Introduction
Systemic lupus erythematosus (SLE) is a multisystem
autoimmune disease with varying patterns of organ involvement.
Lupus nephritis (LN) is a common and severe manifestation of SLE,
and an important cause of acute kidney injury and end-stage renal
disease (1). Immunosuppressive
drugs, including cyclophosphamide and azathioprine, and adrenal
hormones, such as prednisone (PDS), are clinical drugs used in the
treatment of LN (2,3). However, these drugs present significant
side effects with long-term and high-dose application. Therefore,
alternative drugs and low-dose combination therapies have attracted
increasing attention (4).
The multiple benefits of antimalarial therapy for
lupus are currently widely recognized, including reducing damage,
lowering the risk of developing nephritis and improving the outcome
of nephritis treatment (5). Over the
past decades, antimalarial drugs, most notably hydroxychloroquine
(HCQ), have become a key part of SLE treatment strategies. HCQ is a
lipophilic weak base that easily passes across cell membranes and
into the acidic intracellular vesicles, including the lysosomes.
Previous studies have indicated that HCQ may delay the onset of
renal damage and improve the survival rates of patients with LN
(6,7). The HCQ immunomodulatory effects are
mediated by its anti-inflammatory, immunosuppressive and
photoprotective properties, and interfere with lysosomal
degradation (8,9). However, treatment with a high dose of
HCQ may cause toxic retinopathy and induce toxic hepatitis
(10–13).
Artemisinin (ART), extracted from Artemisia
annua L., is a novel type of sesquiterpene lactone compound
that contains a hydroperoxyl radical group. A water-soluble ART
derivative has been demonstrated to exert renoprotective effects,
inhibit the proliferation and activation of B-cells, as well as
reduce the generation of plasma cells in MRL/lpr mice (14). Furthermore, Wu et al (15) have observed that ART presented a
therapeutic sensitization effect on glucocorticoid (GC) treatment
in LN mice by increasing the expression levels of GC receptor α
mRNA and the transcriptional coactivator P300/CREB-binding protein
in renal tissues.
Based on previous studies, it is hypothesized herein
that a combination of ART and a low dose of HCQ may have an
immunosuppressive effect on LN. However, to the best of our
knowledge there have been no previous studies on the effect of HCQ
and ART combination treatment. In order to examine this hypothesis
and identify the underlying mechanism, the present study
investigated and compared the effects of treatment with a high dose
of HCQ alone, as well as of treatment with a low dose of HCQ
combined with ART in LN model mice. The effects on the body weight,
biochemical serum parameters and mRNA expression levels of nuclear
factor-κB (NF-κB) pathway-associated enzymes were examined.
Materials and methods
Animals and reagents
A total of 50 Kunming (KM) female mice (weight,
18–22 g; age, 4 weeks; special pathogen-free; certificate no. SCXK
2013–0020) were obtained from the Laboratory Animal Services Centre
of Guangzhou University of Chinese Medicine (Guangzhou, China). The
animals were maintained on a 12-h light/12-h dark cycle under room
temperature (22±2°C) and humidity of 50±10%, and fed with standard
forage and clean water. The Guangzhou University of Chinese
Medicine Science's Administrative Panel on Laboratory Animal Care
approved all experimental procedures. All animal experiments were
performed in accordance with institutional guidelines and ethics
(16), and every effort was made to
minimize animal suffering. HCQ was purchased from Zhejiang Kangle
Pharmaceutical Co., Ltd. (Wenzhou, China), ART was supplied by
Tongrentai Pharmaceutical Co., Ltd. (Sichuan, China), and PDS was
purchased from the Second Affiliated Hospital, Guangzhou University
of Chinese Medicine (Guangzhou, China).
Animal LN model and experimental
groups
At 3 days prior to model establishment the splenic
lymphocytes of KM mice were extracted by aseptic operation and
immediately incubated at 37°C with 8 µg/ml concanavalin A (cat. no.
C-2010; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 72 h.
The splenic lymphocytes of KM mice were subsequently adjusted to a
concentration of 2×107/ml by 1640 complete medium and
injected subcutaneously into each mouse once weekly for 4 weeks in
order to establish the LN model, as previously described (17). The sham group was injected with
normal saline, not spleen cells. At the beginning of week 5, 50
adult female Kunming mice were randomly divided into five groups as
follows: Sham, model, PDS (10 mg/kg), high-dose HCQ (H-HCQ; 33.33
mg/kg) and low-dose HCQ combined with ART (L-HCQ + ART; 16.6 and
5.55 mg/kg, respectively) groups. There were 10 mice in each group.
The mice were orally administered distilled water in the sham and
model groups, or the corresponding treatment drugs in the PDS,
H-HCQ and L-HCQ + ART groups, once daily for 8 weeks. The weights
of the mice were measured once a week.
Blood, renal tissue and spleen tissue
sample collection and preparation
Following 8 weeks of treatment the mice were
anesthetized using ether (initial concentration, 10–15%;
maintenance, 3–5%) in an anesthesia device and then sacrificed by
cervical dislocation, blood was collected from the eyes. The serum
was isolated by centrifugation at 2,000 × g at 4°C for 15 min for
biochemical analysis. In addition, the kidneys and spleens of each
rat were isolated and divided into two parts. One part of the
tissue was fixed in 10% (v/v) neutral formalin phosphate buffer for
hematoxylin-eosin (HE) staining, while the remaining tissue was
quickly frozen in liquid nitrogen and stored at −80°C prior to use
in quantitative polymerase chain reaction (qPCR) analysis.
Biochemical analysis of serum
parameters and urine
The day prior to the end of the experiment (prior to
sacrifice), urine was collected using a urine collection device.
The enzymatic activity of urine albumin (UALB) was detected using a
Bradford Protein Assay kit (Beyotime Institute of Biotechnology,
Haimen, China). In addition, ELISA kits were used to detect the
concentrations of serum creatinine (SCr; AD2561Mo), urea nitrogen
(BUN; AD1254Mo), anti-double stranded DNA (anti-ds-DNA; AD3384Mo),
antinuclear antibodies (ANA; AD1565Mo), immunoglobulin G (IgG;
AD2864Mo), interferon γ (IFN-γ; AD3373Mo), tumor necrosis factor-α
(TNF-α; AD3051Mo) and transforming growth factor β1 (TGF-β1;
AD2732Mo) (all Beijing Andy Huatai Technology Company, Ltd.,
Beijing, China). The kits were used according to the manufacturer's
protocol.
RT-qPCR for Kruppel-like factor 15
(KLF15) and NF-κB mRNA expression
The expression levels of KLF15 and NF-κB were
determined in the renal tissue samples. The total RNA was extracted
by using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), the concentration of total RNA was
measured by microultraviolet spectrophotometer and 1.5 µg of total
RNA was used for cDNA reverse transcription (at 37°C for 15 min)
using a Reverse Transcriptase kit (D2639A; Takara Biotechnology
Co., Ltd., Dalian, China). The total reaction mixture was 20 µl,
made up of 10 µl SYBR Premix Ex Taq™ II X2 (cat. no.
