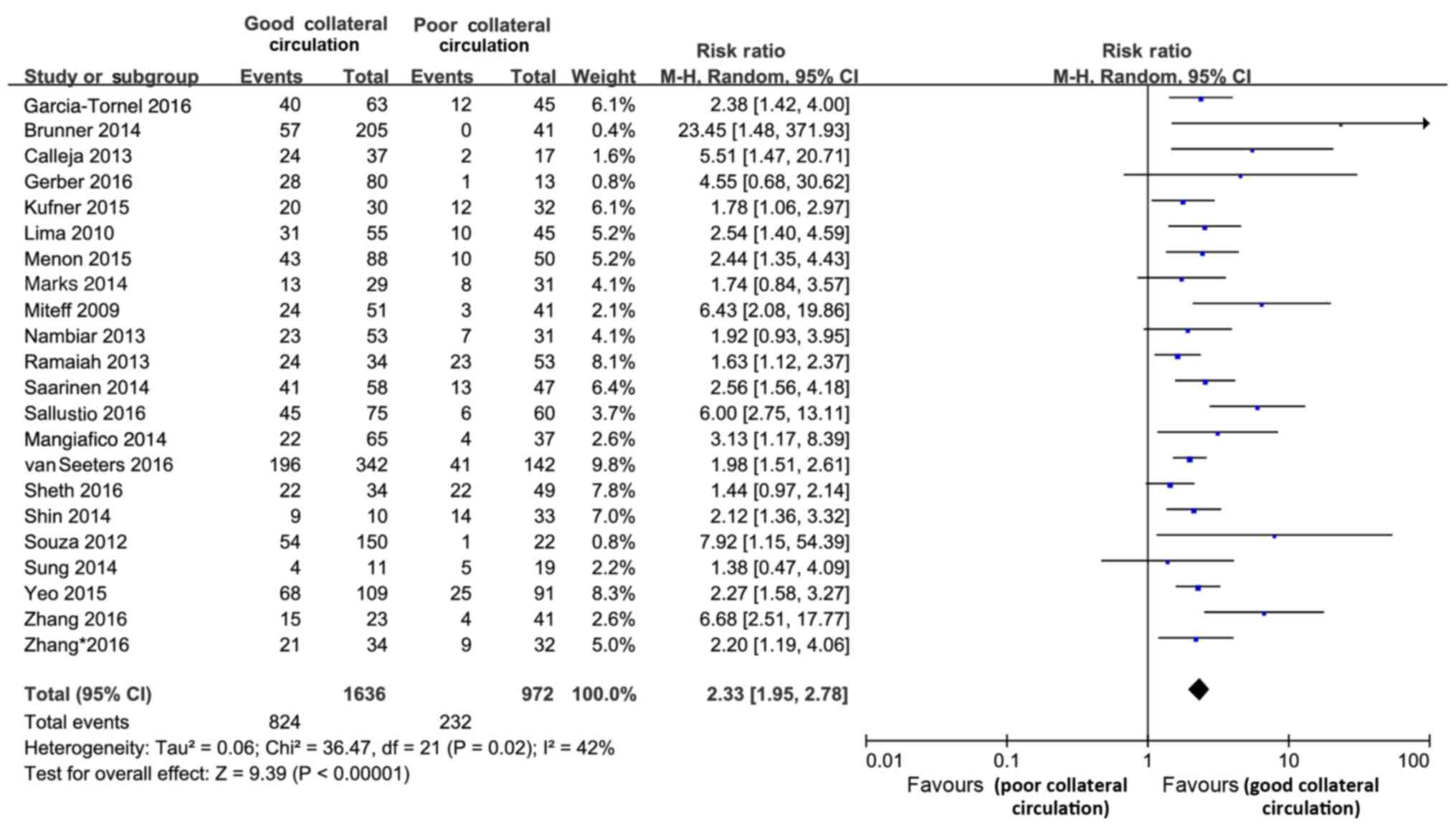
The present review included 22 studies (comprising
2,608 subjects) that reported on functional outcome. The rate of
favorable functional outcome [modified Rankin scale (mRS) 0–3 at
3–6 months] was doubled in the GC statue group as compared with
that in the PC group (RR=2.33; 95% CI, 1.95–2.78; P<0.001;
Fig. 2). This effect of good
collateral circulation did not differ significantly between the
studies (Cochran's Q=36.47; P=0.02; І2=42%). In the
subgroup analyses presented in Table
II, the beneficial effect of good collateral circulation did
not differ significantly between the subgroups of studies
stratified by a median NIHSS at baseline of ≤ or >13 (Cochran's
Q=0.48; P=0.49; І2=0%); a sample size of < or ≥100
subjects (Cochran's Q=1.56; P=0.21; І2=35.8%); a mean
(or median) age of < or ≥70 years (Cochran's Q=1.94; P=0.16;
І2=48.6%); treatment type (IVT alone, IVT+ET or ET
alone; Cochran's Q=0.75; P=0.69; І2=0%); or duration of
IVT treatment (4.5, 4.5–6 h or >6 h; Cochran's Q=2.46; P=0.29;
І2=18.6%).
 | Table II.Subgroup analyses for favorable
functional outcome at 3–6 months. |
Table II.
