Introduction
The immune system, a complex self-regulation system,
is responsible for the defense function of organism to prevent
outside pathogens, remove the waste material and maintain the
monitoring function for malignant cells (1). The immune system is highly sensitive
(2), and shows impaired immune
responsiveness to pathogen invasion, during which, immune cells are
activated and secret pro-inflammatory mediators including
interleukins (ILs) and interferons (INFs) are increased (3,4). And
immunity closely related to oxidative stress, which could lead to
aberrant expression of inflammatory cytokines and chemokines
(5). Cyclophosphamide monohydrate
(CTX), a cancer chemotherapeutic agent, facilitates cell apoptosis
and decreases the homeostatic proliferation of regulatory T cells
(6), which has been widely used in
the establishment of immunosuppressive animal models (7–9).
Previous researchers focus their studies on
searching immunomodulatory agents for years (10). However, the drugs performed in
clinic, especially single-component chemicals, display severe
adverse effects including general malaise and/or neurotoxicity
(11,12), which is hard to meet the requirements
of immunosuppressive patients. Dietary polysaccharides from natural
products are getting more attention due to their health benefits
and low toxicity (13,14), which have been confirmed to exhibit
multiple immunomodulatory effects (15,16).
Gloeostereum incarnatum, a precious edible mushroom,
parasitizes broad-leaved wood in northern Japan and northern China
(17). Traditionally, G.
incarnatum preparation is widely used for the treatment for
enteritis, dysentery and gastric ulcer (18). Polysaccharides obtained from G.
incarnatum fruiting body have been reported to possess
anti-tumor and anti-bacterial activities (17). However, the immunomodulatory effect
of G. incarnatum has not been investigated yet.
The objective of this study aims to investigate
whether polysaccharide compositions of G. incarnatum possess
immunomodulatory and immuno-enhancing effects in a CTX-induced
BALB/c mice model. Data demonstrated that G. incarnatum
polysaccharides (GIPS) enhanced immunologic function in CTX-induced
immunosuppressed mice by promoting the production of
immune-associated factors in spleen, elevating serum immunoglobulin
(Ig) levels, and restoring the imbalance of anti-oxidation and
oxidation.
Materials and methods
GIPS preparation
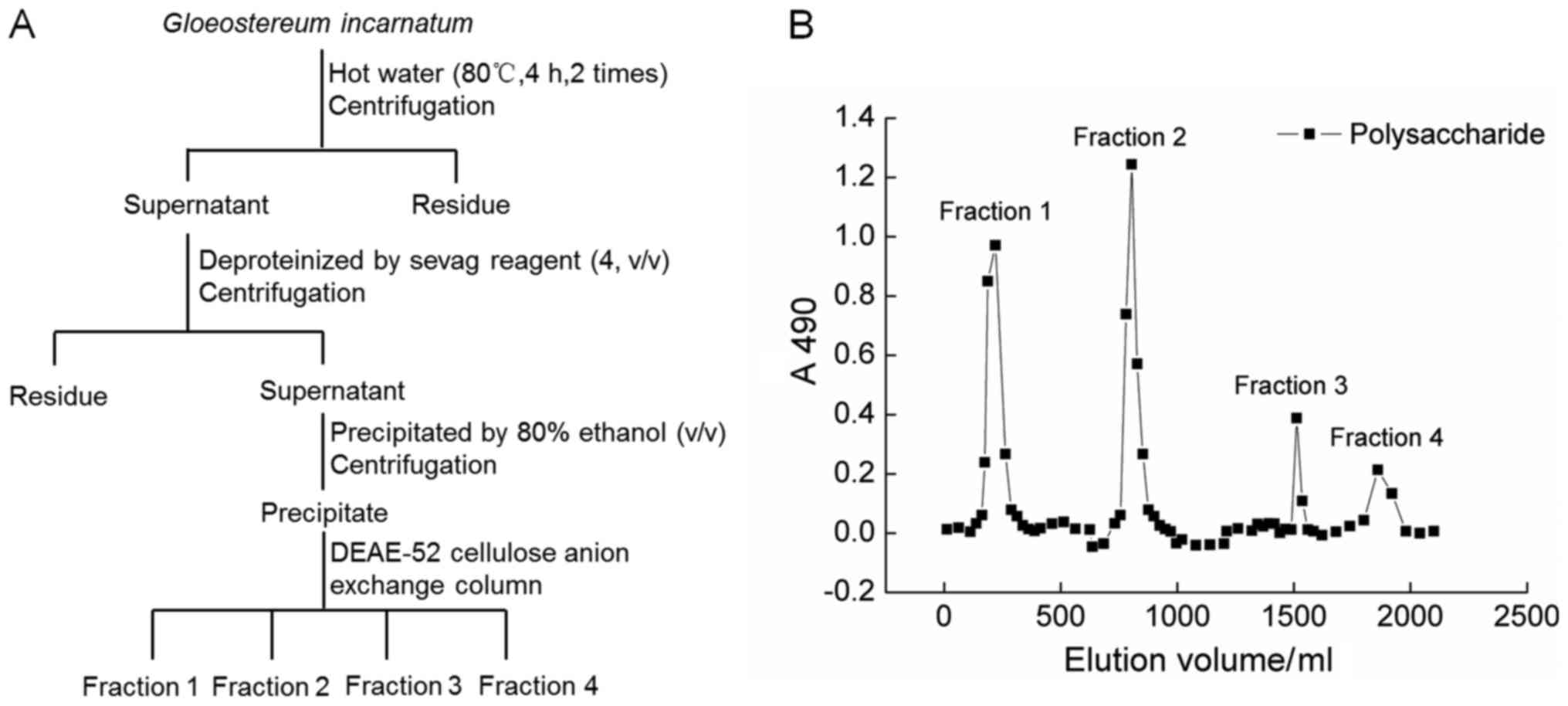
G. incaratum fruiting body (Yunnan, China)
was smashed and extracted with 20-fold double distilled (D.D.)
