Introduction
Cilostazol
(6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril,
CLZ) is known to exert anti-platelet aggregation and vasodilatory
effects with minimal cardiac effects (1). Therefore, CLZ has been used as a
therapeutic agent for the improvement of symptoms in conditions
such as cancer (2), pain
accompanying chronic arterial obstruction (3), and for the amelioration (4) and, prevention of cerebral infarction
(5). The agent is only administered
orally because of its low water-solubility (6). However, most patients with cerebral
infarction have serious secondary conditions, such as impaired
consciousness or aphagia (7). Thus,
sufficient blood concentration and effects cannot be obtained with
commercially available CLZ tablets.
Cataplasms can be classified into two classes a
transdermal absorption type and a locally acting type (8). In particular, the transdermal drug
delivery patch systems have been used for various clinical
treatments, such as asthma, angina, and smoking cessation (9). Transdermal absorption preparations
offer several advantages: They are not subjected to the first-pass
effect, but are simply applied and switched on or off in the body;
ensure sustained release; and improve patient quality of life
through sustained effects (8). The
skin consists of the cuticle, the corium the tela subcutanea and
nerves, blood vessels, and lymph vessels (10). The transdermal drug delivery patch
system acts by penetrating the stratum corneum, after which the
medicine is absorbed into the blood.
Many methods have been used to enhance the
bioavailability of sparingly water-soluble medicaments, in
particular, and nanoscale systems such as liposomes, micelles,
nanocrystals, and dendrimers have been proposed as drug carriers
for transdermal drug delivery systems (11).
Recently, a transdermal delivery system using
nanoparticles was reported. The available pathways for the
penetration of the drug through the stratum corneum are the
transcellular, intracellular, and transaccessory pathways (12,13). The
transcellular pathway appears to be the main pathway (12,13). The
spaces between the cells are reported to be 50–70 nm (14). Therefore, the solid drug particles
that are approximately the same size as the spaces between the
cells, pass through the space and into peripheral blood vessels
(15). Thus, nanocrystals improve
bioavailability. After consideration of these reports, we aimed to
prepare aqueous gel patches containing CLZ nanocrystals
(CLZnano) with a particle size of <100 nm.
In this study, we designed topical formulations
containing CLZnano and investigated the penetration and
the retention of CLZ in rat blood after the administration of
CLZnano gel patches in comparison with gelling agents
for general-purpose bases. Moreover, we clarified the mechanism of
the transport system of CLZnano through rat skin.
Materials and methods
Materials
CLZ powder (CLZmicro) was kindly provided
by Otsuka Pharmaceutical Co., Ltd. (Tokyo, Japan).
2-Hydoxypropyl-β-cyclodextrin (HPβCD) was purchased from Nihon
Shokuhin Kako Co., Ltd. (Tokyo, Japan), low-substituted
methylcellulose (MC) was provided by Shin-Etsu Chemical Co., Ltd.
(Tokyo, Japan), and docusate sodium (DS) was obtained from Sigma
Co., Inc. (Tokyo, Japan). All other chemicals used were of the
highest purity commercially available.
Animals
Seven-week-old male Wistar rats were used in this
study (SLC Inc., Shizuoka, Japan). The animals were housed under
standard conditions (12 h/day of fluorescent light (7:00 a.m.-7:00
p.m.) and 25±1°C) and allowed free access to a commercial diet
(CR-3; Clea Japan Inc., Tokyo, Japan) and water. All procedures
were approved by and performed in accordance with the Kindai
University School of Pharmacy Committee for the Care and Use of
Laboratory Animals (approval no. KAPS-25-002).
Preparation of the CLZnano
formulation
Recrystallized CLZ was prepared as follows (16): CLZ (0.5 g) was dissolved in 50%
ethanol (50 ml) at 120°C, and the extracted CLZ was placed in a
sonicator (Yamato Science Co., Ltd, Tokyo, Japan) for 5 sec and
allowed to stand for 24 h. Thereafter, CLZ was collected by
filtration (recovery rate: 92.3%). Nanocrystals of CLZ
(CLZnano) were prepared by using zirconia balls and a
Pulverisette 7 Planetary Micro Mill (Fritsch Corp., Kanagawa,
Japan). The zirconia balls (diameter: 10 mm) were added to
recrystallized CLZ containing sodium docusate and low-substituted
MC and then crushed with the Pulverisette 7 for 24 h (400 rpm). The
experiment was conducted at 18–21°C (room temperature) (16).
Measurement of particle size, number
and image in CLZ
The CLZ nanoparticle was evaluated using a
nanoparticle size analyzer (SALD-7100; Shimadzu Co., Kyoto, Japan;
refractive index 1.60–0.10i), a nanoparticle tracking size analyzer
(Nanosight LM10; Malvern Instruments Ltd., Worcestershire, UK) and
a scanning probe microscope (SPM-9700; Shimadzu Corp., Kyoto,
Japan). In the SALD-7100, the total distribution in
CLZmicro and CLZnano was 48.3±0.312 mm,
0.064±0.047 mm, respectively (mean particle size ± SD) The particle
size and number by the Nanosight was measured following:
purification system of the water containing 100 µg
CLZnano (500 µl) was injected into the sample chamber of
the unit (Nanosight LM14; Malvern Instruments Ltd.) with a syringe.
The suspension temperature in the sample chamber was 25°C, the
wavelength of the embedded laser was 405 nm (blue), the measurement
time was 60 sec, and the viscosity of the suspension was
0.904–0.906 cP (water). The images of CLZnano were
created by SPM-9700, and the particle size was measured from a
cross section of the images of CLZnano.