Subgroup analyses for favorable
functional outcome at 3–6 months.
|
|
|
|
| Inter-study
heterogeneity | Inter-subgroup
heterogeneity |
|---|
|
|
|
|
|
|
|
|---|
| Subgroup | Number of
studies | Number of
subjects | RR (95% CI) | Cochran's Q
statistics | P-value | І2
statistics (%) | Cochran's Q
statistics | P-value | І2
statistics (%) |
|---|
| Mean (or median)
age (years) | 19 | 2,413 | 2.68
(2.33,3.09) | 37.58 | 0.004 | 52 | 1.94 | 0.16 | 48.6 |
|
≥70 | 5 | 562 | 3.38
(2.35,4.87) |
|
|
|
|
|
|
|
<70 | 14 | 1,851 | 2.55
(2.19,2.97) |
|
|
|
|
|
|
| Median (or mean)
baseline NIHSS | 17 | 2,143 | 2.45
(2.12,2.84) | 23.93 | 0.09 | 33 | 0.48 | 0.49 | 0 |
|
≥13 | 11 | 1,274 | 2.59
(2.09,3.20) |
|
|
|
|
|
|
|
<13 | 6 | 869 | 2.33
(1.91,2.84) |
|
|
|
|
|
|
| Sample size | 22 | 2,608 | 2.46
(2.16,2.79) | 36.47 | 0.02 | 42 | 1.56 | 0.21 | 35.8 |
|
≥100% | 10 | 1,790 | 2.61
(2.20,3.08) |
|
|
|
|
|
|
|
<100 | 12 | 818 | 2.22
(1.83,2.68) |
|
|
|
|
|
|
| Prescribed duration
of treatment (h) | 15 | 1,856 | 2.32
(1.92,2.80) | 18.03 | 0.21 | 22 | 2.46 | 0.29 | 18.6 |
|
≤4.5 | 5 | 635 | 2.24
(1.61,3.12) |
|
|
|
|
|
|
|
4.5–6 | 5 | 367 | 3.18
(1.90,5.35) |
|
|
|
|
|
|
|
>6 | 5 | 854 | 2.03
(1.63,2.54) |
|
|
|
|
|
|
| Treatment type | 22 | 2,608 | 2.33
(1.95,2.78) | 36.47 | 0.02 | 42 | 0.75 | 0.69 | 0 |
| IVT
alone | 7 | 797 | 2.67
(1.87,3.80) |
|
|
|
|
|
|
| IVT +
ET | 13 | 1,666 | 2.22
(1.75,2.81) |
|
|
|
|
|
|
| ET
alone | 2 | 145 | 2.27
(1.51,3.41) |
|
|
|
|
|
|
HT
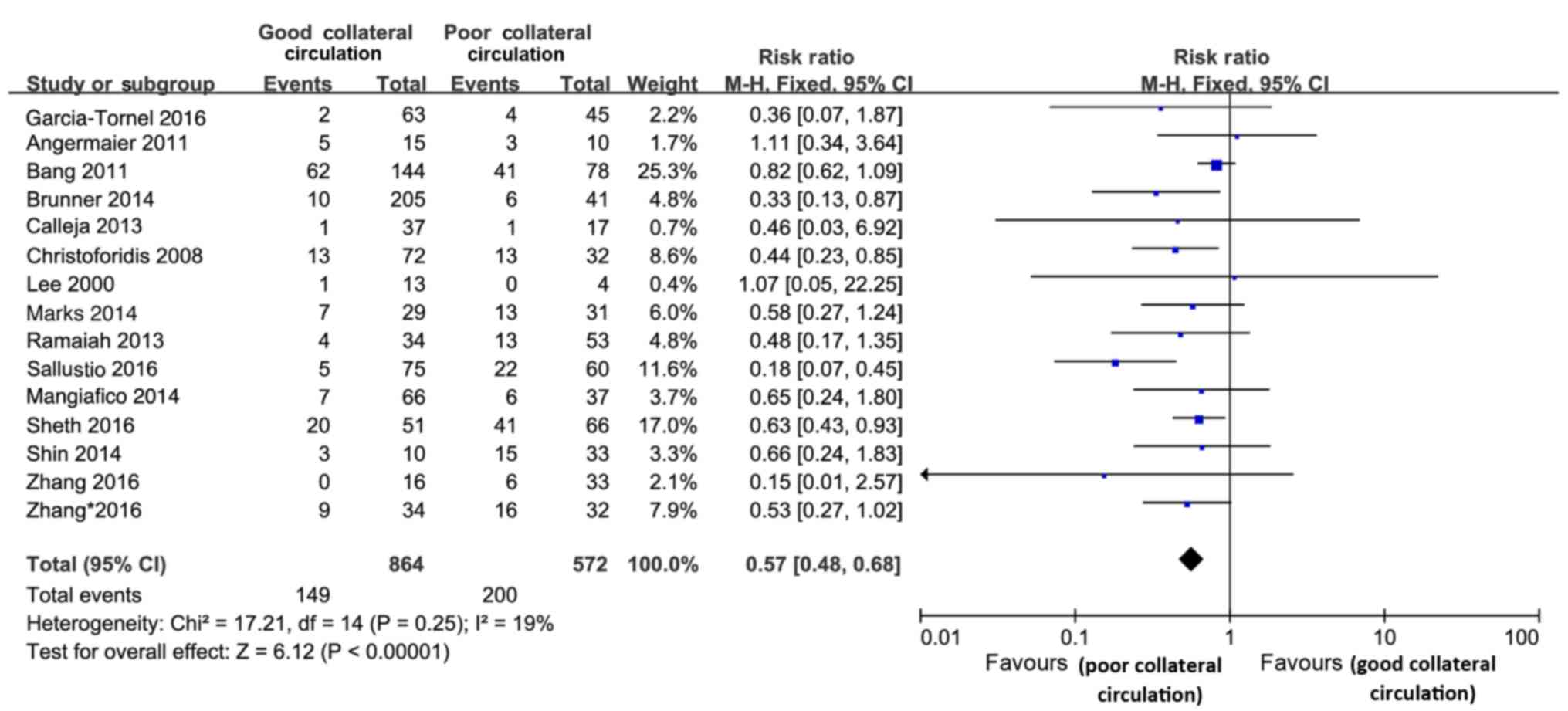
In 8 of the studies (12,13,15,29,30,33,36,37), HT
was defined as symptomatic intracranial hemorrhage in cases with a
4-fold increase in the NIHSS score (ECASS II study) (39), while it was defined differently in 3
studies (7,16,24);
however, the definition was unclear in the 4 remaining studies
(8,20,27,32). In
a total of 1,436 subjects, HT was evaluated from any follow-up CT
or magnetic resonance imaging performed within 24 h after treatment
until the time-point of discharge. The risk of HT was decreased in
the pretreatment good collateral circulation group (RR=0.57; 95%
CI, 0.48–0.68; P<0.001; Fig. 3),
and no significant heterogeneity was observed between studies in
the analysis of HT (Cochran's Q=17.21, P=0.25; І2=19%)
(Fig. 3). Subgroup analyses were
performed to assess the outcomes of the different treatment types
in patients with good and poor collateral circulation (Table III). The most significant effect
was observed in patients administered IVT treatment alone
(Cochran's Q=6.63; P=0.04; І2=69.8%). The RRs of good vs. poor
collateral status for HT were 0.43, 0.47 and 0.73 for studies with
treatment types IVT alone, IVT and ET and ET alone,
respectively.
