Introduction
Neuregulin-1 (NRG-1) is a member of the epidermal
growth factor family. Its receptors are the ErbB family of tyrosine
kinase transmembrane receptors, including the ErbB2, ErbB3 and
ErbB4. In the heart, NRG-1 is synthesized and released by the
endocardial and cardiac microvascular endothelium (1). NRG-1 is essential for the development
of the cardiovascular system and the maintenance of adult heart
function (2,3). Recently, growing evidence indicates
that NRG-1 is a positive regulator of angiogenesis (4,5). Russell
et al reported that NRG-1 and ErbB receptors are expressed
in vascular endothelial cells, and NRG-1 treatment can induce
angiogenesis of endothelial cells in vitro (6). Hedhli et al reported that NRG-1
of endothelial production is necessary for angiogenesis and
arteriogenesis induced by femoral artery ligation, and exogenous
administration of NRG-1 can enhance this process (7). Xiao et al reported elevated
expression of NRG-1 can increase the number of micro-vessels formed
in the ischemic myocardium (8).
Angiogenesis is a highly regulated process requiring
coordinated signaling events among a variety of angiogenic factors.
Vascular endothelial growth factor (VEGF), angiopoietin-1 (Ang-1)
and angiopoietin-2 (Ang-2) play essential roles in angiogenesis.
Both Flk1 and Tie-2 receptors are exclusively expressed in
endothelial cells. The VEGF/Flk1 signal system takes the lead in
new vessel formation, and Ang-1/Tie-2 signal system plays a
critical role in vascular maturation and stabilization. Notably
Ang-2 represents a natural Ang1/Tie2 inhibitor, may cause
destabilization and initiate neovascularization (9).
Our previous study demonstrated that serum NRG-1β
levels are positively correlated with serum VEGF and Ang-1 levels
in patients with diabetes or unstable angina pectoris (10). It has been reported that NRG-1
stimulation can increase the expression and secretion of VEGF in
tumor cells (11) and endothelial
cells (12). Nakaoka et al
reported that NRG-1 stimulation can increase mRNA expression of
Ang-1 in the heart (13). There may
be a relationship between NRG-1 and these angiogenic factors.
However, it is not clear whether stimulation with these angiogenic
factors can increase NRG-1 production in endothelial cells. With
this possibility in mind, the aim of this study was to investigate
the effects of angiogenic factors treatment on the expression and
secretion of NRG-1 in human cardiac microvascular endothelial cells
(HCMECs) under normal or hypoxia/serum deprivation (Hypo/SD)
culture conditions.
Materials and methods
Cells and reagents
HCMECs were obtained from ScienCell Research
Laboratories (Carlsbad, CA, USA); anti-ErbB2 rabbit monoclonal
antibody, anti-ErbB3 rabbit monoclonal antibody and anti-ErbB4
rabbit monoclonal antibody were obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA); anti-NRG-1 rabbit polyclonal
antibody was obtained from R&D Systems (Minneapolis, MN, USA);
anti-GAPDH rabbit polyclonal antibody was obtained from Signalway
Antibody LLC (College Park, MD, USA); VEGF and Ang-1 were obtained
from ProSpec-Tany TechnoGene Ltd. (Nessziona, Israel); Ang-2 was
obtained from Peprotech (Oak Park, CA, USA); NRG-1β ELISA kit was
obtained from RayBiotech (Norcoss, GA, USA). Myocardial tissues of
rat were obtained from our laboratory.
HCMECs culture and grouping
Primary HCMECs from liquid nitrogen were completely
thawed in a 37°C water bath. The cells were cultured in 25
cm2 culture flasks with endothelial cell medium
(ScienCell Research Laboratories) supplemented with 10% fetal
bovine serum (FBS) in a 37°C and 5% CO2 incubator.
Medium was changed every 3 days. At confluence the cells were
passaged. In all experiments, cells from the 4th passage were used.
According to the need of tests, cells were stimulated with VEGF
(100 ng/ml), Ang-1 (100 ng/ml) or Ang-2 (100 ng/ml) under normal or
Hypo/SD conditions for 24 h. Hypo/SD represents both of components
in ischemia in vivo. In our model of Hypo/SD, cells were
incubated in a modular incubator chamber and infused with mixed gas
(95% N2 and 5% CO2) at 37°C and O2
<0.5%. Under normal culture condition, experimental cells were
divided into four groups, including control group, VEGF treatment
group, Ang-1 treatment group and Ang-2 treatment group. Under
Hypo/SD condition, experimental cells were divided into five
groups, including control group, Hypo/SD group, Hypo/SD+VEGF
treatment group, Hypo/SD+Ang-1 treatment group and Hypo/SD+Ang-2
treatment group.