water at 90°C for 4 h twice. The water extracts were concentrated
and deproteinated by Sevag agents (n-butanol:chloroform=1:4)
(19). After centrifugation, the
supernatant were collected, added 4-fold volume of ethanol, and
placed at 4°C for overnight. The precipitation was dissolved in
D.D. water and subjected to DEAE-52 cellulose anion exchange column
(2.6×35 cm) (20). The fractions
(fraction 1 to 4) of crude GIPS were collected together, freeze
dried and stored at −80°C. The detailed information can be found in
Fig. 1A and B.
Protocols for immunosuppression mice
development and agents administration
The experimental protocol was approved by Animal
Ethics Committee in Jilin University (20160402). 72 of Balb/c male
mice (8-week old, 18–20 g) [SCXK (JI)-2014-0003] were housed in
cages with a 12 h light/12 h dark period under a constant
temperature (23±2°C) and humidity-controlled conditions (50–60%),
and allowed free access to solid feed and tap water during the
experiments.
After one-week acclimatization, mice were divided
into six groups randomly as follows. Control group (n=12): 3-day
normal saline injection following with 14-day normal saline gastric
gavages. Cyclophosphamide (CTX)-injected group (model group; n=12):
3-day CTX (75 mg/kg; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
injection following with 14-day normal saline gastric gavages.
GIPS-treated groups (n=12/group): 3-day CTX (75 mg/kg) injection
following with 14-day GIPS (0.1, 0.3 and 0.9 g/kg; dissolved in
normal saline) gastric gavages. Thymosin α1 (Tα1) (positive
drug)-treated group (n=12): 3-day CTX (75 mg/kg) injection
following with 14-day Tα1 (0.16 mg/kg; Jiangsu Hengrui Medicine
Co., Ltd., Lianyungang, China) injection. General health and body
weights of mice were monitored during the whole experimental
period. At 24 h after the last administration, all mice were
weighted, and killed. Blood and organs (spleen and thymus) were
sampled immediately. The thymus index was calculated according to
the formula: Index (mg/10 g)=(Weight of thymus)/10 × (body
weight).
Lymphocyte proliferation assay
Spleen of each mouse was aseptically removed and
grounded into a single cell suspension (21), and seeded into a 96-well plate at
density of 1×106/100 µl mixed with 10 µl of RPMI-1640
(Control group) or 10 µl of 200 µg/ml concanavalin (ConA;
Sigma-Aldrich; Merck KGaA). Then incubated under a humidified
atmosphere containing 5%/95% CO2/air at 37°C for 48 h
similar as previous study (22),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich; Merck KGaA) assay were performed to analysis the
changes of cell viability. The stimulation index (SI) of T
lymphocyte transformation was calculated as following equation:
SI=OD490 ConA/OD490 control.
ELISA assay
The spleens were homogenized with D.D. water, and
the protein concentrations were detected by a bicinchoninic acid
protein assay kit (Merck KGaA). The levels of superoxide dismutase
(SOD; 43125), ROS (43355), IgA (42791), IgG (42793) and IgM (42794)
in serum and spleen, and the levels of ILs [IL-2 (42903), −3
(42902) and −6 (42912)], INFs [IFN-α (43102) and IFN-γ (42918)] and
monocyte chemotactic protein 1 (MCP-1; 42818) in spleen were
detected by commercial ELISA kits (Shanghai Yuanye Bio-Technology
Co., Ltd., Shanghai, China).
Statistical analysis
The statistical values are presented as the mean ±
standard error of the mean (SEM). Statistically comparisons were
analyzed using a one-way analysis of variance (ANOVA) followed by
Dunn's test via SPSS 16.0 software (IBM Corp., Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of GIPS on bodyweight, thymus
index and lymphocyte proliferation
CTX strongly reduced the bodyweights of mice after
3-day injection (P<0.05; Table
I); in contrast, GIPS and Tα1 improved the weight gain in
immunosuppressed mice after 7-day administration (P<0.05;
Table I). Compared to normal mice,
extremely low thymus index was observed in CTX-injected mice
(P<0.001; Table I). GIPS at doses
of 0.1, 0.3 and 0.9 g/kg and Tα1 at 0.16 mg/kg significantly
enhanced the thymus index after 14-day treatment (P<0.05;
Table I).
 | Table I.Regulatory effects of GIPS and Tα1 on
body weight and organ indexes. |
Table I.