Preparation of aqueous gel patches,
gel and ointment containing CLZnano
The gel patches containing CLZnano
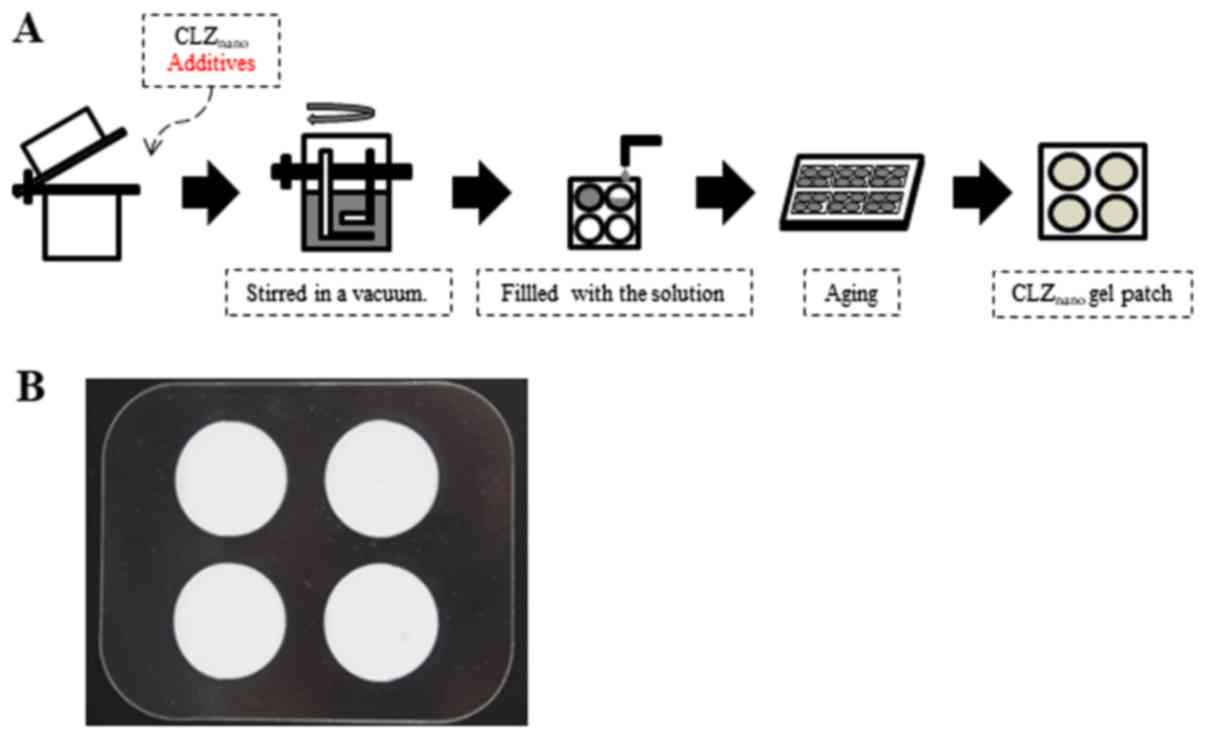
(CLZnano gel patch) were prepared as described in
Fig. 1A. The formulation of the
CLZnano gel patches is described in Table I. In Fig.
1B, the completed aqueous gel patch containing
CLZnano is shown. The CP gel and PEG ointment containing
0.5% CLZmicro and 0.5% CLZnano were prepared
by using Crbopol® 934 (1.5 w/w%) and polyethylene glycol
(94.4 w/w%, PEG400: PEG 4,000=1:1), respectively.
 | Table I.Formulations of the
CLZnano gel patch. |
Table I.
Formulations of the
CLZnano gel patch.
| Formulation | Content (w/w
%) |
|---|
| Glycerin | 15.00 |
| Sodium polyacrylic
acid |
3.01 |
| Aluminum
hydroxide |
0.98 |
| Butylen glycol |
0.61 |
| Isostearic acid
glyceres-25 |
0.31 |
| Tartaric acid |
0.24 |
|
Etylenediamintetraanmin acid-2Na |
0.61 |
| Methyl paraben |
0.03 |
| Propyl paraben |
0.01 |
| HPβCD |
5.00 |
| SM-4 |
0.50 |
| Sodium
docusate |
0.20 |
| CLZnano
crystals |
0.50 |
In vitro skin penetration of CLZ gel
patches
The in vitro skin penetration of CLZ from gel
patches, CP gel, and PEG ointment experiment was evaluated by using
the Franz diffusion cell (17). One
day before the experiment, the hair on the abdominal area of
7-week-old Wistar rats was carefully shaved by using an electric
clipper and razor. On the day of the experiment, sections of
full-thickness abdominal skin (area: 3×3 cm2) were
extracted from the rats and the subcutaneous fat and other visceral
debris were removed from the undersurface. The dermal side of the
full-thickness skin was soaked in buffer (0.85% NaCl-10 mM
phosphate buffer, pH 7.4) for 12 h at 4°C to equilibrate the skin.
Then, 0.3 g of 0.5% CLZ gel patches, CP gel, and PEG ointment was
uniformly applied to the stratum corneum of the skin, which was
then mounted on a Franz diffusion cell (reservoir volume, 12.2 ml;
i.d. O-ring flange, 1.6 cm) and occluded with aluminum foil. The
diffusion cells were maintained at a constant temperature of 37°C
for 48 h. The in vitro skin penetration experiment was
performed as described above without a membrane filter. The amount
of CLZ in the filtrates was determined by HPLC. Fifty microliters
of the sample was added to 100 µl methanol containing 100 µg
benzophenone (internal standard) and centrifuged at 15,000 rpm for
20 min. Ten µl of this solution was injected into an ODS column (3
µm, column size: 2.0×50 mm; Inertsil ODS-3; Shimadzu Co.) by using
a Shimadzu LC-10AD system equipped with a CTO-6A column oven
(Shimadzu Corp., Kyoto, Japan). The mobile phase consisted of a
mixture of acetonitrile/methanol/water (35/15/50, v/v/v). The flow
rate was 0.25 ml/min, the column temperature was 35°C, and the
wavelength used for detection was 254 nm. The obtained data were
analyzed by using the following equations (equations 1 and 2):
Jc=QA(t-τ)=D·Km·Ccδ=Kp·Cc
D=δ26τ
where Jc, Km,
Kp, D, τ, d, Cc and
A are the CLZ penetration rate, skin/preparation partition
coefficient, penetration coefficient through the skin, diffusion
constant within the skin, lag time, thickness of the skin (0.071
cm, mean of five independent rats), amount of CLZ at time t,
and the effective area of skin (2 cm2). A nonlinear
leastsquares computer program (MULTI) was used for the
calculations.
In vitro skin penetration of CLZnano
from gel patches
The in vitro skin penetration of
CLZnano was analyzed Franz diffusion cell (17). In addition, a membrane filter
(Durapore® Membrane Filter, pore size: 0.45 µm) was set
under the skin to remove the debris from the skin. The number of
CLZnano in the filtrates was determined as described
above A calibration curve was generated from the relationship
between the number of CLZnano and the amount of CLZ
measured by HPLC method described above. The release of
CLZnano was evaluated by the experimental method
described above without the rat skin. The prepared analytical curve
was used for the evaluation of the sample concentration from the
ratio of the number of particles.