Western blot analysis
Cells were harvested into 1.5 ml micro-tubes, RIPA
buffer with PMSF (0.1 mM) was added immediately, then protein
extraction and quantification were performed. Total protein was
fractionated by SDS gel electrophoresis and transferred to a
visualization membrane. The membrane was blocked with 5% nonfat
milk for 1 h at room temperature followed by overnight incubation
at 4°C with primary antibodies. Primary antibody binding was
detected using a horseradish peroxidase conjugated secondary
antibody and an enhanced chemiluminescence detection system (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Membrane bands were
analyzed using Image-analysis software (Quantity One; Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
ELISA analysis
The same quantity of HCMECs were plated in each 25
cm2 culture flask and cultured in an incubator as
previously described. After 48 h, the culture medium (the same
volume each flask) was changed. Under normal or Hypo/SD culture
conditions, cells were cultured with VEGF, Ang-1 or Ang-2 treatment
for 24 h. This conditioned medium was then collected and
concentrated using 3kD Amicon Ultra-15 Centrifugal Filter Units
(EMD Millipore, Billerica, MA, USA). The remaining concentrated
medium (250 µl) was transferred to another tube and stored at −80°C
until use. NRG-1β secreted in the HCMECs culture medium was
detected by a commercially available ELISA kit according to the
manufacturer's protocol. Standards or samples (100 µl), detection
reagent, substrate solution and stop solution were sequentially
pipette into wells and incubated with repeated washing. The
reaction product was analyzed spectrophotometrically at 450 nm with
