Introduction
Although >100 thousand fungal species are
recognized, only a couple of hundred have been suggested to be
pathogenic to humans and 10–15% of pathological fungi produce
systemic and/or central nervous system (CNS) mycosis (1).
Species of Candida may cause intracranial
abscesses and multiple cases have been described in the literature.
The most common identified strain that causes infections is
Candida albicans (C. albicans) (2–16);
however, C. parapsilosis (17–19),
C. tropicalis (20), C.
encephalitis (21) and C.
lusitanea (22) infections have
also been recorded. In a retrospective analysis of 128 cases of
Candida bloodstream infections, the most frequently isolated
species were C. albicans (64%), C. glabrata (20%),
C. tropicalis (9%) and C. parapsilosis (5%) (23). Intracranial abscesses caused by
fungal infections may present with various clinical syndromes,
including basal meningitis, space occupying lesions, stroke
syndromes, hydrocephalus and spinal infections (1). Compared to viral, bacterial or
parasitic CNS disorders, symptomatic CNS fungal infection carries
higher risks of morbidities and mortality (1). Different from other Candida
species, C. glabrata is found as blastoconidia as a
commensal and a pathogen, and infections are difficult to treat and
typically resistant to various azole antifungal agents,
particularly fluconazole (24–26).
Consequently, C. glabrata infections have a high mortality
rate in compromised, at-risk hospitalized patients (27). Although C. glabrata has been
identified as causing infections of the bloodstream, to the best of
our knowledge, the current report is the first to describe an
intracranial abscess caused by C. glabrata.
Itraconazole has in vitro activity against
many of the non-albicans Candida species, specifically C.
glabrata; furthermore, an improvied response rate to
itraconazole has been indicated compared with fluconazole (27). The present case study reports the
successfully treated cerebral abscess case due to C.
glabrata specifiying the surgical approach and itraconazole
treatment. Furthermore, the present study reviewed the literature
regarding this infection.
Case report
Approval regarding the use of human data for
experimental and clinical studies was obtained from the Ethics
Committee of the China-Japan Union Hospital of Jilin University
(Jilin, China) and written informed consent was obtained from the
patient's family. The principles expressed in the Declaration of
Helsinki were adhered to in order to maintain the integrity of the
data.
The current study describes the case of a
25-year-old female patient, weighing 40 kg who presented with fever
(38.7–39.0°C), phlegmy cough and numbness in the right hand. Within
24 h onset of these symptoms, the patient was unable to speak or
move the upper and lower limbs on the right-hand side.
The patient had undergone an anal polypectomy on
November 10, 2014 at the Dehui Municipal Hospital (Jilin, China) to
remove a flat, soft, polyp 5 cm in size that had been present
outside the anus for >7 years. Pathology indicated that it was a
benign papilloma. Relevant medical history included frequent colds
and fevers ranging between 38–39°C, psoriasis involving the scalp,
abdomen and all limbs from the age of 8 and a 12-year history of
smoking (~20 cigarettes/day).
On December 29, 2014, the patient was admitted to
the Neurology Clinic at the First Hospital of Jilin University with
lethargy, uncooperativeness during physical examination, motor
aphasia, left central facial paralysis, strong nuchal rigidity,
level 1right limb muscle strength, increased muscle tone,
hyperreflexia and level 5 left limb muscle strength. The patient
exhibited a positive bilateral Kernig sign, bilateral positive
Babinski signs and bilateral positive Chaddock sign, which were
indicative of meningeal irritation and pyramidal sign positive, is
the clinical features of meningitis and pyramidal tract damage.
However, peripheral blood and cerebrospinal fluid cultures tested
negative for bacteria and fungi. An ultrasonograpic examination
indicated no perianal abscess. A computerized tomography (CT) scan
of the lungs identified minor inflammatory changes in the right
lung in all lobes and the lingula and lower lobe of the left lung.
A CT scan of the head revealed multiple low-density patches of
different sizes in the right frontal lobe, left corona radiate and
left half-oval center. The CT value was 16–26 HU and the midline
had not shifted. Minor inflammation was present on the bilateral
maxillary sinuses and mastoid inflammation was evident in the
middle ears. The patient was hospitalized on December 29, 2014 and
began treatment with 125 ml mannitol three times a day via
intravenous injection.