 | Table III.Subgroup analyses for the outcomes of
different treatment types in patients with good vs. poor collateral
circulation. |
Table III.
Subgroup analyses for the outcomes of
different treatment types in patients with good vs. poor collateral
circulation.
|
|
|
|
| Inter-study
heterogeneity | Inter-subgroup
heterogeneity |
|---|
|
|
|
|
|
|
|
|---|
| Outcome | Number of
studies | Number of
subjects | RR (95% CI) | Cochran's Q
statistics | P-value | І2
statistics (%) | Cochran's Q
statistics | P-value | І2
statistics (%) |
|---|
| HT | 15 | 1,436 | 0.57 (0.48,
0.68) | 17.21 | 0.25 | 19 | 6.63 | 0.04 | 69.8 |
| IVT
alone | 5 | 432 | 0.43 (0.25,
0.72) |
|
|
|
|
|
|
| IVT +
ET | 5 | 507 | 0.47 (0.34,
0.63) |
|
|
|
|
|
|
| ET
alone | 5 | 497 | 0.73 (0.57,
0.93) |
|
|
|
|
|
|
| Mortality | 9 | 1,108 | 0.29 (0.22,
0.37) | 16.41 | 0.04 | 51 | 3.66 | 0.16 | 45.4 |
| IVT
alone | 2 | 310 | 0.24 (0.14,
0.42) |
|
|
|
|
|
|
| IVT +
ET | 6 | 696 | 0.27 (0.20,
0.36) |
|
|
|
|
|
|
| ET
alone | 1 | 103 | 0.53 (0.27,
1.03) |
|
|
|
|
|
|
| Recanalization | 13 | 1,265 | 1.48 (1.31,
1.68) | 20.33 | 0.06 | 41 | 0.63 | 0.73 | 0 |
| IVT
alone | 1 | 54 | 2.02 (0.92,
4.42) |
|
|
|
|
|
|
| IVT +
ET | 9 | 1,041 | 1.47 (1.29,
1.67) |
|
|
|
|
|
|
| ET
alone | 3 | 170 | 1.46 (0.94,
2.28) |
|
|
|
|
|
|
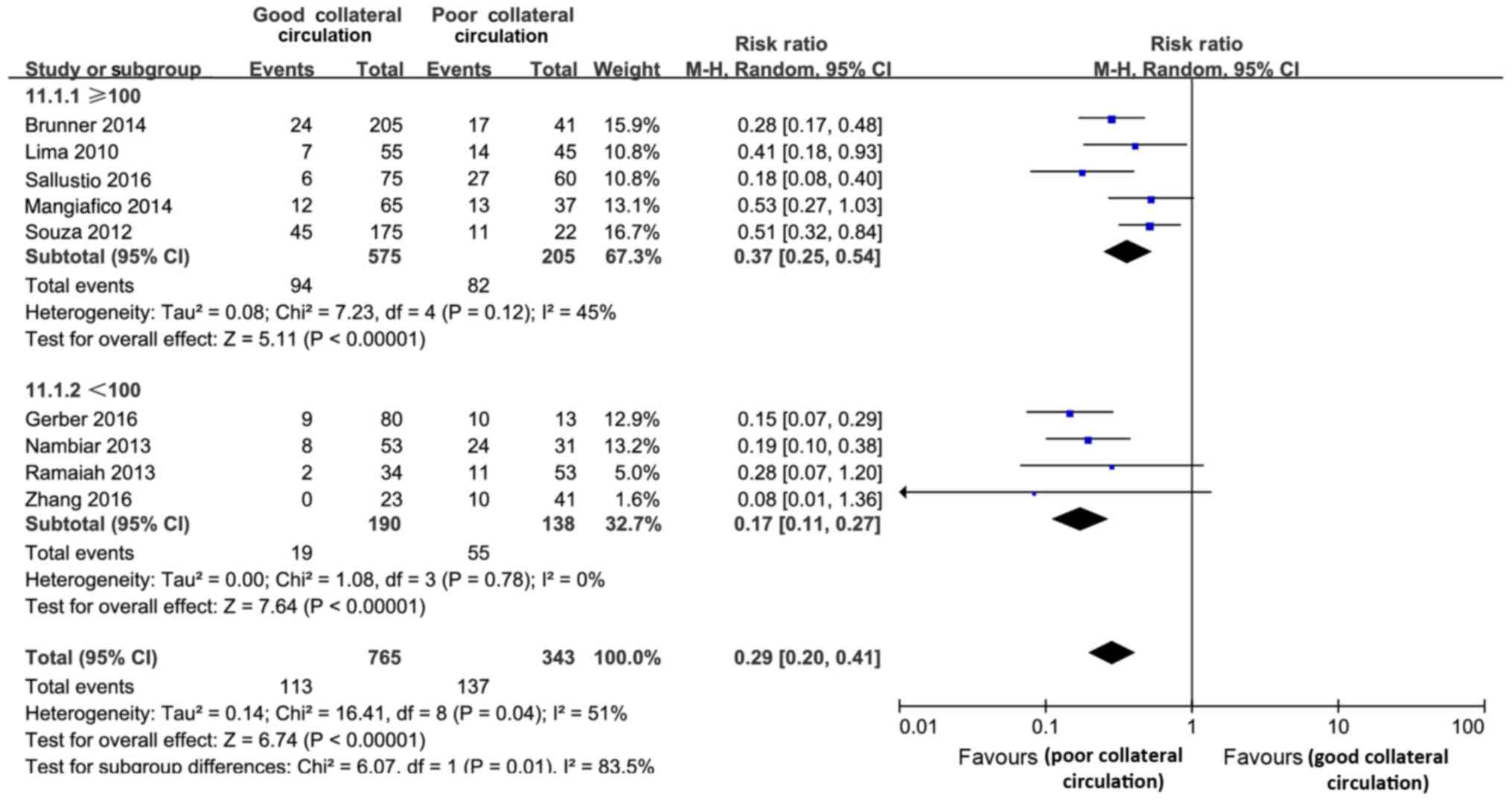
Mortality
The present review included 9 studies (1,108
subjects) that assed mortality. Compared with the PC group, a good
collateral circulation status significantly lowered the risk of
mortality by thrombolysis treatment (RR=0.29, 95% CI, 0.22–0.37;
P<0.01). This effect differed significantly between the studies
(Cochran's Q=16.41; P=0.04; І2=51%). According to the
subgroup analyses, such effects were more significant in studies
with a sample size of <100 subjects (Cochran's Q=6.07; P=0.01;
І2=83.5%), as the RRs of good vs. poor collateral
circulation status for mortality were 0.17 and 0.37, respectively
(Fig. 4).
