Introduction
Tuberculosis (TB), a worldwide infectious disease
caused by Mycobacterium tuberculosis (Mtb), continues to be
a major threat to human health (1,2).
Unfortunately, BCG (bacillus Calmette-Guérin), the only vaccine
widely used against TB, provides varied protective efficacy (0-80%
in randomized control trials) (3–5).
Recently, the emergence of drug-resistant Mtb stains as well as
HIV-TB co-infection poses numerous complexities for TB control
(6,7). More effective vaccines and more potent
immune strategies against TB are now in urgent need.
Therapeutic vaccines shed light on TB control by
inducing antigen specific immune responses against Mtb in
vivo (8), and Mtb potent antigen
encoding genes are delivered into host cells via plasmid,
adenovirus or lentivirus for in vivo gene expression
(9,10). As a novel immunotherapy strategy,
therapeutic vaccine has been successful for TB control not only in
latent TB infection model but also in acute infection model
(11,12).
Ag85B-Rv3425 (A3) is a fusion protein of Ag85B and
Rv3425, a member of the PE (Pro-Glu) and PPE (Pro-Pro-Glu) family,
located in RD11 region which is absent in BCG strains (13,14). It
has been reported rBCG::Ag85B-Rv3425 vaccine, formed by
co-expressing Rv3425 and Ag85B in BCG, provides better protective
efficacy against Mtb challenge compared with BCG (15). Recently, it was reported that A3
delivered into mice via lentivirus with one single dose
administration confers post-infection resistance to acute TB
infection (11).
In this study, we constructed a recombinant plasmid
based on lentiviral vector expressing fusion antigen Ag85B-Rv3425
(A3) and assessed its immunogenicity and treatment effect in TB
mice. We found that in vivo expression of A3 could induce
more IL-2 as well as TNF-α compared with immunization using A3
purified from E. coli, which is recombinant fusion protein of
Ag85B-Rv3425 purified from recombinant E. coli. Moreover,
in vivo expression of A3 provides immunity to acute Mtb
infection characterized by reduced Mtb burden in lung and spleen
and attenuated pathology in lung tissue.
Materials and methods
Animals
A total of 6–8 week-old female C57BL/6 mice were
purchased from SLAC Laboratory Animal Center (Shanghai, China). All
mice were maintained under specific-pathogen-free (SPF) conditions
in animal facilities at Animal Biosafety Level (ABSL)-III lab of
Wuhan University and given sterile water, mouse chow and bedding.
All mice experiments were performed in accordance with
recommendations in the Wuhan University Research Council Guide for
Care and Use of Laboratory Animals. Animal study protocols were
also reviewed and approved by the Wuhan University Institutional
Animal Care and Use Committee.
Plasmid and proteins
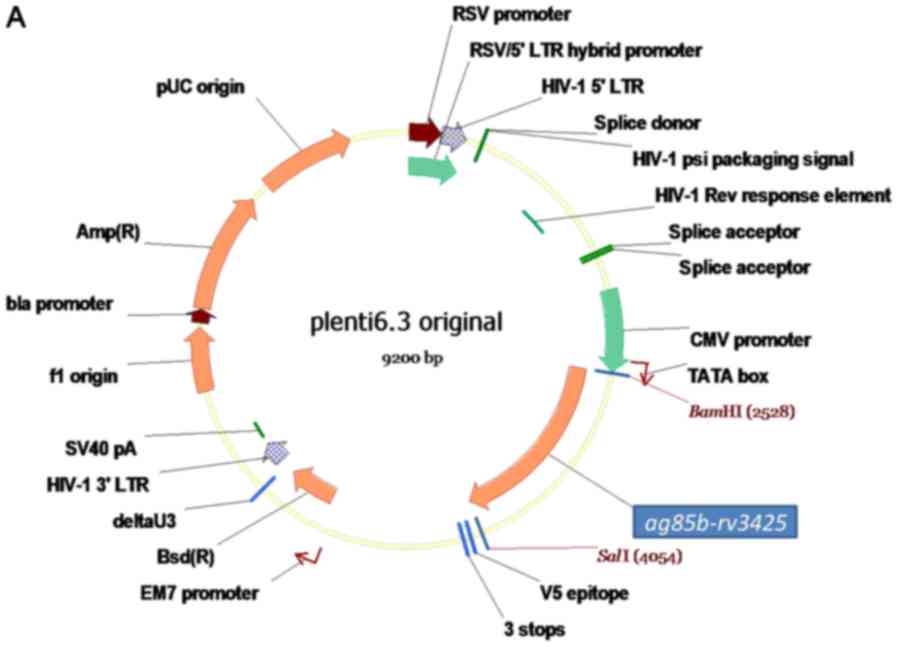
pLenti 6.3 plasmid was purchased from Biomiga (San
Diego, CA, USA). The cloning of ag85b-rv3425 fusion gene
into pLenti6.3 plasmid was our described previously (11). A3 protein were purified as our
described previously (13).
