Introduction
Paraquat (PQ) is an effective and commercially
important herbicide widely used throughout the world. However, the
mortality rate of PQ poisoning has been reported to range from 50
to 90% when the pesticide is ingested either accidentally or
intentionally as a suicide attempt (1). A previous study suggested that absorbed
PQ requires removal from the bloodstream in order to improve the
survival rate of patients with severe PQ poisoning (2).
PQ is not metabolized to any extent and is rapidly
excreted in the urine after PQ poisoning even at low doses. The
kidneys are effective at eliminating PQ but are vulnerable to PQ
injury (3,4). Therefore, elimination of PQ simply
relying on the kidney is slow, and removal of PQ from the blood by
activated charcoal hemoperfusion (HP) in the first 12–15 h
following ingestion may be beneficial (5). Various studies have indicated that HP
is more efficient in the clearance of plasma PQ than the kidneys
(2). Therefore, activated charcoal
HP is widely used in the treatment of PQ poisoning in China
(6).
Although the kidneys and HP are the two major routes
of eliminating PQ following ingestion (7,8), renal
excretion is considered to be the major natural pathway of PQ
elimination. Therefore, the initial renal function is an important
factor for survival.
Application of HP has been reported to accelerate
the removal of PQ from the blood. However, to the best of our
knowledge, no previous study has focused on the effect of HP on
renal function and PQ elimination by the kidneys. The present study
determined the effect of HP on renal function and PQ elimination
via the kidneys.
Materials and methods
Patients
A total of 19 patients with PQ poisoning were
respectively observed in the present study. The subject selection
criteria included the following: i) Oral PQ poisoning; ii) no acute
kidney injury on admission; and iii) age of <65 years and <18
years. Potential participants were excluded if they were initially
treated at a different hospital, or had any known cardiac,
pulmonary or other chronic disease associated with a certain degree
of renal failure as an underlying condition of PQ poisoning. Acute
kidney injury was defined by a serum creatinine (SCr) level of
>97 µmol/l and blood urea nitrogen (BUN) levels of >8.3
mmol/l.
Treatment of the patients
All patients received HP therapy (Braun Diapact CRRT
machine; Braun GmbH, Kronberg im Taunus, Germany) until plasma PQ
levels became undectable, and then received continuous veno-venous
hemofiltration therapy for 12 h after HP. To prevent absorption of
PQ via the gastrointestinal tract, gastric lavage was performed via
a nasogastric tube using 1 g/kg activated charcoal in 500 ml 0.9%
saline for one time every 4 h. Furthermore, Smecta (Beaufour Ipsen
Pharmacy Co., Ltd., Tianjin, China) and magnesium sulfate (Huairen
Pharmacy Co., Ltd., Tianjin, China) powder were placed into 20%
mannitol (Shuanghe Pharmacy Co., Ltd., Tianjin, China) was
administered via the anus.
Other treatments included intravenous infusion of
cyclophosphamide (Guangdong Qingping Pharmacy Co., Ltd., Guangzhou,
China), methylprednisolone sodium succinate injection (Pfizer,
Inc., New York, NY, USA) and intravenous injections of
dexamethasone (Jilin Extrawell Changbaishan Pharmaceutical Co.,
Ltd., Jilin, China). Vitamin E capsules (Xinyi Pharmaceutical Co.,
Ltd., Shanghai, China), metoprolol (Astra Zeneca, London, UK) and
vitamin E injections (Zhongjing Biotechnology Co., Ltd., Harbin,
China) were also administered.
Data collection
The following data were collected: Demographical
factors (age, sex and medical history), initial BUN, SCr, plasma
and urine PQ concentration within 12 h of admission to the
intensive care unit. Samples of plasma and urine were collected
every 3 h while HP was performed. BUN and SCr were measured with a
full automatic biochemical analyzer (AU5800; Beckman Coulter, Inc.,
Brea, CA, USA). Quantitative analysis of the plasma and urine PQ
concentration was performed at the hospital laboratory by a gas
chromatography method (9).
Statistical analysis
SPSS statistical software package 20.0 (IBM Corp.,
Armonk, NY, USA) and GraphPad Prism v4.0 (GraphPad Software, Inc.,
La Jolla, CA, USA) were used to perform statistical analysis.
Values are expressed as the mean ± standard deviation. Categorical
variables are expressed as numbers or percentages for each item.