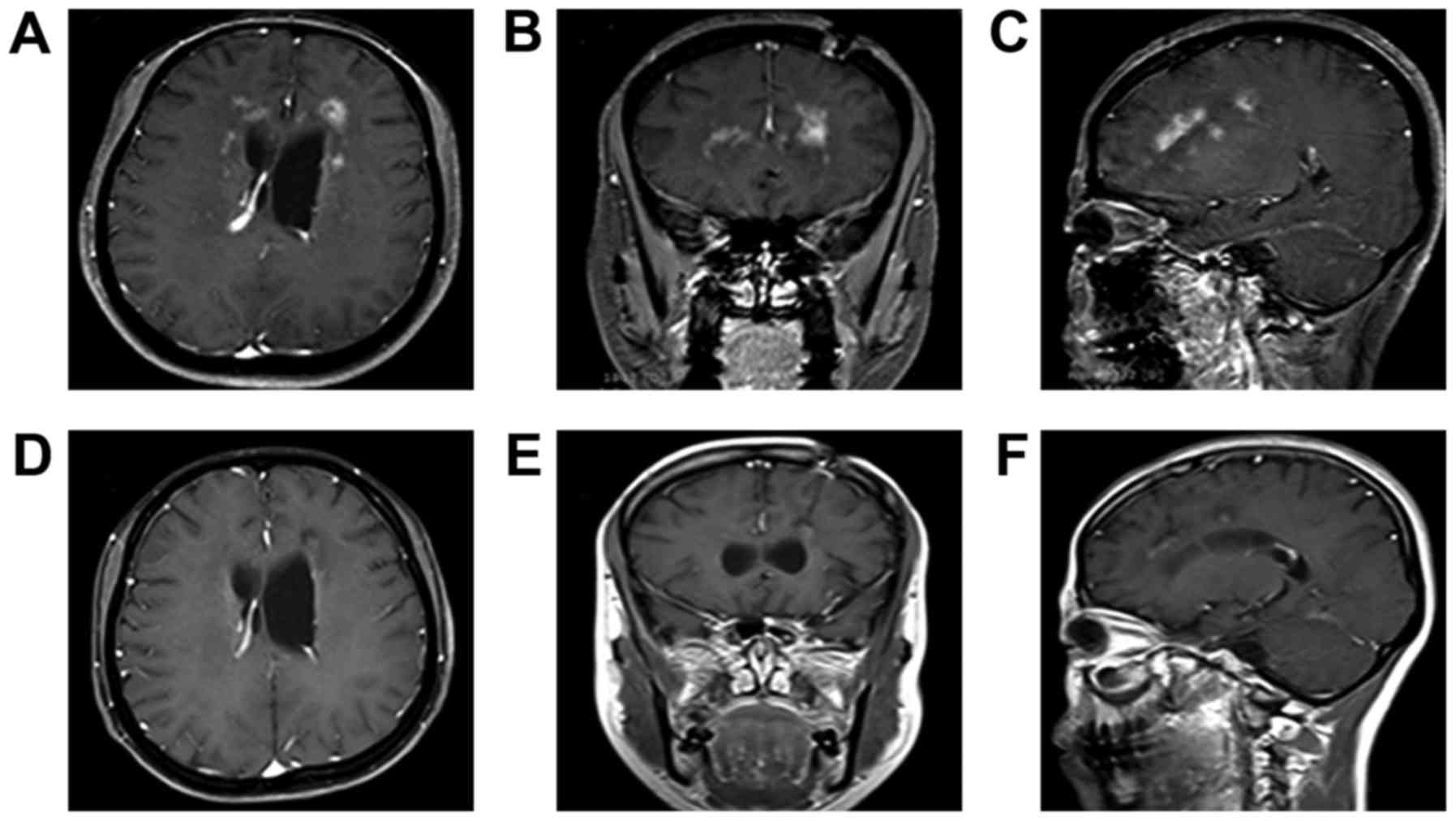
On December 31, 2014, the patient underwent a
magnetic resonance imaging (MRI) scan of the head at the First
Hospital of Jilin University. The results revealed multiple lesions
and abnormal enhancements in the intracranial bilateral frontal and
temporal lobes. Furthermore, the midline was shifted slightly to
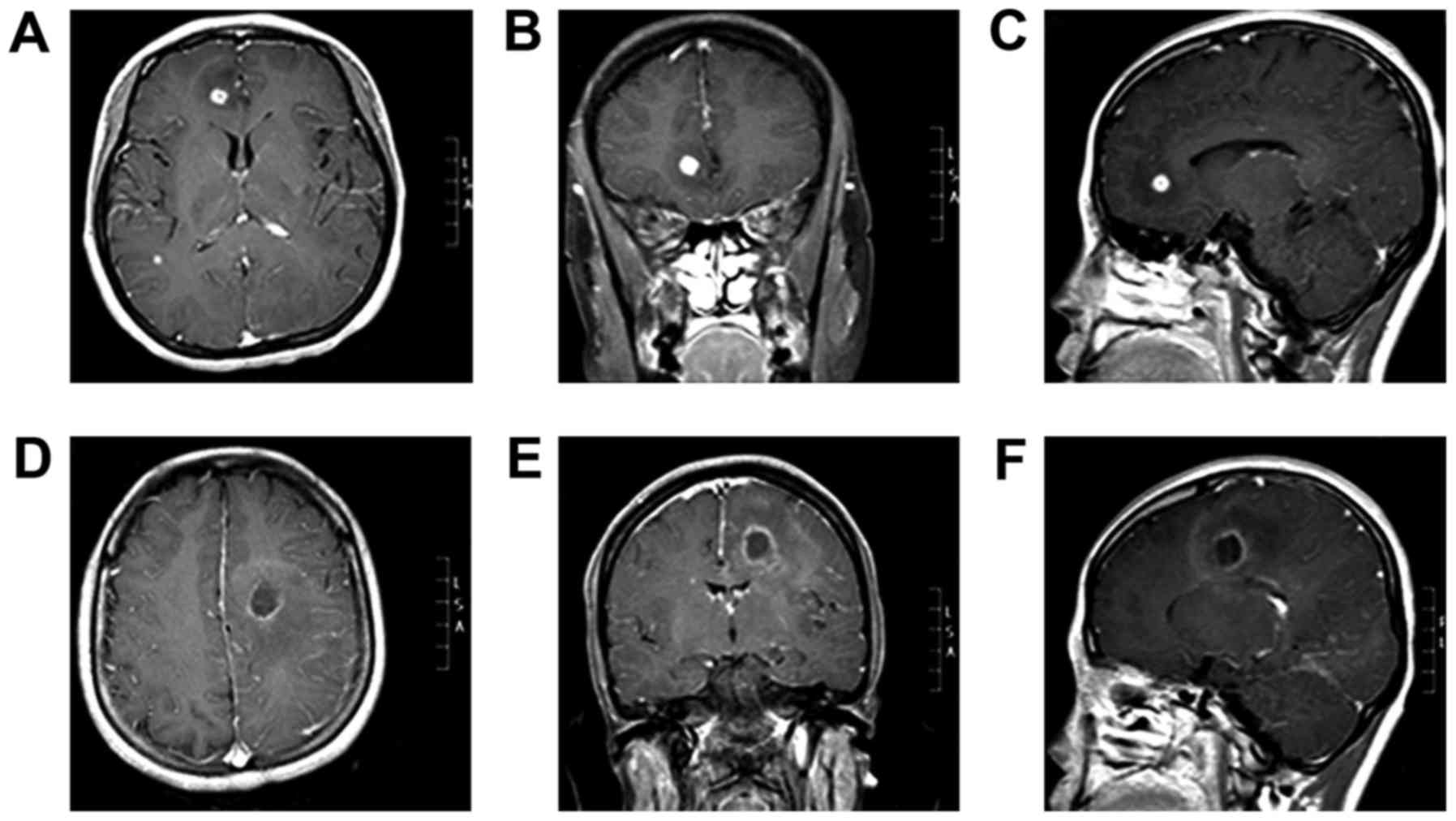
the right. Lesions were detected in the right frontal lobe (size,
8×8×8 mm; volume, 0.2 cm3; Fig. 1A-C) and left lateral temporal lobe
(size, 19×19×20 mm; volume, 2.9 cm3; Fig. 1D-F). Cerebrospinal fluid (CSF)
examination (3 tubes were collected, 2 ml per tube) identified
colorless CSF with a pressure of 110 mmH2O (normal
range, 80–180 mmH2O). The protein, glucose and chlorine
levels in the CSF of the patient were 0.46 g/l (normal range,
0.12–0.60 g/l), 3.49 mmol/l (normal range, 2.2–3.9 mmol/l) and
129.3 mmol/l (normal range, 120.0–132.0 mmol/l), respectively. The
patient exhibited a weakly positive Pandy's reaction. The patient's
white blood cell (WBC) and erythrocyte count in the CSF was
599×106/l (normal range, 0–8×106/l) and
200×106/l (normal range, 0×106/l),
respectively and multinucleate cell and monocyte levels were 9 and
91%, respectively. The level of immunoglobulin (Ig) G in the CSF
was 15.90 mg/l (normal range, 0–34.0 mg/l) and the level of
high-sensitivity C-reactive protein (CRP) was 7.51 mg/l (normal
range, 0.0–5.0 mg/l). Hematological evaluation was performed in the
4-ml blood sample extracted from the patient. The sample was
centrifuged at 1,342 × g at 4°C for 10 min before a WBC count of
11.12×109/l (normal range, 4.0–10.0×109/l),
neutrophil percentage of 79% (normal range, 50–70%) and absolute
neutrophil count of 8.73×109/l (normal range,