RR420A; Takara Biotechnology Co., Ltd.), 0.8 µl PCR forward primer
(10 µM), 0.8 µl PCR reverse primer (10 µM), 0.4 µl ROX reference
dye II X50, 6 µl UdH2O and 2 µl cDNA. The RT-qPCR
cycling program was set at one cycle of pre-denaturation at 95°C
for 30 sec, followed by 40 cycles at 95°C for 5 sec, 60°C for 34
sec, 95°C for 15 sec, 60°C for 60 sec and 95°C for 15 sec. The
primer sequences were designed with the Primer Premier version 5.0
software (Premier Biosoft International, Palo Alto, CA, USA), and
are presented in Table I. The
relative levels of KLF15 and NF-κB mRNA were normalized to the
β-actin levels, and calculated using the 2−ΔΔCq formula
(18).
 | Table I.Sequences of the primers used in
polymerase chain reaction. |
Table I.
Sequences of the primers used in
polymerase chain reaction.
| Genes | Sense primers
(5′→3′) | Antisense primers
(5′→3′) | Product (bp) |
|---|
| KLF15 |
GTATGACCCAGAGCCACCAT |
GAAGGCACAAAGGCACAAG | 20 |
| NF-κB |
TCAATGGCTACACAGGACCA |
CGCTTCTTCACACACTGGAT | 19 |
| β-actin |
TGGAATCCTGTGGCATCCATGAAAC |
TAAAACGCAGCTCAGTAACAGTCCG | 26 |
Western blot analysis of KLF15 and
NF-κB protein expression levels
The kidney tissue samples were lysed using
radioimmunoprecipitation lysis buffer (Beyotime Institute of
Biotechnology) and the total protein concentrations were detected
using the Micro BCA protein assay kit (Guangzhou Youdi
Biotechnology Company, Guangzhou, China). Total cell lysates (50
µg) were loaded into each lane and resolved by 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis, and then transferred to
PVDF membranes (Thermo Fisher Scientific, Inc.). The PVDF membranes
were blocked with 5% non-fat dry milk at room temperature for 1 h
and immunoblotted with anti-KLF15 (ab2647; dilution 1:200) and
anti-NF-κB (ab32360; dilution 1:1,000) at 37°C for 2 h, followed by
incubation with the secondary antibodies (ab6789; dilution 1:5,000)
for another 1 h at room temperature. All antibodies were purchased
from Abcam (Cambridge, MA, USA) Visualization was then performed
using an enhanced chemiluminescence western blotting detection
reagent (Thermo Fisher Scientific, Inc.). Finally, the protein
bands were scanned and quantified using a ChemiDoc™ MP