Analysis of pharmacokinetics of CLZ in
rats
On the day prior to the experiment, the hair on the
abdominal area of the 7-week-old Wistar rats was carefully shaved
with an electric clipper and razor, and a cannula filled with 30
µg/ml heparin (silicone tubing; i.d., 0.5 mm, o.d., 1.0 mm) was
inserted into the right jugular vein of the rats under
pentobarbital anesthesia (40 mg/kg, intraperitoneally). A sheet of
CLZ gel patches (0.3 g) and gel ointment was fixed on the shaved
abdominal skin with an adhesive and immediately occluded with
adhesive tape. Venous blood (100 µl) was collected from the jugular
vein through the cannula between 0 and 48 h after the application
of the CLZ gel patches and gel ointment (18). The blood was centrifuged at 15,000
rpm for 20 min at 4°C to obtain, plasma, which was stored at −80°C
until analysis. The CLZ concentrations in the samples were
determined by the HPLC method described above.
CLZnano gel patches, CLZnano
CP gel, and CLZnano PEG ointment were applied to Wistar
rats (n=3) at a dose of 0.15 g/sheet. The rats were allowed free
access to water and food throughout the experiment. The blood
samples (0.1 ml) were collected from the jugular vein at 0
(pre-dose), 2, 4, 6, 8, 24, 27, 30, 33, and 48 h after the
administration. The serum samples were obtained by centrifugation
at 15,000 rpm for 20 min and then stored at 4°C until analysis. The
CLZ concentration in the samples was determined by the HPLC method
described above. The obtained data were analyzed by the using the
following formula (equations 3 and 4):
CCLZ=Aeαt+Beβt
CCLZ=AKakaαeαt+BKakaβeβt-(AKakaa+Bkakaβ)ekat
where CCLZ is the CLZ
concentration of blood, A and B are the contribution
rates, ka is the rate content, and α and β are
the elimination rate constants. The obtained data were analyzed by
the simplex method and the damped Gauss-Newton method (18).
Statistical analysis
Unpaired Student's t-tests were used for statistical
analysis and P-values less than 0.05 were considered significant.
All data are expressed as the mean ± standard deviation (SD) or
standard error of the mean (SE).
Results
Preparation of CLZnano
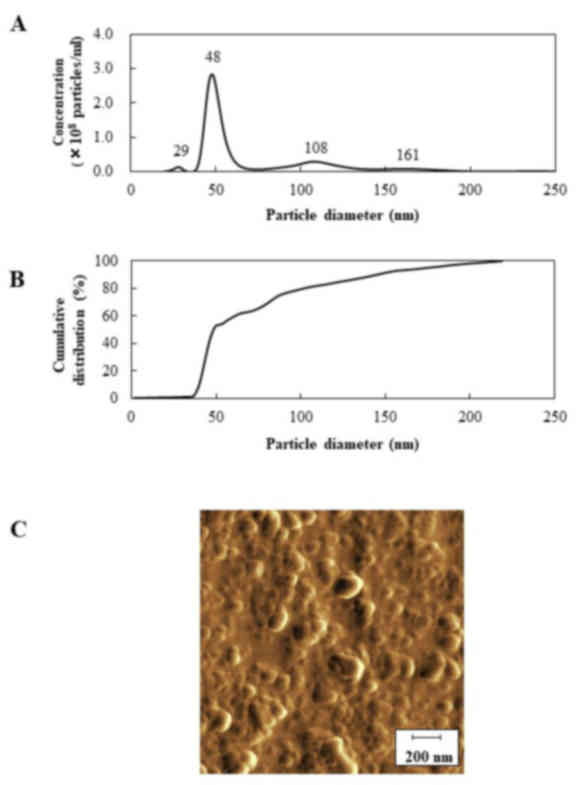
The particle size distribution of CLZnano
is shown in Fig. 2A and B, and the
particle size distribution of CLZnano is shown in
Table II. The mean and mod particle
sizes of CLZnano were 74.5±6.2 and 44.2±4.0 nm,
respectively. The occupation percentage of nanocrystals with a
diameter <200 nm diameter in CLZnano and
CLZmicro was 100 and 21%, respectively. Moreover, the
percentage of nanocrystals with a diameter less than 100 nm
CLZnano was 80±3.2%, (mean ± SE, n=5). The SPM images of
the CLZnano dispersion are shown in Fig. 2C. The particle size of
CLZnano was 66.45±5.35 nm (mean ± SE, n=5). Because
ground CLZnano showed no aggregation, the similarity of
the particle sizes between SPM and the nanoparticle tracking
analyzer provided an indication of uniform quality of the
nanocrystals in CLZnano.
 | Table II.Ratio of nanoparticles (<1 µm) in
CLZnano and CLZmicro. |
Table II.
Ratio of nanoparticles (<1 µm) in
CLZnano and CLZmicro.
| Distribution | CLZnano
(nm) | CLZmicro
(nm) |
|---|
| Mean | 74.5±6.2 | 236.6±35.4 |
| Mode | 44.2±4.0 | 144.5±26.7 |
| SD | 47.1±5.0 | 131.9±12.7 |
| D10 | 40.0±4.1 | 93.4±30.3 |
| D50 | 48.4±4.6 | 215.0±36.7 |
| D90 | 144.8±10.6 | 447.4±44.4 |
| Occupation % of
nanoparticles number (<200 nm) | 100 | 21 |
Percutaneous penetration of CLZ
released from CLZmicro and CLZnano gel
patches
The penetration profiles of CLZ through rat skin
after the application of CLZmicro and CLZnano
gel patches and ointments are shown in Fig. 3, and the pharmacokinetic parameters
calculated from the in vitro skin penetration data are
summarized in Table III. Regarding
particle size, a significant difference was found in the CLZ gel
patches and ointment between the CLZnano and
CLZmicro groups; with respect to gel properties, a
significant difference was found in the pharmacokinetic parameters.
