Introduction
Mesenchymal stem cells (MSCs), which belong to the
pluripotent stem cells, were initially identified in the bone
marrow. Due to their various characteristics, including
multidifferentiation potential, hematopoiesis support, stem cell
implantation promotion, immune regulation and self-renewal
(1,2), MSCs are currently a research focus. In
addition, MSCs have become an attractive cell source for use in
bone repair and tissue engineering due to their capacity for
self-renewal and differentiation into osteoblasts (3).
Semaphorins (Semas) are a large family of conserved
guidance proteins that regulate cellular shape and function
(4). Semas were first identified as
axon guidance factors during nervous system development, while they
were found to be regulators of various developmental processes,
including the heart, bone, kidney, lung and immune development, as
well as angiogenesis (5–9). Previous studies have indicated that
Semas serve important roles in osteoporosis, cardiovascular
diseases, cancer and immune-mediated diseases (10–12). As
a member of class 3 Semas, Sema3A serves a role in suppressing the
progression of various types of cancer by inhibiting angiogenesis
(13–16). More recently, Sema3A has been found
to serve key roles in bone metabolism, at the same time, Sema3A
could promote osteoblast differentiation and inhibit osteoblast
activity, and is the hotspot in research of bone diseases (17,18).
However, the role of Sema3A in the osteogenic differentiation of
human alveolar bone marrow MSCs (hABMMSCs) remains unclear.
Therefore, in the present study, the fundamental
functions of Sema3A in hABMMSC osteogenic differentiation were
investigated and the underlying mechanism was analyzed.
Materials and methods
Materials
The α-minimum essential medium (MEM) culture medium,
fetal bovine serum (FBS), streptomycin, penicillin and L-glutamine
were supplied by Thermo Fisher Scientific, Inc. (Gibco; Waltham,
MA, USA). Tryptase was obtained from Amresco, LLC (Solon, OH, USA).
Dexamethasone, β-glycerin sodium phosphate, ascorbic acid, dimethyl
sulfoxide and Alizarin Red S were purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). The flow cytometer was purchased
from Beckman Coulter, Inc. (Brea, CA, USA), while the enzyme-linked
immune detector was supplied by BioTek Instruments, Inc. (Winooski,
VT, USA). The ultraviolet spectrophotometer instrument
(BioSpectrometer) and the polymerase chain reaction (PCR)
instrument (Mastercycler nexus) were supplied by Eppendorf
(Hamburg, Germany).
Separation and purification of
hABMMCs
The present study was approved by the Ethics
Committee of the Stomatological Hospital of Jiangsu Province
(Nanjing, China), and written informed consent was obtained from
each patient. hABMMSCs were isolated and expanded as described by
Zhang et al (19). Between
January 2014 and December 2015, a total of 15 patients (male 8,
female 7; aged 18–22 years; mean age: 20.5 years), were admitted at
the Department of Oral and Maxillofacial Surgery in Stomatological
Hospital of Jiangsu Province. All patients with systemic or
metabolic disease were excluded from the present study and received
orthognathic surgery due to malocclusion. All the bone collected
from these patients were fresh and healthy. Briefly, healthy jaw
cancellous bone was collected from patients during the orthognathic
surgery under sterile conditions. Subsequently, the samples were
washed and centrifuged with 1 mol/l PBS at 800 × g for three or
four times for 5 min each time at 4°C. The collected endothelial
cells (5×104 cells per well) and bone fragments were
then seeded into a 6-well plate and cultured in α-MEM medium
supplemented with 10% FBS, 100 units/ml penicillin, 100 µg/ml
streptomycin and 2 mM L-glutamine and incubated in a 5%
CO2 incubator at 37°C. The culture medium was replaced
every 3 days, and cells were passaged until 80% confluence was
reached. Next, first generation log-phase cells were seeded into
6-well plates (300–450 cells per well) and cultured for 7–10 days
prior to the observation of visible hABMMSC colonies. Subsequently,
the cells were harvested with using 0.25% trypsin and then cultured
in maintenance medium consisting of 10% FBS, 100 units/ml
penicillin, 100 µg/ml streptomycin and 2 mM L-glutamine at 37°C in
an atmosphere containing 5% CO2, and third to fifth
generation cells were used in subsequent experiments.
