Introduction
Cognitive dysfunction or impairment is prevalent in
older adults with Alzheimer's disease (1,2), or
older individuals receiving anesthesia and surgery (3,4). It is
reported that 10–60% of elderly surgical patients have suffered
with postoperative delirium and cognitive dysfunction in various
surgical procedures (5). However,
there is still no effective treatment to prevent or alleviate this
complication. Similar to other diseases, cognitive impairments are
caused by the abnormal accumulation of genetic or non-genetic
alterations, and the therapy of cognitive impairments in older
adults is a long and arduous process with limited apparent results
(6). Anesthesia-induced cognitive
impairments are associated with dysregulation of gene expression
and neuron cell apoptosis. For instance, inactivation of the
phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) signaling
pathway significantly increases the ratio of B-cell
lymphoma-2-associated X protein (Bax)/B-cell lymphoma-2 (Bcl-2)
resulting in increased hippocampal apoptosis (7). Certain microRNAs (miRs) are also
involved in this procedure. For example, miR-132 mediates
stress-inducible cognitive deficits via acetylcholinesterase
(8), and miR-137 variants serve as
negative predictors of impaired cognitive subtype of schizophrenia
(9). Cell apoptosis is a complicated
process involving various genetic factors, including brain-derived
neurotrophic factor (10),
postsynaptic density protein 95 (PSD95) (11), cAMP-response element binding protein
(CREB) (12,13), miR-34a (14), miR-383 (15), and the PI3K/Akt signaling pathway
(16,17). The PI3K/Akt signaling pathway is
essential for cell apoptosis, and its inhibition has been proven to
contribute to cancer cell apoptosis (16–18).
This pathway is associated with cognitive impairments induced by
diabetes, neonatal hypoxic ischemic brain damage, cardiovascular
disease and anesthesia (19–21). Furthermore, miR-383 has been
previously identified as an underexpressed miRNA in medulloblastoma
(22,23), and its downregulation promoted cancer
cell proliferation and invasion (24). The in vitro effects of miR-383
appear to be partially dependent on p21Cip1 (23), which is a cell cycle inhibitor that
serves a crucial role in several biological processes, including
cell cycle and apoptosis (25).
Additionally, Chakraborty et al (26) have demonstrated that miR-383 was
associated with PI3K/Akt signaling pathway. However, to the best of
our knowledge, no studies have examined the influence of propofol
anesthesia-induced cognitive impairment on miR-383 expression, or
focused on the association between propofol anesthesia-associated
miR-383 expression and the PI3K/Akt signaling pathway.
In the present study, with the aim to investigate
the effect of miR-383 expression on propofol-induced learning and
memory impairment, a cognitive impairment rat model was established
using propofol anesthesia. The effect of miR-383 expression on
propofol anesthesia-induced cognitive impairment was analyzed using
constructed lentivirus vectors expressing miR-383 mimics. Cell
apoptosis, rat learning and memory abilities, and the expression of
genetic factors were also examined. The current study attempted to
provide information on the association between anesthesia-induced
cognitive impairment and miR-383 expression, and assist in the
development of a therapeutic strategy focusing on miR-383 for
cognitive impairment.
Materials and methods
Animal anesthesia model
A total of 48 male Sprague-Dawley rats (7-week-old;
weight, 250±10 g) were obtained from Vital River Laboratory Animal
Technology Co., Ltd. (Beijing, China). Prior to experiments,
animals were housed at 22°C and 50% humidity under a controlled
12-h light-dark cycle with ad libitum access to food and
water for 1 week for acclimatization. Rats were then randomly
divided into four groups (n=12 each): Control group, and three
propofol-anesthetized groups that were untreated (propofol group),
treated with miR-383 mimics (mimic + propofol), and treated with
miR-383 scramble (scramble + propofol). Animals in the three
propofol groups were anesthetized for 6 h, between 9:00 a.m. and
3:00 p.m., by intraperitoneal injection of 300 mg/kg body weight
propofol (833 µg/kg/min; AstraZeneca plc, London, UK) for 7 days.
The control rats were anesthetized with the same conditions (37°C),
administrated with normal saline at 1 ml/h between 9:00 a.m. and
3:00 p.m., and allowed to breath regular air for 6 h. Animals were
allowed to recover for 7 days after anesthesia, and were then
subjected to further tests or sacrifice. All protocols of animal
experiments were reviewed and approved by the Institutional Animal
Care and Use Committee at the Chinese People's Liberation Army No.
94 Hospital (Nanchang, China).
In vivo hippocampal injection of
miR-383 lentivirus
For the induction of hippocampal miR-383 expression,
the lentiviral construct was used. Briefly, the purchased coding
oligonucleotides of antisense mouse miR-383 mimics and scramble
sequence (Guangzhou RiboBio Co., Ltd., Guangzhou, China) were
cloned and inserted into a lentivirus expression vector,
Pcdh-CMV-MCS-EF1-copGFP (System Biosciences, Inc., Morrisville, PA,
USA). The miR-383 mimics and scramble viral particles were produced
in 293T cells (CRL-3216; ATCC, Manassas, VA, USA) via lentivirus
expression vector co-expressed with pPACK packaging system (Systems
Biosciences, Palo Alto, CA, USA). For the miR-383 transfection,
rats were administrated with lentiviruses containing miR-383 mimics
or scramble at 24 h before the first administration of propofol. A
total volume of 2 µl lentivirus-containing mimics or scramble
vectors were injected to the hippocampus of the rats through a
drilled hole (0.05 mm in diameter) on the right cortex just above
the dorsal hippocampus, via a Hamilton syringe under a surgical
microscope.