a plate reader, and sample values were calculated using a standard
curve.
Statistical analysis
Numerical values are expressed as the mean ±
standard deviation. The data were analyzed using the SPSS 16.0
statistic software package (SPSS, Inc., Chicago, IL, USA). One-way
analysis of variance was performed, and post hoc multiple
comparisons were conducted with S-N-K. P<0.05 was considered to
indicate a statistically significant difference.
Results
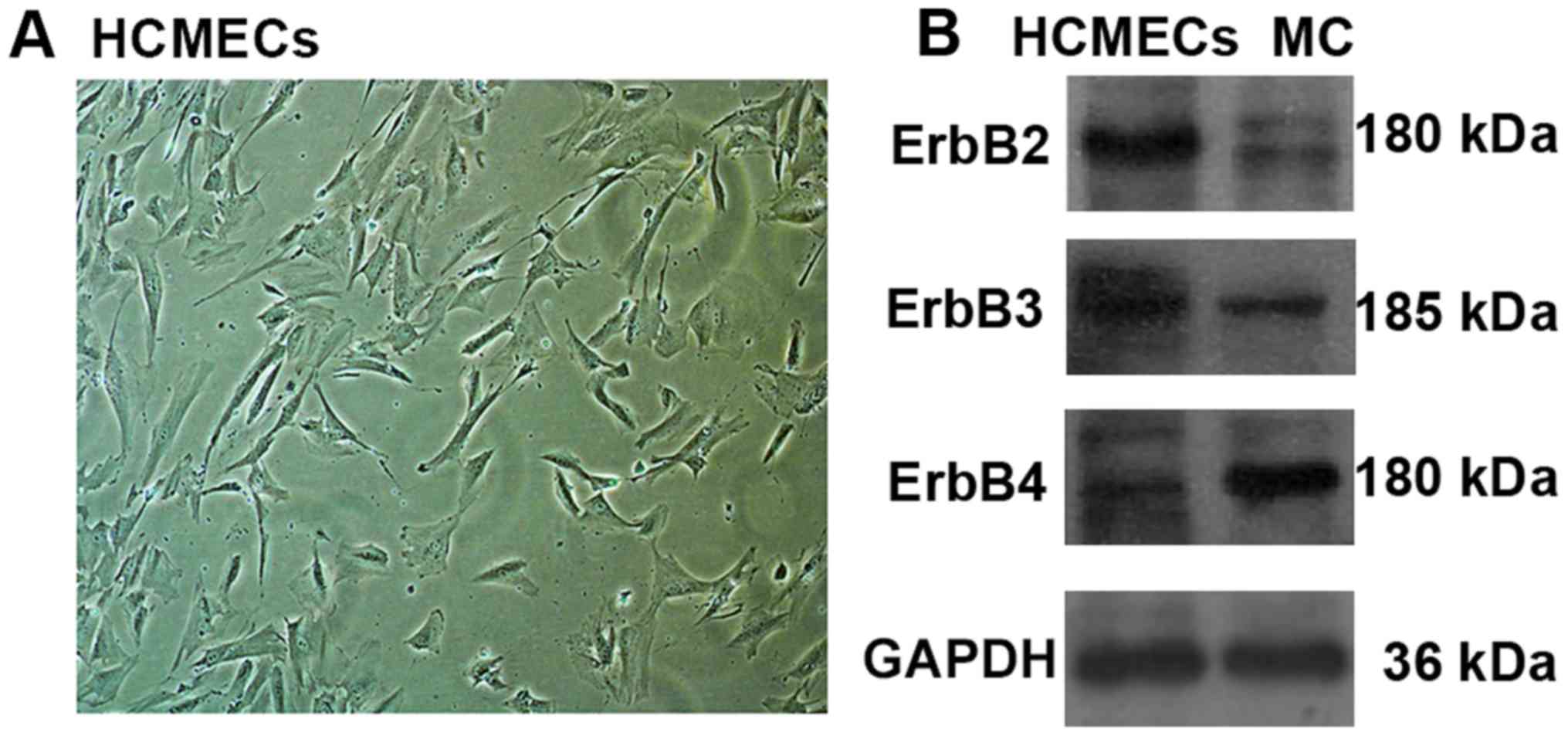
ErbB receptors were expressed in
HCMECs
It is well established that NRG-1 is expressed in
HCMECs, but there are no studies reporting whether ErbB receptors
are expressed in HCMECs. To this end, we screened for the
expression of ErbB2, ErbB3 and ErbB4 receptors in HCMECs using
western blot. Since ErbB receptors are expressed in myocardial
tissues, so myocardial tissues of rat were used as a positive
contrast. Western blot results showed all three ErbB receptors were
expressed in HCMECs, and ErbB2 expression levels were much higher
than ErbB3 and ErbB4 expression levels. However, ErbB4 expression
levels were much higher than ErbB2 and ErbB3 expression levels in
myocardial tissues (Fig. 1).
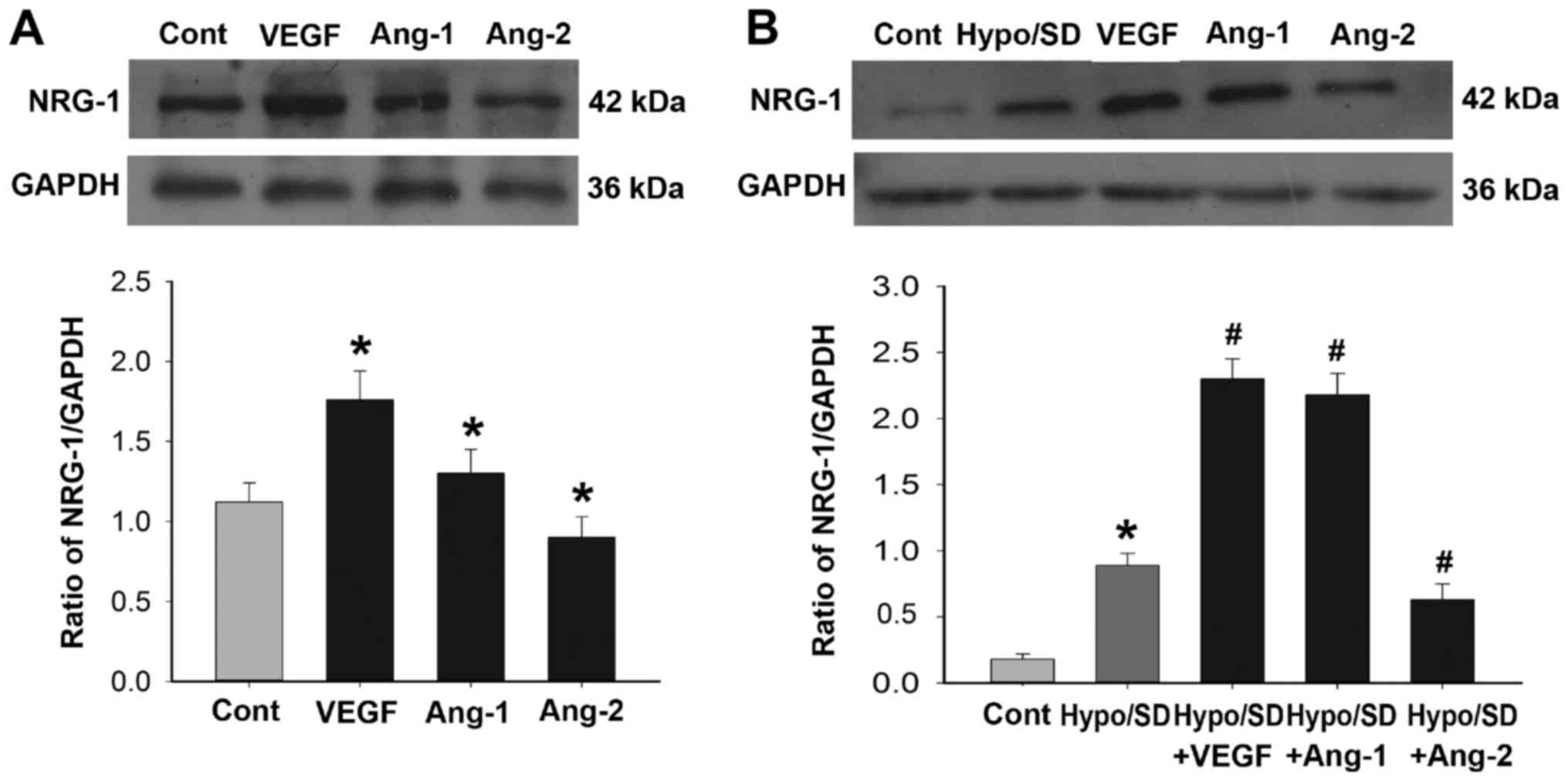
NRG-1 expression was regulated by
angiogenic factors
We examined the effects of VEGF, Ang-1 and Ang-2
treatment on NRG-1 expression in HCMECs under normal or Hypo/SD
conditions. Under normal culture condition, NRG-1 expression was
significantly increased in the VEGF treatment group and Ang-1
treatment group, but significantly decreased in Ang-2 treatment
group as compared with that of the control group (P<0.05;
Fig. 2A). Under Hypo/SD condition,