Regulatory effects of GIPS and Tα1 on
body weight and organ indexes.
|
|
| CTX (mg/kg) | GIPS (g/kg) | Tα1 (mg/kg) |
|---|
|
|
|
|
|
|
|---|
| Measurement | CTRL | 75 | 0.1 | 0.3 | 0.9 | 0.16 |
|---|
| Body weight
(g) |
|
|
|
|
|
|
| 1st
day |
26.4±0.4 |
25.8±0.4 |
27.1±0.3 |
25.0±0.4 |
27.4±0.6 |
26.9±0.4 |
| 4th
day |
25.8±0.3 |
22.6±0.5a |
22.4±0.4 |
22.8±0.7 |
22.8±0.6 |
22.5±0.4 |
| 11th
day |
27.9±0.4 |
24.6±0.3a |
25.6±0.5 |
25.3±0.8 |
26.9±0.5c |
24.8±0.6 |
| 18th
day |
27.3±
0.6 |
23.1±0.4a |
25.1±0.2 |
24.7±0.9 |
26.0±0.8c |
25.9±1.1c |
| Thymus index (mg/10
g) |
0.23±0.02 |
0.09±0.01b |
0.14±0.02d |
0.13±0.03c |
0.17±0.03d |
0.15±0.03d |
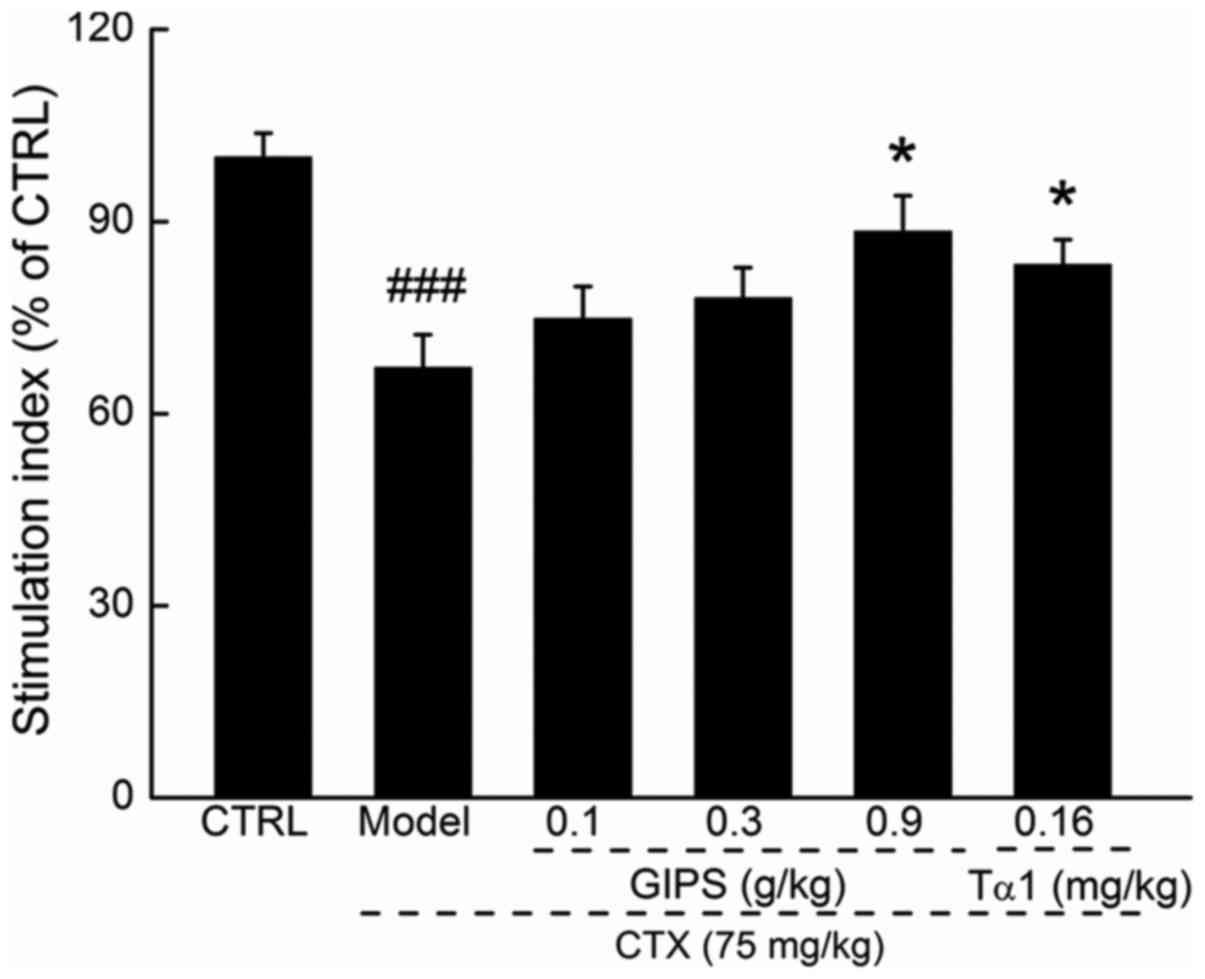
Lymphocyte proliferation is commonly used to reflect
the immune function (23). Compared
to CTX-induced immunosuppressant mice, GIPS at 0.9 g/kg, but not
Tα1 promoted over 21.3% T lymphocyte proliferation (P<0.05;
Fig. 2).