Morris water maze (MWM) test
Subsequent to treatment and propofol anesthesia, the
MWM test was used to examine the spatial learning and memory of
rats. At 7 days after anesthesia (recovery), half of the animals in
each group were subjected to the test. The MWM, placed in a room
with dimmed lights, was composed of a circular water pool (colored
with black ink; 100 cm in diameter, 50 cm in height) with 30-cm
depth of water and a platform at 1 cm below the water surface (12
cm in diameter). The maze was divided into four quadrants. Animals
attempted to find the platform by swimming in each of the four
quadrants every day for 5 consecutive days. After 5 days of
training, swimming data was recorded on day 6. At the beginning of
the trail, animals were immersed in the center of one of the three
quadrants without the hidden platform. A maximum of 60 sec was
allowed for finding the hidden platform, and at the end of each
swim, rats were allowed to rest for 30 min. A video camera on the
ceiling was used to videotape the performance of the rats in
association with their swimming ability. The time taken to reach
the hidden platform (latency; recorded in sec), path length (cm)
and swimming speed (cm/s) were analyzed using image tracking
software (HVS Image 2020 Plus tracking system; HVS Image Software
Ltd., Buckingham, UK). The latency was positively correlated with
the rats' spatial memory.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
The apoptosis of hippocampal neurons was detected
using TUNEL assay. Briefly, at 7 days after anesthesia, rats were
sacrificed and 4-µm paraffin-embedded hippocampal sections were
prepared and de-paraffinized. A colorimetric TUNEL kit (EMD
Millipore, Billerica, MA, USA) was then used for in situ
apoptosis detection of apoptotic hippocampal neurons following the
manufacturer's instructions. The apoptotic hippocampal neurons were
stained a brown coloration when reacted with DAB. Cells in five
arbitrarily selected fields were counted, and the percentage of
TUNEL positive cell number was calculated.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
For RT-qPCR analysis, total RNA was extracted from
hippocampus tissues 7 days after anesthesia using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Then, 2 µg of total RNA was used as a template to synthesize the
first-strand cDNA using iScriptTM Reverse Transcription Supermix
(Bio-Rad Laboratories, Inc., Hercules, CA, USA), and the mRNA and
miRNA expression levels were determined using a Ssofast EvaGreen
Supermix kit (Bio-Rad Laboratories, Inc.) with an ABI 7500 Fast
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) and a miRCURY LNA microRNA Array kit (version 14.0; Exiqon;
Qiagen, Hilden, Germany). The primers (Table I) were synthesized by Shanghai Sangon
Biologic Engineering Technology and Services Co., Ltd. (Shanghai,
China). GAPDH was used as the internal control for mRNA
quantifications and U6 was used as the internal control for miRNA
quantification. The reaction conditions were as follows: 95°C 10
min, followed by 95°C for 30 sec, 60°C for 40 sec and 72°C for 10
sec for 40 cycles. All reactions were run in triplicate. The
relative mRNA expression level was calculated by the
2−ΔΔCq method (27).
 | Table I.Primers used for reverse
transcription-quantitiative polymerase chain reaction. |
Table I.
Primers used for reverse
transcription-quantitiative polymerase chain reaction.
| Gene | Primer
sequence |
|---|
| miR-383 | Forward,
5′-CTTTCCCAAGAGTTTCACT-3′ |
|
| Reverse,
5′-CCACTCCAGTCCACCAAAT-3′ |
| U6 | Forward,
5′-CGCTTCGGCAGCACATATAC-3′ |
|
| Reverse,
5′-TTCACGAATTTGCGTGTCAT-3′ |
| Bax | Forward,
5′-TTTGCTTCAGGGTTTCATCC-3′ |
|
| Reverse,
5′-ATCCTCTGCAGCTCCATGTT-3′ |
| Bcl-2 | Forward,
5′-ATTGTGATGGTCATATTATTG-3′ |
|
| Reverse,
5′-ATATCATATGTCCTTTATGCA-3′ |
| GAPDH | Forward,
5′-GAAGGTGAAGGTCGGAGTCA-3′ |
|
| Reverse,
5′-TTGAGGTCAATGAAGGGGTC-3′ |
Western blot analysis
Animals were sacrificed 7 days after anesthesia, and
the hippocampus tissues were extracted using lysis buffer [300 mM
NaCl, 50 mM Tris-HCl (pH 7.5), 5 mM EDTA, 0.1% NP-40, 1 mM DTT, 1
mM phenylmethanesulphonyl fluoride, and 1:100 protease inhibitor
cocktail], homogenized and centrifuged at 4°C at 12,000 × g for 10
min to collect the supernatants. Concentration of protein was
determined using the bicinchoninic acid method. Hippocampus
proteins were mixed with and boiled in sodium dodecyl sulfate (SDS)
sample buffer for 10 min, then 10 µg protein was prepared for
western blot analysis. Next, 10% SDS-PAGE was used to separate
proteins, which were then electrophoretically transferred to
polyvinylidene difluoride membranes (Invitrogen; Thermo Fisher
Scientific, Inc.). Subsequent to blocking with bovine serum albumin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at room temperature
for 1 h, membranes were incubated at 4°C overnight with primary
antibodies against the following: Bcl-2 (1:5,000, catalog no.
4223), Bax (1:1,000, catalog no. 610982), PSD95 (1:2,000, catalog
no. 610495), phosphorylated (p)-CREB (1:1,000, catalog no. 7722),
CREB (1:1,000, catalog no. 9197), p-PI3K (1:1,000, catalog no.