2.0–7.0×109/l) were determined. The results of tests for
syphilis and human immunodeficiency virus (HIV) were negative. The
patient was diagnosed with a brain abscess, purulent meningitis and
bilateral pneumonia. Vancomycin (1.0 g, twice a day) and meropenem
(1.0 g, 6 times a day) were administered via intravenous injection.
However, 11 days after this treatment was initiated, symptoms had
worsened and the patient was unable to eat.
On January 19, 2015, the patient was transferred
back to Dehui Municipal Hospital. The patient continued to receive
treatment with vancomycin but not meropenem until a rash developed
10 days later and antibiotic therapy was stopped.
On January 29, 2015, the patient was hospitalized
and examined at the neurosurgery clinic of the China-Japanese Union
Hospital of Jilin University. Physical examination demonstrated
that the patient was in a vegetative state. The patient was able to
open their eyes but was unable to move them and unable to speak.
The patient exhibited high tension in the lower limb muscles and
stiff muscles. An MRI scan (non-contrast and contrast-enhanced)
conducted 48 h after admittance to the China-Japanese Union
Hospital revealed sheet-like and patch-like lesions on T1 and mixed
isointense areas on T2 in the bilateral frontal lobe, parietal
lobe, bilateral corona radiata area, semi-oval center and right
occipital lobe. The internal signals were uneven, exhibiting a
number of round-shaped hypointense lesions on T1 and hyperintense
lesions on T2. The left frontal lobe exhibited an abnormal signal
consisting of linear-shaped lesions that may have been caused by
bleeding. The enhanced scan suggested that the lesions were diffuse
and consisted of small patchy areas of ring-like enhancement
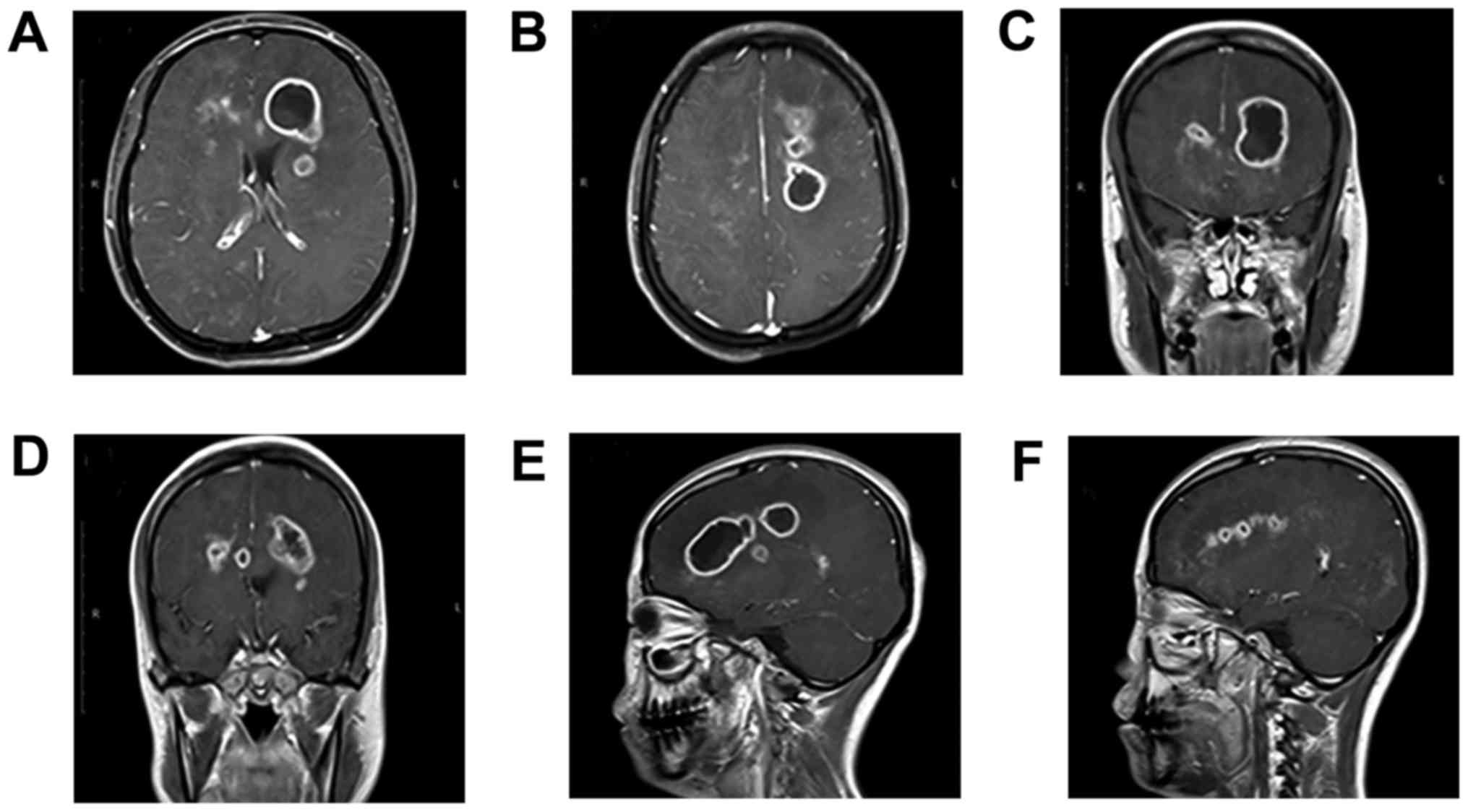