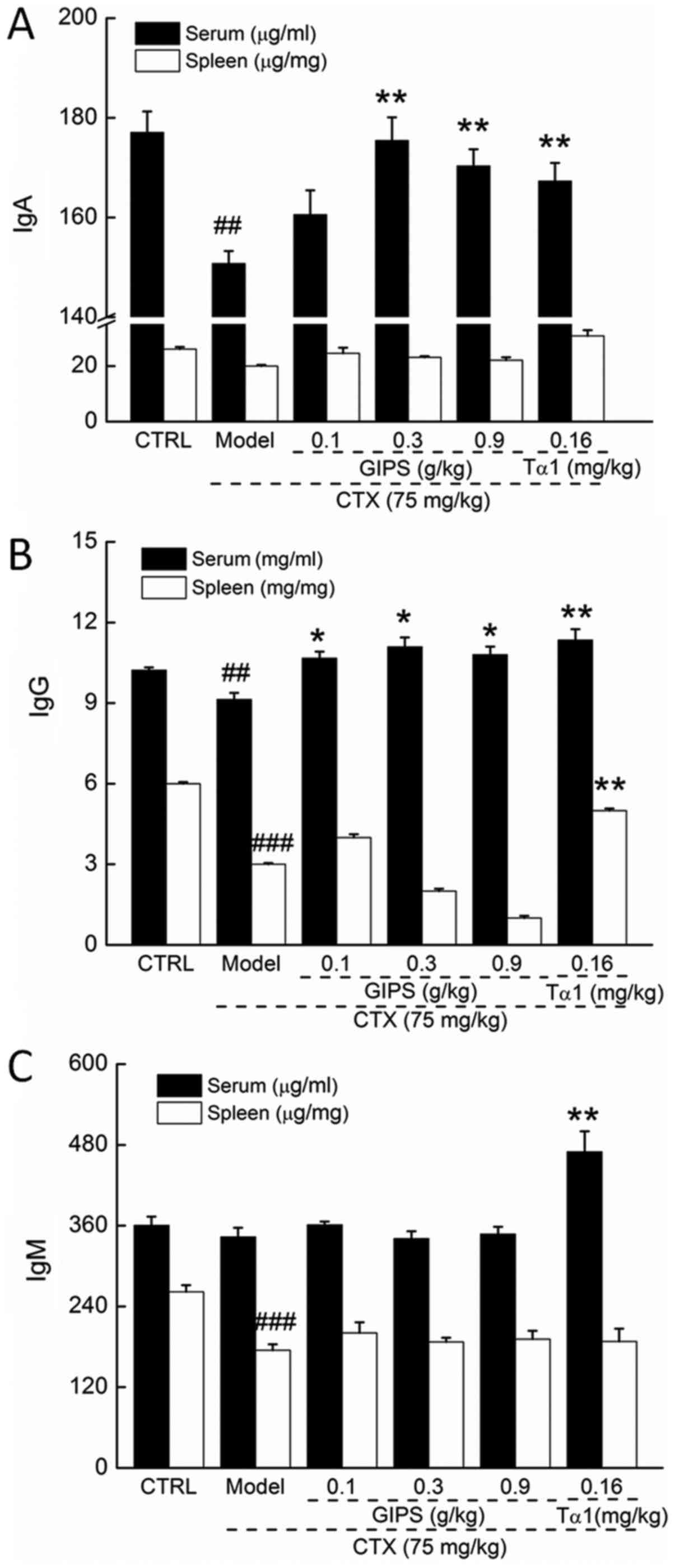
GIPS enhanced immune globulin levels
in the serum and spleen of immunosuppressed mice
CTX injection suppressed the levels of IgA and IgG
in serum (P<0.01; Fig. 3A and B),
and reduced the levels of IgG and IgM in spleen (P<0.01;
Fig. 3B and C) of mice. Compared to
CTX-induced immunosuppressant mice, Tα1 at 0.16 mg/kg significantly
enhanced the levels of IgA, IgG and IgM in serum (P<0.01;
Fig 3), and the levels of IgG in
spleen (P<0.01; Fig. 2B).
Differently, GIPS failed to increase the spleen levels of immune
globulin in CTX-induced immunosuppressant mice (Fig. 3). In contrast, GIPS administration
resulted in >13.0% enhancement on IgA levels except for 0.1 g/kg
of GIPS (P<0.001; Fig 3A) and
>16.8% enhancement on IgG levels (P<0.05; Fig 3B) in serum of CTX-induced
immunosuppressant mice.