4228), PI3K (1:2,000, catalog no. 9655), p-Akt (1:1,000, catalog
no. 4060), Akt (1:1,000, catalog no. 4691) and GAPDH (1:1,000,
catalog no. 560792). The anti-BCL-2, p-CREB, CREB, p-PI3K, PI3K,
p-Akt and Akt antibodies were obtained from Cell Signal Technology,
Inc. (Danvers, MA, USA), while anti-Bax-L, PSD95 and
GAPDH antibodies were obtained from BD Transduction Laboratories
(Breda, The Netherlands). Subsequently, the membranes incubated
with primary antibodies were washed and incubated with horseradish
peroxidase-conjugated secondary antibodies (anti-mouse: 1:1,000,
catalog no. 61-6000; anti-Rabbit: 1:1,000, catalog no. A21253,
Invitrogen; Thermo Fisher Scientific, Inc.) for 1 h. The
polypeptide bands were visualized using a Tanon-5200
Chemiluminescent Imaging System (Tanon Science & Technology
Co., Ltd. Shanghai, China). Images were quantified using ImageJ
software version 1.44 (National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 19.0; IBM Corp., Armonk, NY, USA). All data are
expressed as the mean ± standard deviation. Differences between two
groups were assessed by Tukey's test, while those among more groups
were assessed using analysis of variance. P<0.05 was considered
to indicate differences that were statistically significant.
Results
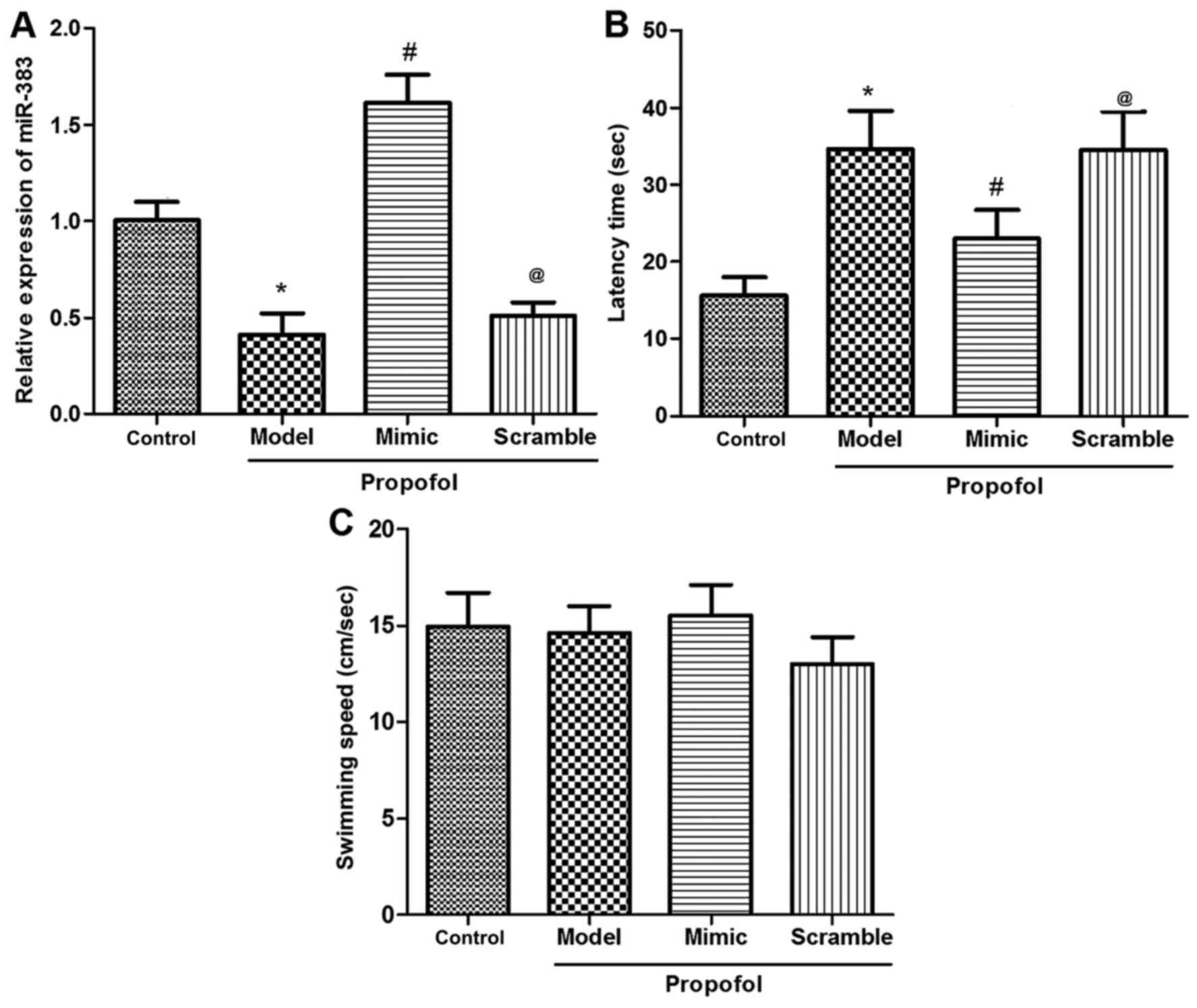
Expression of miR-383 in the
hippocampus following propofol anesthesia
Rats were divided into the untreated control,
propofol anesthesia (propofol group, n=12), and miR-383 mimic or
scramble treatment prior to propofol anesthesia (mimic/scramble +
propofol) groups. Initially, the expression of miR-383 in the
hippocampus of animals in the control or three propofol groups was
determined using RT-qPCR. The results demonstrated that the
expression of miR-383 in the propofol group was significantly
reduced by propofol administration in comparison with the control
group (P<0.05; Fig. 1A). However,
miR-383 expression in the miR-383 mimic + propofol group was
elevated following injection of the lentivirus expression vector,
when compared with the expression in the control, propofol model
and scramble + propofol groups (P<0.05; Fig. 1A). Furthermore, no significant
difference was observed in miR-383 expression between the miR-383
mimic + propofol and the miR-383 scramble + propofol groups. These
data suggested that the miR-383-expressing lentivirus vector was
successfully constructed.
miR-383 protects against reduced
animal learning and memory ability induced by propofol
anesthesia
MWM tests were performed to determine the influence
of propofol, as well as the protective effect of miR-383, on animal
learning and memory ability. Data from MWM test revealed that
propofol administration markedly lengthened the time that rats
required to find the hidden platform compared with the control
group (P<0.05; Fig. 1B).