Hypo/SD stimulation significantly increased NRG-1 expression, and
VEGF or Ang-1 treatment significantly further increased NRG-1
expression, but Ang-2 treatment significantly decreased NRG-1
expression (P<0.05; Fig. 2B).
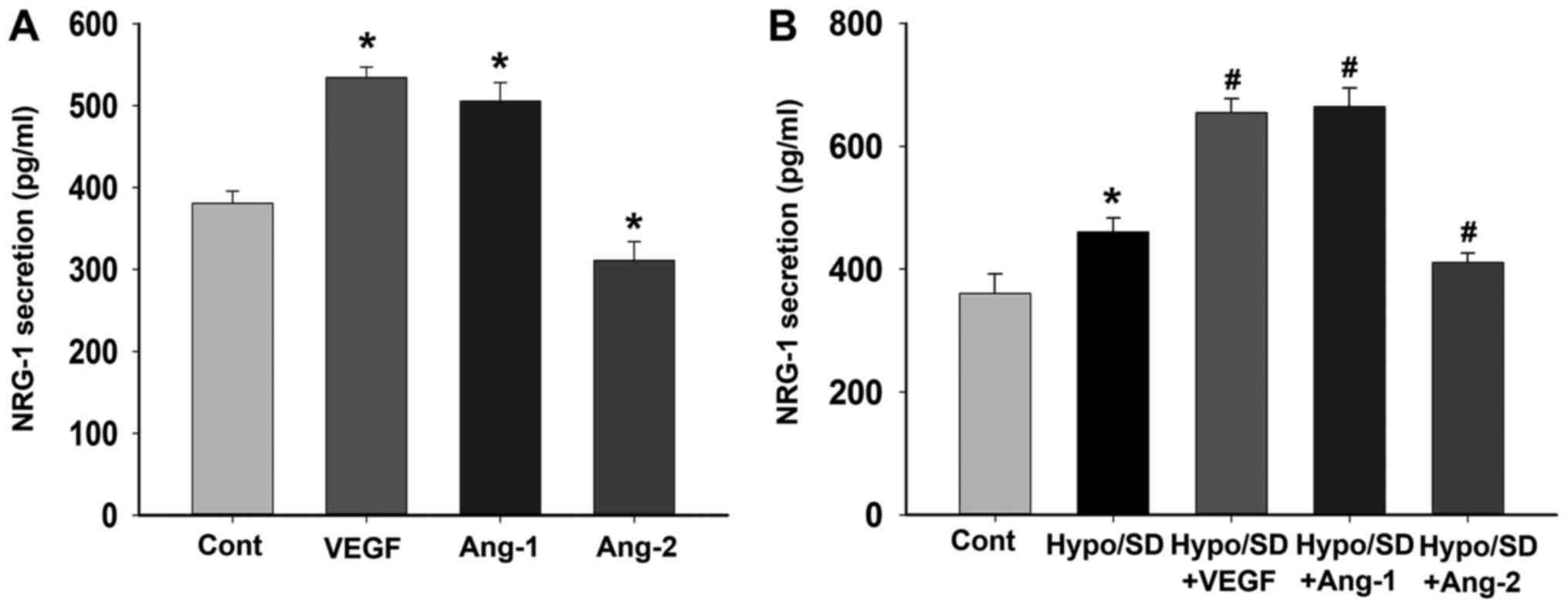
NRG-1 secretion was regulated by
angiogenic factors
We further examined the effects of VEGF, Ang-1 and
Ang-2 treatment on NRG-1β secretion in HCMECs under normal or
Hypo/SD conditions. Under normal culture condition, the results
demonstrated that VEGF or Ang-1 treatment significantly increased
NRG-1β secretion, but Ang-2 treatment significantly decreased
NRG-1β secretion (P<0.05; Fig.
3A). Under Hypo/SD condition, the results demonstrated that
Hypo/SD stimulation significantly increased NRG-1β secretion, and
VEGF or Ang-1 treatment significantly further increased NRG-1β
secretion, but Ang-2 treatment significantly decreased NRG-1β
secretion (P<0.05; Fig. 3B).
Discussion
This study reported, for the first time, the effects
of angiogenic factors treatment on the expression and secretion of
NRG-1 in HCMECs. Results demonstrated that ErbB2, ErbB3 and ErbB4
receptors were expressed in HCMECs, and the expression and
secretion of NRG-1 in HCMECs were upregulated by Hypo/SD, VEGF or
Ang-1 treatment, but were downregulated by Ang-2 treatment.
HCMECs can directly interact with adjacent
cardiomyocytes and are the main cells-type involved in
angiogenesis. Through autocrine and paracrine regulation, HCMECs
can increase cardiac angiogenesis and of myocyte survival. Growth
factors, such as Angs, VEGF and NRG-1, are involved in these
interactions. In the heart, NRG-1 is synthesized and released by
the endocardial and cardiac microvascular endothelium (1,2). Hedhli