Effects of GIPS on immune factors in
the spleen of immunosuppressed mice
Compared to normal mice, the levels of ILs (IL-2, −3
and −10), INFs (IFN-α and IFN-γ) and MCP-1 in spleen were
significantly lowered in CTX-induced immunosuppressant mice
(P<0.05; Table II), which were
all significantly restored by 14-day Tα1 administration at 0.16
mg/kg (P<0.05; Table II). GIPS,
especially at 0.9 g/kg, increase 191.1% of IL-2 (P<0.01), 94.7%
of IL-3 (P<0.01), 136.1% of IL-6 (P<0.001), 68.7% of IFN-α
(P<0.05), 50.6% of IFN-γ (P<0.05), and 77.8% of MCP-1
(P<0.001) in spleen compared to CTX-injected mice (Table II).
 | Table II.Regulatory effects of GIPS and Tα1 on
interleukins, interferons and MCP-1 in spleen of CTX-induced
immunosuppressed mice. |
Table II.
Regulatory effects of GIPS and Tα1 on
interleukins, interferons and MCP-1 in spleen of CTX-induced
immunosuppressed mice.
|
|
|
| GIPS (g/kg) | Tα1 (mg/kg) |
|---|
|
|
|
|
|
|
|---|
| Protein | CTRL | Model | 0.1 | 0.3 | 0.9 | 0.16 |
|---|
| IL-2 (pg/mg) |
21.2±1.0 |
6.7±0.8b |
9.0±0.4 |
9.5±1.0 |
19.5±4.0d |
12.0±0.7c |
| IL-3 (pg/mg) |
6.4±0.37 |
3.8±0.4a |
4.7±0.5 |
5.2±0.4 |
7.4±1.3d |
6.0±0.7c |
| IL-6 (pg/mg) |
9.7±0.7 |
3.6±0.5b |
5.7±0.4 |
6.6±0.6c |
8.5±1.1e |
7.2±0.7d |
| IFN-α (pg/mg) |
2.6±0.2 |
1.6±0.1a |
2.4±0.1 |
2.6±0.3 |
2.7±0.1c |
3.6±0.5d |
| IFN-γ (pg/mg) |
15.4±0.8 |
7.9±0.6b |
9.5±0.8 |
9.2±0.5 |
11.9±1.3c |
12.4±1.2d |
| MCP-1 (pg/mg) |
1.3±0.1 |
0.9±0.1b |
1.2±0.1d |
1.5±0.1e |
1.6±0.1e |
1.3±0.1d |
Effects of GIPS on the oxidative
stress factors in serum and spleen of immunosuppressed mice
Compared to normal mice, CTX injection strongly
reduced the levels of SOD in serum and spleen (P<0.001; Table III), but failed to significantly
influence ROS levels (Table III).
Tα1 only enhanced the levels of SOD (P<0.01; Table III), but not ROS, in serum and
spleen of CTX-injected mice. Compared to immunosuppressed mice,
14-day GIPS administration resulted in >14.6% and >51.5%
enhancement on SOD levels in serum (except for 0.1 g/kg GIPS) and
spleen, respectively (P<0.05; Table
III). GIPS showed no effects on serum ROS levels (Table III); in contrast, GIPS at doses of
0.3 and 0.9 g/kg significantly reduced the levels of ROS in spleen
compared to immunosuppressed mice (P<0.01; Table III).
 | Table III.Regulatory effects of GIPS and Tα1 on
the levels of SOD and ROS in serum and spleen of CTX-induced
immunosuppressed mice. |
Table III.
Regulatory effects of GIPS and Tα1 on
the levels of SOD and ROS in serum and spleen of CTX-induced
immunosuppressed mice.
|
|
|
| GIPS (g/kg) | Tα1 (mg/kg) |
|---|
|
|
|
|
|
|
|---|
| Group | CTRL | Model | 0.1 | 0.3 | 0.9 | 0.16 |
|---|
| Serum |
|
|
|
|
|
|
| SOD
(U/ml) |
150.5±6.1 |
113.3±4.0a |
119.0±3.1 |
129.8±1.6b |
136.8±3.9c |
138.0±3.1c |
| ROS
(U/ml) |
32.6±0.6 |
33.3±0.6 |
32.0±1.0 |
31.75±1.6 |
31.2±1.4 |
34.1±1.0 |
| Spleen |
|
|
|
|
|
|
| SOD
(U/mg) |
30.5±2.2 |
16.5±1.2a |
25.0±2.3b |
26.1±0.6b |
27.8±2.5c |
32.4±2.1d |
| ROS
(U/mg) |
39.8±2.7 |
42.7±7.1 |
30.7±1.9 |
28.2±2.9b |
23.1±2.4c |
30.4±2.9 |
Discussion
In this study, we evaluated the immunoregulation
effect of GIPS in CTX-induced immunosuppressed mice, and this model
is recognized as a well characterized model. There are reports
that, CTX shows immunosuppressive effect mainly through lymphocytes
toxicity via disturbing DNA and RNA function (24,25).