Furthermore, the animals treated with miR-383-expressing vector
presented shorter latency time in comparison with the propofol
model or scramble + propofol groups (P<0.05; Fig. 1B). However, there were no significant
differences in the swimming speed among the various groups
(P>0.05; Fig. 1C). These results
revealed that the miR-383-expressing lentivirus vector treatment
protected against propofol-induced damage in animal learning and
memory ability.
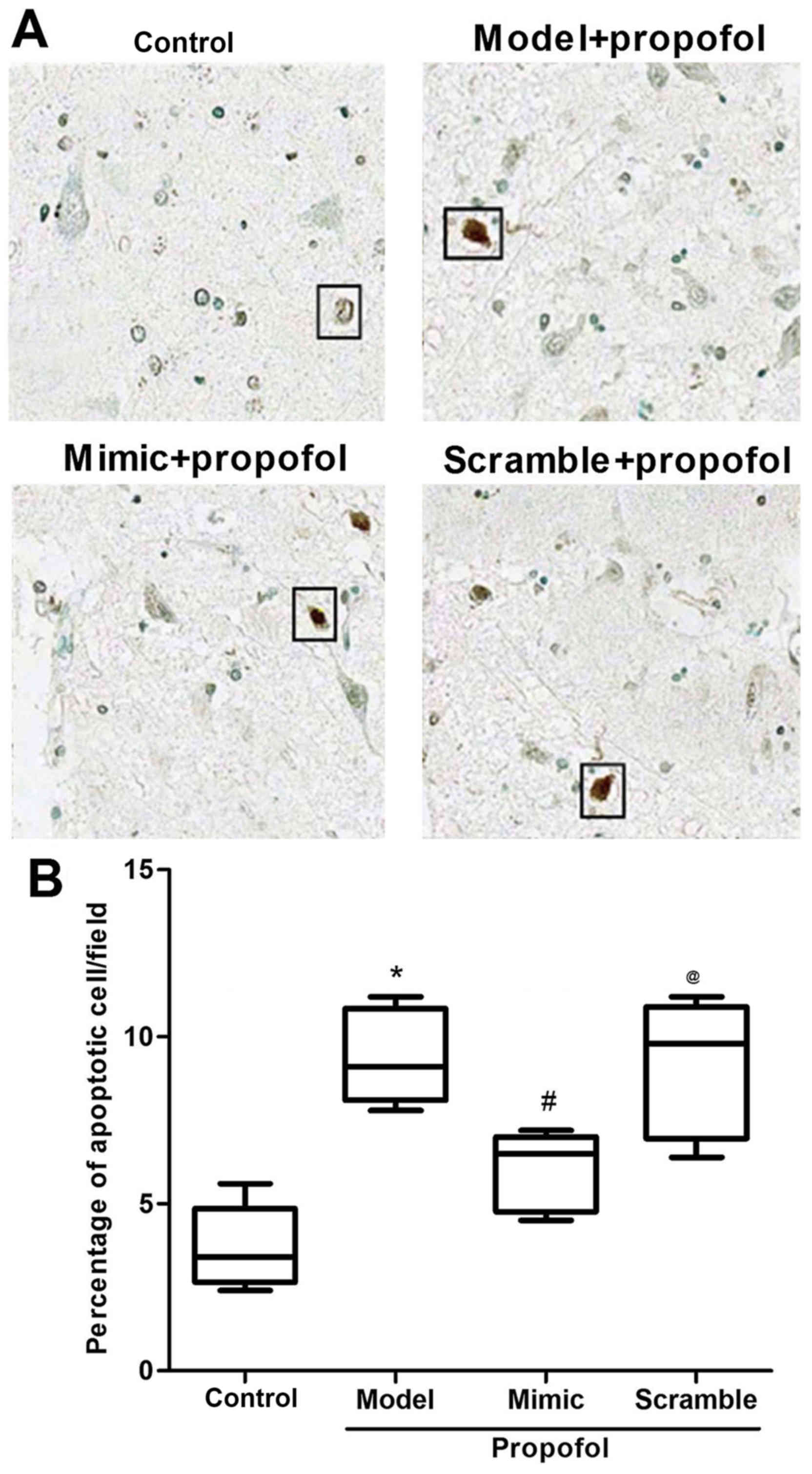
miR-383 inhibits propofol
anesthesia-induced neuron apoptosis
At 7 days after the anesthesia, 4-µm
paraffin-embedded hippocampal sections were prepared for TUNEL
assay. The results from TUNEL assay revealed that propofol
anesthesia significantly increased neuron apoptosis, in comparison
with the control group (P<0.05; Fig.
2). As expected, there was a reduction in neuron apoptosis in
rats treated with miR-383-expressing lentivirus vector, when
comparing with the control rats (P<0.05; Fig. 2). No significant difference was
observed in the neuron apoptosis rate between the propofol model
and the miR-383 scramble + propofol groups. These findings suggest
that miR-383 may have inhibited the propofol anesthesia-induced
damage on animal learning and memory ability by inhibiting neuron
apoptosis.