surrounding them. The abscess in the left frontal cavity had
increased to a volume of 13.0 cm3 (size, 26×26×35 mm;
Fig. 2A, C, E and F). The abscess in
the left semi-oval center cavity had increased to a volume of 9.0
cm3 (size, 25×22×30 mm; Fig.
2B, D-F). Magnetic resonance spectroscopy (MRS) was conducted
and the solid entity on the cavity wall was scanned. The results
identified an N-acetyl aspartate peak, a significant decrease in
the creatine peak, an elevated choline peak, dual lactate (Lac)
peaks (1.1–1.5 ppm) and a visible inositol peak (3.56–4.06 ppm)
(data not shown). Following previous treatment with vancomycin and
meropenem for 1 month, which had led to the deterioration of the
patient's condition, medical therapy was changed to 1.0 g
vancomycin in combination with 2.0 g ceftriaxone twice a day via
intravenous injection.
A total of 2 days after admission, the patient's
peripheral blood was examined for Ig levels (5 µl blood;
centrifuged at 986 × g, at 4°C for 10 min) and complement proteins
(3 µl blood; centrifuged at 986 × g, at 4°C for 10 min). The levels
of IgG, IgM and IgA were 4.65 g/l (normal range, 8–16 g/l), 0.06
g/l (normal range, 0.5–2.2 g/l) and 0.525 g/l (normal range,
0.7–3.3 g/l), respectively. The levels of complement C3 and
complement C4 were 0.788 g/l (normal range, 0.9–1.5 g/l) and 0.236
g/l (normal range, 0.2–0.4 g/l), respectively. Plasma
1–3-β-D-glucan activity (4 ml blood; centrifuged at 98 6 × g, at
4°C for 1 min), a fungal surrogate marker, was elevated at 225.50
pg/ml (normal range, 100.50–151.50 pg/ml). The patient remained
negative for HIV. Taken together, these results suggested the
possibility of a fungal infection; therefore, the patient was
prepared for stereotactic surgery to target the intracranial
lesions.