The amount of penetrated CLZ increased linearly after the
application of either CLZ gel patches or ointment into the donor
chambers, and the penetration rate (Jc) of
CLZnano gel patch was 1.4-fold higher than that of the
CLZmicro gel patch. The penetration coefficient through
the skin (Kp) and the skin/preparation partition
coefficient (Km) values of the CLZnano
gel patches and ointment were significantly higher than those of
the CLZmicro gel patches and ointment. The lag times (τ)
for the CLZmicro and CLZnano gel patches and
ointments were different. In contrast, the pharmacokinetic
parameters of CLZ in the gel patches were markedly increased
compared with those of the CLZ CP gel and PEG ointment.
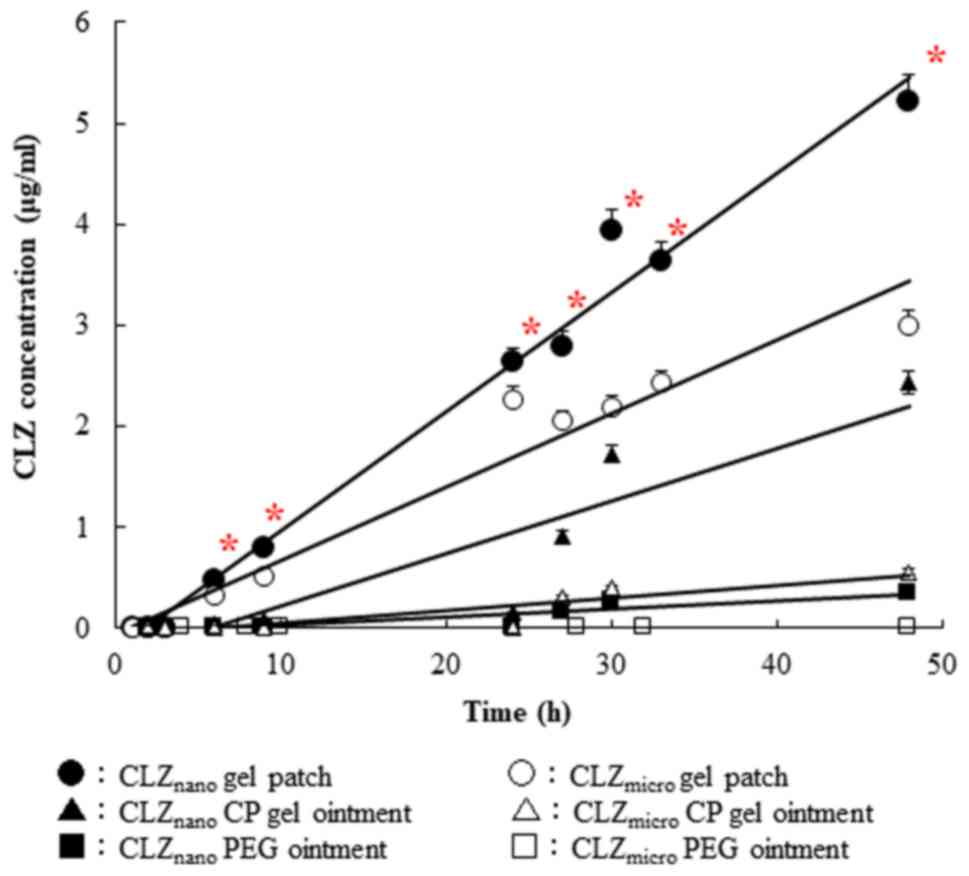 | Figure 3.Changes in CLZ concentration
following the application of CLZ aqueous gel patch, gel and
ointment. CLZmicro gel patch (open circles),
CLZmicro gel patch-applied rat skin; CLZnano
gel patch (closed circles), CLZnano gel patch-applied
rat skin; CLZmicro CP gel (open triangles),
CLZmicro CP gel-applied rat skin; CLZnano CP
gel (closed triangles), CLZnano CP gel-applied rat skin;
CLZmicro PEG ointment (open squares),
CLZmicro PEG ointment-applied rat skin;
CLZnano PEG ointment (closed squares),
CLZnano PEG ointment-applied rat skin. Data are
presented as the mean ± standard error (n=3–5). *P<0.05 vs. the
CLZmicro gel patch groups within each category. CLZ,
cilostazol; CLZnano, CLZ nanocrystals;
CLZmicro, CLZ powder; PEG, polyethylene glycol; CP,
carbopol. |
 | Table III.Pharmacokinetic parameters for in
vitro percutaneous penetration of CLZ from CLZnano
gel patch and carbopol gel and polyethylene glycol ointments. |
Table III.
Pharmacokinetic parameters for in
vitro percutaneous penetration of CLZ from CLZnano
gel patch and carbopol gel and polyethylene glycol ointments.