Endotoxins concentration of A3 protein was tested using the
commercially available Quantitative Chromogenic End-point
Tachypleus Amebocyte Lysate reactivity endotoxin kit (Chinese
Horseshoe Crab Reagent Manufactory, Xiamen, China). A total of
<5.0 EU/mg endotoxins was observed and purified A3 protein was
subsequently subjected to animal immunization.
Immunization
C57BL/6 mice were divided into four groups (20 mice
per group). At week 0, mice in PBS group received a subcutaneous
injection of empty plasmids in PBS and mice in A3-Pro group were
injected subcutaneously with 50 µg recombinant A3 protein in 250 µg
dimethyl dioctadecyl-ammonium bromide (DDA) adjuvant (Sigma). Mice
in A3-Vec group were immunized by an intramuscular injection of 50
µg recombinant pLenti6.3 plasmid harboring ag85b-rv3425
fusion gene. Mice in Vec group were immunized by an intramuscular
injection of 50 µg pLenti6.3 empty plasmid vector. All mice
received 3 injections at a two-week interval. 4 weeks after the
last injection, mice were sacrificed for immune response evaluation
(8 mice per group).
Separation of spleen lymphocytes
Spleen from each mouse was collected under aseptic
condition immediately after sacrifice. The single-cell suspension
(4 ml) was obtained by gently grounding spleen through a 75 µm cell
strainer and underlayed by 4 ml pre-warmed Lymphocyte-M (Cedar Lane
Lab, Burlington, VT, USA). Density-gradient centrifugation was
performed at 1,250 × g for 25 min to isolate splenocytes (16,17).
Cells were counted and diluted to a final concentration of
5×106 cells/ml in RPMI-1640 medium (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10
U/ml penicillin, 10 µg/ml streptomycin and 10% fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc.).
Enzyme-linked ImmunoSpot (ELISPOT)
assay
Filtration plates (96-well; Merck KGaA, Darmstadt,
Germany) were coated with monoclonal antibodies against mouse TNF-α
(U-CyTech, Utrecht, The Netherlands) for 16 h at 4°C. After being
washed with phosphate-buffered saline (PBS), wells were blocked
with 200 µl blocking buffer R for 1 h at 37°C. Spleen lymphocytes
(2.5×105 cells) were mixed with 10 µg/ml A3 protein and added to
each well. Stimulation was completed by incubating cells at 37°C
for 36 h. After being washed with PBST for five times, each well
was filled with 100 µl of biotinylated detection antibodies, and
incubated at 37°C for 1 h. Then, wells were washed five times and
incubated with 100 µl of streptavidin-HRP for 1 h at 37°C. After
being washed, 100 µl of AEC substrate solution was added to each
well and plates were incubated for 25 min at room temperature in
the dark. Color development was stopped by rinsing both sides of
the polyvinylidene fluoride membrane in 96-well filtration plates
with demineralized water. Finally, the plates were air dried and
the amount of positive spots was counted by a dissecting
microscope. The number of TNF-α expressing cell was calculated by
spot forming units per million cells.
Cytokines assay
2.5×106 cells were stimulated with 10
µg/ml A3 fusion protein at 37°C, 5% CO2 and high
humidity for 36 h as described before (18). The supernatant from stimulated spleen
lymphocytes was collected, and the concentrations of TNF-α and IL-2
were measured by BD™ Cytometric Bead Array kit (BD Biosciences,
Franklin Lakes, NJ, USA) according to the manual in the kit.