Cell infection and morphology
observation
The adenovirus expression vector
pAdCMV-SEMA3A-MCS-EGFP, which overexpressed human Sema3A, and the
control vector pCMV-MCS-EGFP were synthesized by Genechem (Shanghai
Genechem Co., Ltd., Shanghai, China). The hABMMSCs were infected
with the pAdCMV-SEMA3A-MCS-EGFP (Sema3A group) or pCMV-MCS-EGFP
(control group) vector in 10 µg/ml hexadimethrine bromide and
incubated for an additional 48 h at 37°C in an atmosphere
containing 5% CO2. The efficiency of infection was
observed under an inverted fluorescence microscope at 48 h after
the infection, and the cell morphology was examined.
Clone formation assay
Log-phase hABMMSCs were harvested with trypsin,
counted with a hemocytometer, and transferred to 75-cm2
cell culture flasks (3×104 cells/cm2; three
replicates per sample). Subsequent to incubation for 10 days, the
cells were carefully rinsed twice with PBS, followed by fixing with
4% paraformaldehyde for 20 min at room temperature and staining
with 0.5% crystal violet for 20 min. Subsequently, the cells were
washed with distilled water and dried naturally. The number of cell
clones with >50 cells was counted under the microscope and the
cloning efficiency was calculated according to the following
formula: Cloning efficiency (%)=(number of clones/number of cells
incubated) ×100% (20).
Cell proliferation assay by cell
counting kit-8 (CCK-8)
Third generation log-phase hABMMSCs were infected
with an empty vector (pCMV-MCS-EGFP) or pAdCMV-SEMA3A-MCS-EGFP, and
then the cells were seeded into a 96-well plate with an initial
density of 2×103 cells per well (three replicates per
sample) and cultured in the osteogenesis-inducing media containing
10% FBS, 100 units/ml penicillin, 100 µg/ml streptomycin, 2 mol/l
dexamethasone, 0.01 mol/l β-glycerin sodium phosphate and 50 µg/ml
ascorbic acid at 37°C in an atmosphere containing 5%
CO2. The viability of these cells was detected on days
1, 2, 3, 4, 5 and 6 according to the protocol of the CCK-8 assay
(Dojindo, Molecular Technologies, Inc., Kumamoto, Japan), and the
results were statistically analyzed.
Alizarin Red S staining
At 24 h after third generation log-phase hABMMSCs
were infected with pCMV-MCS-EGFP or pAdCMV-SEMA3A-MCS-EGFP, the
cells were seeded into 6-well plates (5×104 cells per
well) and grown in osteogenesis-inducing media consisting of α-MEM
medium supplemented with 10% FBS, 10 mmol/l β-glycerin liquid
sodium phosphate, 0.3 mmol/l vitamin C and 1×10−5 mmol/l
dexamethasone. Following incubation for 7, 14 and 21 days, Alizarin
Red S staining was performed as described by Cai et al
(21) with minor modification.
Briefly, the cultured cells in the 6-well plates were rinsed twice
with PBS and fixed with 4% paraformaldehyde for 20 min. The cells
were then washed with PBS and exposed to Alizarin Red S (2%
aqueous) for 5 min. Subsequently, they were washed again with PBS
and observed under a microscope. Positive staining is represented
as a red/purple color.
Reverse transcription-quantitative PCR
(RT-qPCR)
Following infection for 7 or 14 days, RT-qPCR was
performed to detect the mRNA expression level of three
osteogenesis-associated genes, namely Runt-related transcription
factor 2 (Runx2), osteopontin (Opn) and osteocalcin (Ocn). Briefly,
total RNA was extracted from the cell lines using TRIzol reagent
(Takara Bio, Inc., Otsu, Japan) following the manufacturer's
protocol. RNA concentration and quality were measured using a
NanoDrop spectrophotometer (ND-1,000; NanoDrop Technologies,
Wilmington, DE, USA). Next, cDNA was obtained from total RNA using
a cDNA RT kit (Invitrogen; Thermo Fisher Scientific, Inc.)
according to manufacturer's instructions. qPCR was performed to
analyze the synthesized cDNA using a PCR thermal cycler with the
following amplification parameters: 95°C for 10 min, followed by 40
cycles of 95°C for 10 sec, and 60°C for 60 sec. All the primers
used in qPCR were synthesized by Nanjing GenScript Co., Ltd.
(Nanjing, China), and were as follows: Runx-2,
5′-TGGCAGCACGCTATTAAATC-3′ (forward) and 5′-TCTGCCGCTAGAATTCAAAA-3′
(reverse); Opn, 5′-ACGCCGACCAAGGAAAACTC-3′ (forward) and
5′-GTCCATAAACCACACTATCACCTCG-3′ (reverse); Ocn,
5′-CAGACACCATGAGGACCATC-3′ (forward) and 5′-GGACTGAGGCTCTGTGAGGT-3′
(reverse); GAPDH, 5′-GAAGGTGAAGGTCGGAGTC-3′ (forward) and
5′-GAAGATGGTGATGGGATTTC-3′ (reverse). GAPDH served as an internal
control.
