Introduction
Primary liver cancer (PLC) comprises hepatocellular
carcinoma (HCC), hepatoblastoma, intrahepatic cholangiocarcinoma
and other rarer tumors (1).
Hepatoblastoma is the most common type of primary liver tumor in
children <5 years old (2). The
incidence rate of PLC is increasing in Australia, Central Europe,
the United Kingdom, Japan and North America, and this increase will
likely continue due to infection with hepatitis B virus (HBV) and
hepatitis C virus (HCV) (3). For
patients with localized HCC, liver transplantation and resection
are viable, useful treatments option (4). However, curative resection or
transplantation is only possible in 30% of patients, which means
that the overall survival rate of HCC remains poor (5). Transcatheter arterial chemoembolization
(TACE) has been demonstrated to have survival benefits in patients
with intermediate and advanced HCC and hepatoblastoma (6–8). TACE
may reduce tumor burden and significantly improve the prognosis and
survival of patients (6). However,
TACE may also cause local hypoxia in the tumor environment,
resulting in the expression of hypoxia-inducible factor-1α (HIF-1α)
and vascular endothelial growth factor (VEGF), which promote
neovascularization (9). It is
therefore necessary to study the mechanism of hypoxia-induced
angiogenesis to identify novel ways of inhibiting the growth of
PLC.
Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1,
6-heptadiene-3,5-di-one] is a polyphenolic compound isolated from
the rhizomes of Curcuma longa, which has been revealed to
have anti-inflammatory, antioxidant, anti-angiogenesis and
antitumor activities (10,11). Curcumin has also been reported to
protect the liver against the toxic effects of agents, including
galactosamine (12), C-C motif
chemokine 4 (13) and paracetamol
(14). It has previously been
reported that curcumin inhibits human breast carcinoma MCF-7 cell
proliferation by inhibiting the insulin-like growth factor (IGF)-1
axis (15). Patel et al
(16) demonstrated that the
inclusion of curcumin to continued folinic acid, fluorouracil and
oxaliplatin treatment reduced the survival of colon cancer cells
and concurrently reduced the activation of endothelial growth
factor receptor, v-erb-b2 erythroblastic leukemia viral oncogene
homolog 2 (HER-2), IGF-1 receptor (IGF-1R) and protein kinase B
(Akt). However, to the best of our knowledge, there has been little
investigation into whether curcumin affects the angiogenesis of HCC
via the IGF-1R signaling pathway.
Previous studies have revealed that IGF-1R is
overexpressed in the majority of PLC tissues (17,18). The
interaction between IGF-1R and its ligands serves a key role in
regulating the expression of VEGF (17,19).
Therefore, IGF-1R knockout HepG2 cells were constructed to
investigate whether curcumin regulates the expression of HIF-1α and
VEGF via the IGF-1R signaling pathway.
Materials and methods
Cell culture and chemicals
The human hepatoblastoma cell line, HepG2, was
obtained from the American Type Culture Collection (Manassas, VA,
USA). IGF-1R knockout HepG2 cells were constructed and conserved
within the laboratory. HepG2 cells were maintained in Dulbecco's
modified Eagle's medium (DMEM; Gibco, Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(FBS; Gibco, Thermo Fisher Scientific, Inc.), 100 U/ml penicillin
and 100 µg/ml streptomycin at 37°C in an atmosphere containing 5%
CO2. Curcumin and CoCl2 were purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Curcumin was
dissolved in dimethylsulfoxide and CoCl2 was dissolved
in DMEM.
Preparation of IGF-1R knockout
cells
The candidate 20-base guide (g)RNA sequences were
derived from the IGF-1R genome sequence (gene ID: 3480). The cDNA
sequence was obtained from Han's lab (http://hanlab.xmu.edu.cn/cdna/Query.aspx, No. 25207).
The clustered regularly interspaced short palindromic repeats
(CRISPR)/Cas9 design tool from Zhang's lab (crispr.mit.edu/) was used to search the functional
gRNA sequence (5′-GAGAACTGCACGGTGATCGAGGG-3′), which contain the
downstream 3′protospacer-adjacent motif with GG dinucleotide
(N20-NGG). According to the functional gRNA sequence, a pair of
oligo DNA were designed and synthesized (hIGF1R-QC-F,
5′-CACCGAGAACTGCACGGTGATCGA-3′; hIGF1R-QC-R,
5′-AAACTCGATCACCGTGCAGTTCTC-3′). The complementary oligo DNA were
hybridized (50 ng) and then inserted to pGK1.1 (20 ng) (Genloci
Biotechnologies, Inc., Jiangsu, China; http://www.genloci.com) and named
pGK1.1-U6-IGF-1RgRNA. HepG2 cells were seeded in a 24-well plate 24
h prior to transfection and transfected with the
pGK1.1-U6-IGF-1RgRNA plasmid via the electroporation method using a
Neon® Transfection system (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Following transfection, the cells were diluted into 10 96-well
plates and incubated at 37°C in an atmosphere containing 5%
CO2 for 2 weeks. The cells from each 96-well plate were
then transferred to 48-well plates and incubated at 37°C in an
atmosphere containing 5% CO2 for further incubation.
After cell cultures were 70% confluent, they were harvested and the
genomic DNA was extracted using DNA Extraction kits (Genloci
Biotechnologies, Inc.). PCR was conducted to amplify the target
region with genomic DNA derived from the cells. DNA polymerase
(cat. no. R060Q; Takara, Bio, Inc., Otsu, Japan) was use for PCR.
The following primer sequences were used: hIGF1R-seq-F:
5′-GTTTACCCTCTTGTCTCCCT-3′ and hIGF1R-seq-R:
5′-CGGTAATGACCGTGAGCTTG-3′. PCR was performed using the following
thermocycling conditions: 95°C for 10 sec, 60°C for 10 sec, 72°C
for 20 sec for 30 cycles. Amplicons were sent to the Beijing
Genomics Institute (Beijing, China) for deep sequencing.
Hypoxia and CoCl2
incubation
HepG2 cells were plated in 96-well plates (5,000
cells/well) or 6-well plates (3×105 cells/well) in DMEM
supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml
streptomycin at 37°C in an atmosphere containing 5% CO2.
Following 24 h, the medium was changed to fresh serum-free DMEM
with different concentrations of CoCl2 (0, 50, 100, 150,
200 and 400 µM) for different time periods (1, 2, 4, 6 and 8
h).