GIPS significantly reversed the atrophy of immune organs,
especially thymus, and enhanced T lymphocyte proliferation. Spleen,
an important immune organ, is the primary site for the development
of T cells and B cells (26). The
proliferation of T and B lymphocytes in response to microbial
antigen is known as a typical non-specific immune reaction. Thus,
the determination of lymphocyte proliferation can reflect the
immune status (23). Moreover, GIPS
increased the serum Ig levels in CTX-induced immunosuppressed mice.
Ig is the crucial component of organism immune system and plays a
vital role in immune function (27).
IgG is found to be the most abundant Igs and mediates natural
passive immunity including antibacterial, antivirus and antitoxin
(28); meanwhile, IgA shows
bacteriolysis and promotive function in phagocytosis and
aggregation (29).
Immuno-stimulants, such as bacterial antigen lysate, significantly
enhanced IgG and IgA serum levels in children with recurrent
infections (30,31). IgM, one of the largest molecular
weight Ig, accounts or 5–10% of the total serum Ig, which appears
firstly in the humoral immune response of antibodies (32). The detection of serum IgM levels
serves as an index of the early diagnosis of infection. Altogether,
we successfully confirmed the immunoregulation activities of GIPS
in the CTX-induced immunosuppressed mouse model.
GIPS successfully regulated the levels of ILs, INFs
and MCP-1 in spleen of CTX-induced immunosuppressed mice. The
immune response targets various cytokines, some of which directly
influence immunity of organs. T helper (Th) cells, one of subsets
of T cells, mediates the production of ILs, which can active immune
cells during immune response evidencing by huper-levels of IL-2
activates pro-inflammatory CD4+ T cells (33). IL-3 participates in regulating
myeloid cell expansion, autoimmunity and modulate human
CD14+ monocyte response in short-term or long-term
models of trained immunity (34).
IL-6 is a critical factor for maturation, proliferation,
differentiation and maintenance of B cells/plasma cells, which
seems to enhance the development of pro-inflammatory cell and to
inhibit the development T regulatory cells (35). IL-6 promotes the expression of IL-2
in T cells, which further interacts with IL-3 to promote the
differentiation of T cells (36).
Moreover, Th1 cells secretes IFN-α and IFN-γ, which promotes
cytotoxic lymphocytes formation and activity of macrophage
(37,38). IFN-γ, an essential cytokine for host
defense against pathogens, can stimulate macrophages to eliminate
bacterial and tumor cell, activate neutrophil and natural killer
(NK) cell (37). GIPS significantly
enhanced the levels of MCP-1 in spleen of CTX-induced
immunosuppressed mice. MCP-1, a member of the CC chemokine family,
is responsible for recruiting monocytes, T cells and dendritic
cells to inflammatory sites caused by infection (39). All the data obtained in our present
experiment further confirmed the immunoregulation activities of
GIPS in CTX-induced immunosuppressed mice.
On the other hand, ooxidative stress is identified
as one of the pathogenic factor of the immune system in
inflammatory diseases (40), which
has an effect on human T cell differentiation and polarization
(41). The immune cell functions are
mainly associated with the imbalance of the antioxidant/oxidant,
especially the over-generation of ROS (42). Therefore, it is beneficial to prevent
the injury of immune cells and maintain normal immune system
function with adequate amounts of antioxidants (43). The balance between production and
consumption of ROS is a key factor that determines the the
physiological activities and function of T cells (44). SOD, the endogenous anti-oxidant, is
the recognized as the first line of defense of oxidative stress,
especially helping to scavenging ROS (45). GIPSsignificantly enhanced the levels
of SOD in serum and spleen, and reduced the levels of ROS in spleen
of CTX-induced immunosuppressed mice. However, more experiments
need to be performed to investigate the roles of anti-oxidation of
GIPS during its immunoregulation effects.
To our knowledge, we first confirmed the
immunomodulatory effects of G. incaratum in the CTX-induced
immunosuppressed mouse model, and provided the experimental
evidence for the use of GIPS as a potential immunostimulatory
agent. Although we found that GIPS can regulate the levels of ILs,
INFs and oxidative stress factors, the underlying mechanism still
need further investigation.
Acknowledgements
This study was supported by Science and Technology
Key Project in Jilin Province of China (nos. 20160520036JH and
20160204029YY), China Postdoctoral Science Foundation (no.
2016M591495) and the Special Projects of Cooperation between Jilin
University and Jilin Province (grant no. SXGJSF2017-1).