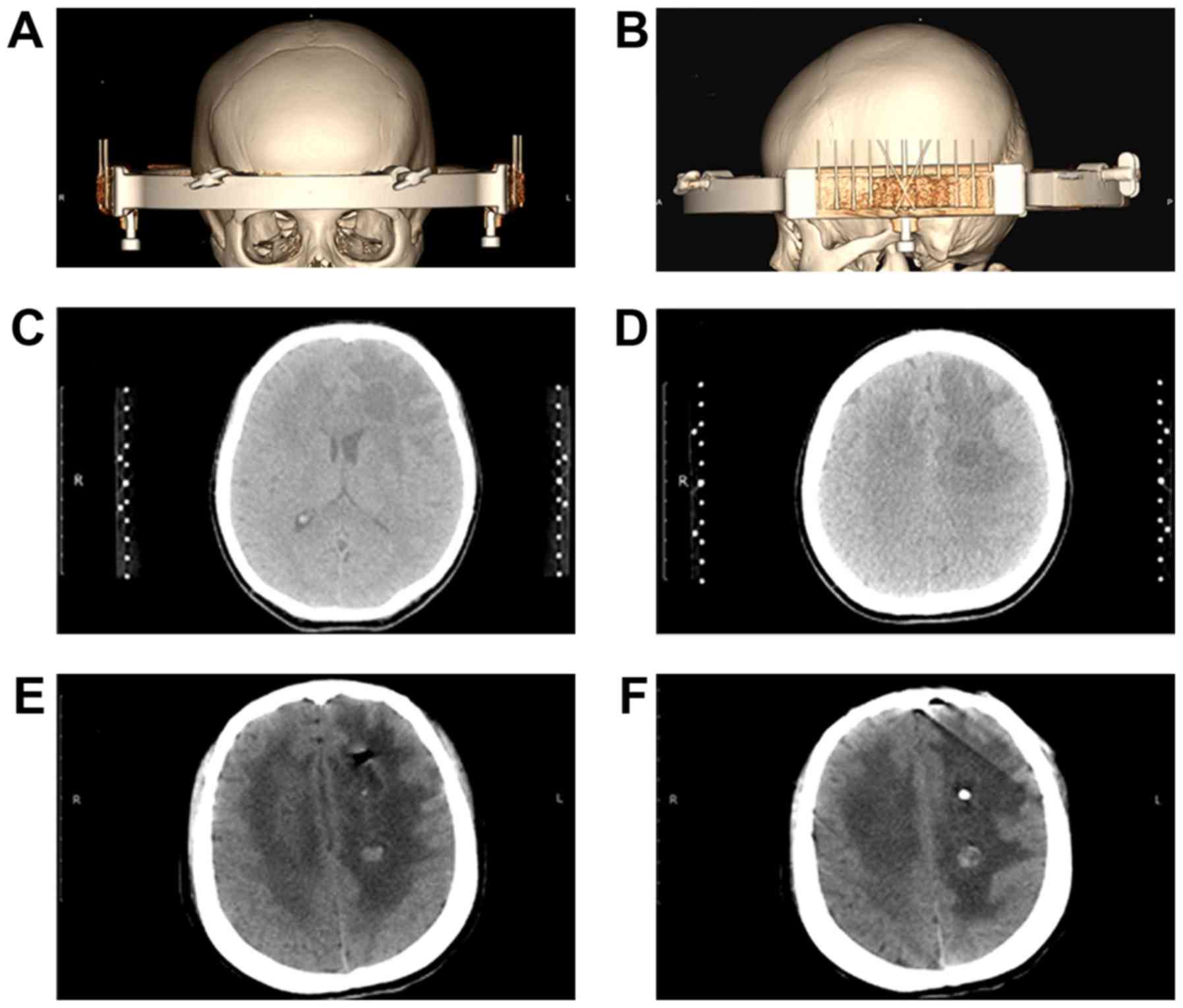
A total of 7 days after admission, the patient
underwent a stereotactic biopsy of aspirate fluid from the
intracranial cyst using Komai's stereotactic instrument (Fig. 3A and B). The CT-Stereotactic Frame
used in the current study was manufactured by Mizuho Medical Co.,
Ltd. (Tokyo, Japan). The left frontal lobe lesion was initially
targeted (Fig. 3C). During the
attempt to puncture the target lesion, the needle encountered
significant resistance prior to reaching the center. The texture
was flexible and resistant and led to the hypothesis that the
resistance encountered was the lesion wall. Following successful
insertion of the needle into the abscess cavity, a syringe was used
to aspirate 5.0 ml pale green fluid and 3.0 ml off-white,
toothpaste-like cystic tissue. A silicone tube was then placed to
for further drainage. The target was adjusted and subsequently, the
lesion in the left semi-oval center was punctured (Fig. 3D). Using a spiral tissue biopsy
needle, the lesion wall tissue, which looked like an off-white,
cheese-like substance to the naked eye, was partially removed. A
total of 3.0 ml yellow-white, granular, millet-like content was
also removed. None of the aspirated materials exhibited a noxious
odor.
Following this procedure, it was considered that the
patient may have a fungal infection. Following surgery, antibiotic
treatment was attenuated and itraconazole solution (200 mg/day;
Janssen Pharmaceutica N.V., Beeerse, Belgium) antifungal treatment
was administered twice daily via a nasogastric tube.
A CT scan of the head was conducted 1 day following
the stereotactic surgery. This confirmed that the abscess in the
left frontal cavity was notably smaller and a shadow of gas was
present in the brain parenchyma (Fig.
3E). The drainage tube was positioned at the center of the
abscess. The abscess in the left semi-oval center was also
noticeably smaller (Fig. 3F). A
round, high-density shadow was present in the cavity, potentially
as a result of minimal bleeding following the biopsy on the wall of
the abscess cavity (Fig. 3F).
Tissue and aspirated fluids were sent to the
pathology laboratory for analysis. Tissue from the cavity wall
indicated local brain tissue gliosis and the presence of scattered
inflammatory cells. Cystic fluid was cultured in CHROMagar
Candida chromogenic medium (Chromagar; Paris, France).