Cell survival assay
HepG2 cells were plated in a 96-well plate in 100 µl
medium at a density of 5,000 cells/well and incubated using
standard culture conditions overnight. The medium was removed
carefully and the purple formazan was dissolved with 150 µl
dimethyl sulfoxide. Cell viability was determined by an MTT assay
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) according to the
manufacturer's protocol. The optical density was determined at 570
nm by SpectraMax M2 (Molecular Devices, LLC, Sunnyvale, CA,
USA).
Western blot analysis
Total protein was isolated from cells using a
radioimmunoprecipitation buffer reagent (cat. no. P0013B, Beyotime
Institute of Biotechnology, Shanghai, China). The lysates were
ultrasonicated and centrifuged at 12,000 × g and at 4°C for 10 min.
Supernatants were collected and stored at −70°C. Protein
concentrations were determined using a Bicinchoninic Acid Protein
Assay kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Protein (50 µg per lane) was separated by
10% SDS-PAGE and electroblotted onto a nitrocellulose membrane. The
membrane was blocked with Tris-buffered saline (TBS)/5% nonfat dry
milk at room temperature for 2 h and the membranes were
subsequently incubated with primary antibodies overnight at 4°C.
Following three washes with TBS+Tween-20 (TBST), the membranes were
incubated with goat polyclonal immunoglobulin G horseradish
peroxidase-conjugated secondary antibodies (cat. no. sc-2004;
1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 45
min at room temperature. The membranes were washed with TBST three
times prior to visualization using a Western Blotting Luminol
Reagent (cat. no. sc-2048, Santa Cruz Biotechnology, Inc.). Image J
1.6 software (National Institutes of Health, Bethesda, MD, USA) was
used to detect and analyze the relative densitometry. The primary
antibodies used in the experiment were as follows: Rabbit
anti-IGF-1R (cat. no. 9750), rabbit anti-HIF-1α (cat. no. 3716),
rabbit anti-VEGF (cat. no. 2463), rabbit anti-Akt (cat. no. 9272),
rabbit anti-phosphorylated (p)-Akt (cat. no. 5012), rabbit
anti-extracellular signal-regulated kinases (Erk1/2; cat. no.
4695), rabbit anti-p-Erk1/2 (cat. no. 4377) (all 1:1,000; Cell
Signaling Technology, Inc., Danvers, MA, USA) and rabbit
anti-β-actin (cat. no. sc-7210; 1:4,000; Santa Cruz Biotechnology,
Inc.).
Statistical analysis
Data are presented as the mean ± standard deviation
and were analyzed using SPSS version 19.0 (IBM Corp., Armonk, NY,
USA). Differences between the groups at each time point were
examined for statistical significance using one-way analysis of
variance and a least significant difference post-hoc comparison.
The correlation between HIF-1α and VEGF protein levels was
evaluated statistically using Pearson's correlation coefficient
analysis if the quantitative data had a normal distribution; if the
data was not normally distributed, the Spearman rank correlation
coefficient analysis was used. P<0.05 was considered to indicate
a statistically significant difference.
Results
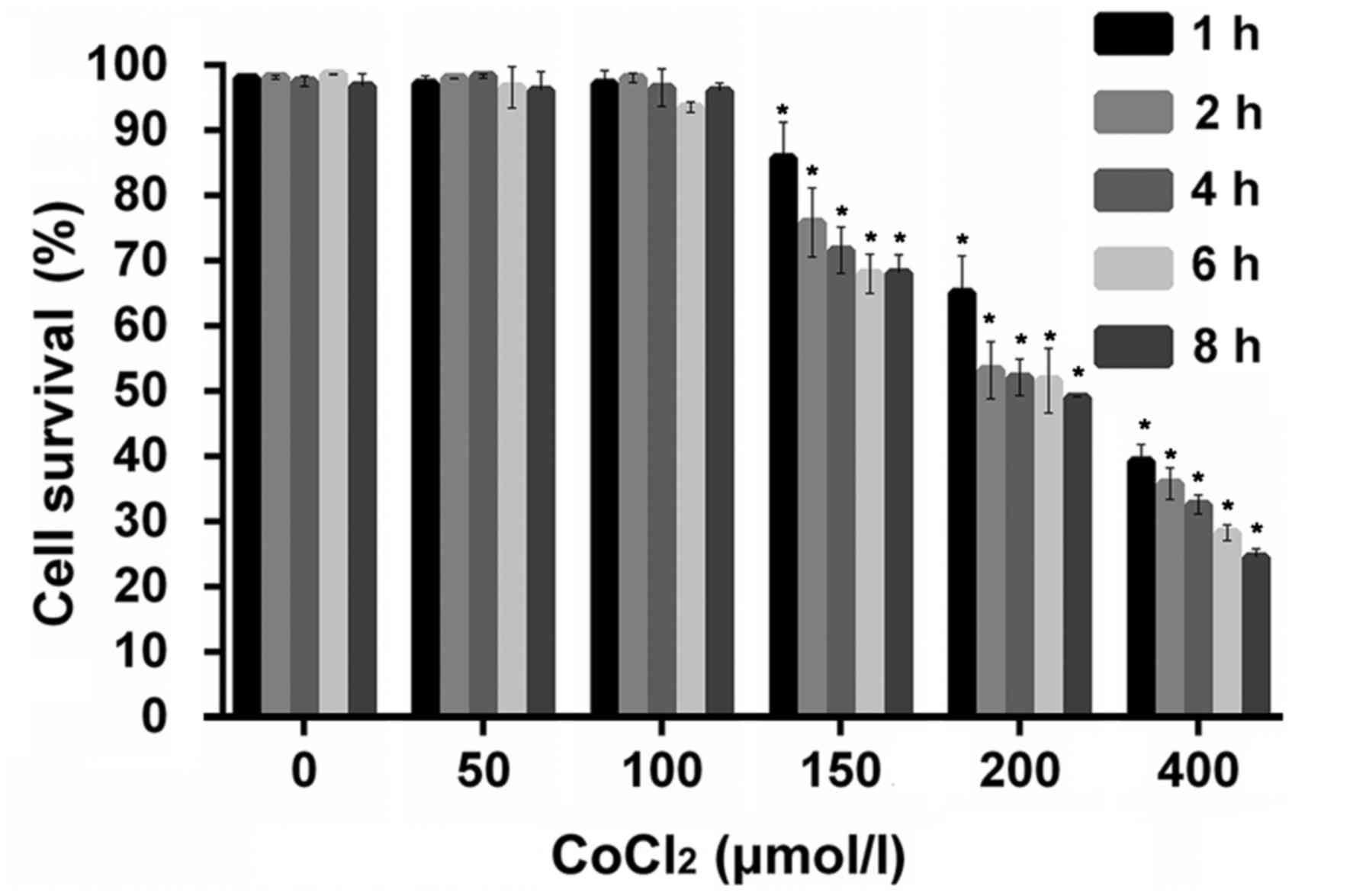
Effect of hypoxia on the viability of
IGF-1R knockout HepG2 cells
An IGF-1R knockout HepG2 cell line was successfully
constructed using a CRISPR/Cas9 genome-editing system. To
investigate the effect of hypoxia on the IGF-1R knockout HepG2 cell
line, cells were treated with different concentrations of
CoCl2 for 1–8 h and the viability was subsequently
analyzed using an MTT assay. No significant differences were
observed between the control cells and those treated with low
CoCl2 doses (50–100 µM; Fig.