References
|
1
|
Loh L, Wang ZF, Sant S, Koutsakos M,
Jegaskanda S, Corbett AJ, Liu L, Fairlie DP, Crowe J, Rossjohn J,
et al: Human mucosal-associated invariant T cells contribute to
antiviral influenza immunity via IL-18-dependent activation. Proc
Natl Acad Sci USA. 113:pp. 10133–10138. 2016; View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Collopy KT, Kivlehan SM and Snyder SR: The
impaired immune system. How is it suppressed and what does it mean
for EMS? EMS World. 42:115–118. 2013.
|
|
3
|
Yoshimura A, Suzuki M, Sakaguchi R, Hanada
T and Yasukawa H: SOCS, inflammation and autoimmunity. Front
Immunol. 3:202012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferlazzo G, Tsang ML, Moretta L, Melioli
G, Steinman RM and Münz C: Human dendritic cells activate resting
natural killer (NK) cells and are recognized via the NKp30 receptor
by activated NK cells. J Exp Med. 195:343–351. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reuter S, Gupta SC, Chaturvedi MM and
Aggarwal BB: Oxidative stress, inflammation, and cancer: How are
they linked? Free Radic Biol Med. 49:1603–1616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goldstein M, Roos WP and Kaina B:
Apoptotic death induced by the cyclophosphamide analogue
mafosfamide in human lymphoblastoid cells: Contribution of DNA
replication, transcription inhibition and Chk/p53 signaling.
Toxicol Appl Pharmacol. 229:20–32. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frey BM: Mechanism of action of
immunosuppressive agents. Ther Umsch. 50:71–76. 1993.(In German).
PubMed/NCBI
|
|
8
|
Gassmann W, Uharek L, Wottge HU, Schmitz
N, Löffler H and Mueller-Ruchholtz W: Comparison of
cyclophosphamide, cytarabine, and etoposide as immunosuppressive
agents before allogeneic bone marrow transplantation. Blood.
72:1574–1579. 1988.PubMed/NCBI
|
|
9
|
Fan Y, Ma L, Zhang W, Xu Y, Suolangzhaxi,
Zhi X, Cui E and Song X: Microemulsion can improve the
immune-enhancing activity of propolis flavonoid on
immunosuppression and immune response. Int J Biol Macromol.
63:126–132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao HY, Li GY, Huang J, Han Y, Sun FZ, Du
XW, An LJ, Wang HY and Wang JH: Protective effects of Zhuyeqing
liquor on the immune function of normal and immunosuppressed mice
in vivo. BMC Complement Altern Med. 13:2522013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Płonka PM: Hair pigmentation disorders or
50 years of German-Polish alliance for study on a severe side
effect of chemotherapy: Kostanecki's legacy. Exp Dermatol.
24:10–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Markman M: Chemotherapy-associated
neurotoxicity: An important side effect-impacting on quality,
rather than quantity, of life. J Cancer Res Clin Oncol.
122:511–512. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ren L, Perera C and Hemar Y: Antitumor
activity of mushroom polysaccharides: A review. Food Funct.
3:1118–1130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ben Jeddou K, Chaari F, Maktouf S,
Nouri-Ellouz O, Helbert CB and Ghorbel RE: Structural, functional,
and antioxidant properties of water-soluble polysaccharides from
potatoes peels. Food Chem. 205:97–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou L, Liu Z, Wang Z, Yu S, Long T, Zhou
X and Bao Y: Astragalus polysaccharides exerts immunomodulatory
effects via TLR4-mediated MyD88-dependent signaling pathway in
vitro and in vivo. Sci Rep. 7:448222017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng Y, Wang Q, Zhuang W, Lu X, Miron A,
Chai TT, Zheng B and Xiao J: Cytotoxic, antitumor and
immunomodulatory effects of the water-soluble polysaccharides from
lotus (Nelumbo nucifera Gaertn.) seeds. Molecules. 21:pii:
E14652016. View Article : Google Scholar
|
|
17
|
Asai R, Mitsuhashi S, Shigetomi K,
Miyamoto T and Ubukata M: Absolute configurations of (−)-hirsutanol
A and (−)-hirsutanol C produced by Gloeostereum incarnatum. J
Antibiot (Tokyo). 64:693–696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bunbamrung N, Intaraudom C, Dramae A,
Boonyuen N, Veeranondha S, Rachtawee P and Pittayakhajonwut P:
Antimicrobial activity of illudalane and alliacane sesquiterpenes
from the mushroom Gloeostereum incarnatum BCC41461. Phytochem Lett.
20:274–281. 2017. View Article : Google Scholar
|
|
19
|
Yan H, Zhu D, Xu D, Wu J and Bian X: A
study on Cordyceps militaris polysaccharide purification,
composition and activity analysis. Afr J Biotechnol. 7:4004–4009.
2008.
|
|
20
|
Zhang AL, Lu Jh, Zhang N, Zheng D, Zhang
GR and Teng LR: Extraction, purification and anti-tumor activity of
polysaccharide from mycelium of mutant cordyceps militaris. Chem
Res Chin Univ. 26:798–802. 2010.
|
|
21
|
Jia D, Lu W, Wang C, Sun S, Cai G, Li Y,
Wang G, Liu Y, Zhang M and Wang D: Investigation on
immunomodulatory activity of calf spleen extractive injection in
cyclophosphamide-induced immunosuppressed mice and underlying
mechanisms. Scand J Immunol. 84:20–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roehm NW, Rodgers GH, Hatfield SM and
Glasebrook AL: An improved colorimetric assay for cell
proliferation and viability utilizing the tetrazolium salt XTT. J
Immunol Methods. 142:257–265. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cho CW, Han CJ, Rhee YK, Lee YC, Shin KS,
Shin JS, Lee KT and Hong HD: Cheonggukjang polysaccharides enhance
immune activities and prevent cyclophosphamide-induced
immunosuppression. Int J Biol Macromol. 72:519–525. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Misra RR and Bloom SE: Roles of dosage,
pharmacokinetics, and cellular sensitivity to damage in the
selective toxicity of cyclophosphamide towards B and T cells in
development. Toxicology. 66:239–256. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fraiser LH, Kanekal S and Kehrer JP:
Cyclophosphamide toxicity. Characterising and avoiding the problem.