Imaging system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
HE staining of renal and spleen
tissues
The renal and spleen tissue samples were fixed in
10% (v/v) neutral formalin phosphate buffer, dehydrated in a graded
series of alcohol and embedded in paraffin. Next, 5-µm sections
were stained with hematoxylin for 3 min, washed and then stained
with 0.5% eosin for a further 3 min. The morphological alterations
in the kidney and spleen were blindly evaluated under a light
microscope (Nikon Eclipse TE2000; Nikon Corporation, Tokyo, Japan)
by an experienced pathologist.
Statistical analysis
All data are presented as the mean ± standard
deviation. The values obtained in the same group prior to and
following drug administration were compared using a paired t-test.
Comparisons between groups were conducted using one-way analysis of
variance followed by Duncan's test. P<0.05 was considered to
indicate a difference that was statistically significant.
Results
Body weight, UALB and serum
biochemical parameters
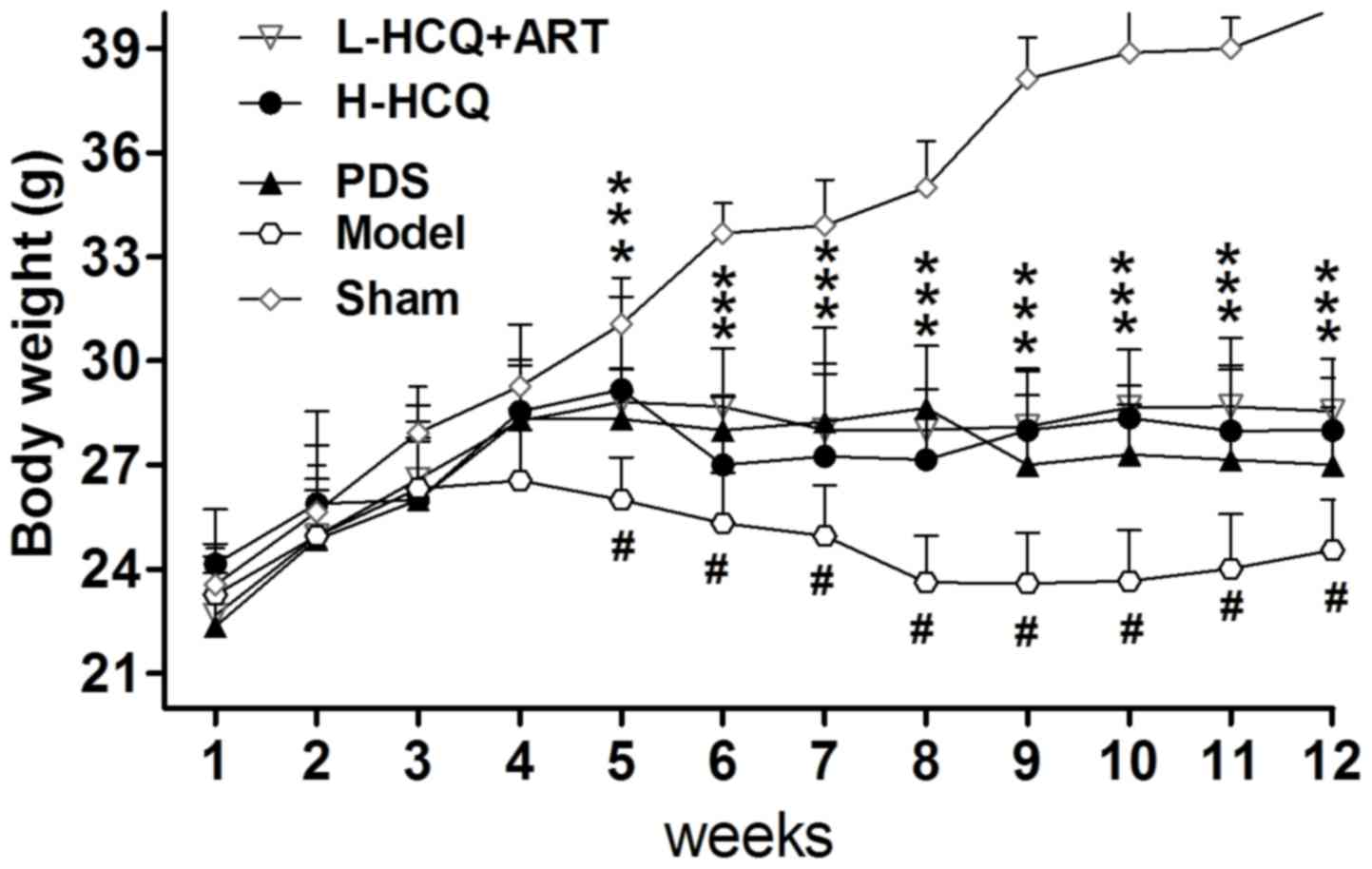
As shown in Fig. 1,
the body weight of LN animals was significantly decreased compared
with the sham group (P<0.01). However, after 5 weeks, treatment
in the PDS, H-HCQ and L-HCQ + ART groups helped to stabilize the
body weight, and these animals presented a significantly higher
weight compared with the model group (P<0.05). Furthermore, the
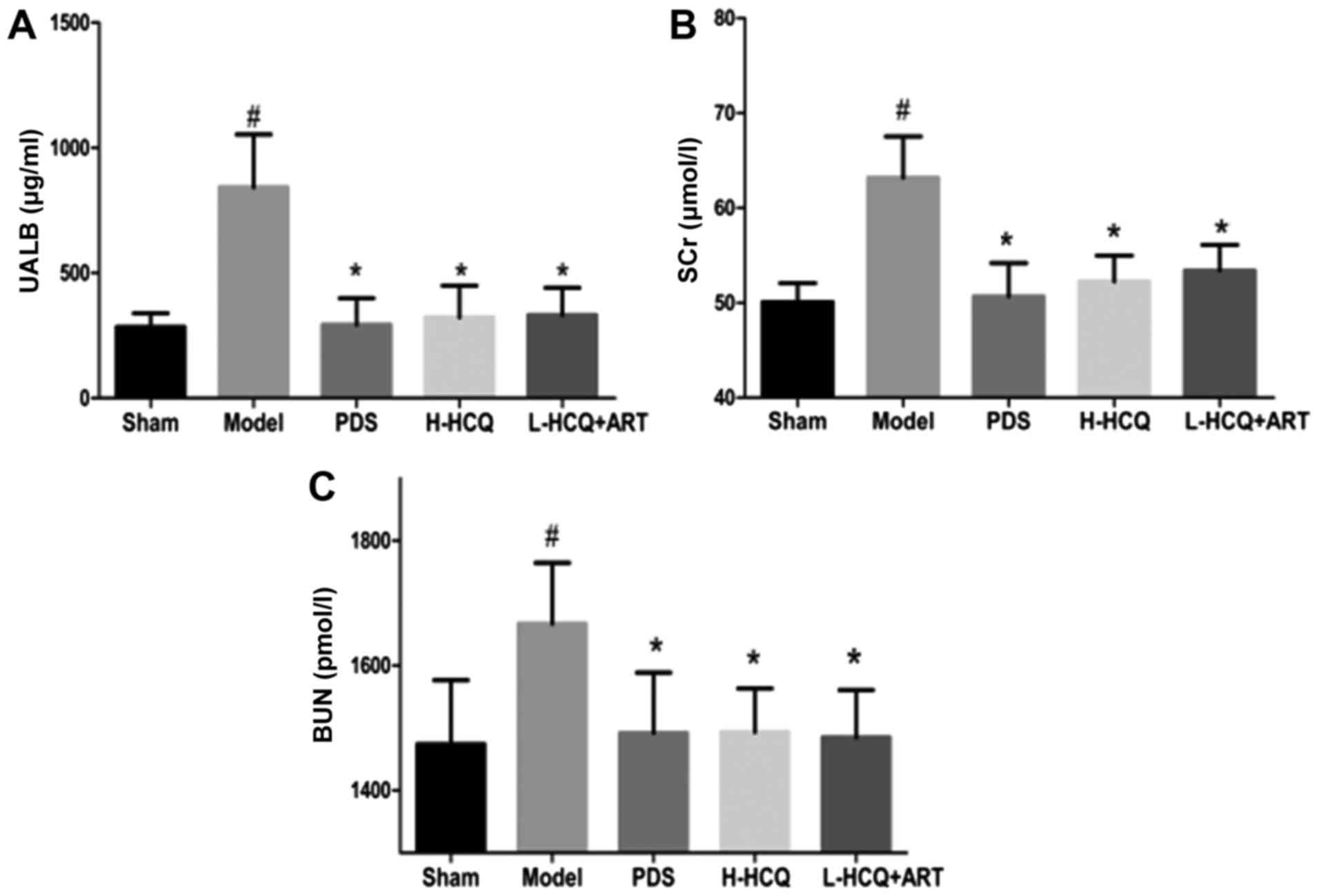
L-HCQ + ART treatment significantly reduced the LN-induced UALB,
SCr and BUN levels, as compared with the model group (P<0.05),
which was similar to the effect of PDS and H-HCQ, with no
significant difference observed among the three treatments
(Fig. 2).
L-HCQ + ART treatment decreases the
anti-ds-DNA, ANA and IgG levels
As shown in Fig. 3,
the anti-ds-DNA, ANA and IgG levels in the model group were
significantly increased compared with the sham group (P<0.05).
However, treatment of LN mice with PDS, H-HCQ and L-HCQ + ART was
able to significantly decrease the levels of anti-ds-DNA, ANA and
IgG (P<0.05). These data indicated that L-HCQ + ART treatment
had a similar immunosuppressive effect with that of H-HCQ
treatment.