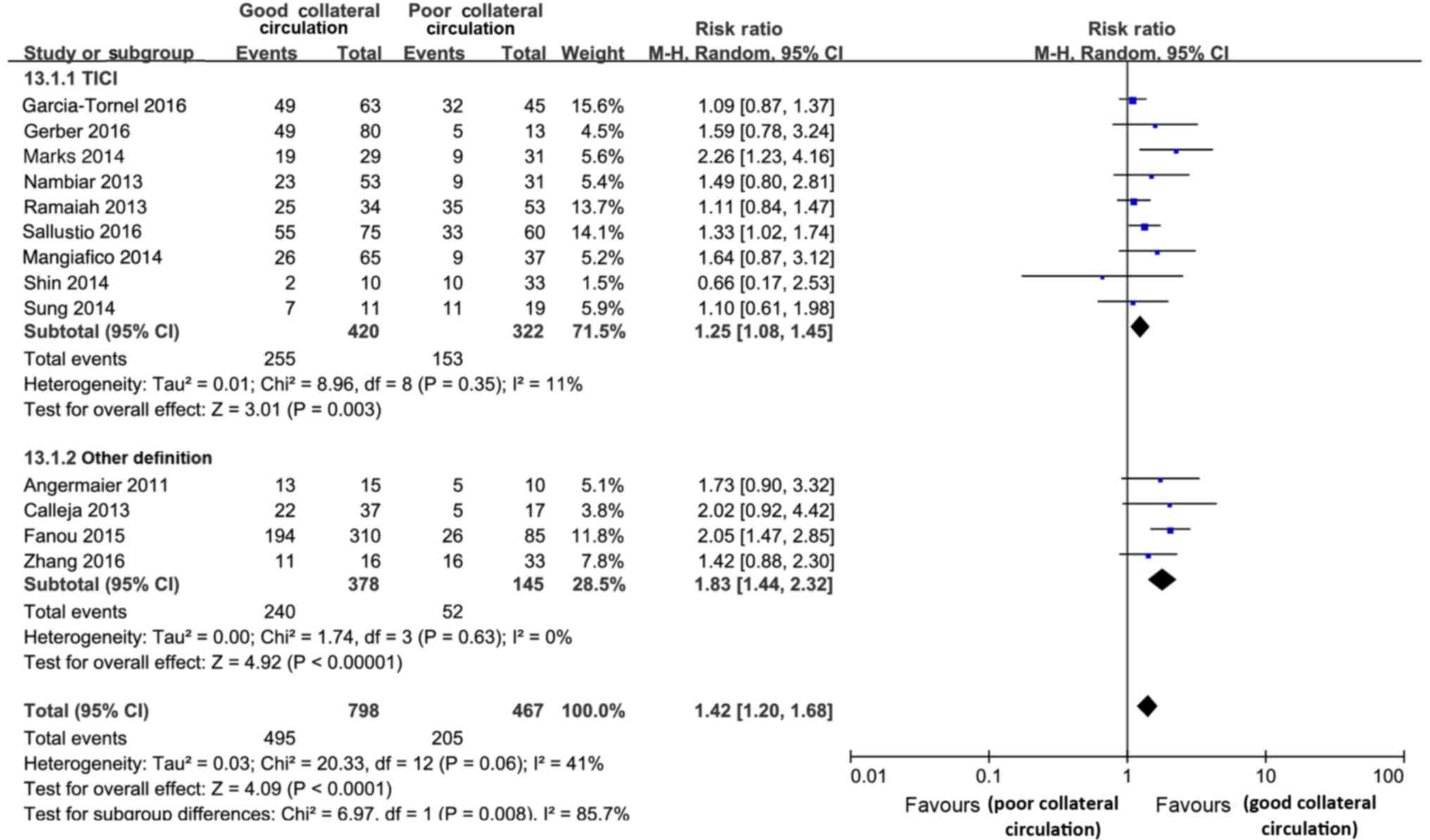
Recanalization or reperfusion
The present review included 13 studies (comprising
1,265 subjects) that reported on recanalization or reperfusion. In
comparison with the poor collateral circulation status, good
collateral circulation was significantly associated with elevated
the rates of good recanalization/reperfusion (RR=1.42; 95% CI,
1.20–1.68; P<0.001; Fig. 5), and
this effect was significantly different between the studies
(Cochran's Q=20.33; P=0.06; І2=41%). According to the
subgroup analyses, such effects were more significant in studies
with successful recanalization (thrombolysis in cerebral infarction
score of 2b/3 or other definitions; Cochran's Q=6.97; P=0.008;
І2=85.7%; Fig. 5).