1). However, the viability of IGF-1R knockout HepG2
cells was significantly reduced in the 150, 200 and 400 µM
CoCl2 treated groups compared with the control (no
CoCl2 treatment) at the same time points (P<0.05;
Fig. 1).
Effect of CoCl2-induced
hypoxia on HIF-1α, IGF-1R and VEGF expression in IGF-1R knockout
HepG2 cells
Western blot analysis was performed to examine the
effects of CoCl2-induced hypoxia on HIF-1α, IGF-1R and
VEGF protein expression in IGF-1R knockout HepG2 cells. The cells
were treated with 0, 50, 100, 150, 200 or 400 µM CoCl2
for 6 h and the expression of HIF-1α, IGF-1R and VEGF was
determined by western blotting. The protein expression of HIF-1α
and VEGF was significantly higher in cells treated with 150 or 200
µM CoCl2 compared with the control group (P<0.05;
Fig. 2A and B). Correlation analysis
revealed that the expression of the HIF-1α was positively
correlated with the expression of VEGF (r=0.85, P<0.05) in a
dose-dependent manner (data not shown).
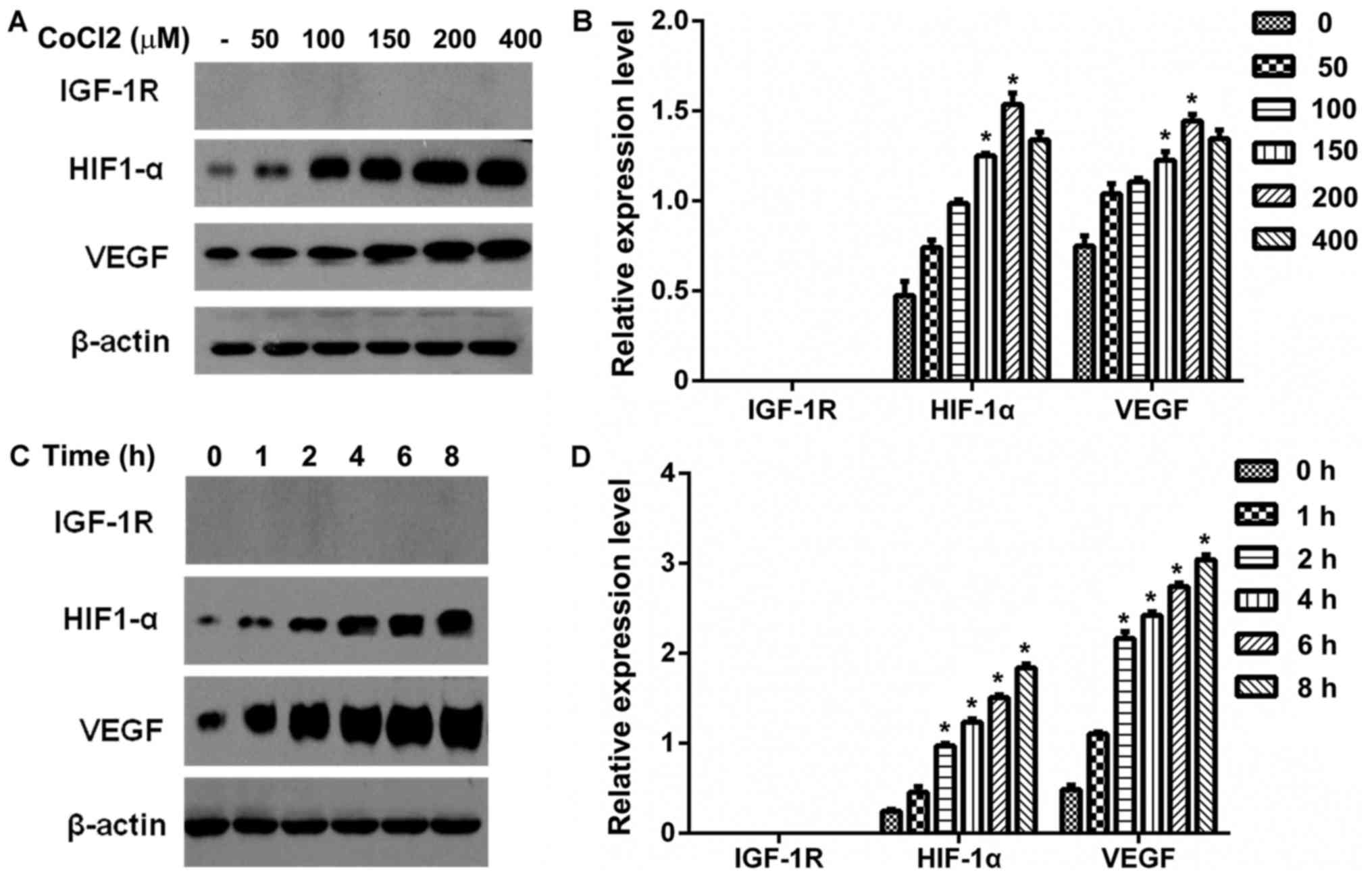 | Figure 2.Effect of CoCl2-induced
hypoxia on IGF-1R, HIF-1α and VEGF protein expression in HepG2
cells. (A) Serum-starved IGF-1R knockout HepG2 cells were treated
with different concentrations of CoCl2 (0, 50, 100, 150,
200 and 400 µM) for 6 h. IGF-1R, HIF-1α and VEGF protein expression
were detected by western blot analysis. (B) Western blot analysis
data was quantified and the protein expression of IGF-1R, HIF-1α
and VEGF are presented as a bar graph. *P<0.05 vs. the control
group (0 uM). (C) Serum-starved IGF-1R knockout HepG2 cells were
treated with CoCl2 (150 µM) for the indicated time (0–8
h). Total cell protein was extracted and IGF-1R, HIF-1α and VEGF
protein expression was determined by western blot analysis. (D)
Graphic representation of the relative density of IGF-1R, HIF-1α
and VEGF protein expression levels, which were normalized to those
of β-actin. *P<0.05 vs. the control group (0 h). IGF-1R,
insulin-like growth factor-1 receptor; VEGF, vascular endothelial
growth factor; HIF-1α, hypoxia-inducible factor-1α;
CoCl2, cobalt chloride. |
The IGF-1R knockout HepG2 cells were
treated with 150 µM CoCl2 for the indicated time periods
and the protein expression of HIF-1α, IGF-1R and VEGF was
determined by western blotting (Fig.