Statistical analysis
All data are displayed as the mean ± standard
deviation. Statistical comparisons between two groups were
conducted with the Student's t-test. Statistical analysis was
performed using the SPSS version 18.0 statistical software package
(SPSS, Inc., Chicago, IL, USA). Values of P<0.05 were considered
to indicate a difference that was statistically significant.
Results
Cell morphology
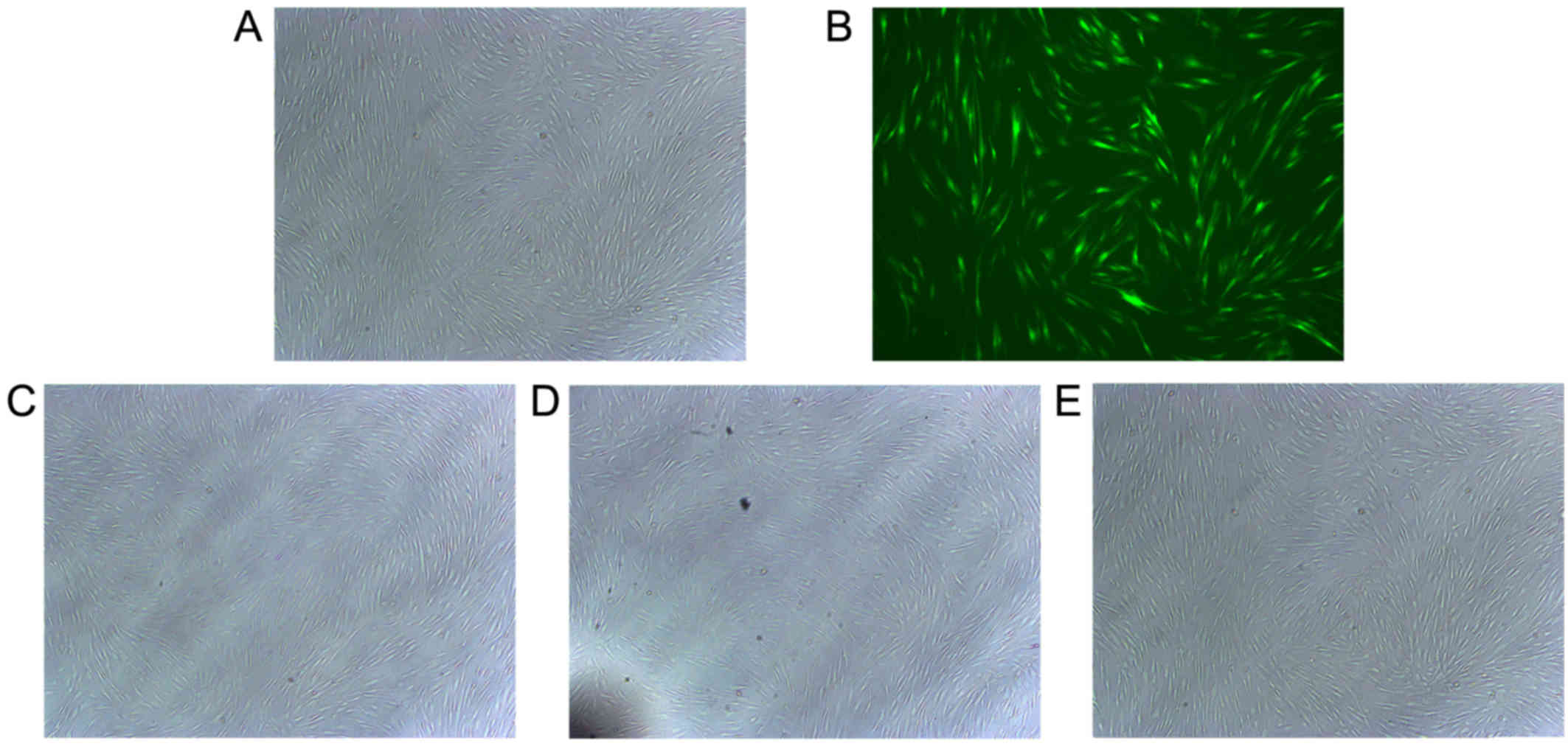
hABMMCs were separated and purified as described
previously (22). The majority of
the hABMMCs were spindle shaped, there was an abundant cytoplasm
and only a few hABMMCs were oval-shaped. The efficiency of
infection and cell morphology were examined under an inverted
fluorescence microscope at 48 h after the infection. The results
revealed that the cell transfection efficiency was >60%, while
the cell morphology of the infected cells was stable and exhibited
no significant alterations when compared with the normal control
group (Fig. 1).