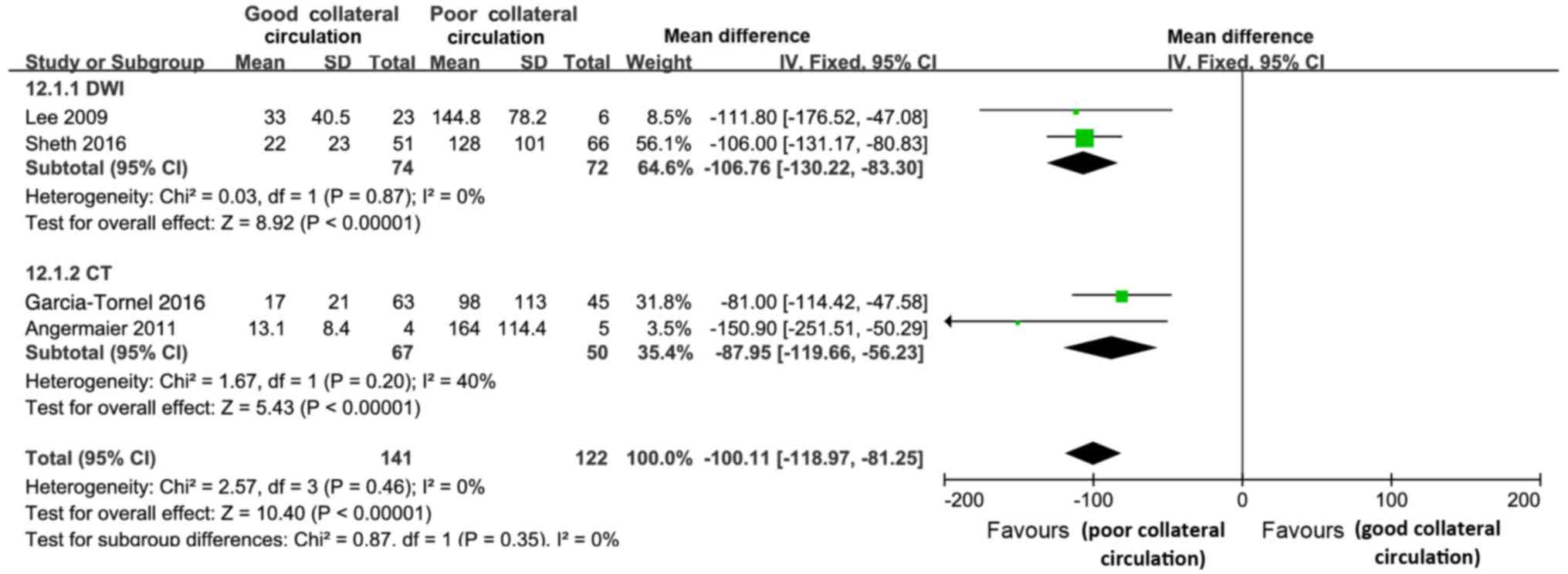
Final infarct volume
The final infarct volume was determined by
diffusion-weighted imaging in the studies by Sheth et al
(32) and Lee et al (21) and by CT in the studies by
García-Tornel et al (12) and
Angermaier et al (13). The
good collateral circulation group displayed a significantly lower
final infarct volume than the poor collateral circulation group
[mean difference=−100.11; 95% CI, -(118.97–81.25); P<0.01];
however, no significant heterogeneity was identified between the
studies (Cochran's Q=2.57; P=0.046; І2=0%) or between
the subgroups (Cochran's Q=0.87; P=0.35; І2=0%)
(Fig. 6).
Discussion
The collateral circulation connects the cerebral
arteries to maintain penumbra perfusion and provide alternative
routes for blood flow prior to achievement
recanalization/reperfusion in patients with AIS. The predictive
value of the collateral circulation status regarding the outcome in
such patients has been demonstrated in various studies (6,7,35,40–42);
however, these studies display certain differences, including
imaging evaluation methods and types of thrombolysis therapy. The
literature, including baseline features of collateral status and
effects of determining outcomes, was systematically reviewed in the
present study. The beneficial effects of good collateral
circulation regarding the favorable functional outcome at 3–6
months have been demonstrated (12,16,26,32,43–45),
without any significant difference among various durations of
thrombolysis (4.5 h, 4.5–6 h or >6 h) and among different
treatment types (ET alone, ET+IVT or IVT alone). These phenomena
may be explained by the following two points: First, good
collateral circulation may have improved the delivery of
thrombolysis to both sides of the thrombus, while limiting any
extension of the occlusion (25),
facilitated adequate preservation of the penumbra until effective
reperfusion was reached and increased the efficacy of aggressive ET
applied to patients beyond 6 h as per the clinical risk of futile
recanalization (46). Second,
patients with poor collateral circulation status had a relatively
low rate of favorable functional outcomes due to the low amount of
salvageable tissue to be reperfused beyond 6 h.
To the best of our knowledge, the present study was
the first to systematically review and meta-analyze the correlation
between collateral circulation status and final infarct volume. The
results indicated that the collateral circulation score predicted
the final infarct volume, which was negatively associated with an
increased collateral blood supply that improved oligemic
peri-infarct tissue perfusion to reduce infarct core expansion and
maintain the viable tissue eligible for recovery of function for a
prolonged duration, as also outlined in a previous study (47). According to the results of the
present study, good collateral circulation also lowered the risk of
HT and mortality and enhanced the rate of
recanalization/reperfusion. Such effects were independent of
variability in estimation methods of collateral circulation, type
of thrombolysis therapy and treatment duration, which was in
disagreement with the results provided by McVerry et al
(48), who indicated that
inconsistencies in evaluating the collateral blood flow may lead to
an underestimation of the effect of the collateral circulation on
the outcome of patients with AIS.