Drugs. 42:781–795. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kuwana M, Okazaki Y, Kaburaki J, Kawakami
Y and Ikeda Y: Spleen is a primary site for activation of
platelet-reactive T and B cells in patients with immune
thrombocytopenic purpura. J Immunol. 168:3675–3682. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Clem LW and Small PA Jr: Phylogeny of
immunoglobulin structure and function. V. Valences and association
constants of teleost antibodies to a haptenic determinant. J Exp
Med. 132:385–400. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Quast I, Peschke B and Lünemann JD:
Regulation of antibody effector functions through IgG Fc
N-glycosylation. Cell Mol Life Sci. 74:837–847. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Herich R: Is the role of IgA in local
immunity completely known? Food Agric Immunol. 28:1–15. 2016.
|
|
30
|
Sanad MM and Al-Furaeihi LM: Effect of
some immunomodulators on the host-parasite system in experimental
Hymenolepiasis nana. J Egypt Soc Parasitol. 36:65–80.
2006.PubMed/NCBI
|
|
31
|
Quezada A, Maggi L, Pèrez MA and Rodríguez
J: Effect of bacterial antigen lysate on IgG and IgA levels in
children with recurrent infections and hypogammaglobulinemia. J
Investig Allergol Clin Immunol. 9:178–182. 1999.PubMed/NCBI
|
|
32
|
Maverakis E, Kim K, Shimoda M, Gershwin
ME, Patel F, Wilken R, Raychaudhuri S, Ruhaak LR and Lebrilla CB:
Glycans in the immune system and the altered glycan theory of
autoimmunity: A critical review. J Autoimmun. 57:1–13. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu J, Yamane H and Paul WE:
Differentiation of effector CD4 T cell populations (').
Annu Rev Immunol. 28:445–489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Borriello F, Iannone R, Di Somma S,
Loffredo S, Scamardella E, Galdiero MR, Varricchi G, Granata F,
Portella G and Marone G: GM-CSF and IL-3 modulate human monocyte
TNF-α production and renewal inin vitromodels of trained immunity.
Front Immunol. 7:6802016.PubMed/NCBI
|
|
35
|
Tvedt THA, Ersvaer E, Tveita AA and
Bruserud Ø: Interleukin-6 in allogeneic stem cell transplantation:
Its possible importance for immunoregulation and as a therapeutic
target. Front Immunol. 8:6672017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dienz O and Rincon M: The effects of IL-6
on CD4 T cell responses. Clin Immunol. 130:27–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cope A, Le Friec G, Cardone J and Kemper
C: The Th1 life cycle: Molecular control of IFN-γ to IL-10
switching. Trends Immunol. 32:278–286. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ma JJ, Yang BY, Yu SF, Zhang Y, Zhang X,
Lao S, Chen X, Li B and Wu C: Tuberculosis antigen-induced
expression of IFN-α in tuberculosis patients inhibits production of
IL-1β. Faseb J. 28:3238–3248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu LL, Warren MK, Rose WL, Gong W and Wang
JM: Human recombinant monocyte chemotactic protein and other C-C
chemokines bind and induce directional migration of dendritic cells
in vitro. J Leukoc Biol. 60:365–371. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Egea J, Fabregat I, Frapart YM, Ghezzi P,
Görlach A, Kietzmann T, Kubaichuk K, Knaus UG, Lopez MG,
Olaso-Gonzalez G, et al: European contribution to the study of ROS:
A summary of the findings and prospects for the future from the
COST action BM1203 (EU-ROS). Redox Biol. 13:94–162. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
King MR, Ismail AS, Davis LS and Karp DR:
Oxidative stress promotes, polarization of human T cell
differentiation toward a T helper 2 phenotype. J Immunol.
176:2765–2772. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
De la Fuente M: Effects of antioxidants on
immune system ageing. Eur J Clin Nutr. 56 Suppl 3:S5–S8. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gutteridge JM and Mitchell J: Redox
imbalance in the critically ill. Br Med Bull. 55:49–75. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen X, Song M, Zhang B and Zhang Y:
Reactive oxygen species regulate T cell immune response in the
tumor microenvironment. Oxid Med Cell Longev. 2016:15809672016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Islam MT: Oxidative stress and
mitochondrial dysfunction-linked neurodegenerative disorders.
Neurol Res. 39:73–82. 2017. View Article : Google Scholar : PubMed/NCBI
|