Mtb infection and immunotherapy
For immunotherapy, mice were infected intravenously
via lateral caudal vein with 6.8×105 live Mtb strain
H37Rv. At 4 weeks after infection, immunotherapy was applied and
injection strategy was the same as immune responses evaluation
listed above. At 4 weeks later at week 12, mice were sacrificed for
colony-forming unit (CFU) counts (6 mice per group) and
histopathological analysis in lung and spleen (6 mice per
group).
Histopathology analysis
Each lung was excised and fixed in 4%
neutral-buffered paraformaldehyde solution for 24 h. Then lung
tissue was embedded with paraffin. Series of sections with a
thickness of 4–7 µm were then cut and stained with haematoxylin and
eosin under standard methods. Double blind analysis was then made
by board-certified pathologists and more than 10 slides of each
lung were evaluated.
Statistical analysis
Statistical analysis was performed using SPSS
Statistics 17.0 for Windows software package. Results were
subjected to one-way Anova test followed by multi-comparison
testing. P<0.05 was considered to indicate a statistically
significant difference.
Results
Fusion antigen A3 delivered by plasmid
pLenti6.3 could be expressed in mice and induce immune
response
To make fusion Mtb antigen A3 expressed
appropriately in eukaryotic cells, we constructed a recombinant
plasmid expressing A3 (A3-Vec) by cloning ag85b-rv3425
fusion gene fragment and inserting it into pLenti6.3 vector, whose
CMV promoter upstream could drive the transcription of
ag85b-rv3425 fusion gene in eukaryotic cells. Then, 50 µg
endotoxin-free recombinant plasmids (A3-Vec) were injected into
mice for 3 times at a two-week interval. As controls, 50 µg
endotoxin-free empty plasmids (Vec) and 50 µg recombinant proteins