According to recent RCT studies, ET has achieved a
higher efficiency as compared with IVT (49–53). The
putative factors for this change are the use of stent-retriever
device technology, a reduction in time delay between admission and
groin puncture and the use of neuroimaging modalities for
documenting vessel occlusion and patient selection (54); however, the impact of collateral
status on the outcome in different treatment types had remained to
be subjected to a comparative analysis. The present study revealed
that good collateral circulation may have beneficial effects in
both treatment types; although ET may be more effective and
reliable owing to technological developments, while IVT alone also
achieved favorable functional outcomes for patients with a good
collateral circulation status.
Evaluation of the collateral circulation status and
penumbra area prior to treatment are critical for identifying
patients with AIS are likely to benefit from thrombolysis
treatment. In addition, collateral circulation scores and the
penumbra area should be evaluated alongside the application of the
imaging methods for improved accuracy and a timely decision
regarding the application of IVT. In addition, certain assessment
manuals, including the Houston Intraarterial Therapy (54) and the Totaled Health Risks in
Vascular Events score (55), should
be jointly considered for improving the selection of patients for
whom thrombolysis treatment is likely to be beneficial rather than
harmful.
However, the present meta-analysis had several
limitations: First, the patients included in this systematic review
did not only have anterior circulation ischemic stroke but also
with posterior circulation ischemic stroke, which may have resulted
in prognosis evaluation bias; second, the present study had other
heterogeneities, including differences in ethnicity and treatment
compliance after recanalization, and the effects of good collateral
circulation are probably restricted to certain subgroups of
patients, which requires further exploration. Third, a sampling
bias may have been present, as an English language restriction was
imposed on the literature search due to which the studies published
in other languages were neither identified nor included, thereby
reducing the broadness of the analysis. Also, in the present study
the effect was more significant in the subgroup of other
definitions (TIMI, TIBI or AOL scores) than in the TICI subgroup.
The number of studies included in the two groups was different,
therefore resulting in a bias between the two groups. Unifying the
evaluation and reporting standards for revascularization would
benefit horizontal and longitudinal comparisons among IVT or ET
studies in the future.
In conclusion, a good collateral circulation status
may lead to a favorable 3–6-month functional outcome, a better
recanalization or reperfusion rate, a smaller infarct core, a lower
HT rate and a lower risk of mortality after thrombolysis treatment
for various durations in patients with AIS. In clinical practice,
it may be worth considering the collateral circulation status,
penumbra assessment and onset-to-treatment time for optimally
identifying suitable patients who are likely to benefit from
thrombolysis treatment.
References
|
1
|
Hankey GJ: Stroke. Lancet.
389:641–6542016.
|
|
2
|
Angermaier A, Langer S, Kirsch M, Kessler
C, Hosten N and Khaw AV: CT-angiographic collateralization predicts
final infarct volume after intra-arterial thrombolysis for acute
anterior circulation ischemic stroke. Cerebrovasc Dis. 31:177–184.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shuaib A, Butcher K, Mohammad AA, Saqqur M
and Liebeskind DS: Collateral blood vessels in acute ischaemic
stroke: A potential therapeutic target. Lancet Neurol. 10:909–921.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ribo M, Flores A, Rubiera M, Pagola J,
Sargento-Freitas J, Rodriguez-Luna D, Coscojuela P, Maisterra O,
Piñeiro S, Romero FJ, et al: Extending the time window for
endovascular procedures according to collateral pial circulation.
Stroke. 42:3465–3469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bang OY, Goyal M and Liebeskind DS:
Collateral circulation in ischemic stroke: Assessment tools and
therapeutic strategies. Stroke. 46:3302–3309. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bang OY, Saver JL, Kim SJ, Kim GM, Chung
CS, Ovbiagele B, Lee KH and Liebeskind DS: Collateral flow predicts
response to endovascular therapy for acute ischemic stroke. Stroke.
42:693–699. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Christoforidis GA, Karakasis C, Mohammad
Y, Caragine LP, Yang M and Slivka AP: Predictors of hemorrhage
following intra-arterial thrombolysis for acute ischemic stroke:
The role of pial collateral formation. AJNR Am J Neuroradiol.
30:165–170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bang OY, Saver JL, Kim SJ, Kim GM, Chung
CS, Ovbiagele B, Lee KH and Liebeskind DS; UCLA-Samsung Stroke
Collaborators, : Collateral flow averts hemorrhagic transformation
after endovascular therapy for acute ischemic stroke. Stroke.
42:2235–2239. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leng X, Lan L, Liu L, Leung TW and Wong
KS: Good collateral circulation predicts favorable outcomes in
intravenous thrombolysis: A systematic review and mate-analysis.
Eur J Neurol. 23:1738–1749. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moher D, Liberati A, Tetzlaff J and Altman
DG; PRISMA Group, : Preferred reporting items for systematic
reviews and meta-analyses: The PRISMA statement. Ann Intern Med.
151:264–269, W64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stroup DF, Berlin JA, Morton SC, Olkin I,
Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA and Thacker
SB: Meta-analysis of observational studies in epidemiology: A
proposal for reporting. Meta-analysis of observational studies in
epidemiology (MOOSE) group. JAMA. 283:2008–2012. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
García-Tornel A, Carvalho V, Boned S,
Flores A, Rodríguez-Luna D, Pagola J, Muchada M, Sanjuan E,
Coscojuela P, Juega J, et al: Improving the evaluation of
collateral circulation by multiphase computed tomography
angiography in acute stroke patients treated with endovascular
reperfusion therapies. Interv Neurol. 5:209–217. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Angermaier A, Langner S, Kirsch M, Kessler
C, Hosten N and Khaw AV: CT-angiographic collateralization predicts
final infarct volume after intra-arterial thrombolysis for acute
anterior circulation ischemic stroke. Cerebrovasc Dis. 31:177–184.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Berkhemer OA, Jansen IG, Beumer D, Fransen
PS, van den Berg LA, Yoo AJ, Lingsma HF, Sprengers ME, Jenniskens
SF, Lycklama À, Nijeholt GJ, et al: Collateral status on baseline
computed tomographic angiography and intra-arterial treatment
effect in patients with proximal anterior circulation stroke.