| A, Patch |
|---|
|
|---|
| Preparation | τ (h) | D
(×10−4 cm2/h) |
Jc
(ng/cm2/h) |
Kp (×10−4
cm/h) |
Km |
|---|
| CLZnano
gel |
1.75±0.46a |
4.81±1.83a |
686±51.90a,b |
1.37±0.10a,b |
2.03±0.66a,b |
| CLZmicro
gel | 1.82±0.46 | 4.61±1.83 | 474±112.7 | 0.95±0.23 | 1.46±0.36 |
|
| B,
Ointment |
|
|
Preparation | τ (h) | D
(×10−4 cm2/h) |
Jc
(ng/cm2/h) |
Kp (×10−4
cm/h) |
Km |
|
| CLZnano
CP gel | 3.75±1.78 | 3.04±2.33 | 306±67.40 | 0.88±0.19 | 0.03±0.01 |
| CLZmicro
CP gel | 5.52±1.95 | 1.83±1.10 | 104±29.20 | 0.30±0.08 | 0.01±0.01 |
| CLZnano
PEG | 6.75±0.64 | 1.47±0.16 | 160±11.80 | 0.46±0.03 | 0.02±0.01 |
| CLZmicro
PEG | N.D. | N.D. | N.D. | N.D. | N.D. |
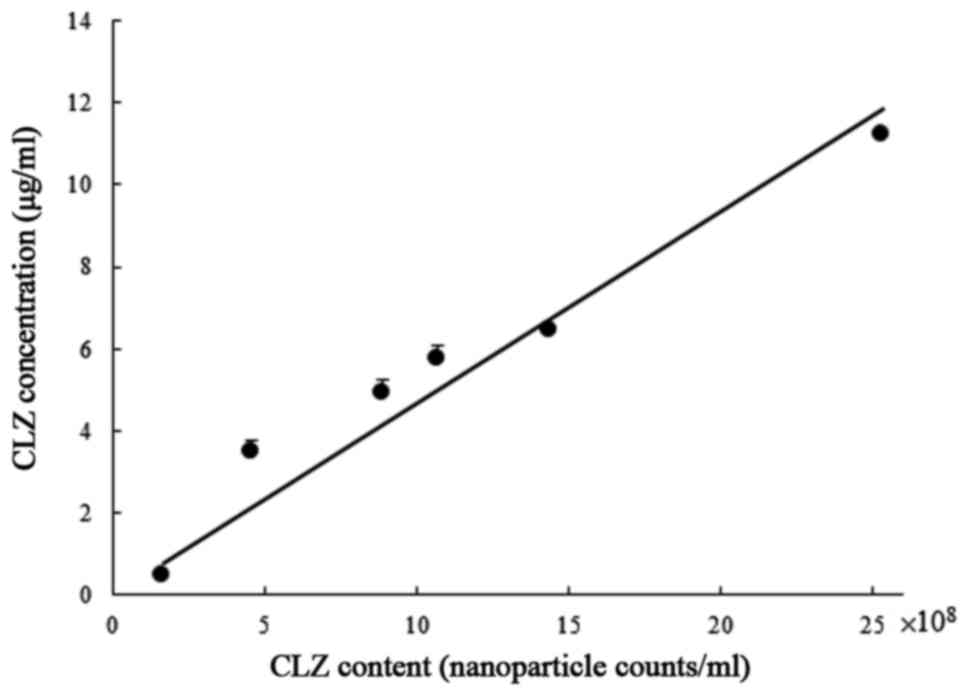
The calibration curve between the number of
CLZnano with an average particle diameter of 200 nm and
the concentration of CLZ is shown in Fig. 4. The calibration curve was shown as a
straight line with the slope of 4.68×10−8 mg/particle
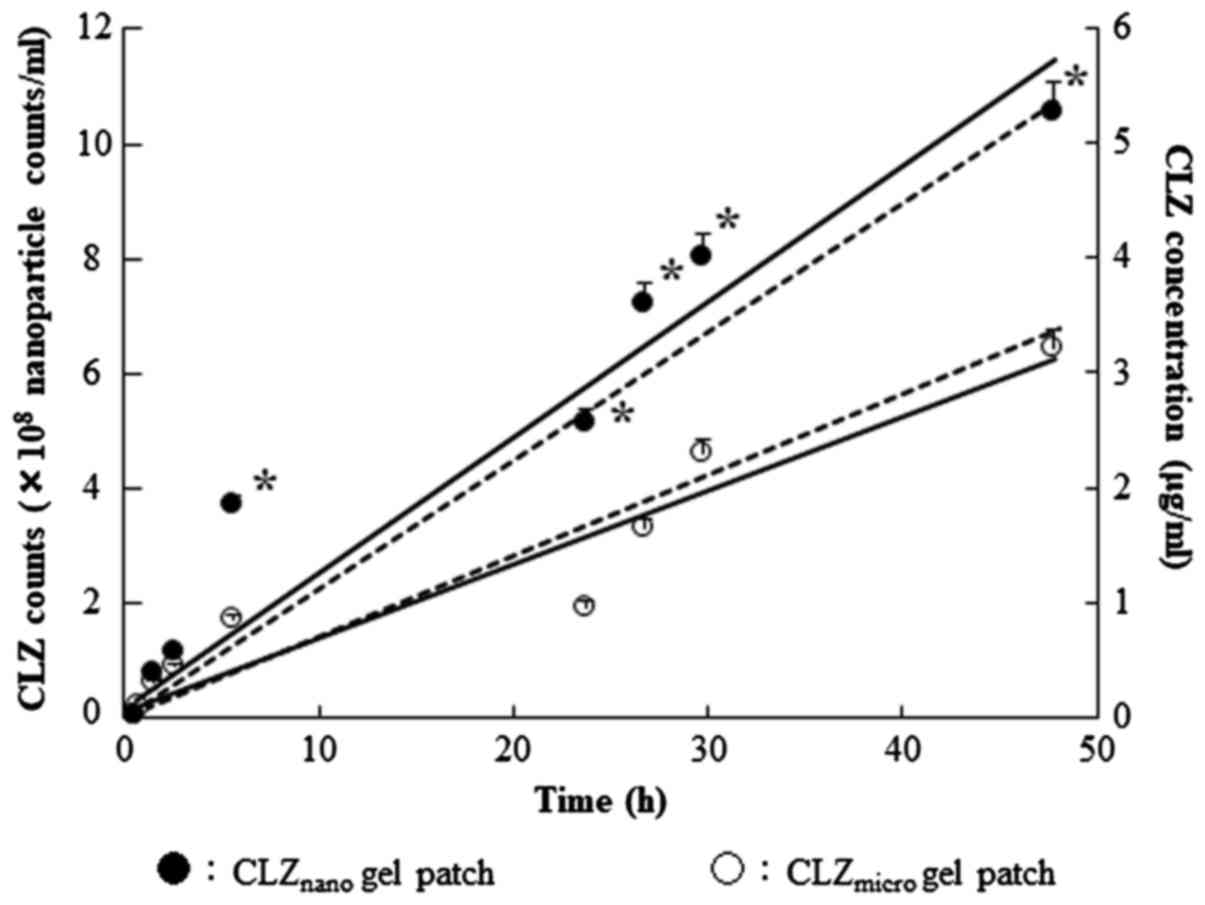
number and correlation coefficient (r)=0.972. In Fig. 5, the profiles of the penetrated
CLZnano in the in vitro skin penetration
experiment are shown. The nanocrystals with an average particle
diameter <200 nm were detected in a reservoir chamber, and the
number of nanocrystals increased with the incubation time. As shown
in Fig. 5, the penetration rate
(Jc) of the CLZnano gel patch and the
CLZmicro gel patch was 769.9 and 367.6
ng/cm2/h, respectively. Therefore, 98% of penetrated CLZ
after the application of CLZnano gel patch to rat skin
was observed in the nanocrystal form; for the CLZmicro
gel patch, the value was 96%.