A3 (A3-Pro) purified from E. coli were also injected for 3 times at
a two-week interval, respectively. In the 4th week after the last
immunization, mice were sacrificed for immune response analysis. We
have detected A3 fusion protein specific antibody IgG response in
A3-Vec group and found that injection of recombinant plasmids
A3-Vec, as well as recombinant proteins A3, induced A3 specific
antibody Immunoglobulin G (IgG) production (Fig. 1). It indicates that Mtb fusion gene
ag85b-rv3425 delivered by pLenti6.3 plasmid could be
expressed and translated into fusion protein in mice and then
recognized by immune system.
A3 delivered by pLenti6.3 plasmid
increased the production of Th1-type cytokines
Next, we want to know whether A3 delivered by
plasmid could induce protective immune response since it could be
expressed in mice. As Th1-type immune responses plays important
role in protection against Mtb (18,19). To
identify whether A3-Vec plasmid could induce Mtb-specific Th1-type
immune responses, we evaluated the production of Th1-type cytokines
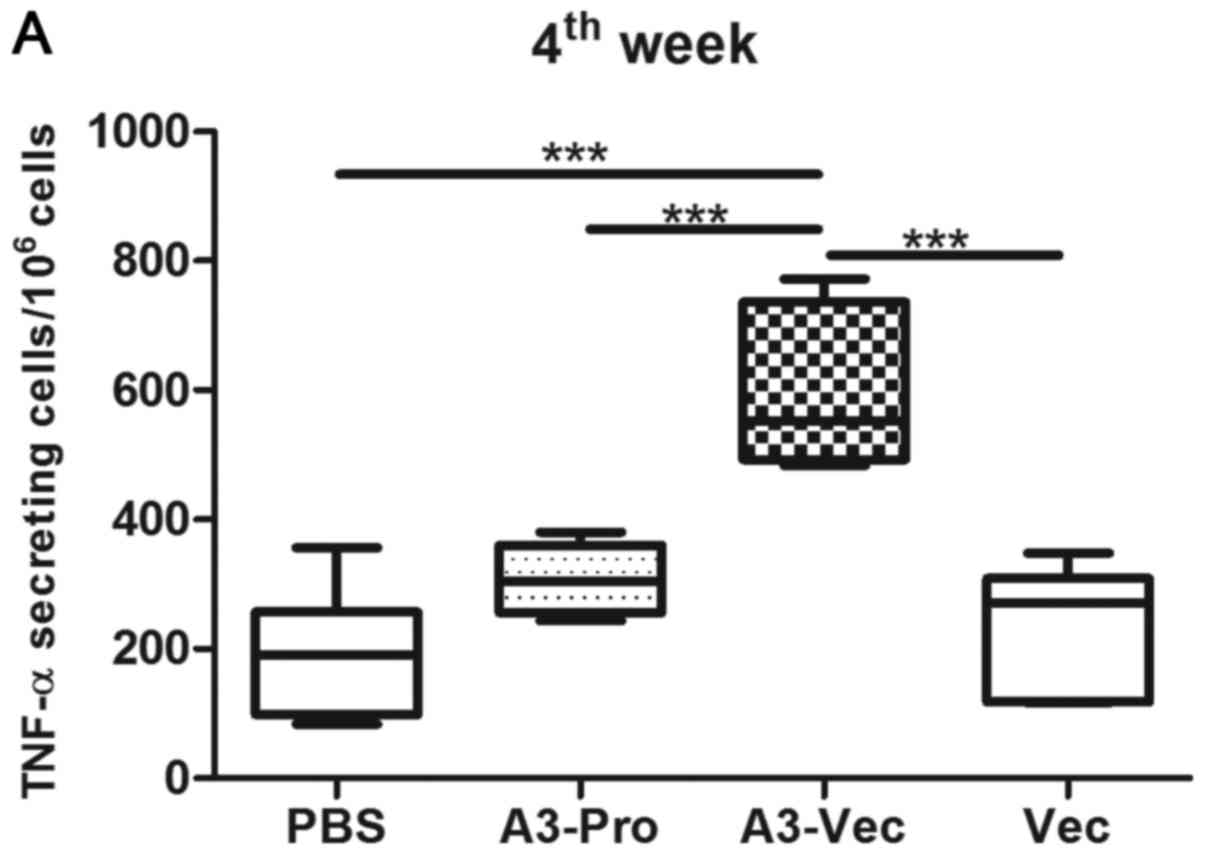
in mice. ELISPOT results show that there are more TNF-α producing
cell in mice of A3-Vec group than those in A3-Pro group, Vec (empty
plasmids) group and PBS group (Fig.
2A). And we found that the concentration of TNF-α in culture
supernatant of spleen lymphocytes from A3-Vec immunized mice was
higher than that from other groups determined with enzyme linked
immunosorbent assay (ELISA) methods with recombinant A3 protein
stimulation in vitro (Fig.
2B).
Moreover, we detected a much higher concentration of
IL-2 production in spleen lymphocytes from A3-Vec group upon A3
protein stimulation (Fig. 2C). It
seems that A3-Vec immunization exhibited higher ability in inducing