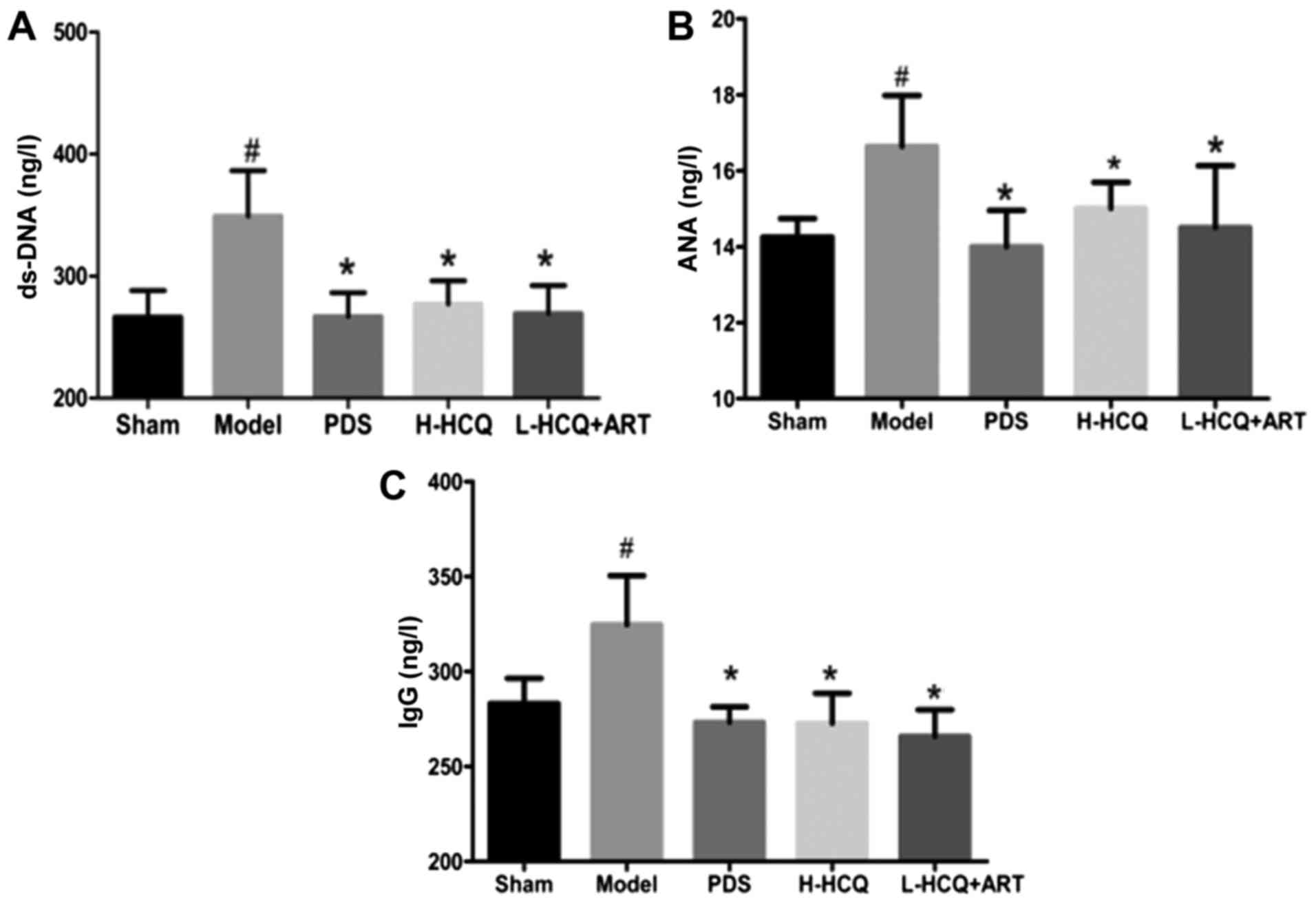 | Figure 3.Serum contents of (A) ds-DNA, (B) ANA
and (C) IgG in each group, as determined by ELISA. The values are
expressed as the mean ± standard deviation (n=10 mice). #P<0.01,
vs. the sham group; *P<0.05 vs. the model group. PDS,
prednisone; H-HCQ, high-dose hydroxychloroquine; L-HCQ, low-dose
hydroxychloroquine; ART, artemisinin; ds-DNA, double stranded DNA;
ANA, antinuclear antibodies; IgG, immunoglobulin G. |
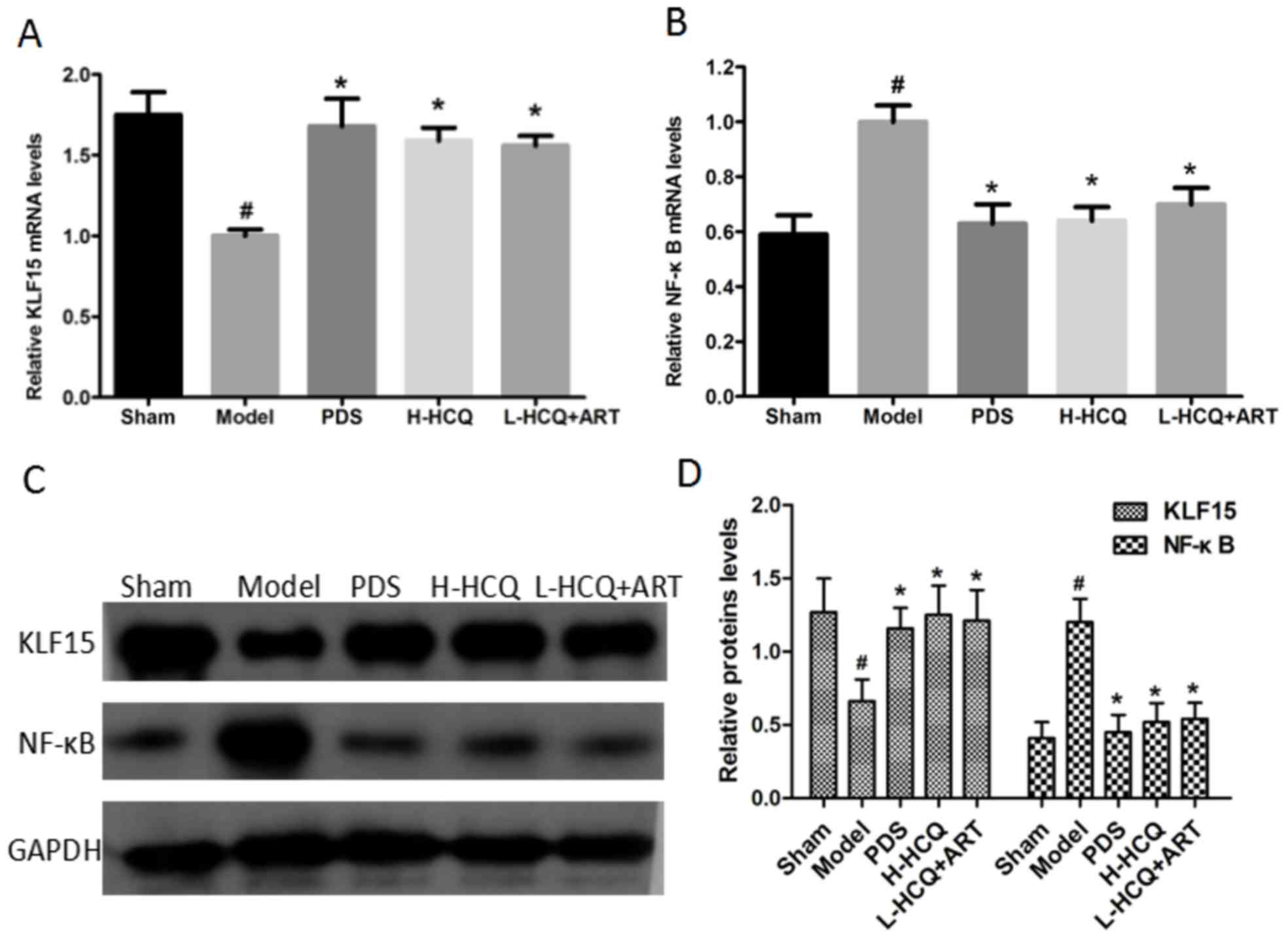
L-HCQ + ART regulates the KLF15 and
NF-κB expression levels
As shown in Fig. 4,
the model group exhibited significantly lower expression of KLF15
and higher expression of NF-κB at the mRNA and proteins levels, as
compared with the sham group (P<0.05). However, PDS, H-HCQ and
L-HCQ + ART treatment significantly increased the expression of
KLF15 and decreased the expression of NF-κB to varying degrees
(P<0.05). The results revealed that L-HCQ + ART in LN mice was
able to reverse the inflammatory response by downregulating NF-κB
and increasing the level of KLF15 expression.