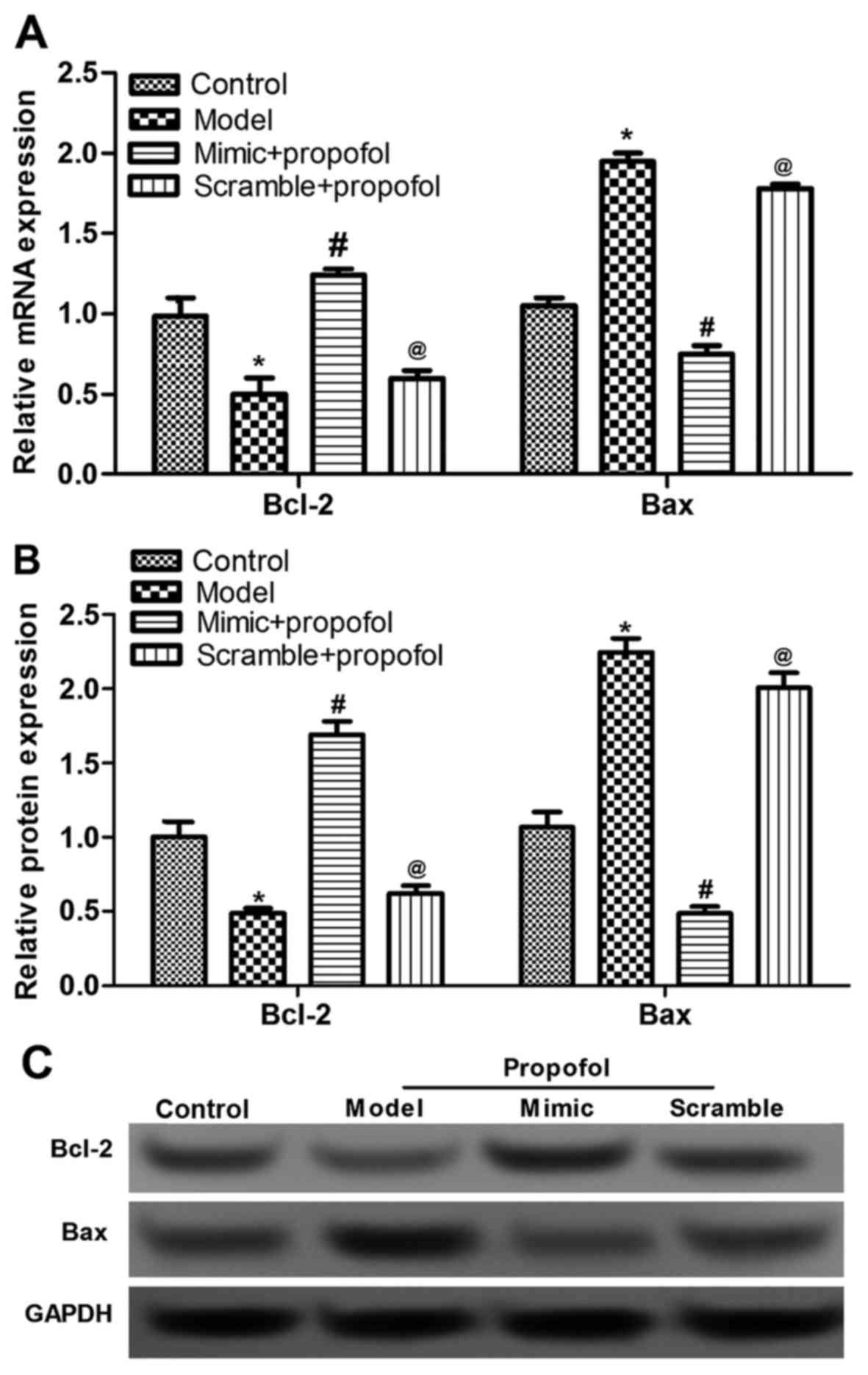
miR-383 inhibits propofol
anesthesia-induced dysregulation of apoptotic proteins
Based on the TUNEL assay, it was speculated that
both propofol and miR-383 affected the expression of
apoptosis-associated proteins. Thus, the present study detected the
expression of two key apoptotic factors, Bcl-2 and Bax (28,29). The
mRNA and protein expression levels of Bcl-2 were significantly
reduced, while those of Bax were significantly upregulated, by
propofol anesthesia compared with the levels in the control group
(P<0.05; Fig. 3). Furthermore,
the propofol-induced dysregulation of Bcl-2 and Bax was evidently
inhibited in the miR-383-treated group, compared with the propofol
anethesia model group (P<0.05; Fig.
3). However, no significant difference was observed in the
expression levels of Bcl-2 and Bax between the propofol model and
miR-383 scramble + propofol groups. These data suggested that
miR-38 mimic inhibited neuron apoptosis via regulating Bcl-2 and
Bax expression.