Cystic fluid was streaked onto sterile Petri dishes which contain
10 ml CHROMagar Candida chromogenic medium, and incubated in
aerobic conditions at 35°C.) and purple colony growth was present
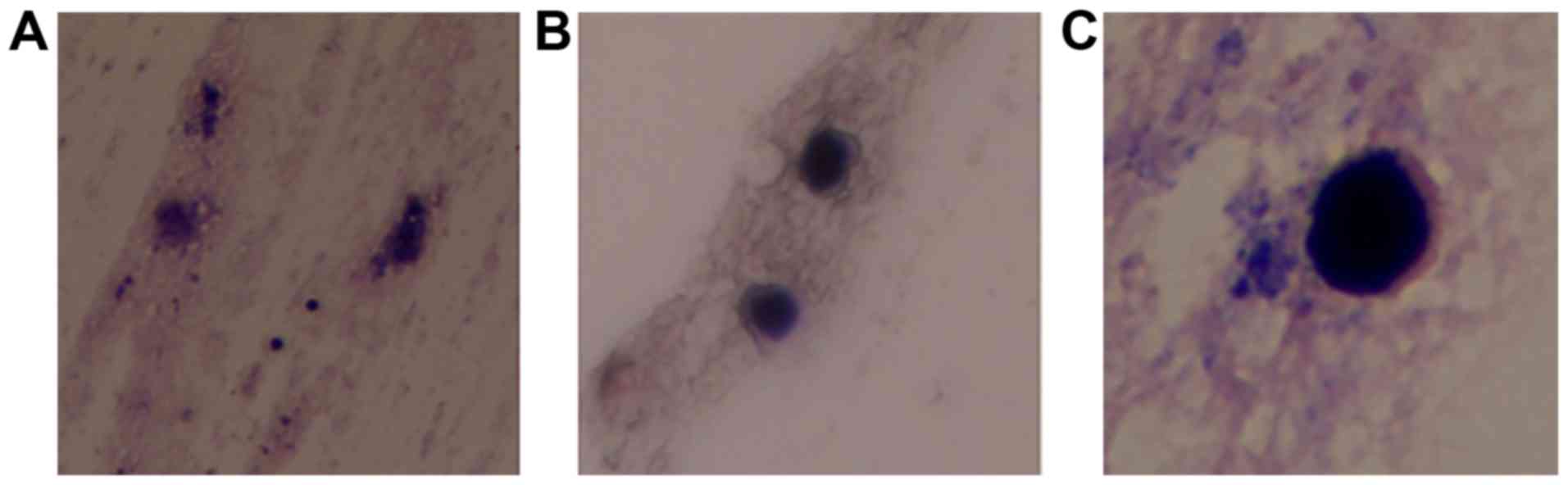
following 7 days in culture. Following 2 weeks of culture, the
medium exhibited a scattered, gram-staining, oval yeast that was
identified as C. glabrata (Fig.
4). The steps of gram-staining were as follows: The colony was
smeared on the slide, stained with crystal violet for 1 min, rinsed
gently with water, saturated with iodine for 1 min, rinsed with
water again and decolorized with 75% alcohol for 20 sec.
Subsequently, the slide was rinsed with water, counterstained with
safranin for 1 min and rinsed with water again. The slide was
observed under a light microscope. The cultured C. glabrata
did not present any blastospores or mycelium, suggesting that the
strain was in a low-activity state.
A total of 4 days following surgery, the patient
regained consciousness and regained the ability to speak. Right
limb muscle strength improved from level 2 to level 5. Levels of
plasma 1–3-β-D-glucan decreased to 88.20 pg/ml by February 14,
2015. The patient was discharged 10 days following surgery and
began eating the following day. After a further 9 days, the patient
was able to move the limbs on their right side. On March 5, 2015,
the patient regained the ability to speak in complete sentences and
was able to walk again on Apri 12, 2015.
A follow-up examination on April 20, 2015 included
an MRI scan. A mild ring-like enhancement indicated the presence of
a 0.7 cm3 (12×11×10 mm) single lesion in the left
frontal lobe. There was no enhancement shadow in place of the
original lesions (Fig. 5A-C). The
patient underwent 20-day, 1-month, 2-month and 2.5-month follow-up
examinations to confirm full recovery of speech and mobility.
During these follow-ups the patient reported a change in their
eating habits, with an increased preference for meat instead of
vegetables and also gained weight. An additional follow-up MRI scan
conducted 8 months after surgery, which indicated the disappearance
of all lesions (Fig. 5D-F),
confirming treatment success. Up to now (24-month follow-up), there
was no recurrence of C. glabrata infection.
Discussion
Candida infections occur most often in
patients with severe immune function defects (28), such as HIV infections (29). However, some patients develop brain
abscesses following bone marrow transplantation (30). Although the patient in the current
report did not have a history of compromised immune function,
examination of peripheral blood for Ig levels and complement tests
revealed lower than normal levels of IgG, IgM and complement C3,
indicating an immune system abnormality. Cerebral abscesses
secondary to Candida infection have been reported in
previous studies (3,8,16);
however, in the current study, it is considered that the patient's
brain abscesses were primarily caused by C. glabrata.
The patient was initially diagnosed with a brain
abscess by MRI and CSF examinations. It was confirmed following
hospitalization that infection was not present in any other organs
and there was no history of antibiotic use within 6 months of
hospitalization. Following treatment with antibiotics, the
patient's condition deteriorated. The patient lost the ability to
eat and talk, and subsequently fell into a vegetative state.
Additional tests were conducted, including tests for CRP and the
fungal surrogate marker, plasma 1–3-β-D-glucan. The results of
these tests suggested the possibility of a fungal infection. An MRI
scan of the head identified that the abscesses had increased in
size; therefore, the patient was prepared for stereotactic surgery
to target the intracranial lesions. A section of the cystic wall
was removed and aspirated fluid and tissue were removed from
intracranial lesions with no complications. The patient underwent
treatment with itraconazole and 1 day following surgery, the
lesions were noticeably smaller. The patient's plasma
1–3-β-D-glucan value decreased to within normal limits following
treatment. The patient regained consciousness, right limb muscle
strength and was able to speak 4 days following stereotactic
surgery. Tissues and fluid were cultured for 2 weeks and C.
glabrata was identified.
Although there have been numerous advances in
diagnostic procedures and therapeutic strategies, patients with
fungal infections of the brain typically have a poor prognosis
(2). Factors associated with the
poor prognosis of patients with Candida meningitis include:
At >2 weeks prior to diagnosis of Candida meningitis,
onset of intracranial hypertension, appearance of focal neurologic
deficits and decreased glucose levels in the CSF (<35 mg/dl) was
indicated (31). Successful
management of patients requires practitioners to have a high degree
of suspicion of a cerebral fungal infection, even as the severity
of symptoms varies (12,32,33).
Diagnosis is often difficult, as the results of neuroradiological
investigations, including CT scans, MRS, radiography and laboratory
assessments are often inconclusive (11,12).