2C). The relative expression of each protein was calculated and
it was observed that the protein expression of HIF-1α and VEGF was
significantly increased at 2–8 h compared with the control group
(P<0.05; Fig. 2D). In addition,
HIF-1α expression was positively correlated with the expression of
VEGF (r=0.71, P<0.05) in a time-dependent manner (data not
shown).
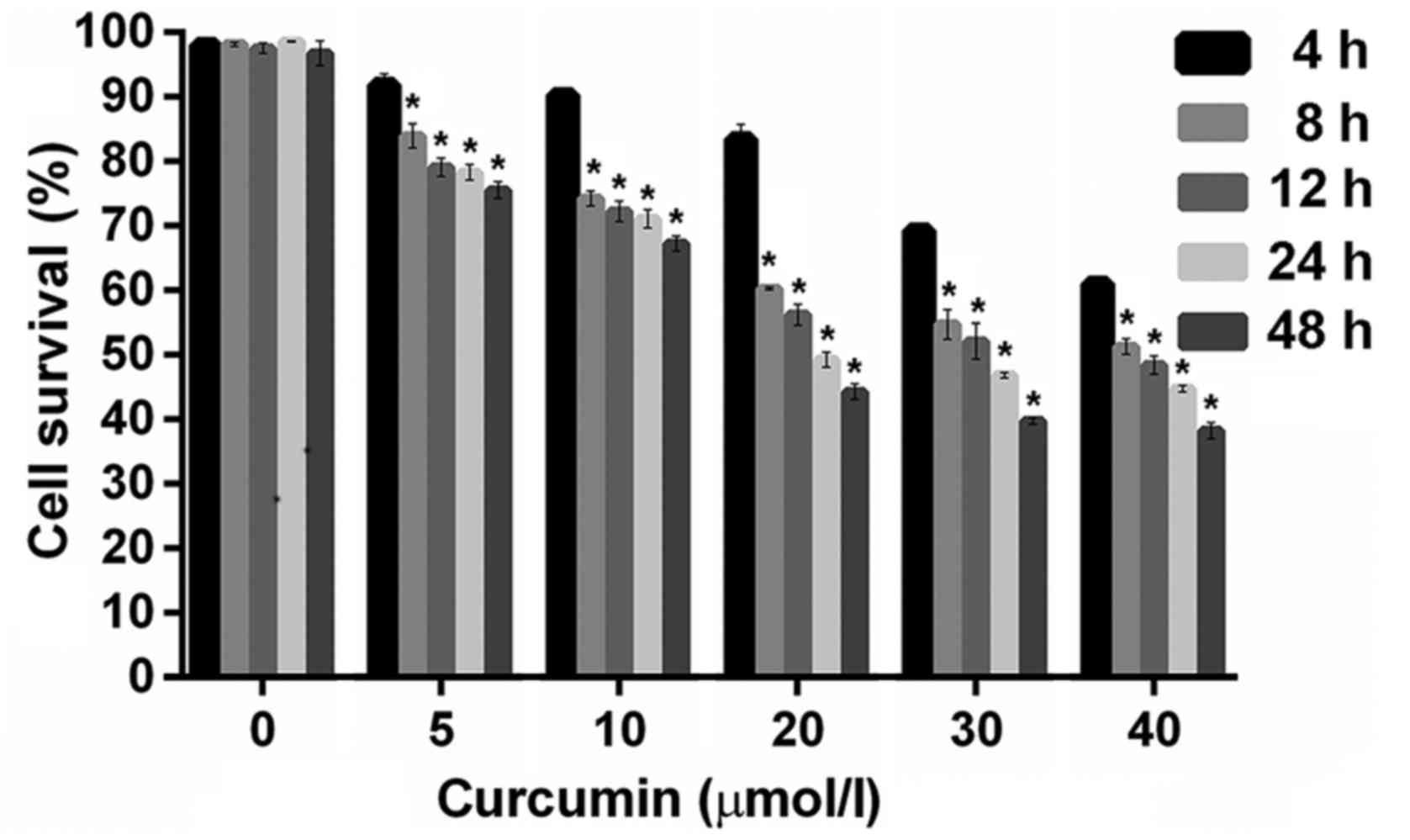
Effect of curcumin on IGF-1R knockout
HepG2 cell viability under CoCl2-induced hypoxia
IGF-1R knockout HepG2 cells were incubated with
increasing concentrations of curcumin (5, 10, 20, 30 and 40 µM) in
the presence of 150 µM CoCl2. It was observed that the
cell survival rate decreased as the incubation time or dose
increased, and all doses of curcumin caused a reduction in cell
survival (Fig. 3). The incubation of
cell cultures with different concentrations of curcumin at each
time point (8, 12, 24 and 48 h) had significant inhibitory effects
on the survival of IGF-1R knockout HepG2 cells compared with the
control group (P<0.05; Fig.
3).
Effect of curcumin on IGF-1R, p-Akt,
p-Erk1/2, HIF-1α and VEGF protein expression in IGF-1R knockout
HepG2 cells in the presence of CoCl2
HepG2 cells and IGF-1R knockout HepG2 cells were
treated with or without curcumin (20 µM) under
CoCl2-induced hypoxic conditions. Following 24 h of
treatment, total cell protein was extracted and analyzed using
western blotting (Fig. 4A). The
expression of IGF-1R, p-Akt, p-Erk1/2, HIF-1α and VEGF proteins was
significantly reduced in HepG2 cells treated with curcumin compared
with untreated cells (P<0.05; Fig.