Stroke. 47:768–776. 2016.PubMed/NCBI
|
|
15
|
Brunner F, Tomandl B, Hanken K,
Hildebrandt H and Kastrup A: Impact of collateral circulation on
early outcome and risk of hemorrhagic complications after systemic
thrombolysis. Int J Stroke. 9:992–998. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Calleja AI, Cortijo E, García-Bermejo P,
Gómez RD, Pérez-Fernández S, Del Monte JM, Muñoz MF,
Fernández-Herranz R and Arenillas JF: Collateral circulation on
perfusion-computed tomography-source images predicts the response
to stroke intravenous thrombolysis. Eur J Neurol. 20:795–802. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fanou EM, Knight J, Aviv RI, Hojjat SP,
Symons SP, Zhang L and Wintermark M: Effect of collaterals on
clinical presentation, baseline imaging, complications, and outcome
in acute stroke. AJNR Am J Neuroradiol. 36:2285–2291. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gerber JC, Petrova M, Krukowski P, Kuhn M,
Abramyuk A, Bodechtel U, Dzialowski I, Engellandt K, Kitzler H,
Pallesen LP, et al: Collateral state and the effect of endovascular
reperfusion therapy on clinical outcome in ischemic stroke
patients. Brain Behav. 6:e005132016. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kufner A, Galinovic I, Ambrosi V, Nolte
CH, Endres M, Fiebach JB and Ebinger M: Hyperintense vessels on
FLAIR: Hemodynamic correlates and response to thrombolysis. AJNR Am
J Neuroradiol. 36:1426–1430. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee KH, Lee SJ, Cho SJ, Na DG, Byun HS,
Kim YB, Song HJ, Jin IS and Chung CS: Usefulness of triphasic
perfusion computed tomography for intravenous thrombolysis with
tissue-type plasminogen activator in acute ischemic stroke. Arch
Neurol. 57:1000–1008. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee KY, Latour LL, Luby M, Hsia AW, Merino
JG and Warach S: Distal hyperintense vessels on FLAIR: An MRI
marker for collateral circulation in acute stroke? Neurology.
72:1134–1139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lima FO, Furie KL, Silva GS, Lev MH,
Camargo EC, Singhal AB, Harris GJ, Halpern EF, Koroshetz WJ, Smith
WS, et al: The pattern of leptomeningeal collaterals on CT
angiography is a strong predictor of long-term functional outcome
in stroke patients with large vessel intracranial occlusion.
Stroke. 41:2316–2322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Menon BK, Qazi E, Nambiar V, Foster LD,
Yeatts SD, Liebeskind D, Jovin TG, Goyal M, Hill MD, Tomsick TA, et
al: Differential effect of baseline computed tomographic
angiography collaterals on clinical outcome in patients enrolled in
the interventional management of stroke III trial. Stroke.
46:1239–1244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marks MP, Lansberg MG, Mlynash M, Olivot
JM, Straka M, Kemp S, McTaggart R, Inoue M, Zaharchuk G, Bammer R,
et al: Effect of collateral blood flow on patients undergoing
endovascular therapy for acute ischemic stroke. Stroke.
45:1035–1039. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miteff F, Levi CR, Bateman GA, Spratt N,
McElduff P and Parsons MW: The independent predictive utility of
computed tomography angiographic collateral status in acute
ischaemic stroke. Brain. 132:2231–2238. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nambiar V, Sohn SI, Almekhlafi MA, Chang
HW, Mishra S, Qazi E, Eesa M, Demchuk AM, Goyal M, Hill MD and
Menon BK: CTA collateral status and response to recanalization in
patients with acute ischemic stroke. AJNR Am J Neuroradiol.
35:884–890. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ramaiah SS, Churilov L, Mitchell P,
Dowling R and Yan B: The impact of arterial collateralization on
outcome after intra-arterial therapy for acute ischemic stroke.
AJNR Am J Neuroradiol. 35:667–672. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saarinen JT, Rusanen H and Sillanpää N:
Collateral score complements clot location in predicting the
outcome of intravenous thrombolysis. AJNR Am J Neuroradiol.
35:1892–1896. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sallustio F, Motta C, Pizzuto S, Diomedi
M, Giordano A, D'Agostino VC, Samà D, Mangiafico S, Saia V,
Legramante JM, et al: CT angiography-based collateral flow and time
to reperfusion are strong predictors of outcome in endovascular
treatment of patients with stroke. J Neurointerv Surg. 9:940–943.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mangiafico S, Saia V, Nencini P, Romani I,
Palumbo V, Pracucci G, Consoli A, Rosi A, Renieri L, Nappini S, et
al: Effect of the interaction between recanalization and collateral
circulation on functional outcome in acute ischaemic stroke. Interv
Neuroradiol. 20:704–714. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
van Seeters T, Biessels GJ, Kappelle LJ,
van der Graaf Y and Velthuis BK; Dutch acute stroke study (DUST)
investigators, : Determinants of leptomeningeal collateral flow in
stroke patients with a middle cerebral artery occlusion.
Neuroradiology. 58:969–977. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sheth SA, Sanossian N, Hao Q, Starkman S,
Ali LK, Kim D, Gonzalez NR, Tateshima S, Jahan R, Duckwiler GR, et
al: Collateral flow as causative of good outcomes in endovascular
stroke therapy. J Neurointerv Surg. 8:2–7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shin NY, Kim KE, Park M, Kim YD, Kim DJ,
Ahn SJ, Heo JH and Lee SK: Dual-phase CT collateral score: A
predictor of clinical outcome in patients with acute ischemic
stroke. PLoS One. 9:e1073792014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Souza LC, Yoo AJ, Chaudhry ZA, Payabvash
S, Kemmling A, Schaefer PW, Hirsch JA, Furie KL, González RG,
Nogueira RG and Lev MH: Malignant CTA collateral profile is highly
specific for large admission DWI infarct core and poor outcome in
acute stroke. AJNR Am J Neuroradiol. 33:1331–1336. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sung SM, Lee TH, Cho HJ, Kang TH, Jung DS,
Park KP, Park MK, Lee JI and Ko JK: Functional outcome after
recanalization for acute pure M1 occlusion of the middle cerebral
artery as assessed by collateral CTA flow. Clin Neurol Neurosurg.