Th1-type cytokines production than immunization of A3-proteins or
empty plasmids.
We also compared the percentages/numbers of T cell
subsets (CD3+, CD4+ and CD8+ T cells subsets) in spleen lymphocytes
among these groups, and found no significant difference (data not
shown).
Administration of A3-Vec plasmids
effectively reduces Mtb burdens in lungs and spleens of Mtb
infected mice
Since A3-Vec could induce antigen specific Th1-type
cytokines production, we want to know whether A3-Vec could relieve
symptoms of TB in mice. To address this, we evaluated the
therapeutic effect of A3-Vec injection in Mtb-infected murine
model. Mice were challenged by intravenous injection with Mtb H37Rv
to establish acute infection. After 4 weeks of infection, we began
to inject A3-Vec plasmids, A3-Pro, Vec (empty plasmids) and PBS
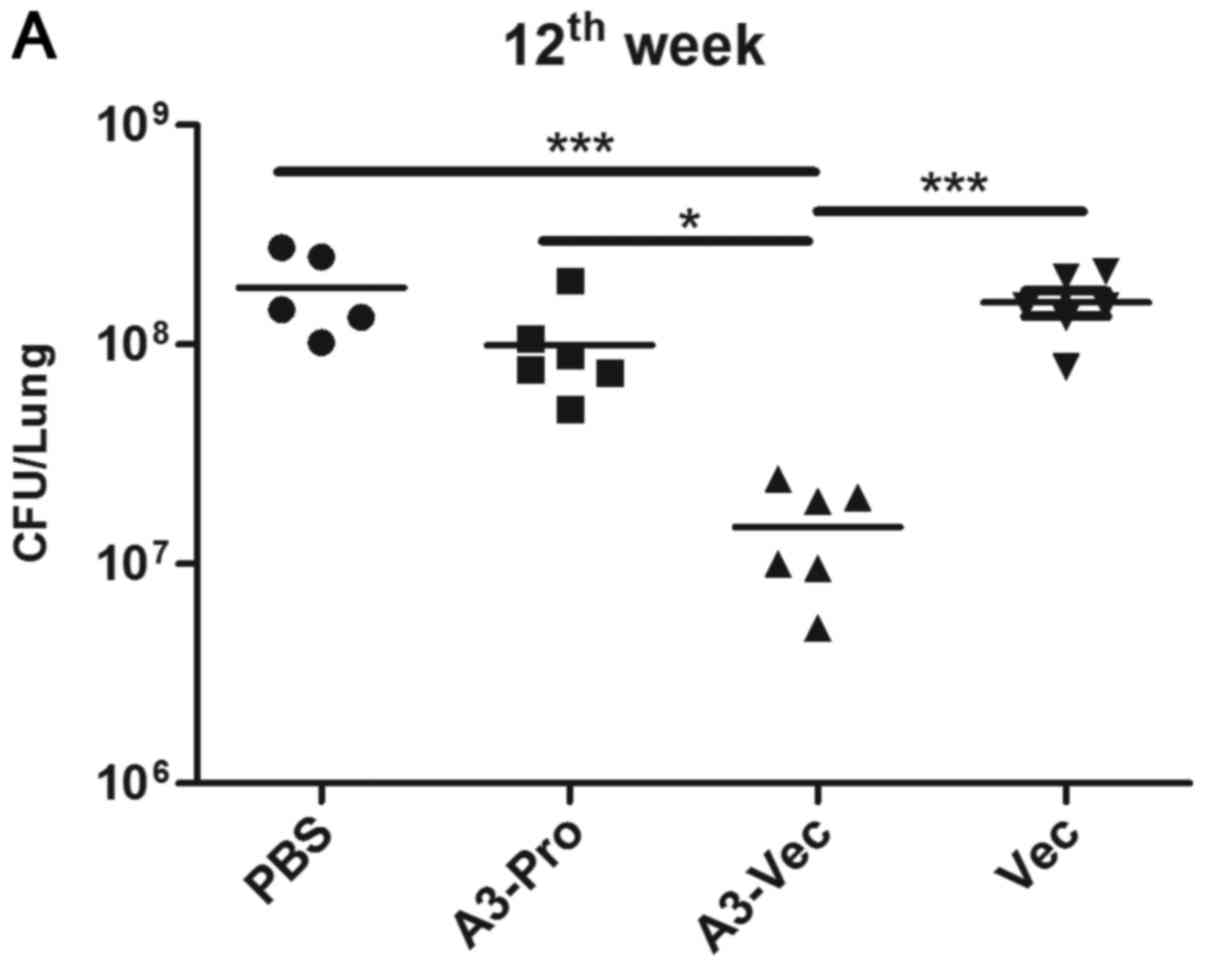
control into TB mice according to the schedules listed in Table I. At the 12th week, mice were
sacrificed, and bacteria burdens in lungs and spleens were counted.
We found that there was a statistically significant CFU burden
reduction in lung, as well as spleen, of A3-Vec treated mice
comparing with Vec and PBS controls or even A3-Pro treated mice
(Fig. 3A). And mice in A3-Vec
present the lowest CFU counts in spleen (Fig. 3B). Acid-fast staining results showed
that many Mtb bacteria aggregating were found in lung tissue from
control groups (PBS and empty plasmid-Vec) as well as from A3-Pro
treated group, while fewer Mtb bacteria were seen in slide from
A3-Vec group (Fig. 3C). These
results demonstrate that A3-Vec administration could provide immune
protection to acute Mtb infection through inhibiting Mtb growth
in vivo.
 | Table I.Schedule of therapy experiments
against Mtb challenge. |
Table I.