Statistically significant differences between the two groups were
analyzed using the independent two-samples t-test or the
Mann-Whitney U test. The Chi-square test was used to assess the
association between treatment protocols and survival rate.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Demographic and laboratory data
Demographic and laboratory results of the patients
are summarized in Table I. A total
of 19 patients were analyzed, including 8 females and 11 males
(mean age, 40.37±12.55 years; range, 25–63 years). A total of 7
patients died from pulmonary fibrosis and 12 survived. There were
no significant inter-group differences in age or time between
poisoning and HP therapy.
 | Table I.Clinical status of each of the 19
patients. |
Table I.
Clinical status of each of the 19
patients.
|
|
|
|
|
|
|
| SCr (µmol/l) | BUN (mmol/l) |
|
|---|
| Subject no. | Sex | Age (years) | Initial PPC
(mg/l) | Initial UPC
(mg/l) | Time between
poisoning and HP therapy (h) | Duration of activated
charcoal HP therapy (h) | Prior to HP | After HP | At discharge | Prior to HP | After HP | At discharge | Outcome |
|---|
| 1 | Male | 39 | 5.7 | 9.2 | 7 | 18 | 69 | 62 | 258 | 5.1 | 4.5 | 12.9 | Death |
| 2 | Male | 28 | 6.3 | 12.1 | 5 | 17 | 64 | 94 | 100 | 4.0 | 6.5 | 6.8 | Death |
| 3 | Female | 30 | 10.1 | 21.7 | 6 | 20 | 63 | 91 | 228 | 3.5 | 5.1 | 14.1 | Death |
| 4 | Female | 54 | 6.5 | 15.7 | 6 | 17 | 68 | 57 | 135 | 6.5 | 9.5 | 13.1 | Death |
| 5 | Male | 30 | 2.1 | 4.4 | 8 | 14 | 76 | 79 | 99 | 5.8 | 5.1 | 13.5 | Survival |
| 6 | Female | 39 | 4.6 | 11.2 | 10 | 16 | 32 | 112 | 53 | 4.2 | 8.5 | 7.0 | Survival |
| 7 | Female | 49 | 2.3 | 6.2 | 8.5 | 17 | 31 | 45 | 78 | 3.5 | 2.6 | 10.9 | Survival |
| 8 | Female | 56 | 1.6 | 2.8 | 9 | 16 | 49 | 86 | 84 | 6.3 | 7.8 | 9.4 | Survival |
| 9 | Male | 58 | 9.8 | 18.6 | 5.5 | 23 | 85 | 84 | 208 | 7.9 | 9.3 | 13.5 | Death |
| 10 | Male | 25 | 11.8 | 23.7 | 7 | 16 | 57 | 109 | 153 | 4.0 | 6.4 | 10.6 | Death |
| 11 | Female | 48 |
1.45 | 3.5 | 5 | 8 | 46 | 38 | 51 | 5.7 | 5.3 | 6.8 | Survival |
| 12 | Male | 47 | 4.1 | 6.1 | 10.5 | 17 | 70 | 101 | 188 | 5.1 | 8.9 | 11.2 | Death |
| 13 | Female | 25 | 0.1 | 0.7 | 4.5 | 6 | 45 | 38 | 49 | 3.7 | 7.0 | 3.5 | Survival |
| 14 | Female | 29 | 3.1 | 4.5 | 6.5 | 12 | 72 | 57 | 86 | 5.4 | 6.8 | 8.1 | Survival |
| 15 | Male | 33 | 2.2 | 3.5 | 8 | 14 | 45 | 46 | 46 | 2.9 | 2.4 | 6.3 | Survival |
| 16 | Male | 63 | 3.6 | 6.1 | 9 | 18 | 45 | 74 | 145 | 5.4 | 7.0 | 14.1 | Survival |
| 17 | Male | 36 | 3.5 | 6.9 | 5.5 | 14 | 78 | 75 | 82 | 4.6 | 4.7 | 6.3 | Survival |
| 18 | Male | 50 | 1.1 | 1.7 | 11 | 8 | 48 | 49 | 62 | 3.7 | 4.6 |
5.6 | Survival |
| 19 | Male | 26 |
4.3 | 16.7 | 6.5 | 6 | 69 | 92 | 88 | 4.5 | 3.5 |
7.6 | Survival |
The renal function of all patients was normal on
admission, and that of 16 patients remained normal after HP.
However, non-oliguric renal failure occurred in all of the 7
patients who died. Among the 12 surviving patients, 10 had a normal
renal function, while 2 (subjects nos. 5 and 16) developed
non-oliguric renal failure (Table
I).