4B-F). IGF-1R knock out cells treated with curcumin also
exhibited markedly reduced p-Akt, p-Erk1/2, HIF-1α and VEGF protein
expression compared with the IGF-1R knock out cells not treated
with curcumin (P<0.05). Knockdown IGF-1R also inhibited the
expression of HIF-1α, VEGF, p-Akt and p-Erk proteins in
CoCl2-induced HepG2 cells (P<0.05, Fig. 4C-F). Additionally, the difference
between control group (lane A) and HepG2 cells with
CoCl2 plus curcumin (lane B) seems greater than the
difference between IGF-1R knockout HepG2 cells with
CoCl2 (lane C) and IGF-1R knockout HepG2 cells with
CoCl2 plus curcumin (lane D).
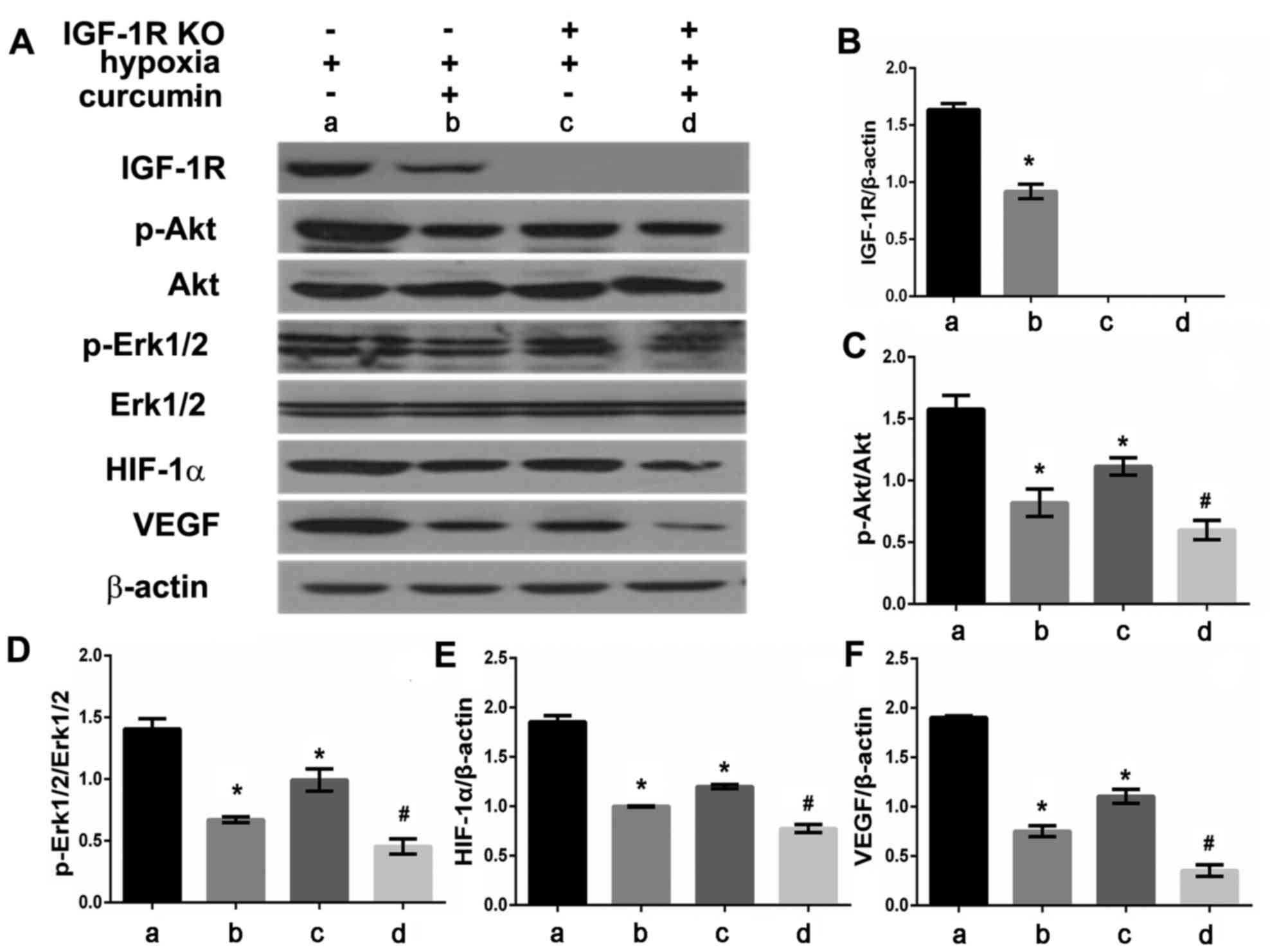 | Figure 4.Effect of curcumin and hypoxia on
IGF-1R, p-Akt, p-Erk1/2, HIF-1α, VEGF protein expression in IGF-1R
knockout HepG2 cells. (A) IGF-1R, p-Akt, p-Erk1/2, HIF-1α, VEGF
protein expression were detected by western blot analysis. Graphic
representation of relative density of (B) IGF-1R, (C) p-Akt, (D)
p-Erk1/2, (E) HIF-1α and (F) VEGF protein levels, which were
normalized to those of β-actin, Akt or Erk1/2, respectively.
*P<0.05 vs. the control group (vs. group A),
#P<0.05 vs. IGF-1R knockout HepG2 cells with
CoCl2 treatment group (vs. group C). IGF-1R,
insulin-like growth factor-1 receptor; Akt, protein kinase B; p,
phosphorylated; Erk, extracellular signal-regulated kinases; VEGF,
vascular endothelial growth factor; HIF-1α, hypoxia-inducible
factor-1α. |
Discussion
Angiogenesis serves a crucial role in tumor growth,
invasion and metastasis (20). Tumor
neovascularization is a complex process that is regulated by
angiogenic activators and inhibitors (20). Hypoxia is an important stimulus and
key regulator of angiogenesis in cancer, and tumor hypoxia may
occur due to increased metabolic activity and oxygen consumption by
rapidly proliferating tumor cells (19). Hypoxia may be induced by TACE,
transarterial embolization, portal venous embolizations or other
treatments for PLC (21).
TACE may reduce the tumor burden and improve the
prognosis and survival rate of patients with PLC; however, TACE
also induces hypoxic conditions, which promotes the secretion of
HIF-1α and VEGF and leads to tumor angiogenesis, recurrence and
metastasis (8). Several previous
studies have confirmed that VEGF is one of the most reliable
prognostic markers of HCC (22–24).
VEGF may be useful in monitoring the response to treatment in
patients with HCC undergoing TACE, and angiogenesis in these
patients may be a target for adjuvant treatments with drugs
interfering with the process (25).