131:72–76. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yeo LL, Paliwal P, Teoh HL, Seet RC, Chan
BP, Ting E, Venketasubramanian N, Leow WK, Wakerley B, Kusama Y, et
al: Assessment of intracranial collaterals on CT angiography in
anterior circulation acute ischemic stroke. AJNR Am J Neuroradiol.
36:289–294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang S, Chen W, Tang H, Han Q, Yan S,
Zhang X, Chen Q, Parsons M, Wang S and Lou M: The prognostic value
of a four-dimensional CT angiography-based collateral grading scale
for reperfusion therapy in acute ischemic stroke patients. PLoS
One. 11:e01605022016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang S, Zhang X, Yan S, Lai Y, Han Q, Sun
J, Zhang M, Parsons MW, Wang S and Lou M: The velocity of
collateral filling predicts recanalization in acute ischemic stroke
after intravenous thrombolysis. Sci Rep. 6:278802016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hacke W, Kaste M, Fieschi C, von Kummer R,
Davalos A, Meier D, Larrue V, Bluhmki E, Davis S, Donnan G, et al:
Randomised double-blind placebo-controlled trial of thrombolytic
therapy with intravenous alteplase in acute ischaemic stroke (ECASS
II). Second European-Australasian acute stroke study investigators.
Lancet. 352:1245–1251. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ito M, Yoshimoto T, Kawabori M, Fujimoto
S, Yamauchi T, Yamaguchi H, Tokuda K and Kaneko S: Diagnostic
impact of baseline cerebral blood flow in patients with acute
ischemic stroke prior to intravenous recombinant tissue plasminogen
activator therapy. Clin Neurol Neurosurg. 115:1464–1469. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liebeskind DS: Imaging the future of
stroke: I. Ischemia. Ann Neurol. 66:574–590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liebeskind DS and Alexandrov AV: Advanced
multimodal CT/MRI approaches to hyperacute stroke diagnosis,
treatment, and monitoring. Ann N Y Acad Sci. 1268:1–7. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mangiafico S, Saia V, Nencini P, Romani I,
Palumbo V, Pracucci G, Consoli A, Rosi A, Renieri L, Nappini S, et
al: Effect of the interaction between recanalization and collateral
circulation on functional outcome in acute ischaemic stroke. Interv
Neuroradiol. 20:704–714. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Beyer SE, Thierfelder KM, von Baumgarten
L, Rottenkolber M, Meinel FG, Janssen H, Ertl-Wagner B, Reiser MF
and Sommer WH: Strategies of collateral blood flow assessment in
ischemic stroke: Prediction of the follow-up infarct volume in
conventional and dynamic CTA. AJNR Am J Neuroradiol. 36:488–494.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ernst M, Forkert ND, Brehmer L, Thomalla
G, Siemonsen S, Fiehler J and Kemmling A: Prediction of infarction
and reperfusion in stroke by flow- and volume-weighted collateral
signal in MR angiography. AJNR Am J Neuroradiol. 36:275–282. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jickling GC, Liu D, Stamova B, Ander BP,
Zhan X, Lu A and Sharp FR: Hemorrhagic transformation after
ischemic stroke in animals and humans. J Cereb Blood Flow Metab.
34:185–199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Arnold M, Schroth G, Nedeltchev K, Loher
T, Remonda L, Stepper F, Sturzenegger M and Mattle HP:
Intra-arterial thrombolysis in 100 patients with acute stroke due
to middle cerebral artery occlusion. Stroke. 33:1828–1833. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
McVerry F, Liebeskind DS and Muir KW:
Systematic review of methods for assessing leptomeningeal
collateral flow. AJNR Am J Neuroradiol. 33:576–582. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Goyal M, Demchuk AM, Menon BK, Eesa M,
Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL,
et al: Randomized assessment of rapid endovascular treatment of
ischemic stroke. N Engl J Med. 372:1019–1030. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Campbell BC, Mitchell PJ, Kleinig TJ,
Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley
TJ, et al: Endovascular therapy for ischemic stroke with
perfusion-imaging selection. N Engl J Med. 372:1009–1018. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Berkhemer OA, Fransen PS, Beumer D, van
den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn
PJ, Wermer MJ, et al: A randomized trial of intraarterial treatment
for acute ischemic stroke. N Engl J Med. 372:11–20. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Saver JL, Goyal M, Bonafe A, Diener HC,
Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, et
al: Stent-retriever thrombectomy after intravenous t-PA vs. t-PA
alone in stroke. N Engl J Med. 372:2285–2295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jovin TG, Chamorro A, Cobo E, de Miquel
MA, Molina CA, Rovira A, Román LS, Serena J, Abilleira S, Ribó M,
et al: Thrombectomy within 8 h after symptom onset in ischemic
stroke. N Engl J Med. 372:2296–2306. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hallevi H, Barreto AD, Liebeskind DS,
Morales MM, Martin-Schild SB, Abraham AT, Gadia J, Saver JL; UCLA
Intra-Arterial Therapy Investigators, ; Grotta JC and Savitz SI:
Identifying patients at high risk for poor outcome after
intra-arterial therapy for acute ischemic stroke. Stroke.
40:1780–1785. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Flint AC, Kamel H, Rao VA, Cullen SP,
Faigeles BS and Smith WS: Validation of the totaled health risks in
vascular events (THRIVE) score for outcome prediction in
endovascular stroke treatment. Int J Stroke. 9:32–39. 2014.
View Article : Google Scholar : PubMed/NCBI
|