et al have reported that NRG-1 plays an important role in
cardiac myocyte protection and angiogenic responses to ischemia
injury, and NRG-1 secretion is significantly increased in cardiac
endothelial cells in response to hypoxia (14). Our study also showed the expression
and secretion of NRG-1 were increased in HCMECs with Hypo/SD
stimulation. Hypo/SD mimics the environments of ischemia in
vivo. This result is also consistent with the findings of
previous in vivo studies. Kuramochi et al reported
that NRG-1 released from the endothelium is acutely promoted in
models of ischemia/reperfusion (15). Ky et al reported that
circulating NRG-1 levels are increased in chronic heart failure of
ischemic but not of non-ischemic etiology (16). Geisberg et al reported plasma
NRG-1β levels were statistically higher in patients with
stress-induced ischemia, and NRG-1β levels were inversely
correlated with ischemia severity (17). These findings suggest that ischemia
may be an important trigger for endothelial NRG-1 synthesis and
release.
Our results further illustrate that VEGF or Ang-1
treatment can increase the expression and secretion of NRG-1 in
HCMECs, but Ang-2 treatment has opposite effect. Regulated cleavage
and release of transmembrane growth factors have been recognized as
a common and important mechanism for autocrine and paracrine
signaling. Kalinowski et al reported that pro-forms of NRG-1
can be cleaved by TNF converting enzyme in response to the
inflammatory cytokines IL-6 and IFN (18). Lemmens et al reported that
mechanical strain increases endothelial NRG-1 synthesis and
release, but angiotensin II and adrenergic agonists decrease
endothelial NRG-1 synthesis and release (1,19).