L-HCQ + ART decreases the serum IFN-γ,
TNF-α and TGF-β1 levels
As shown in Fig. 5,
the contents of IFN-γ, TNF-α and TGF-β1 in the serum were markedly
increased in the model group compared with the sham group
(P<0.05). However, these alterations were reversed in the three
treatment groups, and PDS, H-HCQ and L-HCQ + ART treatments were
observed to markedly decrease the levels of IFN-γ, TNF-α and TGF-β1
in the LN mice (P<0.05). These data indicated that L-HCQ + ART
improved the inflammatory status of LN mice, similarly to the
treatments with PDS and H-HCQ.
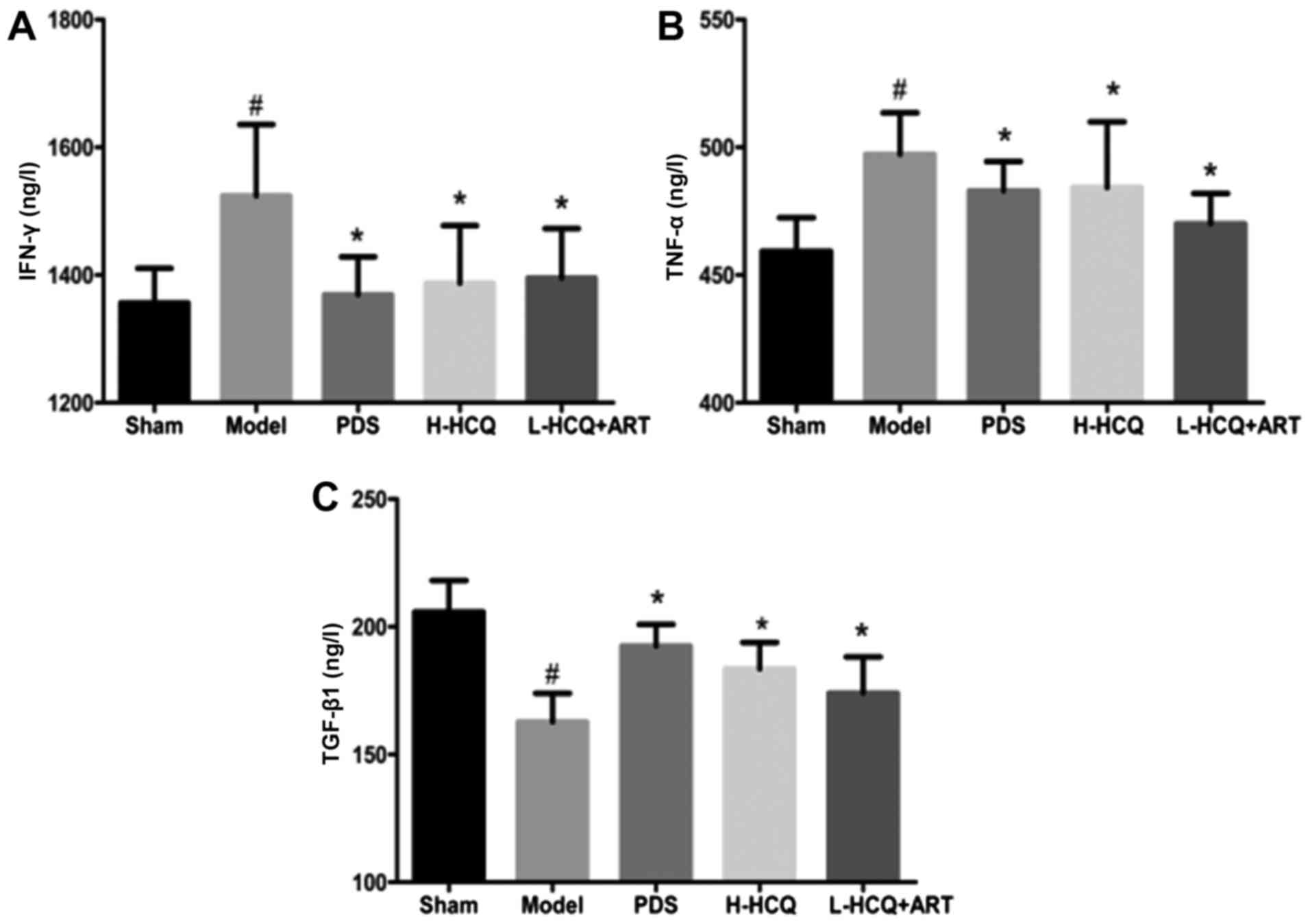 | Figure 5.Serum concentrations of (A) IFN-γ, (B)
TNF-α and (C) TGF-β1 in each group, as determined by ELISA. The
values are expressed as the mean ± standard deviation (n=10 mice).
#P<0.01, vs. the sham group; *P<0.05 vs. the model group.