Liapi and Geschwind (26) reviewed
several anti-angiogenic agents combined with TACE for the treatment
of HCC, including sorafenib, bevacizumab. Sorafenib as a
complementary treatment acting on VEGF improved overall survival,
time to progression and objective response rate when administrated
prior to or in conjunction with TACE (27). Sorafenib was also reported to inhibit
CoCl2-induced HIF-1α and VEGFA expression in hepatoma
cells (28). However, the incidence
of adverse reactions was higher in the TACE + sorafenib group
compared with the TACE only group; these adverse reactions included
hepatotoxicity, hypertension and abdominal pain (27,29).
These results suggest that sorafenib may reduce the overexpression
of VEGF when combined with TACE, although it cause liver injury.
Curcumin was reported to have protective effects on the liver and
anticancer effects (30), whereas
its effects on angiogenesis under hypoxic conditions are poorly
understood.
HIF-1α is the major transcription factor
specifically activated during hypoxia (19). It regulates the expression of
molecules associated with glucose transport, glycolysis,
erythropoiesis, iron transport and angiogenesis (31). In the present study, CoCl2
was utilized to mimic hypoxia as it enhances the stability of
HIF-1α. It was observed that CoCl2 at a concentration of
150, 200 and 400 µM suppressed the survival ability of IGF-1R
knockout HepG2 cells; this effect may be due to its cytotoxicity at
high concentrations. Additionally, the expression of HIF-1α and
VEGF was increased in a time- and dose-dependent manner and
correlation analysis revealed that the expression of HIF-1α was
positively correlated with the expression of VEGF. These results
are consistent with a previous study by the authors (32), which concluded that hypoxia induced
the accumulation of IGF-1R and HIF-1α and regulated the expression
of VEGF in HepG2 cells. In the present study, IGF-1R knockout HepG2
cells were constructed and it was revealed that curcumin inhibits
the IGF-1R signaling pathway under hypoxic conditions.
The IGF system has emerged as an important pathway
in the development and progression of hepatoblastoma and represents
a potential therapeutic target (33,34). The
anti-IGF-1R antibody has been reported to be effective in reducing
cell proliferation and promoting apoptosis, as well as having a
synergistic effect with doxorubicin (35). CoCl2 is a chemical inducer
of HIF-1 and active HIF-1 induces the expression of VEGF (36). In the present study, IGF-1R knockdown
inhibited the expression of HIF-1α, VEGF p-Akt and p-Erk proteins.
Curcumin also significantly suppressed the expression of IGF-1R,
HIF-1α, VEGF p-Akt and p-Erk proteins. The IGF-1R signaling pathway
consists of IGF-1, IGF-1R and its downstream signaling targets
(37). Akt and mitogen-activated
protein kinase are two main downstream signaling targets of the
IGF-1R signaling pathway (37). In
the present study, the addition of curcumin reduced the expression
of HIF-1α, VEGF, p-Akt and p-Erk, which indicates that curcumin may
inhibit HIF-1α and VEGF in an IGF-1R-independent manner. These
results suggest that curcumin may suppress the expression of HIF-1α
and VEGF by inhibiting IGF-1R or its downstream signaling pathways
(Fig. 5).
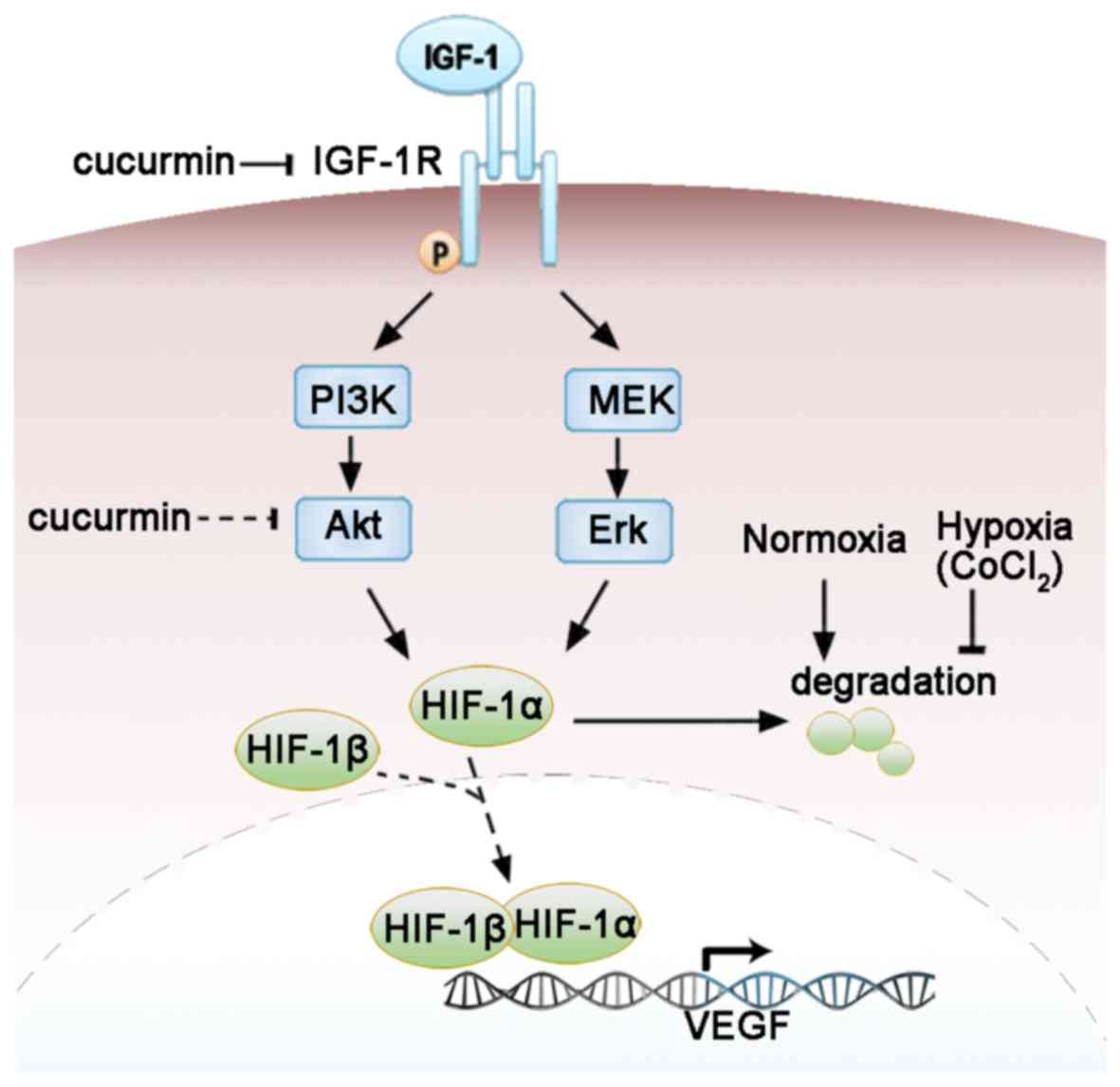 | Figure 5.Molecular mechanisms by which
curcumin regulates the IGF-1R signaling pathway and the expression
of VEGF in HepG2 cells under CoCl2-induced hypoxia. The
arrow and blocked arrow (no arrowhead) indicate stimulation and
inhibition, respectively. The dotted arrow indicates the
translocation of HIF-1α and HIF-1β. The dotted blocked arrow
indicates the potential effects of curcumin not directly examined
in the present study. It was concluded that curcumin may suppress
the expression of HIF-1 and VEGF by targeting IGF-1R or its
downstream signaling pathways. VEGF, vascular endothelial growth
factor; HIF-1α, hypoxia-inducible factor-1α; HIF-1β,
hypoxia-inducible factor-1β; IGF-1, insulin-like growth factor-1;
IGF-1R, insulin-like growth factor-1 receptor; PI3K,
phosphoinositide-3-kinase; Akt, protein kinase B; MEK,
mitogen-activated protein kinase-Erk kinase; Erk, extracellular
signal-regulated kinase. |
The present study was not without limitations.