The group of patients who did not survive had a
higher initial plasma PQ concentration and longer time of activated
charcoal HP therapy than the group of survivors. All patients
exhibited a progressive increase in SCr and BUN levels during
hospitalization. However, there were no significant inter-group
differences in BUN at baseline, as well as SCr and BUN after HP.
However, the group of non-survivors had a higher SCr and BUN than
the survivors at discharge (Table
II).
 | Table II.Demographic and laboratory data of
patients stratified by outcome. |
Table II.
Demographic and laboratory data of
patients stratified by outcome.
|
|
|
|
|
|
| SCr (µmol/l) | BUN (mmol/l) |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Group | n | Age (years) | Initial PPC
(mg/l) | Delay between
poisoning and HP therapy (h) | Time of activated 1
charcoa HP therapy (h) | Prior to HP | After HP | At discharge | Prior to HP | After HP | At discharge |
|---|
| Death | 7 | 40.43±13.53 | 7.75±2.81 | 6.71±1.82 | 18.29±2.43 | 68.00±8.72 | 85.43±19.42 | 181.43±55.34 | 5.16±1.57 | 7.17±2.06 | 11.74±2.51 |
| Survival | 12 | 40.33±12.56 | 2.50±1.35 | 7.63±2.04 | 12.42±4.34 | 53.00±16.45 | 65.91±23.90 | 76.92±27.97 | 4.68±1.13 | 5.44±2.00 | 8.26±3.18 |
| P-value |
| 0.988 | <0.001 | 0.339 | 0.005 | 0.040 | 0.449 | 0.085 | 0.090 | <0.001 | 0.024 |
Effect of activated charcoal HP on the
kidney toxicokinetics of paraquat
Plasma and urine PQ concentrations prior to and
during HP are presented in Fig. 1.
The plasma PQ concentration decreased rapidly when HP was
performed. The concentrations of PQ in urine were associated with
the plasma PQ concentration. When the plasma PQ concentration was