Schedule of therapy experiments
against Mtb challenge.
| Group | 0 week | 4th week | 6th week | 8th week | 12th week |
|---|
| PBS | Infection | PBS | PBS | PBS | Necropsy |
| A3-Pro | Infection | A3-Pro | A3-Pro | A3-Pro | Necropsy |
| A3-Vec | Infection | A3-Vec | A3-Vec | A3-Vec | Necropsy |
| Vec | Infection | Vec | Vec | Vec | Necropsy |
Administration of A3-Vec plasmids
confers immune resistance to TB lesions
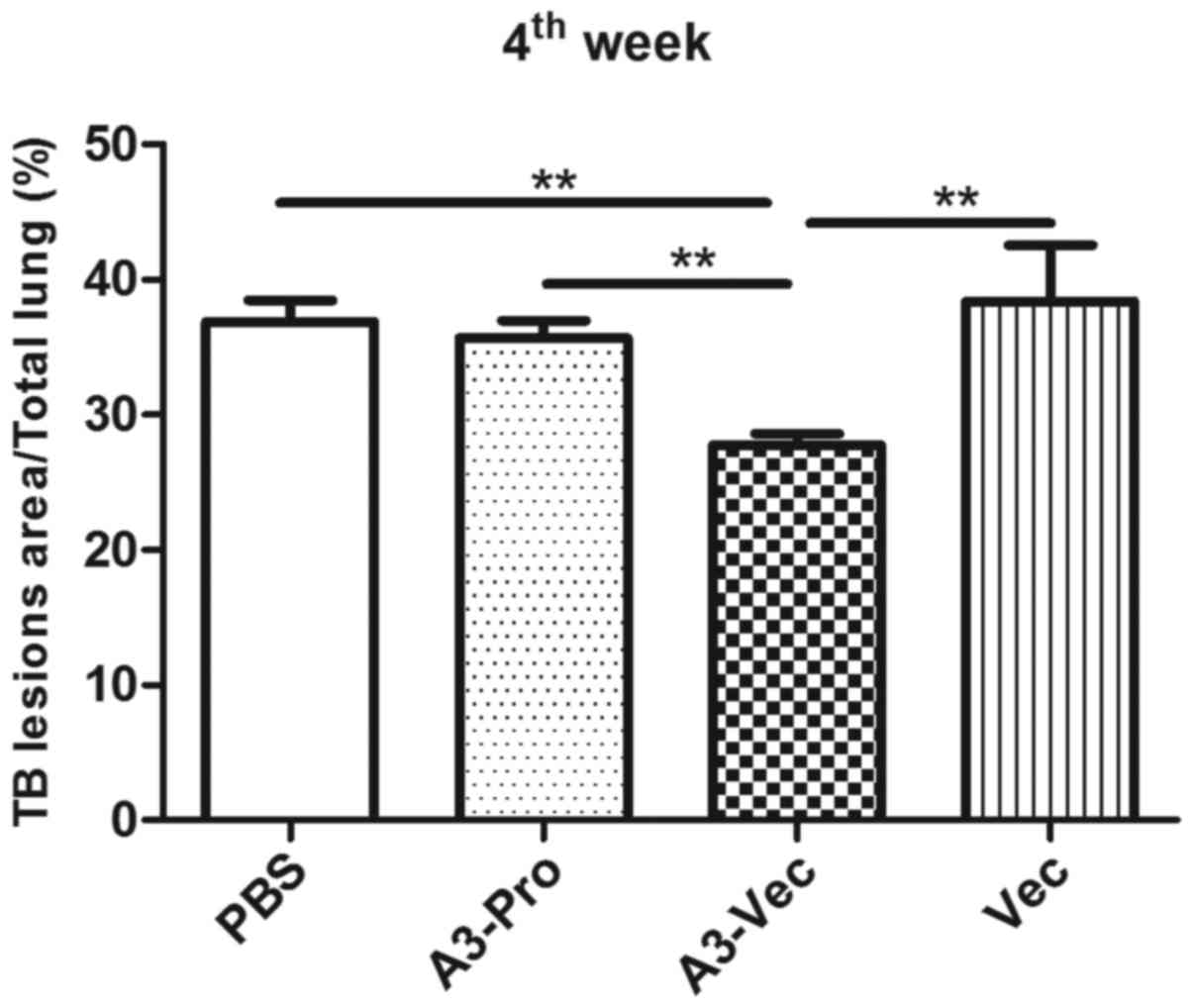
To identify whether administration of A3-Vec plasmid
could decrease TB lesions in lungs caused by Mtb infection, we
performed histopathological analysis to compare gross pathology in
lungs of mice in different groups. TB lesions area percentage was
calculated by double blind pathologists. We found that A3-Vec group
exhibits the least lesion area among these four groups (Fig. 4). More detailed histopathological
images illustrated mild lesion with small area of epithelioid cells
and lymphocytes hyperplasia in A3-Vec group, while there were large
area of lymphocytes, eosinophils and macrophages hyperplasia in
pulmonary alveoli of the other three groups (data not shown). It
means that administration of A3-Vec in mice conferred immune
resistance to lesions caused by TB acute infection.