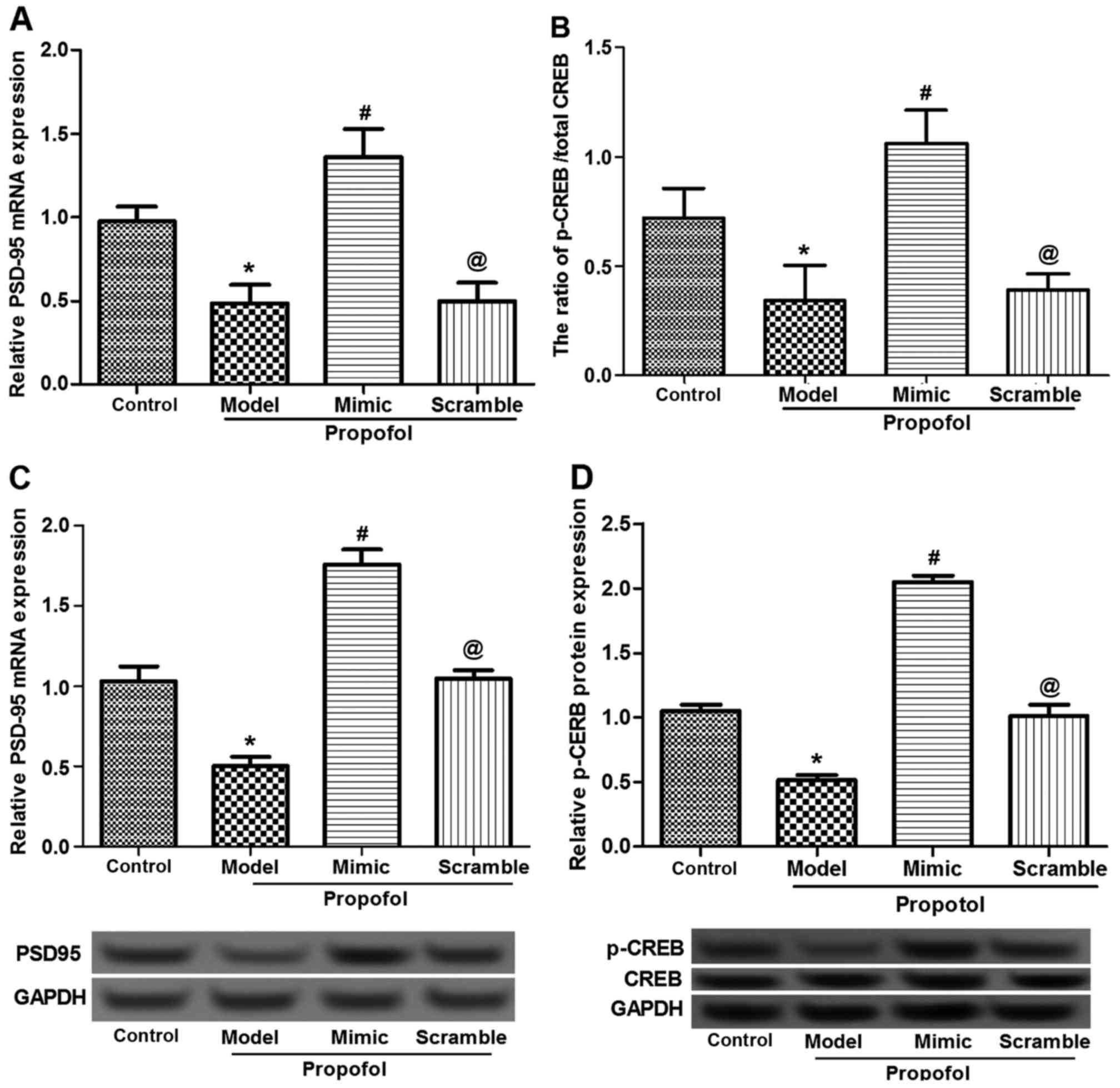
Effects of propofol and miR-383
expression on neurodevelopment-associated protein expression
As previously reported, PSD95 and CREB are
associated with and serve crucial roles in the development of
hippocampal neurons (30,31). Thus, the present study detected the
effect of propofol administration, as well as of miR-383-expressing
vectors, on the expression levels of PSD95 and CREB. Data from
RT-qPCR and western blot analysis revealed that the mRNA and
protein expression levels, respectively, of PSD95 and CREB were
significantly inhibited by propofol administration (P<0.05;
Fig. 4). In the mimic-treated group,
however, the expression levels of PSD95 and CREB in the rat
hippocampus were significantly upregulated compared with those of
rats in the propofol, scramble + propofol and control groups
(P<0.05; Fig. 4). This
demonstrated that miR-383 mimic administration contributed to the
promotion of the propofol-damaged development of hippocampal
neurons.
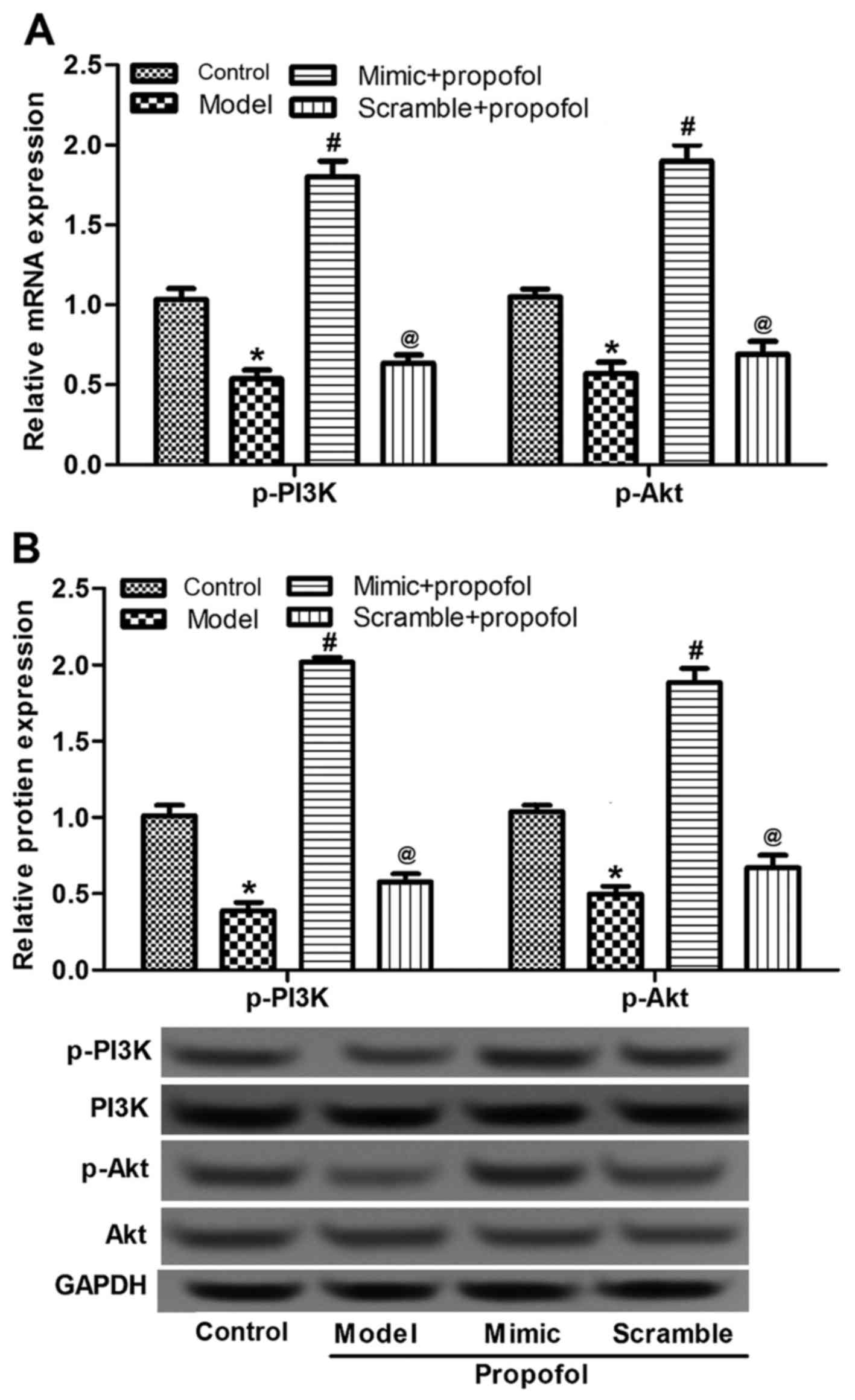
Effects of propofol and miR-383
expression on PI3K/Akt signaling pathway-associated protein
expression
Since the PI3K-Akt signaling pathway is essential
for cell apoptosis (32,33), the expression and activated status of
this pathway in animals were detected in the current study in order
to examine the action mechanism of propofol and miR-383 expression
on neuron apoptosis. The expression of p-PI3K and p-Akt mRNA and
protein levels in the propofol-anesthetized group were
significantly reduced in comparison with those in the control group
(P<0.05; Fig. 5). By contrast,
the miR-383-expressing vector triggered the expression of p-PI3K
and p-Akt, in comparison with the propofol group (P<0.05;
Fig. 5). There were no notable
differences in the expression of total PI3K and Akt among the
various groups, or in the expression of p-PI3K and p-Akt between
rats treated with propofol and with miR-383 or scramble + propofol
(Fig. 5B). These data demonstrated
that propofol and miR-383 may regulate the activation of PI3K-Akt
signaling pathway.
Discussion
Anesthesia-induced cognitive impairment is a
recognized clinical phenomenon and possibly the most frequent type
of postoperative cognitive impairment (3,4). miRNAs
had been reported to be associated with cognitive ability (34,35). The
present study aimed to investigate the effect of miR-383 expression
on propofol-induced learning and memory impairment. The current
results revealed that propofol anesthesia induced the
downregulation of miR-383 expression. Further experiments