>0.5 mg/l, the urine PQ concentration decreased in parallel with
the plasma PQ concentration. Fig. 1
also revealed that urinary PQ concentrations were almost 2 times
greater than plasma PQ concentrations when the plasma PQ
concentration was >0.5 mg/l (P<0.05). When the plasma PQ
concentration was <0.5 mg/l, the urine PQ concentration tended
to be equal to the plasma PQ concentration.
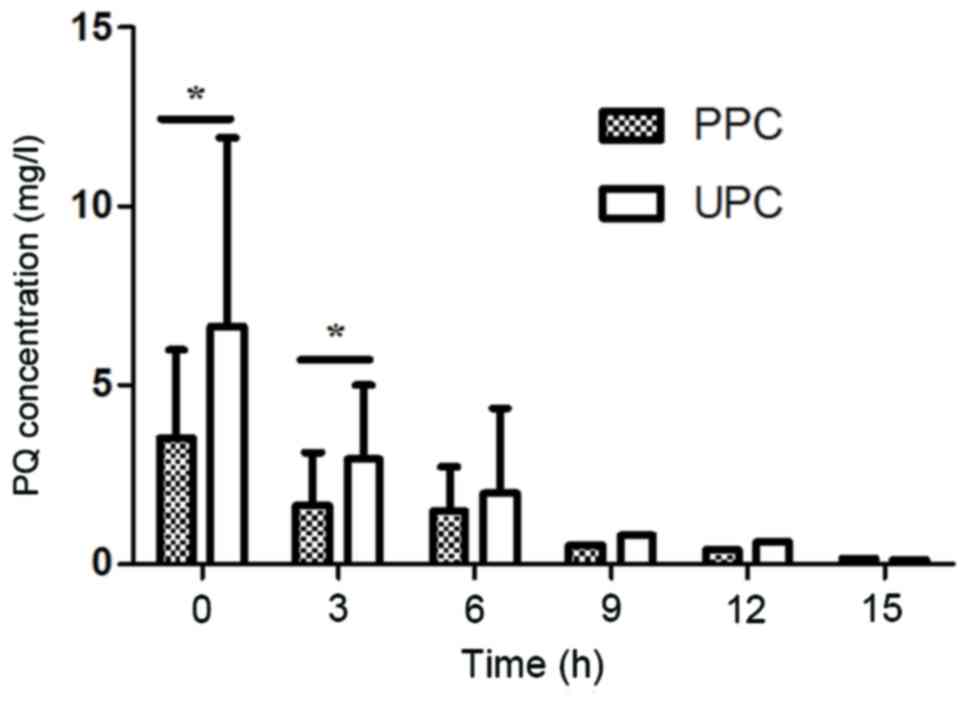 | Figure 1.PPC and UPC at various time-points
during HP therapy. Prior to HP therapy (0 h): PPC, 3.50±2.493 mg/l;
UPC, 6.66±5.25 mg/l (n=19)-; 3 h after HP therapy: PPC, 1.65±1.477
mg/l; UPC, 2.94±2.085 mg/l (n=17); 6 h after HP therapy: PPC,
1.48±1.228 mg/l; UPC, 1.98±2.362 mg/l (n=10); 9 h after HP therapy:
PPC, 0.50 mg/l; UPC, 0.80 mg/l (n=3); 12 h after HP therapy: PPC,
0.40 mg/l; UPC, 0.60 mg/l (n=1); 15 h after HP therapy: PPC, 0.15
mg/l; UPC, 0.1 mg/l (n=1). *P<0.05 as indicated. UPC, urine
paraquat concentration; PPC, plasma paraquat concentration; HP,
charcoal hemoperfusion. |
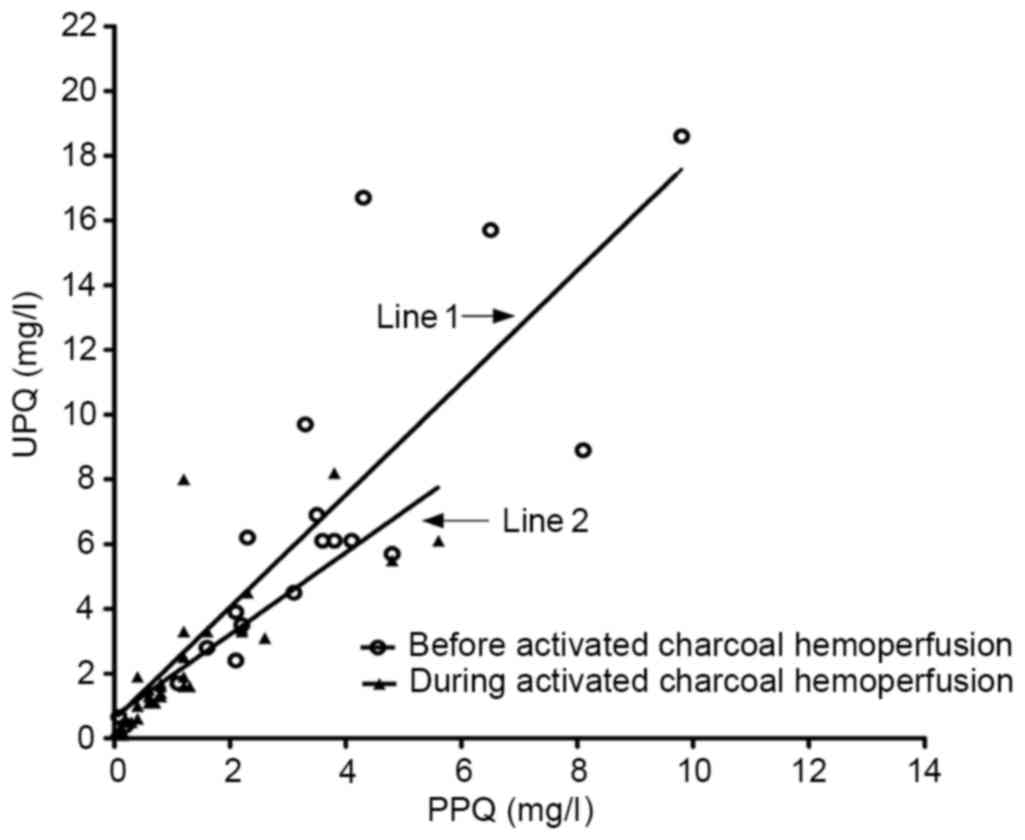
The association between the plasma and urine PQ
concentration prior to and during HP is presented in Fig. 2. At baseline, there was a linear
association between plasma and urine PQ concentration. The equation
parameters together with the correlation coefficient on admission
were as follows: Y=0.5820+1.7348X (R2=0.678; F=35.768;
P<0.0001). However, during HP, there was also a linear
correlation between blood and urine PQ concentration. The equation
parameters together with the correlation coefficient were as
follows: Y=0.6827+1.2649X (R2=0.626; F=50.308;
P<0.0001).