The mechanisms of the inverse relationship of Ang-2
on NRG-1 are not clear. Although Ang-1 and Ang-2 share similar
binding affinities for the Tie2 receptor, they have opposing
effects on receptor activation. Ang-1 induces receptor
phosphorylation and contributes to blood vessel stabilization. The
roles of Ang-2 in angiogenesis are complicated, depending on the
availability of VEGF. In general, Ang-2 antagonizes the actions of
Ang-1 and is associated with blood vessel growth or regression
(20). Previous studies have
reported that NRG-1 stimulation can increase the expression of VEGF
and Ang-1 in different kinds of cells (10–12).
This is the first report regarding the effect of angiogenic factors
treatment on the expression and secretion of NRG-1, but the
limitation of this study is that we only investigated the effect of
an angiogenic factor alone. These results suggest VEGF and Ang-1
may increase myocardial angiogenesis and survival via NRG-1/ErbB
signaling.
Russell et al demonstrated that ErbB2, ErbB3
and ErbB4 are expressed in human umbilical vein endothelial cells
and the ErbB2 expression levels are the highest among three ErbB
receptors (6). Lok et al
reported ErbB2 and ErbB3 receptors are expressed in brain
endothelial cells, but ErbB4 was not detected (21). Zhao et al reported that ErbB4
expression is quite prominent in cardiac myocytes, while ErbB2 is
only barely detectable and ErbB3 is not detectable (22). This study reported, for the first
time, that three ErbB receptors are expressed in HCMECs, and ErbB2
expression levels are higher than the expression levels of ErbB3
and ErbB4. However, ErbB4 expression levels were much higher than
ErbB2 and ErbB3 expression levels in myocardial tissues. This
results are consistent with previous studies.
NRG-1 binds with high affinity only to ErbB3 and
ErbB4, but can very efficiently activate ErbB2 as a heterodimer
with either receptor. Hedhli et al found three ErbB
receptors are expressed in human coronary artery smooth muscle
cells (SMC) (4), and this study
found HCMECs express both NRG-1 and ErbB receptors. So these
findings indicate HCMECs and SMC can participate in both autocrine
and paracrine regulation via NRG-1/ErbB signaling, an important
potential mechanism for regulation of vascular responses. ErbB2 and
ErbB4 are expressed in cardiomyocytes, the crosstalk between
endothelial cells and cardiomyocytes via this pathway can have
profound effects on myocardial function and survival (23).
In conclusion, the expression and release of NRG-1
are increased in HCMECs in the presence of VEGF or Ang-1,
demonstrating that VEGF and Ang-1 may regulate myocardial
angiogenesis and survival via NRG-1/ErbB signaling.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81460063) and Guangxi
Natural Science Foundation (grant no. 2014GXNSFDA118024).
References
|
1
|
Lemmens K, Segers VF, Demolder M and De
Keulenaer GW: Role of neuregulin-1/ErbB2 signaling in
endothelium-cardiomyocyte crosstalk. J Biol Chem. 281:19469–19477.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Odiete O, Hill MF and Sawyer DB:
Neuregulin in cardiovascular development and disease. Circ Res.
111:1376–1385. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rupert CE and Coulombe KL: The roles of
neuregulin-1 in cardiac development, homeostasis, and disease.
Biomark Insights. 10 Suppl 1:S1–S9. 2015.
|
|
4
|
Hedhli N, Kalinowski A and S Russell K:
Cardiovascular effects of neuregulin-1/ErbB signaling: Role in
vascular signaling and angiogenesis. Curr Pharm Des. 20:4899–4905.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hedhli N and Russell KS: Cytostatic drugs,
neuregulin activation of erbB receptors and angiogenesis. Curr
Hypertens Rep. 12:411–417. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Russell KS, Stem DF, Polverini PJ and
Bender JR: Neuregulin activation of ErbB receptors in vascular
endothelium leads to angiogenesis. Am J Physiol. 277:H2205–H2211.