demonstrated that miR-383-expressing vector treatment was able to
repair the propofol anesthesia-induced cognitive impairment by
inhibiting neuron cell apoptosis and by modulating the expression
of apoptotic proteins and other factors associated with neuronal
development, as well as by regulating the apoptosis signaling
pathway.
Previous studies have demonstrated that
anesthesia-induced cognitive impairment, including isoflurane,
sevoflurane or propofol exposure, and the associated neuron
apoptosis are recognized clinical phenomena (36,37).
Additionally, studies focusing on the comparison between propofol
and other clinical anesthetics have observed that propofol has a
controversial or dual effect on cognitive impairment (37–39).
Propofol may induce cognitive impairment with a higher severity in
comparison with sevoflurane administration (39). However, propofol may protect against
or reduce cognitive impairment resulting from surgery or
electroconvulsive therapy (38,40). In
the present study, a rat model with cognitive impairment was
successfully established using propofol administration. The
increment in the apoptotic rate of hippocampal neurons (Fig. 2), alterations in apoptosis-associated
protein expression (Figs. 3 and
4), as well as inhibition of the
PI3K/Akt signaling pathway activation following propofol treatment
(Fig. 5), demonstrated that propofol
administration resulted in cognitive impairment in rats.
miR-383 is a disease-associated miRNA, and its
downregulation has been identified in patients with various types
of cancer, infertility and other diseases (23,24).
Ectopic expression of miR-383 has been demonstrated to be associate
with cell growth, apoptosis and expression of apoptosis-associated
proteins (22). In the present
study, miR-383 mimic-expressing lentivirus vectors were
successfully constructed and employed as a treatment for
propofol-induced cognitive impairment. Administration of miR-383
mimics significantly upregulated the reduced expression levels of
miR-383, neuron development factors and PI3K/Akt signaling pathway
factors, p-PI3K and p-Akt, that resulted from propofol
administration. In addition, miR-383 mimics inhibited the
propofol-altered expression of apoptosis-associated proteins and
apoptotic neuron cells. These observations revealed that miR-383
repaired the propofol-induced cognitive impairment in the rat
model, and that it may be a novel target for exploring effective
therapeutic strategies for propofol anesthesia-induced cognitive
impairment.