There have been several cases in which patients have experienced a
loss of consciousness, fever, focal neurological deficits,
headaches and seizures; however, examinations of the CSF,
peripheral blood, urine and/or CT scans have failed to uncover the
underlying cause(s) of their neurological issues (11,12,34). In
addition, it can be difficult to differentiate between patients
with cerebral aspergillosis and cerebral candidiasis since the two
infections manifest with similar clinicopathological features
(32). However, infection of the
sino-nasal tract is more common in patients with cerebral
aspergillosis, whereas infections of the gastrointestinal tract are
more common in patients with cerebral candidiasis (32). In the current report, it was
hypothesized that the initial site of infection was the perineum
and occurred following the patient's anal polypectomy.
A surgical approach combined with medical therapy is
the most successful method of treating suspected fungal central
nervous system (CNS) infections (2).
Patients with intracranial fungal masses may be treated with
stereotactic biopsy and/or partial or radical surgical excision of
the mass(es), coupled with intravenously or intrathecally
administered antifungal therapy (28,35). The
most widely used medications for patients with CNS mycoses include
liposomal amphotericin B, 5-fluorocytosine, fluconazole and
itraconazole (28,31). In the current study, a stereotactic
biopsy was performed on the patient. Tissue samples and aspirated
fluids were sent to a pathology laboratory and after 2 weeks, C.
glabrata was identified in culture. MRS was conducted during
diagnosis of the patient. Although the patient exhibited a Lac
peak, which is indicative of anaerobic metabolism in the lesion and
suggestive of inflammatory processes, the results of MRS were
inconclusive. Therefore, the use of MRS does not replace the
requirement for tissue samples when diagnosing a brain abscess.
The outcome for the majority of patients with CNS
fungal infections is poor. Mortality rates are ~50% which is
attributed to delayed diagnosis and onset of treatment, the
presence of serious underlying diseases or conditions, including
HIV, transplant recipients and hematological malignancies, and the
existence of multi-drug-resistant fungal organisms (5,28). In a
study investigating patients undergoing bone marrow transplantation
with a risk of mortality unrelated to the etiology of the brain
abscess or choice of therapeutic regimen, the mortality rate was
97% (30). In cases where patients
survive fungal CNS infections, they often face long-term
neurological sequelae (28).
Infection with Candida species has become the most prevalent
cerebral mycosis detected at autopsy (2,11,12,31),
indicating ineffective and/or inadequate eradication (19).
In the current study, a final diagnosis of C.
glabrata cerebral mycosis was made following pathological
examination of tissue and fluid samples obtained during
stereotactic biopsy. Treatment with antifungal therapy led to
recovery and the patient experienced no further neurological
sequelae. Assessment of the patient's immunological status during
the course of illness revealed lower than normal levels of IgG, IgM
and complement C3, indicating the presence of an underlying immune
system abnormality. Information gleaned from previous studies and
the current case report have demonstrated that timing,
neuroradiological investigations and typical clinical laboratory
assessments, such as examination of CSF, do not lead to the
diagnosis of cerebral mycosis.
In conclusion, all brain abscesses, including small
abscesses should be biopsied as early as possible so that objective
evidence of the underlying cause of the disease, particularly
regarding the type of mycosis, maybe identified as early as
possible following onset. To the best of our knowledge, the current
case report is the first to be published in English describing a
brain abscess caused primarily by C. glabrata.
Acknowledgements
The present case report was supported by the Science
and Technology Department of Jilin Province, China (grant nos.
20110472 and 20150204072SF).
References
|
1
|
Raman Sharma R: Fungal infections of the
nervous system: Current perspective and controversies in
management. Int J Surg. 8:591–601. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neves N, Santos L, Reis C and Sarmento A:
Candida albicans brain abscesses in an injection drug user patient:
A case report. BMC Res Notes. 7:8372014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang GH, Dai CL, Liu YF and Li YM:
Cerebral and renal abscess and retino-choroiditis secondary to
Candida albicans in preterm infants: Eight case retrospective
study. Clin Exp Obstet Gynecol. 40:519–523. 2013.PubMed/NCBI
|
|
4
|
Ancalle IM, Rivera JA, Garcia I, Garcia L
and Valcárcel M: Candida albicans meningitis and brain abscesses in
a neonate: A case report. Bol Asoc Med P R. 102:45–48.
2010.PubMed/NCBI
|
|
5
|
Njambi S, Huttova M, Kovac M, Freybergh
PF, Bauer F and Muli JM: Fungal neuroinfections: Rare disease but
unacceptably high mortality. Neuro Endocrinol Lett. 28 Suppl
2:S25–S26. 2007.
|
|
6
|
Prabhu RM and Orenstein R: Failure of
caspofungin to treat brain abscesses secondary to Candida albicans
prosthetic valve endocarditis. Clin Infect Dis. 39:1253–1254. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marcinkowski M, Bauer K,
Stoltenburg-Didinger G and Versmold H: Fungal brain abscesses in
neonates: Sonographic appearances and corresponding histopathologic
findings. J Clin Ultrasound. 29:417–421. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gözdaşoĝlu S, Ertem M, Büyükkeçeci Z,
Yavuzdemir S, Bengisun S, Ozenci H, Taçyildiz N, Unal E, Yavuz G,
Deda G and Aysev D: Fungal colonization and infection in children
with acute leukemia and lymphoma during induction therapy. Med
Pediatr Oncol. 32:344–348. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Burgert SJ, Classen DC, Burke JP and
Blatter DD: Candidal brain abscess associated with vascular
invasion: A devastating complication of vascular catheter-related
candidemia. Clin Infect Dis. 21:202–205. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kamitsuka MD, Nugent NA, Conrad PD and
Swanson TN: Candida albicans brain abscesses in a premature infant
treated with amphotericin B, flucytosine and fluconazole. Pediatr
Infect Dis J. 14:329–331. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Radhakrishnan VV, Saraswathy A, Rout D and
Mohan PK: Disseminated intra-cerebral microabscesses: A
clinico-pathologic study. Indian J Pathol Microbiol. 37:171–178.