Discussion
In the present study, HP was demonstrated to
effectively eliminate PQ. The kidney toxicokinetics of PQ during HP
were assessed, which were previously not well assessed in humans.
In the present study, urinary and plasma PQ concentrations were
continuously monitored while HP was performed. A linear correlation
was identified between the plasma and urine PQ concentration while
HP was performed. When the plasma PQ concentration was >0.5
mg/l, urinary concentrations of PQ were nearly 2 times greater than
the plasma concentrations. It was suggested that the kidney
toxicokinetics of PQ at a given time may be interpreted along with
the plasma levels of PQ, and are not associated with HP.
However, these results did not agree with those of
Ikebuchi (10), who reported that
urinary concentrations of PQ were 3.3–4.5 times greater than the
plasma concentrations if renal function was normal. This
discrepancy is likely due to differences in test methods and study
populations. Another reason may be that HP eliminates BUN and SCr,
which affects the judgment of renal function.
Theoretically, accelerated removal of PQ from the
blood should have a protective effect on the kidney. However, SCr
and BUN of all patients displayed a progressive increase during
hospitalization. Non-oliguric acute renal failure still occurred in
9 patients in spite of a rapid decrease in the PQ concentrations
being observed. Not only the 7 patients who died, but also two of
the surviving patients developed non-oliguric renal failure.
However, no severe renal failure occurred in the survival group.
These results suggested that HP has positive effects on avoiding
kidney damage in patients with mild-to-moderate PQ-poisoning, and
is ineffective for patients with severe PQ poisoning. It is likely
that the potentially damaging concentration of PQ had already been
attained in the kidney when HP was performed.
Finally, it should be noted that, even though renal
function is normal in the early stage of PQ poisoning, and the
toxin was removed effectively, this did not affect the clinical
outcome in patients who had ingested a potentially lethal dose of
PQ.
In conclusion, the present study suggested that the
kidney toxicokinetics of PQ were only associated with the plasma PQ
concentration in patients with normal renal function. Activated
charcoal HP had little effect on avoiding acute kidney injury in
patients with severe PQ poisoning.
Acknowledgements
The authors are grateful to the Basic Research
Project of the Logistics University of Chinese People's Armed
Police Force (grant no. WHJ2015020) for financially supporting the
present study.
References
|
1
|
Jones GM and Vale JA: Mechanisms of
toxicity, clinical features, and management of diquat poisoning: A
review. J Toxicol Clin Toxicol. 38:123–128. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hsu CW, Lin JL, Lin-Tan DT, Chen KH, Yen
TH, Wu MS and Lin SC: Early hemoperfusion may improve survival of
severely paraquat-poisoned patients. PLoS One. 7:e483972012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kang MS, Gil HW, Yang JO, Lee EY and Hong
SY: Comparison between kidney and hemoperfusion for paraquat
elimination. J Korean Med Sci. 24 Suppl:S156–S160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pavan M: Acute kidney injury following
paraquat poisoning in india. Iran J Kidney Dis. 7:64–66.
2013.PubMed/NCBI
|
|
5
|
Hawksworth GM, Bennett PN and Davies DS:
Kinetics of paraquat elimination in the dog. Toxicol Appl
Pharmacol. 57:139–145. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu L, Hong G, Ma J, Wang X, Lin G, Zhang X
and Lu Z: Clearance rate and BP-ANN model in paraquat poisoned
patients treated with hemoperfusion. Biomed Res Int.
2015:2982532015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pond SM, Rivory LP, Hampson EC and Roberts
MS: Kinetics of toxic doses of paraquat and the effects of
hemoperfusion in the dog. J Toxicol Clin Toxicol. 31:229–246. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hong SY, Yang JO, Lee EY and Kim SH:
Effect of haemoperfusion on plasma paraquat concentration in vitro
and in vivo. Toxicol Ind Health. 19:17–23. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Posecion NC, Ostrea EM and Bielawski DM:
Quantitative determination of paraquat in meconium by sodium
borohydride-nickel chloride chemical reduction and gas
chromatography/mass spectrometry (GC/MS). J Chromatogr B Analyt
Technol Biomed Life Sci. 862:93–99. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ikebuchi J: Evaluation of paraquat
concentrations in paraquat poisoning. Arch Toxicol. 60:304–310.
1987. View Article : Google Scholar : PubMed/NCBI
|