PDS, prednisone; H-HCQ, high-dose hydroxychloroquine; L-HCQ,
low-dose hydroxychloroquine; ART, artemisinin; IFN-γ, interferon γ;
TNF-α, tumor necrosis factor-α; TGF-β1, transforming growth factor
β1. |
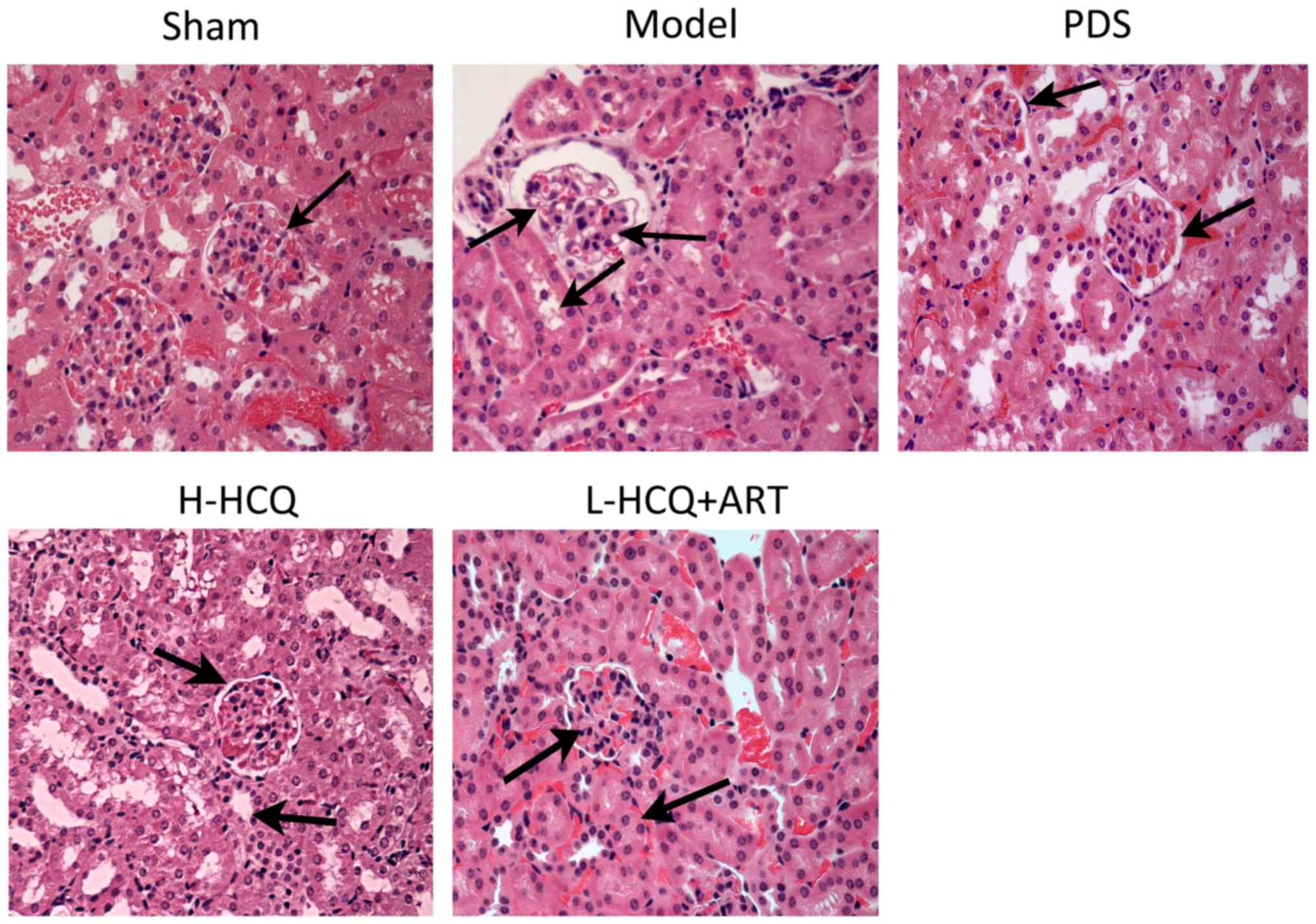
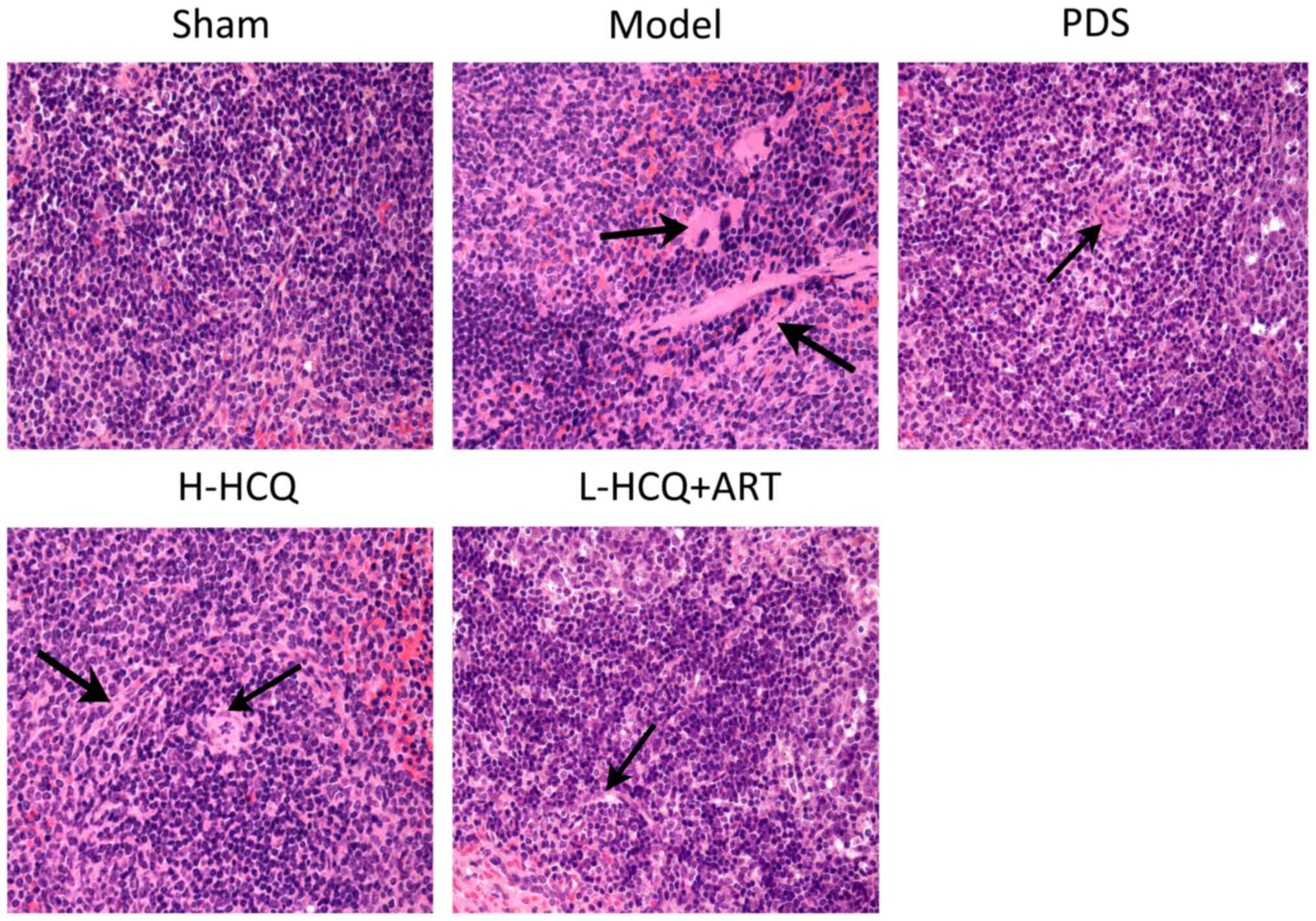
L-HCQ + ART improves the renal and
splenic pathomorphology
The results of the renal and splenic histopathology
examination by HE staining are shown in Figs. 6 and 7, respectively. In the model group, severe
pathological injury was observed in both the renal and splenic
tissues. In the renal tissue, the injury was characterized by
glomerulus enlargement, irregular glomerular basement membrane
thickening and an increase in the glomerular cell number. In the
splenic tissue, injury was characterized by clear cell interstitial
edema, splenic corpuscle hyperplasia, periarterial lymphatic sheath
thickening and partial splenic artery fibrosis. Notably, these
histopathological changes were ameliorated following treatment with
L-HCQ + ART, with a similar effect observed upon treatment with
H-HCQ or PDS. This suggests that the L-HCQ + ART combination
presented a similar effect to that of H-HCQ and PDS treatment,
alleviating the pathological renal and splenic damage in LN
mice.