1994.PubMed/NCBI
|
|
12
|
Pendlebury WW, Perl DP and Munoz DG:
Multiple microabscesses in the central nervous system: A
clinicopathologic study. J Neuropathol Exp Neurol. 48:290–300.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wiethölter H, Thron A, Scholz E and
Dichgans J: Systemic Candida albicans infection with cerebral
abscess and granulomas. Clin Neuropathol. 3:37–41. 1984.PubMed/NCBI
|
|
14
|
Thron A and Wiethölter H: Cerebral
candidiasis: CT studies in a case of brain abscess and granuloma
due to Candida albicans. Neuroradiology. 23:223–225. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Holyst J, Majewski A and Tyszkiewicz S:
Massive cerebellar abscess due to Candida albicans. Neurochirurgia
(Stuttg). 19:126–129. 1976.PubMed/NCBI
|
|
16
|
Black JT: Cerebral candidiasis: Case
report of brain abscess secondary to Candida albicans, and review
of literature. J Neurol Neurosurg Psychiatry. 33:864–870. 1970.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bagheri F, Cervellione KL, Maruf M, Marino
W and Santucci T Jr: Candida parapsilosis meningitis associated
with shunt infection in an adult male. Clin Neurol Neurosurg.
112:248–251. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Andres RH, Guzman R, Weis J, Schroth G and
Barth A: Granuloma formation and occlusion of an unruptured
aneurysm after wrapping. Acta Neurochir (Wien). 149:953–958. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lipton SA, Hickey WF, Morris JH and
Loscalzo J: Candidal infection in the central nervous system. Am J
Med. 76:101–108. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoganathan S, Chakrabarty B, Gulati S,
Kumar A, Kumar A, Singh M and Xess I: Candida tropicalis brain
abscess in a neonate: An emerging nosocomial menace. Ann Indian
Acad Neurol. 17:448–450. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maschke M, Dietrich U, Prumbaum M, Kastrup
O, Turowski B, Schaefer UW and Diener HC: Opportunistic CNS
infection after bone marrow transplantation. Bone Marrow
Transplant. 23:1167–1176. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Antunes NL, Hariharan S and DeAngelis LM:
Brain abscesses in children with cancer. Med Pediatr Oncol.
31:19–21. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schelenz S and Gransden WR: Candidaemia in
a London teaching hospital: Analysis of 128 cases over a 7-year
period. Mycoses. 46:390–396. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hitchcock CA, Pye GW, Troke PF, Johnson EM
and Warnock DW: Fluconazole resistance in Candida glabrata.
Antimicrob Agents Chemother. 37:1962–1965. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Komshian SV, Uwaydah AK, Sobel JD and
Crane LR: Fungemia caused by Candida species and Torulopsis
glabrata in the hospitalized patient: Frequency, characteristics,
and evaluation of factors influencing outcome. Rev Infect Dis.
11:379–390. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Willocks L, Leen CL, Brettle RP, Urquhart
D, Russell TB and Milne LJ: Fluconazole resistance in AIDS
patients. J Antimicrob Chemother. 28:937–939. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fidel PL Jr..Vazquez JA and Sobel JD:
Candida glabrata: Review of epidemiology, pathogenesis, and
clinical disease with comparison to C. albicans. Clin Microbiol
Rev. 12:80–96. 1999.PubMed/NCBI
|
|
28
|
Selby R, Ramirez CB, Singh R, Kleopoulos
I, Kusne S, Starzl TE and Fung J: Brain abscess in solid organ
transplant recipients receiving cyclosporine-based
immunosuppression. Arch Surg. 132:304–310. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pham LV, Quang AT, Ton Nu PA, Duc TT and
Thi HN: Cladophialophora bantiana and Candida albicans mixed
infection in cerebral abscess of an HIV-negative patient. J Infect
Dev Ctries. 2:245–248. 2008.PubMed/NCBI
|
|
30
|
Hagensee ME, Bauwens JE, Kjos B and Bowden
RA: Brain abscess following marrow transplantation: Experience at
the Fred Hutchinson cancer research center, 1984–1992. Clin Infect
Dis. 19:402–408. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sanchez-Portocarrero J, Pérez-Cecilia E,
Corral O, Romero-Vivas J and Picazo JJ: The central nervous system
and infection by Candida species. Diagn Microbiol Infect Dis.
37:169–179. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Larbcharoensub N, Wongwichai S,
Chongtrakool P, Boongird A, Noinang A, Watcharananan SP,
Tunlayadechanont S, Witoonpanich R and Phudhichareonrat S: Cerebral
aspergillosis and cerebral candidiasis; a retrospective analysis of
clinicopathologic features in Ramathibodi Hospital. J Med Assoc
Thai. 93:1443–1450. 2010.PubMed/NCBI
|
|
33
|
Fennelly AM, Slenker AK, Murphy LC,
Moussouttas M and DeSimone JA: Candida cerebral abscesses: A case
report and review of the literature. Med Mycol. 51:779–784. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kaji M, Shoji H and Oizumi K: Intractable
meningitis and intracranial abscess following sinusitis due to
Candida species. Kurume Med J. 45:279–281. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yampolsky C, Corti M and Negroni R: Fungal
cerebral abscess in a diabetic patient successfully treated with
surgery followed by prolonged antifungal therapy. Rev Iberoam
Micol. 27:6–9. 2010. View Article : Google Scholar : PubMed/NCBI
|