Effect of Sema3A on hABMMSC cell
proliferation activity
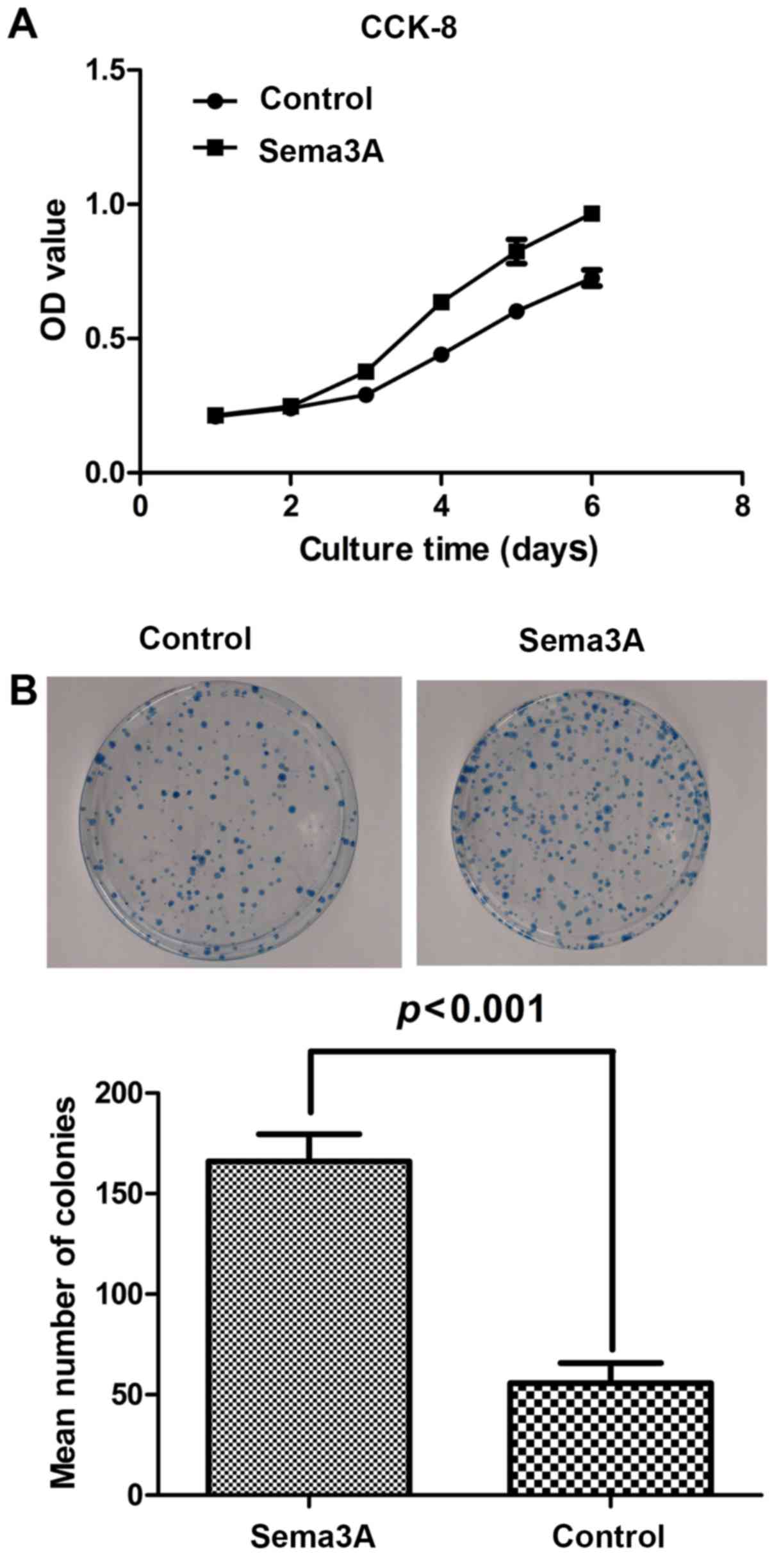
To investigate the effect of Sema3A on the hABMMSC
cell proliferation activity, clone formation assay and cell
proliferation (CCK-8) assays were performed. As shown in Fig. 2A, compared with the control group,
infection of hABMMSCs with the pAdCMV-SEMA3A-MCS-EGFP vector
significantly affected the cell proliferation, while the cell
viability was significantly enhanced. As shown in Fig. 2B, the results of the clone formation
assay suggested that the clone formation ability of
pAdCMV-SEMA3A-MCS-EGFP-infected cells was significantly increased
as compared with that of the pCMV-MCS-EGFP-infected cells. All
these data indicated that Sema3A overexpression significantly
increased the hABMMSC proliferation.