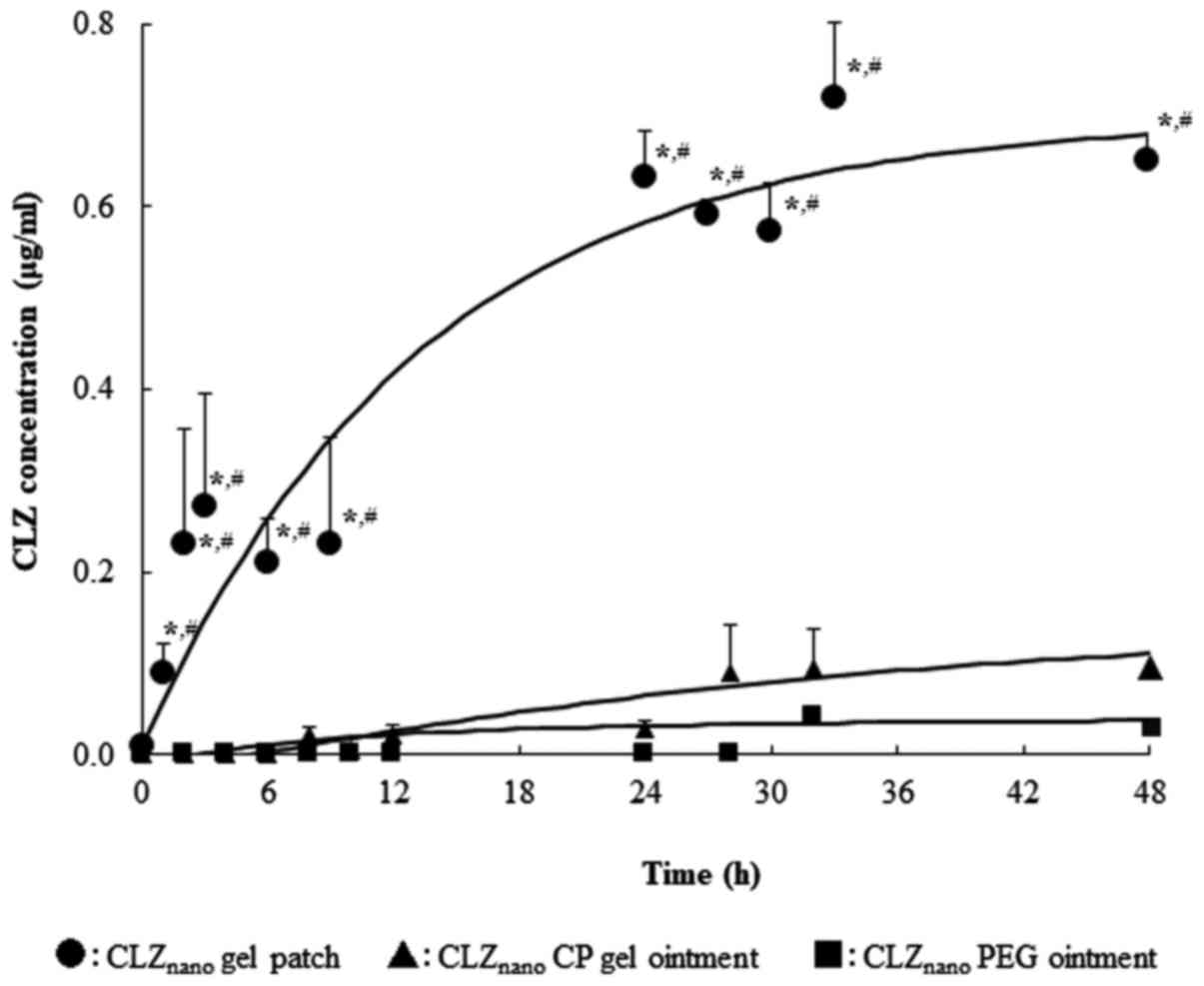
The absorption profiles of CLZ through rat skin
in vivo after the application of CLZnano gel
patches and ointments are shown in Fig.
6; Table IV summarizes the
pharmacokinetic parameters calculated from the in vivo
percutaneous absorption data. The plasma concentration of CLZ
increased after the application of the CLZnano gel
patches, and the apparent absorption rate constant
(ka) and AUC0-48 h values in the skin
of rats administered the CLZnano gel patches were
significantly higher than those of rats administered the
CLZnano gel and ointment, but the lag time was
decreased.
 | Table IV.Pharmacokinetic parameters for in
vivo percutaneous absorption of CLZ following the application
of CLZnano gel patch and ointments. |
Table IV.
Pharmacokinetic parameters for in
vivo percutaneous absorption of CLZ following the application
of CLZnano gel patch and ointments.
| Preparation | τ (h) |
ka (/h) | A
(µg/ml) | B
(µg/ml) | AUC
(µg/ml) | MRT (h) | BA (%) |
|---|
| CLZnano
gel patch |
0.71±0.03a |
19.03±2.08a,b |
0.15±0.16 |
99.08±22.10a,b |
7.51±0.48a,b |
34.36±3.09a,b |
63.3±0.6a,b |
| CLZnano
CP gel ointment |
2.66±1.09 |
11.70±4.08 |
8.06±1.14 |
4.99±1.3 |
2.92±0.94b |
25.05±0.60 |
24.5±1.3b |
| CLZnano
PEG ointment |
5.75±0.55 |
7.06±0.15 |
4.58±0.28 |
3.81±0.47 |
1.53±0.35 |
29.86±2.05 |
12.9±0.2 |
Discussion
Sparingly water-soluble medicaments have poor oral
absorption. We have previously designed drug nanocrystals by a
combination of recrystallization and the breakdown method using a
ball mill (16) and shown that
CLZnano enhanced drug bioavailability in the small
intestine of rats (16).
In this study, we confirmed the diameter of
CLZnano by using a nanoparticle tracking size analyzer
and a scanning probe microscope. Moreover, we designed new
transdermal drug delivery formulations containing
CLZnano using hydrophilic polymeric gelling agents, and
investigated the penetration of CLZ through rat skin.