1999.PubMed/NCBI
|
|
7
|
Hedhli N, Dobrucki LW, Kalinowski A,
Zhuang ZW, Wu X, Russell RR III, Sinusas AJ and Russell KS:
Endothelial-derived neuregulin is an important mediator of
ischaemia-induced angiogenesis and arteriogenesis. Cardiovasc Res.
93:516–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiao J, Li B, Zheng Z, Wang M, Peng J, Li
Y and Li Z: Therapeutic effects of neuregulin-1 gene transduction
in rats with myocardial infarction. Coron Artery Dis. 23:460–468.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Karamysheva AF: Mechanisms of
angiogenesis. Biochemistry (Mosc). 73:751–760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zeng Z, Gui C, Nong Q, Du F and Zhu L:
Serum neuregulin-1β levels are positively correlated with VEGF and
Angiopoietin-1 levels in patients with diabetes and unstable angina
pectoris. Int J Cardiol. 168:3077–3079. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yonezawa M, Wada K, Tatsuguchi A, Akamatsu
T, Gudis K, Seo T, Mitsui K, Nagata K, Tanaka S, Fujimori S and
Sakamoto C: Heregulin-induced VEGF expression via the ErbB3
signaling pathway in colon cancer. Digestion. 80:215–225. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iivanainen E, Paatero I, Heikkinen SM,
Junttila TT, Cao R, Kint P, Jaakkola PM, Cao Y and Elenius K:
Intra- and extracellular signaling by endothelial neuregulin-1. Exp
Cell Res. 313:2896–2909. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakaoka Y, Nishida K, Narimatsu M, Kamiya
A, Minami T, Sawa H, Okawa K, Fujio Y, Koyama T, Maeda M, et al:
Gab family proteins are essential for postnatal maintenance of
cardiac function via neuregulin-1/ErbB signaling. J Clin Invest.
117:1771–1781. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hedhli N, Huang Q, Kalinowski A, Palmeri
M, Hu X, Russell RR and Russell KS: Endothlium-derived neuregulin
protects the heart against ischemic injury. Circulation.
123:2254–2262. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuramochi Y, Cote GM, Guo X, Lebrasseur
NK, Cui L, Liao R and Sawyer DB: Cardiac endothelial cells regulate
reactive oxygen species-induced cardiomyocyte apoptosis through
neuregulin-1beta/erbB4 signaling. J Biol Chem. 279:51141–51147.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ky B, Kimmel SE, Safa RN, Putt ME,
Sweitzer NK, Fang JC, Sawyer DB and Cappola TP: Neuregulin-1 beta
is associated with disease severity and adverse outcomes in chronic
heart failure. Circulation. 120:310–317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Geisberg CA, Wang G, Safa RN, Smith HM,
Anderson B, Peng XY, Veerkamp B, Zhao DX, Blakemore D, Yu C and
Sawyer DB: Circulating neuregulin-1β levels vary according to the
angiographic severity of coronary artery disease and ischemia.
Coron Artery Dis. 22:577–582. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kalinowski A, Plowes NJ, Huang Q,
Berdejo-Izquierdo C, Russell RR and Russell KS: Metalloproteinase
dependent cleavage of neuregulin and autocrine stimulation of
vascular endothelial cells. Faseb J. 24:2567–2575. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lemmens K, Doggen K and De Keulenaer GW:
Role of neuregulin-1/ErbB signaling in cardiovascular physiology
and disease: Implications for therapy of heart failure.
Circulation. 116:954–960. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reiss Y, Droste J, Heil M, Tribulova S,
Schmidt MH, Schaper W, Dumont DJ and Plate KH: Angiopoietin-2
impairs revascularization after limb ischemia. Circ Res. 101:88–96.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lok JL, Sardi SP, Guo S, Besancon E, Ha
DM, Rosell A, Kim WJ, Corfas G and Lo EH: Neuregulin-1 signaling in
brain endothelial cells. J Cereb Blood Flow Metab. 29:39–43. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao YY, Sawyer DR, Baliga RR, Opel DJ,
Han X, Marchionni MA and Kelly RA: Neuregulins promote survival and
growth of cardiac myocytes. Persistence of erbb2 and erbb4
expression in neonatal and adult ventricular myocytes. J Biol Chem.
273:10261–10269. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li B, Xiao J, Li Y, Zhang J and Zeng M:
Gene transfer of human neuregulin-1 attenuates ventricular
remodeling in diabetic cardiomyopathy rats. Exp Ther Med.
6:1105–1112. 2003. View Article : Google Scholar
|