Sema3A facilitates hABMMSC osteogenic
differentiation
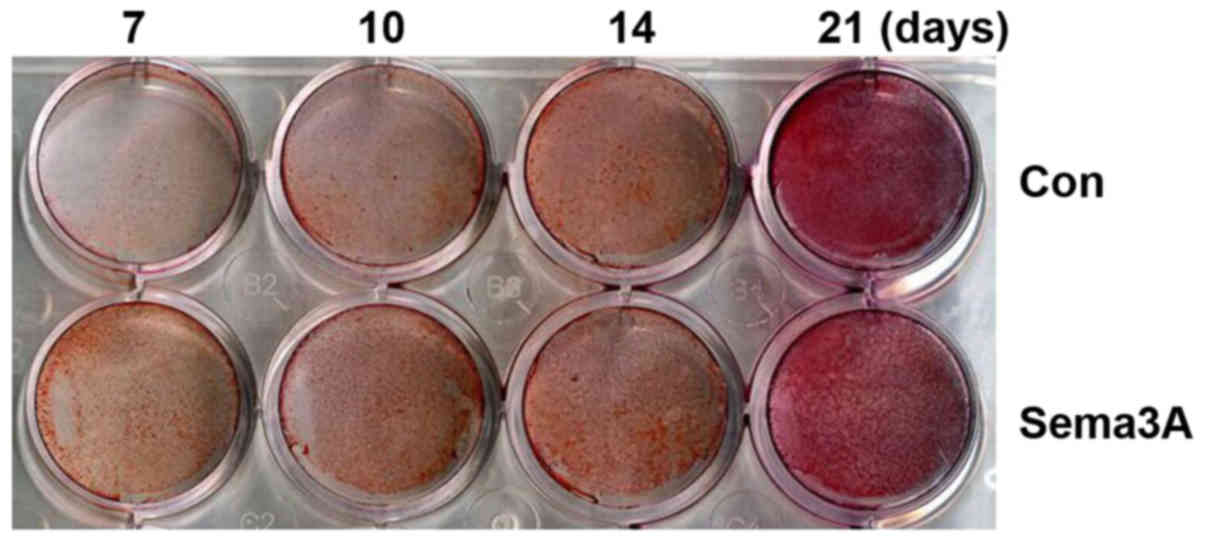
In order to investigate whether Sema3A exhibited an
effect on osteogenic differentiation, pAdCMV-SEMA3A-MCS-EGFP or
pCMV-MCS-EGFP vector was transfected into hABMMSCs. Alizarin Red S
staining was then performed after 7, 10, 14 and 21 days of
culturing in the osteogenesis-inducing media. hABMMSCs transfected
with pAdCMV-SEMA3A-MCS-EGFP demonstrated matrix mineralization with
more intense Alizarin Red S staining when compared with the
pCMV-MCS-EGFP-transfected hABMMSCs (Fig.
3). Notably, the staining was more intense at earlier time
points in Sema3A overexpressed hABMMSCs compared with the control.