Discussion
The emergence of drug-resistant TB strains and
HIV-TB co-infection makes global TB control a real challenge, it's
in urgent need to facilitate worldwide control of TB (20). Immunotherapy is regarded as a
potential approach to eliminate Mtb (21). In this study, we constructed a
recombinant plasmid based on lentiviral vector expressing multiple
antigens and assessed its immune response and treatment effect in
TB mice.
As our previously reported, fusion protein
Ag85B-Rv3425 is proved to be an effective multiple epitopes antigen
(11,13). In our study, we found that
recombinant plasmid A3-Vec, like lentivirus delivered A3 (11), could induce A3 fusion protein
specific antibody IgG response, indicating that it could be
expressed in mice and then recognized by immune system. Moreover,
we have observed immunization of plasmid A3-Vec could induce high
Th1-type cytokines production, such as IL-2 and TNF-α (22), in spleen lymphocytes in A3-Vec
treated mice. Moreover, recombinant plasmid A3-Vec shows potent
effective therapeutic effect in acute Mtb infected mice. After
immunotherapy, mice in A3-Vec group present the lower CFU counts in
lung as well as in spleen than the control groups and exhibits
significantly mild tuberculous lesions, which showed a symbol of
epithelioid cells and lymphocytes hyperplasia combined with
exudative inflammation of pneumonedema.
At the same time, we also compared the
immunogenicity and treatment effects of the same fusion antigen A3
delivered by plasmid and recombinant protein purified from
recombinant E. coli (subunit vaccine) in TB mice. We found
that immunization of plasmid A3-Vec could induce higher Th1-type
cytokines production, such as IL-2 and TNF-α, than immunization of
A3-Pro recombinant protein, though A3-Pro can induce high IgG in
mice. And A3-Vec can decrease the CFU counts in lung as well as in
spleen and alleviate tuberculous lesions in acute Mtb infected mice
comparing to A3-Pro. It implies that plasmid vector might be a more
effective method in antigen presentation than protein subunit
vaccine.
In summary, our results show a good therapeutic
effect of recombinant plasmid A3-Vec treatment against acute TB
infection in mice. Further study might be conducted in non-human
primates or even in humans to assess therapeutic effect of this
recombinant plasmid.
Acknowledgements
This study was supported by the grants from the
National Natural Science Foundation of China (no. 81401711), Nature
Science Foundation of Shanghai Science and Technology Committee
(no. 14ZR1444300).
References
|
1
|
Sulis G, Centis R, Sotgiu G, D'Ambrosio L,
Pontali E, Spanevello A, Matteelli A, Zumla A and Migliori GB:
Recent developments in the diagnosis and management of
tuberculosis. NPJ Prim Care Respir Med. 26:160782016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Raviglione M and Sulis G: Tuberculosis
2015: Burden, challenges and strategy for control and elimination.
Infect Dis Rep. 8:65702016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Jonge MI, Brosch R, Brodin P, Demangel
C and Cole ST: Tuberculosis: From genome to vaccine. Expert Rev
Vaccines. 4:541–551. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang L, Ru HW, Chen FZ, Jin CY, Sun RF,
Fan XY, Guo M, Mai JT, Xu WX, Lin QX and Liu J: Variable virulence
and efficacy of BCG vaccine strains in mice and correlation with
genome polymorphisms. Mol Ther. 24:398–405. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bali P, Tousif S, Das G and Van Kaer L:
Strategies to improve BCG vaccine efficacy. Immunotherapy.