Firstly, CoCl2-induced hypoxia primarily depends on the
regulation of HIF-1α and there are HIF-independent pathways that
are capable of inducing angiogenesis in response to hypoxia
(38). In future studies it would be
preferable to study cells under natural hypoxic conditions.
Secondly, the HepG2 cell line has been reported to be
misidentified; it was originally thought to be an HCC cell line but
was later revealed to be derived from a hepatoblastoma cell line
(39). As ~90% of PLCs are HCC,
experiments on HCC cell lines are required to validate the results
of the present study. Thirdly, intact curcumin was used in the
present cell culture study and it has been reported that, within an
in vivo system, the majority of curcumin is converted to
curcumin glucuronide, the effects of which are weaker than those of
curcumin (40). Whether curcumin
would have the same effects in vivo as in the cultured cells
is not known. Further studies are required to investigate the
effects of curcumin on the IGF-1R signaling pathway in
vivo.
In conclusion, the present study provides
mechanistic insights into the link between IGF-1R and VEGF that
mediates angiogenesis and highlights the important
anti-proliferation and anti-angiogenesis effects of curcumin. The
results of the present study suggest that IGF-1R and its downstream
signals may be targets for anti-angiogenesis treatment of
hepatoblastomas. Curcumin in combination with TACE may be an
effective treatment for liver cancer, considering its liver
protective, pro-apoptosis and anti-angiogenesis effects.
Acknowledgements
This study was supported by grants from the Natural
Science Foundation of Fujian Province (grant no. 2017J01164),
Foundation Supporting the Army of Science and Technology of
Zhangzhou (grant no. zz2016kd06) and Innovation Funds of the
Medical Science and Technology of Military Region of Nanjing (grant
no. 11MA082).
References
|
1
|
Bosman FT: World Health Organization and
International Agency for Research on Cancer: WHO classification of
tumours of the digestive system. 4th. IARC Press; Lyon: 2010
|
|
2
|
Sia D, Villanueva A, Friedman SL and
Llovet JM: Liver cancer cell of origin, molecular class, and
effects on patient prognosis. Gastroenterology. 152:745–761. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bosch FX, Ribes J, Diaz M and Cleries R:
Primary liver cancer: Worldwide incidence and trends.
Gastroenterology. 127 5 Suppl 1:S5–S16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dageforde LA, Fowler KJ and Chapman WC:
Liver transplantation for hepatocellular carcinoma: Current update
on treatment and allocation. Curr Opin Organ Transplant.
22:128–134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fitamant J, Kottakis F, Benhamouche S,
Tian HS, Chuvin N, Parachoniak CA, Nagle JM, Perera RM, Lapouge M,
Deshpande V, et al: YAP inhibition restores hepatocyte
differentiation in advanced HCC, leading to tumor regression. Cell
Rep. Mar 10–2015.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsurusaki M and Murakami T: Surgical and
locoregional therapy of HCC: TACE. Liver Cancer. 4:165–175. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun JJ, Wang K, Zhang CZ, Guo WX, Shi J,
Cong WM, Wu MC, Lau WY and Cheng SQ: Postoperative adjuvant
transcatheter arterial chemoembolization after R0 hepatectomy
improves outcomes of patients who have hepatocellular carcinoma
with microvascular invasion. Ann Surg Oncol. 23:1344–1351. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hirakawa M, Nishie A, Asayama Y, Fujita N,
Ishigami K, Tajiri T, Taguchi T and Honda H: Efficacy of
preoperative transcatheter arterial chemoembolization combined with
systemic chemotherapy for treatment of unresectable hepatoblastoma
in children. Jpn J Radiol. 32:529–536. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu K, Min XL, Peng J, Yang K, Yang L and
Zhang XM: The changes of HIF-1α and VEGF expression after TACE in
patients with hepatocellular carcinoma. J Clin Med Res. 8:297–302.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiao D, Wang J, Lu W, Tang X, Chen J, Mou
H and Chen QY: Curcumin inhibited HGF-induced EMT and angiogenesis
through regulating c-Met dependent PI3K/Akt/mTOR signaling pathways
in lung cancer. Mol Ther Oncolytics. 3:160182016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abdollahi E, Momtazi AA, Johnston TP and
Sahebkar A: Therapeutic effects of curcumin in inflammatory and
immune-mediated diseases: A nature-made jack-of-all-trades? J Cell
Physiol. 233:830–848. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang J, Xu L, Zhang L, Ying Z, Su W and
Wang T: Curcumin attenuates
D-galactosamine/lipopolysaccharide-induced liver injury and
mitochondrial dysfunction in mice. J Nutr. 144:1211–1218. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salem MM, El-Rasheid HGA and Mahmoud AN:
Therapeutic effects of curcumin and royal jelly as natural
antioxidants on some biochemical parameters in hepatotoxicity
induced by carbon tetrachloride (CCl4) in male albino rats. Int J.