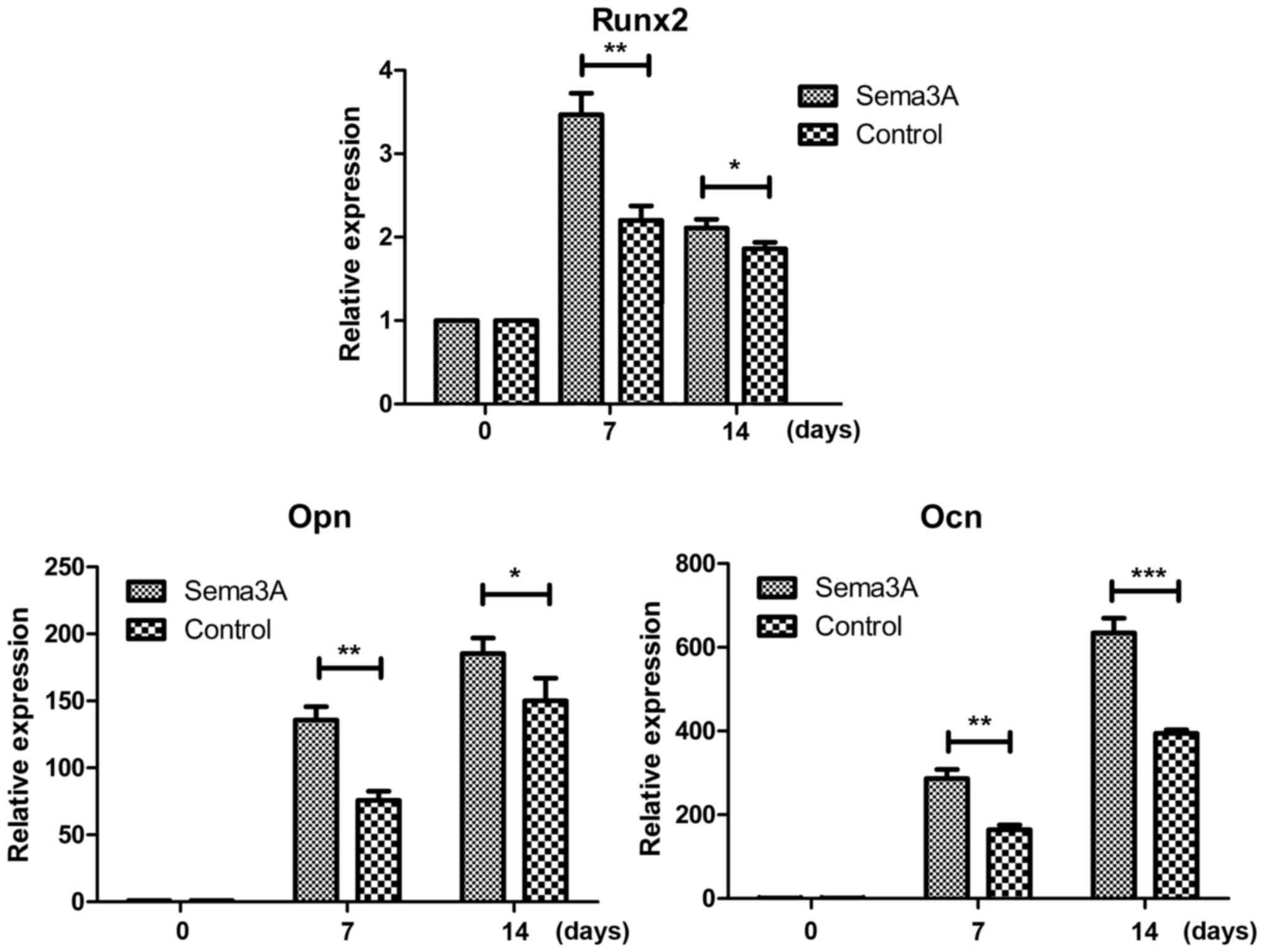
The mRNA expression levels of osteogenesis-associated genes (Runx2,
Opn and Ocn) was also detected on days 0, 7 and 14 during the
osteogenic differentiation using RT-qPCR. The results demonstrated
that the mRNA expression levels of Runx2, Opn and Ocn were all
significantly increased in the Sema3A overexpression hABMMSCs
compared with the control hABMMSCs on days 7 and 14 (Fig. 4). These findings indicated that
Sema3A serves an important role in promoting hABMMSC osteogenic
differentiation.
Discussion
Sema3A has been reported to serve various important
roles in the peripheral nerve, blood vessel and skeletal tissue
development (23–25). In addition, a previous study has
indicated that Sema3A-loaded chitosan intensely improved the
osteogenic differentiation of osteoblasts and may be applied onto
the Ti implant surface (26). In the
present study, the aim was to investigate the role of Sema3A in
hABMMSC osteogenic differentiation.
The process of osteogenic differentiation can be
divided into three parts, including the proliferation,
extracellular matrix (ECM) maturation and mineralization (27). To investigate whether Sema3A affects
hABMMSC proliferation and osteogenic differentiation, hABMMSCs were
initially isolated and expanded, and then a stable
Sema3A-overexpression cell line was generated by infection with a
pAdCMV-SEMA3A-MCS-EGFP vector, while cells infected with a control
vector (pCMV-MCS-EGFP) were used as the negative control. The cell
morphology of the infected cells was observed under a microscope,
and no significant differences were detected between the
Sema3A-overexpression and normal control groups. Subsequently, the
cell proliferation ability of hABMMSCs was investigated using CCK-8
and clone formation assays, and the data suggested that Sema3A
overexpression was able to significantly promote the proliferation
ability of the hABMMSCs. Furthermore, Alizarin Red S staining was
performed to analyze the cell osteogenic differentiation. As
compared with the control group cells, the ossification process of
hABMMSCs overexpressing Sema3A was evidently accelerated.