Discussion
It has been previously reported that LN is
accompanied by renal function failure (19). When LN occurs the normal glomerular
filtration function is damaged, ultimately leading to elevated
levels of SCr and BUN, and the formation of proteinuria (20). In addition, LN is characterized by an
increase in ds-DNA and IgG levels, and a decrease in ANA (21,22). LN
is initiated by the glomerular deposition of IgG and complement in
the majority of cases (23). ds-DNA
is considered to be one of the characteristics of the polyreactive
autoantibody pathogenesis of LN. Anti-DNA antibodies exert their
pathogenic effects through deposition as immune complexes in the
kidney or through the recognition of cross-reactive antigens in the
kidney, while in situ deposition of an immune complex
(composed of DNA, anti-DNA antibody and IgG) in the renal tissue
leads to the activation of the complement system and causes a
series of immune injury (24).
Tubulointerstitial injury is found frequently in lupus nephritis.
Immune complex deposits can occur in the tubular basement membranes
(TBMs), it was proved TBM deposits were common in lupus nephritis
patients and correlated closely with the clinical disease activity
and renal outcome.
In the present study, the results indicated that
treatment with PDS, H-HCQ or L-HCQ + ART significantly decreased
the levels of UALB, SCr, BUN, ds-DNA, ANA and IgG. Treatment with a
low dose of HCQ combined with ART was observed to have a similar
effect to that of a high dose of HCQ. Furthermore, HE staining
indicated that L-HCQ + ART improved the renal pathologic damage in
LN mice, and weight detection indicated that this combined
treatment inhibited the LN-induced weight loss. The aforementioned
results suggested that a low dose of HCQ combined with ART was able
to improve the LN-induced renal function failure.
Podocyte injury resulting from a loss of
differentiation is the hallmark of various glomerular diseases.
KLF15 is a key regulator of podocyte differentiation, and a loss of
KLF15 increases the susceptibility to kidney injury (25). KLF15 is highly expressed in the
normal endothelial and mesangial cells of the kidney (26). NF-κB is a downstream target gene of
KLF15, and KLF15 affects the downstream cascade reaction by
regulating the expression of NF-κB. A previous study has
demonstrated that KLF15 alters the acetylation status of NF-κB and
activates NF-κB by direct interaction with P300 (27). In general, the coactivator P300
interacts with NF-κB p56 to induce the NF-κB acetylation-activated
inflammatory pathway, while KLF15 interferes with the formation of
P300-p56 by competing with P300. Therefore, increasing the
expression level of KLF15 can inhibit the activity of NF-κB,
consequently inhibiting the inflammatory response and improving the
LN (28). In the present study,
KLF15 was significantly decreased and NF-κB was markedly increased
following allogeneic lymphocyte injection for 4 weeks. By contrast,
treatment with PDS, H-HCQ or L-HCQ + ART was able to reverse these
alterations. Thus, the current study proved that L-HCQ combined
with ART may regulate the KLF15-NF-κB signaling pathway.
NF-κB belongs to a family of inducible transcription
factors. Accumulating evidence demonstrates that the transcription
factor NF-κB serves a crucial role in regulating the expression of
numerous pro-inflammatory cytokines during the immune response
(1). TNF-α activates the NF-κB
signaling pathway in order to amplify the inflammatory response and
increases the duration of chronic inflammation (5). In addition, Yazici et al
reported that IFN-γ may have a role in the pathogenesis and
progression of LN (29). T-cell
cytokines, including IFN-γ, have also been detected in nephritic
kidneys (30). Furthermore, TGF-β1,
as an important cytokine, has been demonstrated to promote
extracellular matrix synthesis and secretion, and lead to mesangial
cell proliferation (31). Therefore,
the measurement of inflammatory cytokine IFN-γ, TNF-α and TGF-β1
levels is of great significance for predicting the development of
LN. In the current study, PDS, H-HCQ or L-HCQ + ART was observed to
decrease significantly the levels of IFN-γ, TNF-α and TGF-β1, while
the L-HCQ + ART combined treatment and H-HCQ alone had a similar
anti-inflammatory effect. Additionally, HE staining of tissue from
the spleen, the main immune organ, indicated that L-HCQ + ART
improved the spleen pathologic damage in LN mice. These results
confirmed that anti-inflammatory effect is one of the mechanisms
underlying the action of these treatments. Furthermore, it is
suggested that improvements in the immunologic response and
anti-inflammatory ability are the dominant mechanisms involved in
the attenuation of LN following L-HCQ + ART combined treatment.
In conclusion, the present study demonstrated that
the combination of a low dose of HCQ and ART presented a similar
immunosuppressive effect as that of the PDS and H-HCQ treatments.
The potential mechanisms underlying the effect of L-HCQ + ART
combined treatment may be through regulation of the expression
levels of cytokines, KLF15 and NF-κB. The use of a low dose of HCQ
may help reduce the side effects of H-HCQ, which may provide a
novel method for the treatment of LN.
Acknowledgements
The present study was supported by the YangFan
Innovative and Entrepreneurial Research Team Project (grant no.
2014YT02S008), the China Postdoctoral Science Foundation (grant no.
2015M572294) and the Guangdong Provincial Science and Technology
Department Project (grant no. 2014B050502013).
References
|
1
|
Bhatt D and Ghosh S: Regulation of the
NF-κB-mediated transcription of inflammatory genes. Front Immuno.
5:712014. View Article : Google Scholar
|
|
2
|
Appel GB, Contreras G, Dooley MA, Ginzler
EM, Isenberg D, Jayne D, Li LS, Mysler E, Sánchez-Guerrero J,
Solomons N, et al: Mycophenolate mofetil versus cyclophosphamide
for induction treatment of lupus nephritis. J Am Soc Nephrol.