First, we attempted to measure the diameter of the
CLZnano. Recently, nanocrystals have been thoroughly
evaluated for their potential as a tool to carry drug payloads,
image contrast agents, or gene therapeutics for disease diagnosis
and treatment, with the primary focus on cancer (19–27). It
is known that the behavior of nanoparticles <100 nm is unique
(19). Nanocrystals possess
different physical and chemical properties as well as optical and
electromagnetic characteristics (19). The diameter of CLZnano was
measured by using a nanoparticle tracking size analyzer and a
scanning probe microscope. In this study, the mean diameter of
CLZnano measured by using a tracking size analyzer was
<100 nm, but the cumulative distribution indicated that
approximately 20% of the CLZnano were >100 nm
(Fig. 2A and B). Therefore, we
visually evaluated the images of the CLZnano captured by
using a scanning probe microscope to confirm the size of the
nanocrystals visually. In these images, nanocrystals with a primary
particle size of 30–80 nm were clearly visible and comprised a
majority of the primary particles and a few secondary particles
comprising agglomerated or flocculated primary particles (Fig. 2C). However, the secondary particles
are moved and crushed by the extremely small power of the probe
cantilever of the SPM. This suggests that the secondary particles
are transient and crushed to the primary particles, and that the
number of secondary particles that are >100 nm is almost
negligible.
In this study, we prepared an aqueous gel patch
containing CLZnano, with excellent drug release
properties, skin permeability and skin permeation rate,
quantitative drug release even in long term application and showed
excellent retention. The data indicated that the milled
CLZnano were homogeneous with a narrow particle size
distribution. It is reported that nanoparticles from organic
compounds are able to move through the spaces between the cells
(28). In a previous study, we
reported that recrystallization was suitable for the preparation of
nanocrystals by using mill methods (16). These results suggested that the CLZ
released from the CLZnano gel patches was in a
nanocrystal state.
The successful delivery of a drug across the skin
requires a high-performance drug delivery device (29). Clinically, the most common bases for
transdermal therapeutic systems are CP gel and PEG ointment, which
are used pharmaceutically as lubricants and also as carriers for
many drugs (30,31). Aqueous gel patches using high
molecular weight polymer have attracted attention as a new base in
the cosmetics industry (32).
Therefore, we prepared an aqueous gel patch containing
CLZnano by using high molecular weight polymer
absorbent, which has excellent drug release and skin permeation
properties, skin permeation rate, quantitative release of a drug,
even in long term application and with excellent retention, which
allowed the uniform incorporation of the CLZnano. As
shown by the above results, the gel patch using aqueous high
molecular weight polymer absorbents shows excellent release
properties and sustainability against rat skin and are subsequently
extremely useful as a transdermal absorption base.
These results show that the formula developed in
this study was suitable for the preparation of aqueous gel patches
containing CLZnano. The diffusion constant within the
penetration rate (Jc), the penetration
coefficient through the skin (Kp), and the
skin/preparation partition coefficient (Km) for
the CLZnano gel patch were all significantly higher than
those of the CLZmicro gel patch, CLZnano and
CLZmicro gel ointment. Only lag time of
CLZnano gel patch is shorter than others (Fig. 3, Table
III). These results suggested that the CLZnano gel
patches were exceptionally well suited for the base of percutaneous
absorption type formulation.
In addition to this, we attempted to clarify the
absorption mechanism by counting the number of CLZnano
that permeated the rat skin from the CLZ gel patches. According to
the calibration straight line and the penetration profiles of
CLZnano, a positive correlation was found between the
number of CLZnano and the penetration of CLZ. Moreover,
the predicted values that were calculated from the number of
CLZnano and actual measurements were almost the same
quantities (Figs. 4 and 5). These results suggested that the
CLZnano smaller than 100 nm were transferred through the
spaces in rat skin and into peripheral blood vessels. To the best
of our knowledge, this is the first report to elucidate the
permeation mechanism of nanoparticles in the percutaneous
absorption experiment. Thus, we are now conducting a more detailed
investigation of some details about the absorption mechanism of
CLZnano.
In the in vivo study, the CLZ concentrations
in the plasma of rats administered the CLZnano gel
patches were also significantly higher than those of rats
administered the CLZnano CP gel and PEG ointment
(Table IV, Fig. 6). In this study, we have shown that
the supply of CLZ from the CLZnano gel patches was
higher than that from the CLZnano CP and PEG gel
ointment. Therefore, the abundant supply of CLZ from the
CLZnano gel patches may be related to the CLZ
concentrations in the plasma. These results showed that the
characteristics of the CLZnano gel patches in skin
differ and suggested that the effects of local and systemic therapy
were greater after the application of the CLZnano gel
patches than the CLZnano CP and PEG gel ointment. In
addition, it is important to clarify a suitable formulation for the
transdermal therapeutic system for ischemic stroke symptoms using
CLZnano. Therefore, we are now investigating the
therapeutic effects of transdermal systems by using
CLZnano and various additives on ischemic stroke
symptoms.
In conclusion, we have developed a new aqueous gel
patch system that includes CLZnano by using
recrystallization and a planetary micro mill. The penetration of
CLZ is attributed to the nanoscale crystals. The percutaneous
penetration of CLZ from the CLZnano gel patches through
the rat skin was significantly better than that from the
CLZnano CP gel and PEG ointment. Moreover, we have
clarified the mechanism of the transparency system of
CLZnano through the rat skin. Thus, our findings suggest
that a transdermal therapeutic system using nanocrystals may enable
the application of medications without high systemic levels to
provide an efficient and effective therapy and to spare patients
from unwanted side effects. A transdermal formulation using
CLZnano may provide a delivery option for clinical
treatment of ischemic stroke symptoms.