The current study also attempted to evaluate the
expression levels of three osteogenic markers, Runx 2, Opn and Ocn.
As an important transcription factor, Runx2 is essential for the
initiation of osteoblast differentiation and bone formation
(28). In the present study results,
the relative expression level of Runx2 in hABMMSCs overexpressing
Sema3A was markedly increased compared with that in the control
cells. In addition, the osteoblast-associated proteins Ocn, which
binds to calcium and promotes bone matrix calcification (29), and Opn, which is associated with cell
attachment (30), were also
investigated. These proteins are the main osteogenic genes that
support proliferation, matrix formation and mineralization. The
data of the current study revealed that these two proteins were
increased in hABMMSCs overexpressing Sema3A when compared with the
control cells. This observation confirmed the osteogenetic capacity
of hABMMSCs demonstrated by the highest total protein content and
the increased mRNA expression levels of osteogenic markers.
In conclusion, to the best of our knowledge, the
present study demonstrated for the first time that Sema3A is a key
positive regulator in hABMMSC osteogenic differentiation. These
findings suggested that Sema3A may be a potentially novel
therapeutic agent in bone diseases.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81500823), the Priority
Academic Program Development of Jiangsu Higher Education
Institutions (grant no. 2014-37), a grant supported by Shanghai
Stomatological Hospital (grant no. SSDC-2014-07), and the Natural
Science Foundation of Jiangsu Province of China (grant no.
BK20171057).
References
|
1
|
Tocci A and Forte L: Mesenchymal stem
cell: Use and perspectives. Hematol J. 4:92–96. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Quarto R, Mastrogiacomo M, Cancedda R,
Kutepov SM, Mukhachev V, Lavroukov A, Kon E and Marcacci M: Repair
of large bone effects with the use of autologous bone marrow
stromal cells. N Engl J Med. 344:385–386. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mauney JR, Volloch V and Kaplan DL: Role
of adult mesenchymal stem cells in bone tissue engineering
applications: Current status and future prospects. Tissue Eng.
11:787–802. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tran TS, Kolodkin AL and Bharadwaj R:
Semaphorin regulation of cellular morphology. Annu Rev Cell Dev
Biol. 23:263–292. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hinck L: The versatile roles of ‘axon
guidance’ cues in tissue morphogenesis. Dev Cell. 7:783–793. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Behar O, Golden JA, Mashimo H, Schoen FJ
and Fishman MC: Semaphorin III is needed for normal patterning and
growth of nerves, bones and heart. Nature. 383:525–528. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Singh MK, Degenhardt KR, Lu MM,
Bennett J, Yoshida Y and Epstein JA: Tie2Cre-mediated inactivation
of plexinD1 results in congenital heart, vascular and skeletal
defects. Dev Biol. 325:82–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reidy KJ, Villegas G, Teichman J, Veron D,
Shen W, Jimenez J, Thomas D and Tufro A: Semaphorin3a regulates
endothelial cell number and podocyte differentiation during
glomerular development. Development. 136:3979–3989. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Neufeld G, Sabag AD, Rabinovicz N and
Kessler O: Semaphorins in angiogenesis and tumor progression. Cold
Spring Harb Perspect Med. 2:a0067182012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hota PK and Buck M: Plexin structures are
coming: Opportunities for multilevel investigations of semaphorin
guidance receptors, their cell signaling mechanisms, and functions.
Cell Mol Life Sci. 69:3765–3805. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maione F, Capano S, Regano D, Zentilin L,
Giacca M, Casanovas O, Bussolino F, Serini G and Giraudo E:
Semaphorin3A overcomes cancer hypoxia and metastatic dissemination
induced by antiangiogenic treatment in mice. J Clin Invest.
122:1832–1848. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takamatsu H and Kumanogoh A: Diverse roles
for semaphorin-plexin signaling in the immune system. Trends
Immunol. 33:127–135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chakraborty G, Kumar S, Mishra R, Patil TV
and Kundu GC: Semaphorin 3A suppresses tumor growth and metastasis
in mice melanoma model. PLoS One. 7:e336332012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Serini G, Maione F, Giraudo E and
Bussolino F: Semaphorins and tumor angiogenesis. Angiogenesis.
12:187–193. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maione F, Capano S, Regano D, Zentilin L,
Giacca M, Casanovas O, Bussolino F, Serini G and Giraudo E:
Semaphorin 3A overcomes cancer hypoxia and metastatic dissemination
induced by antiangiogenic treatment in mice. J Clin Invest.