20:1103–1112. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ruiz-Irastorza G, Danza A, Perales I,
Villar I, Garcia M, Delgado S and Khamashta M: Prednisone in lupus
nephritis: How much is enough? Autoimmun Rev. 13:206–214. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Illei GG, Austin HA, Crane M, Collins L,
Gourley MF, Yarboro CH, Vaughan EM, Kuroiwa T, Danning CL, et al:
Combination therapy with pulse cyclophosphamide plus pulse
methylprednisolone improves long-term renal outcome without adding
toxicity in patients with lupus nephritis. Ann Intern Med.
135:248–257. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kawasaki H, Onuki R, Suyama E and Taira K:
Identification of genes that function in the TNF-alpha-mediated
apoptotic pathway using randomized hybrid ribozyme libraries. Nat
Biotechnol. 20:376–380. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng ZH, Zhang LJ, Liu WX, Lei YS, Xing
GL, Zhang JJ, Quan SX, Liu D, Hu DS, Li LL and Liu ZS: Predictors
of survival in Chinese patients with lupus nephritis. Lupus.
21:1049–1056. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pons-Estel GJ, Alarcón GS, McGwin G Jr,
Danila MI, Zhang J, Bastian HM, Reveille JD and Vilá LM; Lumina
Study Group, : Protective effect of hydroxychloroquine on renal
damage in patients with lupus nephritis: LXV, data from a
multiethnic US cohort. Arthritis Rheum. 61:830–839. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee SJ, Silverman E and Bargman JM: The
role of antimalarial agents in the treatment of SLE and lupus
nephritis. Nat Rev Nephrol. 7:718–729. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wallace DJ, Gudsoorkar VS, Weisman MH and
Venuturupalli SR: New insights into mechanisms of therapeutic
effects of antimalarial agents in SLE. Nat Rev Rheumatol.
8:522–533. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Costedoat-Chalumeau N, Dunogué B, Leroux
G, Morel N, Jallouli M, Le Guern V, Piette JC, Brézin AP, Melles RB
and Marmor MF: A critical review of the effects of
hydroxychloroquine and chloroquine on the eye. Clin Rev Allergy
Immunol. 49:317–326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bhavsar KV, Mukkamala LK and Freund KB:
Multimodal imaging in a severe case of hydroxychloroquine toxicity.
Ophthalmic Surg Lasers Imaging Retina. 46:377–379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ding HJ, Denniston AK, Rao VK and Gordon
C: Hydroxychloroquine-related retinal toxicity. Rheumatology
(Oxford). 55:957–967. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abdel Galil SM: Hydroxychloroquine-induced
toxic hepatitis in a patient with systemic lupus erythematosus: A
case report. Lupus. 24:638–640. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Y, He S, Bai B, Zhang L, Xue L, Lin Z,
Yang X, Zhu F, He P, Tang W and Zuo J: Therapeutic effects of the
artemisinin analog SM934 on lupus-prone MRL/lpr mice via inhibition
of TLR-triggered B-cell activation and plasma cell formation. Cell
Mol Immunol. 13:379–390. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu XL, Zhang WG, Shi XM, An P, Sun WS,
Qiao CL and Wang Z: Effect of artemisinin combined with
glucocorticoid on the expressions of glucocorticoid receptor α
mRNA, glucocorticoid receptor β mRNA and P300/CBP protein in lupus
nephritis mice. Chin J Integr Med. 17:277–282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
The People's Republic of China of
Laboratory Animals-Guideline of welfare and ethics. Standardization
Administration of China. 2016.
|
|
17
|
Wen ZK, Xu W, Xu L, Cao QH, Wang Y, Chu YW
and Xiong SD: DNA hypomethylation is crucial for apoptotic DNA to
induce systemic lupus erythematosus-like autoimmune disease in
SLE-non-susceptible mice. Rheumatology (Oxford). 46:1796–1803.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shaharir SS, Ghafor AA, Said MM and Kong
NC: A descriptive study of the factors associated with damage in
Malaysian patients with lupus nephritis. Lupus. 23:436–442. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang ZM, Zhao L, Zhang XL and Wang XF:
Correlation analysis between urinary T lymphocyte subsets and lupus
nephritis disease activity. Int J Clin Exp Med. 10:6061–6070.
2017.
|
|
21
|
Bertsias GK, Tektonidou M, Amoura Z,
Aringer M, Bajema I, Berden JH, Boletis J, Cervera R, Dörner T,
Doria A, et al: Joint European league against rheumatism and
European renal association-European dialysis and transplant
association (EULAR/ERA-EDTA) recommendations for the management of
adult and paediatric lupus nephritis. Ann Rheum Dis. 71:1771–1782.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Imran TF, Yick F, Verma S, Estiverne C,
Ogbonnaya-Odor C, Thiruvarudsothy S, Reddi AS and Kothari N: Lupus
nephritis: An update. Clin Exp Nephrol. 20:1–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tucci M, Stucci S, Strippoli S and
Silvestris F: Cytokine overproduction, T-cell activation and
defective T-regulatory functions promote nephritis in systemic
lupus erythematosus. Bio Med Res Int. 2010:4571462010.
|
|
24
|
Deshmukh US, Bagavant H and Fu SM: Role of
anti-DNA antibodies in the pathogenesis of lupus nephritis.
Autoimmun Rev. 5:414–418. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mallipattu SK, Liu R, Zheng F, Narla G,
Ma'ayan A, Dikman S, Jain MK, Saleem M, D'Agati V, Klotman P, et
al: Kruppel-like factor 15 (KLF15) is a key regulator of podocyte
differentiation. J Biol Chem. 287:19122–19135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hong Q, Li C, Xie Y, Lv Y, Liu X, Shi S,
Ding R, Zhang X, Zhang L, Liu S and Chen X: Kruppel-like factor-15
inhibits the proliferation of mesangial cells. Cell Physiol
Biochem. 29:893–904. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu Y, Zhang L, Liao X, Sangwung P,
Prosdocimo DA, Zhou G, Votruba AR, Brian L, Han YJ, Gao H, et al:
Kruppel-like factor 15 is critical for vascular inflammation. J
Clini Invest. 123:4232–4241. 2013. View
Article : Google Scholar
|
|
28
|
Jain MK, Sangwung P and Hamik A:
Regulation of an inflammatory disease. Arterioscler Thromb Vasc
Biol. 34:499–508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yazici MU, Orhan D, Kale G, Besbas N and
Ozen S: Studying IFN-gamma, IL-17 and FOXP3 in pediatric lupus
nephritis. Pediatr Nephrol. 29:853–862. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Davidson A: What is damaging the kidney in
lupus nephritis? Nat Rev Rheumatol. 12:143–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang L, Sun X, Zhan Y, Liu H, Wen Y, Mao
H, Dong XI and Li P: Yi Qi Qing Re Gao-containing serum inhibits
lipopolysaccharide-induced rat mesangial cell proliferation by
suppressing the Wnt pathway and TGF-β1 expression. Exp Ther Med.
11:1410–1416. 2016. View Article : Google Scholar : PubMed/NCBI
|