3:520–535. 2015.
|
|
14
|
Li G, Chen JB, Wang C, Xu Z, Nie H, Qin
XY, Chen XM and Gong Q: Curcumin protects against
acetaminophen-induced apoptosis in hepatic injury. World J
Gastroenterol. 19:7440–7446. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mitsiades CS, Mitsiades NS, McMullan CJ,
Poulaki V, Shringarpure R, Akiyama M, Hideshima T, Chauhan D,
Joseph M, Libermann TA, et al: Inhibition of the insulin-like
growth factor receptor-1 tyrosine kinase activity as a therapeutic
strategy for multiple myeloma, other hematologic malignancies, and
solid tumors. Cancer Cell. 5:221–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patel BB, Gupta D, Elliott AA, Sengupta V,
Yu Y and Majumdar AP: Curcumin targets FOLFOX-surviving colon
cancer cells via inhibition of EGFRs and IGF-1R. Anticancer Res.
30:319–325. 2010.PubMed/NCBI
|
|
17
|
Samani AA, Yakar S, LeRoith D and Brodt P:
The role of the IGF system in cancer growth and metastasis:
Overview and recent insights. Endocr Rev. 28:20–47. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
El-Serag HB, Davila JA, Petersen NJ and
McGlynn KA: The continuing increase in the incidence of
hepatocellular carcinoma in the United States: An update. Ann
Intern Med. 139:817–823. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liao D and Johnson RS: Hypoxia: A key
regulator of angiogenesis in cancer. Cancer Metastasis Rev.
26:281–290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nishida N, Yano H, Nishida T, Kamura T and
Kojiro M: Angiogenesis in cancer. Vasc Health Risk Manag.
2:213–219. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pleguezuelo M, Marelli L, Misseri M,
Germani G, Calvaruso V, Xiruochakis E, Manousou P and Burroughs AK:
TACE versus TAE as therapy for hepatocellular carcinoma. Expert Rev
Anticancer Ther. 8:1623–1641. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Poon RT, Ho JW, Tong CS, Lau C, Ng IO and
Fan ST: Prognostic significance of serum vascular endothelial
growth factor and endostatin in patients with hepatocellular
carcinoma. Br J Surg. 91:1354–1360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park YN, Kim YB, Yang KM and Park C:
Increased expression of vascular endothelial growth factor and
angiogenesis in the early stage of multistep hepatocarcinogenesis.
Arch Pathol Lab Med. 124:1061–1065. 2000.PubMed/NCBI
|
|
24
|
Yamaguchi R, Yano H, Iemura A, Ogasawara
S, Haramaki M and Kojiro M: Expression of vascular endothelial
growth factor in human hepatocellular carcinoma. Hepatology.
28:68–77. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sergio A, Cristofori C, Cardin R, Pivetta
G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A and
Farinati F: Transcatheter arterial chemoembolization (TACE) in
hepatocellular carcinoma (HCC): The role of angiogenesis and
invasiveness. Am J Gastroenterol. 103:914–921. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liapi E and Geschwind JF: Combination of
local transcatheter arterial chemoembolization and systemic
anti-angiogenic therapy for unresectable hepatocellular carcinoma.
Liver Cancer. 1:201–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang L, Hu P, Chen X and Bie P:
Transarterial chemoembolization (TACE) plus sorafenib versus TACE
for intermediate or advanced stage hepatocellular carcinoma: A
meta-analysis. PLoS One. 9:e1003052014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu M, Zheng YL, Xie XY, Liang JY, Pan FS,
Zheng SG and Lü MD: Sorafenib blocks the HIF-1α/VEGFA pathway,
inhibits tumor invasion, and induces apoptosis in hepatoma cells.
DNA Cell Biol. 33:275–281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu L, Chen H, Wang M, Zhao Y, Cai G, Qi X
and Han G: Combination therapy of sorafenib and TACE for
unresectable HCC: A systematic review and meta-analysis. PLoS One.
9:e911242014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shehzad A, Lee J and Lee YS: Curcumin in
various cancers. Biofactors. 39:56–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
North S, Moenner M and Bikfalvi A: Recent
developments in the regulation of the angiogenic switch by cellular
stress factors in tumors. Cancer Lett. 218:1–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Q, Xu Z, Mao S, Chen W, Zeng R, Zhou S
and Liu J: Effect of hypoxia on hypoxia inducible factor-1α,
insulin-like growth factor I and vascular endothelial growth factor
expression in hepatocellular carcinoma HepG2 cells. Oncol Lett.
9:1142–1148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gray SG, Eriksson T, Ekström C, Holm S,
von Schweinitz D, Kogner P, Sandstedt B, Pietsch T and Ekström TJ:
Altered expression of members of the IGF-axis in hepatoblastomas.
Br J Cancer. 82:1561–1567. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tomizawa M and Saisho H: Signaling pathway
of insulin-like growth factor-II as a target of molecular therapy
for hepatoblastoma. World J Gastroenterol. 12:6531–6535. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yue L, Wang Y, Wang H, Gao H, Liang J, Sui
A, Xiang J, Zhou F, Xu C, Zhao W, et al: Inhibition of
hepatocellular carcinoma cell growth by an anti-insulin-like growth
factor-I receptor monoclonal antibody. Oncol Rep. 28:1453–1460.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rey S and Semenza GL: Hypoxia-inducible
factor-1-dependent mechanisms of vascularization and vascular
remodelling. Cardiovasc Res. 86:236–242. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Simpson A, Petnga W, Macaulay VM,
Weyer-Czernilofsky U and Bogenrieder T: Insulin-like growth factor
(IGF) pathway targeting in cancer: Role of the IGF Axis and
opportunities for future combination studies. Target Oncol.
12:571–597. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arany Z, Foo SY, Ma Y, Ruas JL,
Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J,
Rangwala SM, et al: HIF-independent regulation of VEGF and
angiogenesis by the transcriptional coactivator PGC-1alpha. Nature.
451:1008–1012. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
López-Terrada D, Cheung SW, Finegold MJ
and Knowles BB: Hep G2 is a hepatoblastoma-derived cell line. Hum
Pathol. 40:1512–1515. 2009. View Article : Google Scholar
|
|
40
|
Shoji M, Nakagawa K, Watanabe A, Tsuduki
T, Yamada T, Kuwahara S, Kimura F and Miyazawa T: Comparison of the
effects of curcumin and curcumin glucuronide in human
hepatocellular carcinoma HepG2 cells. Food Chem. 151:126–132. 2014.
View Article : Google Scholar : PubMed/NCBI
|