7:945–948. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ahmed MM, Velayati AA and Mohammed SH:
Epidemiology of multidrug-resistant, extensively drug resistant,
and totally drug resistant tuberculosis in middle east countries.
Int J Mycobacteriol. 5:249–256. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Odone A, Matteelli A, Chiesa V, Cella P,
Ferrari A, Pezzetti F, Signorelli C and Getahun H: Assessing the
impact of defining a global priority research agenda to address
HIV-associated tuberculosis. Trop Med Int Health. 21:1420–1427.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cardona PJ: The progress of therapeutic
vaccination with regard to tuberculosis. Front Microbiol.
7:15362016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bhargava S, Choubey S and Mishra S:
Vaccines against tuberculosis: A review. Indian J Tuberc. 63:13–18.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schnepp BC and Johnson PR: Vector-mediated
in vivo antibody expression. Microbiol Spectr. 2:AID–0016-2014.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang E, Wang F, Xu Y, Wang H, Hu Y, Shen H
and Chen ZW: A lentiviral vector-based therapeutic vaccine encoding
Ag85B-Rv3425 potently increases resistance to acute tuberculosis
infection in mice. Acta Biochim Biophys Sin (Shanghai). 47:588–596.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dheda K, Barry CE III and Maartens G:
Tuberculosis. Lancet. 387:1211–1226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang E, Gu J, Wang F, Wang H, Shen H and
Chen ZW: Recombinant BCG prime and PPE protein boost provides
potent protection against acute Mycobacterium tuberculosis
infection in mice. Microb Pathog. 93:1–7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang J, Qie Y, Zhang H, Zhu B, Xu Y, Liu
W, Chen J and Wang H: PPE protein (Rv3425) from DNA segment RD11 of
Mycobacterium tuberculosis: A novel immunodominant antigen of
Mycobacterium tuberculosis induces humoral and cellular immune
responses in mice. Microbiol Immunol. 52:224–230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang J, Qie Y, Liu W and Wang H:
Protective efficacy of a recombinant BCG secreting antigen
85B/Rv3425 fusion protein against Mycobacterium tuberculosis
infection in mice. Hum Vaccin Immunother. 8:1869–1874. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang E, Lu Y, Xu Y, Liang Q, Wang C, Wang
H and Shen H: Recombinant BCG coexpressing Ag85B, ESAT-6 and
Rv3620c elicits specific Th1 immune responses in C57BL/6 mice.
Microb Pathog 69–70. 1–59. 2014.
|
|
17
|
Shen H, Wang Y, Chen CY, Frencher J, Huang
D, Yang E, Ryan-Payseur B and Chen ZW: Th17-related cytokines
contribute to recall-like expansion/effector function of
HMBPP-specific Vγ2Vδ2 T cells after Mycobacterium tuberculosis
infection or vaccination. Eur J Immunol. 45:442–451. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen H, Wang C, Yang E, Xu Y, Liu W, Yan
J, Wang F and Wang H: Novel recombinant BCG coexpressing Ag85B,
ESAT-6 and mouse TNF-alpha induces significantly enhanced cellular
immune and antibody responses in C57BL/6 mice. Microbiol Immunol.
54:435–441. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim JS, Kim WS, Choi HG, Jang B, Lee K,
Park JH, Kim HJ, Cho SN and Shin SJ: Mycobacterium tuberculosis
RpfB drives Th1-type T cell immunity via a TLR4-dependent
activation of dendritic cells. J Leukoc Biol. 94:733–749. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pontali E, Sotgiu G, Centis R, D'Ambrosio
L, Spanevello A and Migliori GB: Management of drug resistantTB in
patients with HIV co-infection. Expert Opin Pharmacother.
16:2737–2750. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abate G and Hoft DF: Immunotherapy for
tuberculosis: Future prospects. Immunotargets Ther. 5:37–45.
2016.PubMed/NCBI
|
|
22
|
Palavecino CE, Céspedes PF, Gómez RS,
Kalergis AM and Bueno SM: Immunization with a recombinant bacillus
Calmette-Guerin strain confers protective Th1 immunity against the
human metapneumovirus. J Immunol. 192:214–223. 2014. View Article : Google Scholar : PubMed/NCBI
|