122:1832–1848. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gaur P, Bielenberg DR, Samuel S, Bose D,
Zhou Y, Gray MJ, Dallas NA, Fan F, Xia L, Lu J and Ellis LM: Role
of class 3 semaphorins and their receptors in tumor growth and
angiogenesis. Clin Cancer Res. 15:6763–6770. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fukuda T, Takeda S, Xu R, Ochi H, Sunamura
S, Sato T, Shibata S, Yoshida Y, Gu Z, Kimura A, et al: Sema3A
regulates bone-mass accrual through sensory innervations. Nature.
497:490–493. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hayashi M, Nakashima T, Taniguchi M,
Kodama T, Kumanogoh A and Takayanagi H: Osteoprotection by
semaphorin 3A. Nature. 485:69–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang H, Cheng JQ, Huang Q, Yang J, Shen
B, Zhou ZK, Kang PD, Lian YY and Pei FX: Increasing alcohol-induced
osteogenesis of human bone marrow-derived mesenchymal cells using
siRNA transient suppression of peroxisome proliferator activated
receptor gamma: An in vitro experiment study. Zhonghua Yi Xue Za
Zhi 88: 2603–2608, 2008. Zhonghua Yi Xue Za Zhi 88: 2603–2608,
2008. 88: 2603–2608, 2008:2603-2608, 2008–2608, 2008. 2008.(In
Chinese).
|
|
20
|
Farh KK, Grimson A, Jan C, Lewis BP,
Johnston WK, Lim LP, Burge CB and Bartel DP: The widespread impact
of mammalian MicroRNAs on mRNA repression and evolution. Science.
310:1817–1821. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cai Y, Xu MJ, Teng X, Zhou YB, Chen L, Zhu
Y, Wang X, Tang CS and Qi YF: Intermedin inhibits vascular
calcification by increasing the level of matrix
gamma-carboxyglutamic acid protein. Cardiovasc Res. 85:864–873.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim BS, Kim YC, Zadeh H, Park YJ, Pi SH,
Shin HS and You HK: Effects of the dichloromethane fraction of
Dipsaci Radix on the osteoblastic differentiation of human alveolar
bone marrow-derived mesenchymal stem cells. Biosci Biotechnol
Biochem. 75:13–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bates D, Taylor GI, Minichiello J, Farlie
P, Cichowitz A, Watson N, Klagsbrun M, Mamluk R and Newgreen DF:
Neurovascular congruence results from a shared patterning mechanism
that utilizes Semaphorin3A and Neuropilin-1. Dev Biol. 255:77–98.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Serini G, Valdembri D, Zanivan S, Morterra
G, Burkhardt C, Caccavari F, Zammataro L, Primo L, Tamagnone L,
Logan M, et al: Class 3 semaphorins control vascular morphogenesis
by inhibiting integrin function. Nature. 424:391–397. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gomez C, Burt-Pichat B, Mallein-Gerin F,
Merle B, Delmas PD, Skerry TM, Vico L, Malaval L and Chenu C:
Expression of Semaphorin-3A and its receptors in endochondral
ossification: Potential role in skeletal development and
innervation. Dev Dyn. 234:393–403. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fang K, Song W, Wang L, Jia S, Wei H, Ren
S, Xu X and Song Y: Immobilization of chitosan film containing
semaphorin 3A onto a microarc oxidized titanium implant surface via
silane reaction to improve MG63 osteogenic differentiation. Int J
Nanomedicine. 9:4649–4657. 2014.PubMed/NCBI
|
|
27
|
Owen TA, Aronow M, Shalhoub V, Barone LM,
Wilming L, Tassinari MS, Kennedy MB, Pockwinse S, Lian JB and Stein
GS: Progressive development of the rat osteoblast phenotype in
vitro: Reciprocal relationships in expression of genes associated
with osteoblast proliferation and differentiation during formation
of the bone extracellular matrix. J Cell Physiol. 143:420–430.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ducy P, Zhang R, Geoffroy V, Ridall AL and
Karsenty G: Osf2/Cbfa1: A transcriptional activator of osteoblast
differentiation. Cell. 89:747–754. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lian JB, Stein GS, Stein JL and van Wijnen
AJ: Osteocalcin gene promoter: Unlocking the secrets for regulation
of osteoblast growth and differentiation. J Cell Biochem Suppl
30–31. 1–72. 1998.
|
|
30
|
Donzelli E, Salvadè A, Mimo P, Viganò M,
Morrone M, Papagna R, Carini F, Zaopo A, Miloso M, Baldoni M and
Tredici G: Mesenchymal stem cells cultured on a collagen scaffold:
In vitro osteogenic differentiation. Arch Oral Biol. 52:64–73.
2007. View Article : Google Scholar : PubMed/NCBI
|