Glossary
Abbreviations
Abbreviations:
|
CP
|
carbopol
|
|
CLZ
|
cilostazol
|
|
CLZmicro
|
CLZ powder
|
|
CLZnano
|
CLZ nanocrystals
|
|
HPβCD
|
2-hydoxypropyl-β-cyclodextrin
|
|
ka
|
absorption rate constant
|
|
Kp
|
penetration coefficient through the
skin
|
|
MC
|
methylcellulose
|
|
PEG
|
polyethylene glycol
|
|
SPM
|
scanning probe microscope
|
References
|
1
|
Kimura Y, Tani T, Kanbe T and Watanabe K:
Effect of cilostazol on platelet aggregation and experimental
thrombosis. Arzneimittelforschung. 35:1144–1149. 1985.PubMed/NCBI
|
|
2
|
Kanbayashi J, Liu Y, Sun B, Shakur Y,
Yoshitake M and Czerwiec F: Cilostazol as a unique antithrombotic
agent. Curr Pharm Des. 9:2289–2302. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bramer SL and Forbes WP: Relative
bioavailability and effects of a high fat meal on single dose
cilostazol pharmacokinetics. Clin Pharmacokinet. 2(37 Suppl):
S13–S23. 1999. View Article : Google Scholar
|
|
4
|
Jinno J, Kamada N, Miyake M, Yamada K,
Mukai T, Odomi M, Toguchi H, Liversidge GG, Higaki K and Kimura T:
Effect of particle size reduction on dissolution and oral
absorption of a poorly water-soluble drug, cilostazol, in beagle
dogs. J Control Release. 111:56–64. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jinno J, Kamada N, Miyake M, Yamada K,
Mukai T, Odomi M, Toguchi H, Liversidge GG, Higaki K and Kimura T:
In vitro-in vivo correlation for wet-milled tablet of poorly
water-soluble cilostazol. J Control Release. 130:29–37. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rasenack N and Müller BW: Micron-size drug
particles: Common and novel micronization techniques. Pharm Dev
Technol. 9:1–13. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gotoh F, Tohgi H, Hirai S, Terashi A,
Fukuuchi Y, Otomo E, Shinohara Y, Itoh E, Matsuda T, Sawada T, et
al: Cilostazol stroke prevention study: A placebo-controlled
double-blind trial for secondary prevention of cerebral infarction.
J Stroke Cerebrovasc Dis. 9:147–157. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sugibayashi K: Development &
Applications of Transdermal Drug Delivery Systems. CMC publishing
Company; Tokyo: pp. 167–176. 2011
|
|
9
|
Honeywell-Nguyen PL and Bouwstra JA:
Vesicles as a tool for transdermal and dermal delivery. Drug Discov
Today Technol. 2:67–74. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Walters KA and Roberts MS: The structure
and function of the skin. Marcel Dekker; New York, NY: pp. 1–40.
2002
|
|
11
|
Cevc G and Vierl U: Nanotechnology and the
transdermal route: A state of the art review and critical
appraisal. J Control Release. 141:277–299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Scheuplein RJ: Mechanism of percutaneous
absorption. I. Routes of penetration and influence of solubility. J
Invest Dermatol. 45:334–346. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Watanabe T: The present conditions and the
prospects of a skin applications drug. Drug Delivery System.
22:450–457. 2007. View Article : Google Scholar
|
|
14
|
Tsujimoto H, Hara K, Yokoyama T, Yamamoto
H, Takeuchi H, Kawashima Y, Akagi K, Miwa N and Haung CC:
Percutaneous absorption study of biodegradable PLGA nano-spheres
via human skin biopsies. J Soc Powder Technol. 41:867–875. 2004.
View Article : Google Scholar
|
|
15
|
Bal SM, Ding Z, van Riet E, Jiskoot W and
Bouwstra JA: Advances in transcutaneous vaccine delivery: Do all
ways lead to Rome? J Control Release. 148:266–282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshioka C, Ito Y and Nagai N: An oral
formulation of cilostazol nanoparticles enhances intestinal drug
absorption in rats. Exp Ther Med. 15:454–460. 2017.PubMed/NCBI
|
|
17
|
Franz TJ: Percutaneous absorption on the
relevance of in vitro data. J Invest Dermatol. 64:190–195. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nagai N, Iwamae A, Tanimoto S, Yoshioka C
and Ito Y: Pharmacokinetics and antiinflammatory effect of a novel
gel system containing ketoprofen solid nanoparticles. Biol Pharm
Bull. 38:1918–1924. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
DeLouise LA: Applications of
nanotechnology in dermatology. J Invest Dermatol. 132:964–975.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao X, Cui Y, Levenson RM, Chung LW and
Nie S: In vivo cancer targeting and imaging with semiconductor
quantum dots. Nat Biotechnol. 22:969–976. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moghimi SM, Hunter AC and Murray JC:
Nanomedicine: Current status and future prospects. FASEB J.
19:311–330. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Al-Jamal WT, Al-Jamal KT, Tian B,
Cakebread A, Halket JM and Kostarelos K: Tumor targeting of
functionalized quantum dot-liposome hybrids by intravenous
administration. Mol Pharm. 6:520–530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boisselier E and Astruc D: Gold
nanoparticles in nanomedicine: Preparations, imaging, diagnostics,
therapies and toxicity. Chem Soc Rev. 38:1759–1782. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Debbage P: Targeted drugs and
nanomedicine: Present and future. Curr Pharm Des. 15:153–172. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang HC, Barua S, Sharma G, Dey SK and
Rege K: Inorganic nanoparticles for cancer imaging and therapy. J
Control Release. 155:344–357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang X, Peng X, Wang Y, Wang Y, Shin DM,
El-Sayed MA and Nie S: A reexamination of active and passive tumor
targeting by using rod-shaped gold nanocrystals and covalently
conjugated peptide ligands. ACS Nano. 4:5887–5896. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ilbasmiş-Tamer S, Yilmaz S, Banoğlu E and
Değim IT: Carbon nanotubes to deliver drug molecules. J Biomed
Nanotechnol. 6:20–27. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang LW, Yu WW, Colvin VL and
Monteiro-Riviere NA: Biological interactions of quantum dot
nanoparticles in skin and in human epidermal keratinocytes. Toxicol
Appl Pharmacol. 228:200–211. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Garala K, Faldu N, Basu B, Bhalodia R,
Mehta K and Joshi B: Chemical penetration enhancement. J Pharm Res.
2:1804–1808. 2009.
|
|
30
|
Nishihata T, Kamada A, Sakai K, Takahashi
K, Matsumoto K, Shinozaki K, Tabata Y, Keigami M, Miyagi T and
Tatsumi N: Percutaneous absorption of diclofenac in rats and
humans: Aqueous gel formulation. Int J Pharm. 46:1–7. 1988.
View Article : Google Scholar
|
|
31
|
Ahmed TA, Ibrahim HM, Ibrahim F, Samy AM,
Fetoh E and Nutan MT: In vitro release, rheological, and stability
studies of mefenamic acid coprecipitates in topical formulations.
Pharm Dev Technol. 16:497–510. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Goddard ED and Gruber JV: Principles of
polymer science and technology in cosmetics and personal care.
Marcel Dekker, Inc.; New York-Basel: 1999, View Article : Google Scholar
|