Cell apoptosis is a complicate process involved in
numerous signaling pathways, as well as stimulation, genetic and
non-genetic factors (41–44). Cognitive impairments resulting from
propofol or other anesthetics have been demonstrated to involve
hippocampal neuron apoptosis and regulation of apoptosis-associated
proteins and signaling pathways (37,45,46). For
instance, Bcl-2 and Bax are important factors for cell apoptosis,
which is benefited by overexpression of Bax and inhibition of
Bcl-2, or upregulation of the Bax/Bcl-2 ratio (16,17,47,48). In
addition, Bcl-2 expression may be mediated by p-CREB or
CREB-dependent Bcl-2 signaling, which further contributes to cell
apoptosis (49,50). Furthermore, CREB is required for
PSD95 disruption (31), and the
transcription of PSD95 may be directly activated CREB (51). In the current study, it was
determined that the propofol anesthesia upregulated Bax/Bcl-2
ratio, but this was then reduced by miR-383 mimic transfection via
inhibiting Bax and inducing Bcl-2 (Fig.
3). Additionally, propofol anesthesia-inhibited PSD95 and
p-CREB expression levels were upregulated by miR-383 mimic
administration (Fig. 4). These
findings demonstrated that the apoptotic factors Bax and Bcl-2, as
well as PSD95 and p-CREB, responded to the abnormal expression of
miR-383.
PI3K/Akt signaling pathway is a key pathway for cell
apoptosis (16,17). As reported, inhibition of the
PI3K/Akt signaling pathway contributed to cancer cell apoptosis
(18). Yoshii and Constantine-Paton
revealed that the inhibition of MAPK/ERK signaling pathway
disrupted PSD95 expression, and that PI3K is critical for the PSD95
disruption (51). However, there was
no evidence demonstrating the effect of miR-383 mimic on PI3K/Akt
signaling pathway activation in propofol anesthetized animals with
cognitive impairment. Since miR-383 dysregulation and PI3K/Akt
signaling pathway participate in cell apoptosis (16,17,23,24), the
present study speculated that miR-383 mimics may be able to
regulate the expression levels of p-PI3K and p-Akt, and the
activation status factors of PI3K/Akt signaling pathway. As
expected, propofol administration significantly downregulated the
expression levels of p-PI3K and p-Akt proteins (Fig. 5), as well as those of miR-383,
Bax/Bcl-2, PSD95 and p-CREB. However, miR-383 mimic evidently
upregulated the expression of all these factors, revealing that
miR-383 regulated the expression or activation of PI3K/Akt
signaling pathway, thus modulating cell apoptosis.
In conclusion, in the present study, a cognitive
impairment rat model was established using propofol anesthesia.
Propofol induced downregulation of miR-383, lengthened latency
time, apoptosis of hippocampal neurons, upregulation of Bax/Bcl-2,
inhibition of PSD95 and p-CREB expression levels, and inactivation
of PI3K/Akt signaling pathway. However, treatment with miR-383
mimic-expressing lentivirus vector significantly improved the
propofol effects, demonstrating the protective effect of miR-383 on
propofol anesthesia-induced cognitive impairment in the rat model.
The current study also suggested the miR-383 mimics may be used as
a novel target for the development of a therapeutic strategy for
propofol anesthesia-induced cognitive impairment.
References
|
1
|
Parrott MD, Winocur G, Bazinet RP, Ma DW
and Greenwood CE: Whole-food diet worsened cognitive dysfunction in
an Alzheimer's disease mouse model. Neurobiol Aging. 36:90–99.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ng CL, Cheng OY, Kwan SC, Ho WL, Cheng KY,
Chung SK, Lam KSL, Xu A and Chan KH: Adiponectin deficiency induced
cognitive dysfunction and Alzheimer's disease pathogenesis in mice
through the disruption of insulin sensitivity and inactivated AMPK
signaling. Proceedings of the 67th Annual Meeting of the American
Academy of Neurology (AAN 2015). AAN. Washington, DC. 2015;
|
|
3
|
Rundshagen I: Postoperative cognitive
dysfunction. Dtsch Arztebl Int. 111:119–125. 2014.PubMed/NCBI
|
|
4
|
Grape S, Ravussin P, Rossi A, Kern C and
Steiner L: Postoperative cognitive dysfunction. Trends Anaesthesia
Crit Care. 2:98–103. 2012. View Article : Google Scholar
|
|
5
|
Tabaka P, Goodam S, Sommer BR, Maloney W,
Huddleston J and Lemmens HJ: The effect of desdlurane versus
propofol anesthesia on postoperative delirium in elderly obese
patients undergoing total knee replacement: A randomized,
controlled, double-blinded clinical trial. J Clin Anethesia.
39:17–22. 2017. View Article : Google Scholar
|
|
6
|
Vogelstein B and Kinzler KW: Cancer genes
and the pathways they control. Nat Med. 10:789–799. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y, Wu C, Han B, Xu F, Mao M, Guo X
and Wang J: Dexmedetomidine attenuates repeated propofol
exposure-induced hippocampal apoptosis, PI3K/Akt/Gsk-3b siganling
disruption, and juvenile cognitive deficits in neonatal rats. Mol
Med Rep. 14:769–775. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shaltiel G, Hanan M, Wolf Y, Barbash S,
Kovalev E, Shoham S and Soreq H: Hippocampal microRNA-132 mediates
stress-inducible cognitive deficits through its
acetylcholinesterase target. Brain Struct Funct. 218:59–72. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Green M, Cairns M, Wu J, Dragovic M,
Jablensky A, Tooney PA, Scott RJ and Carr VJ; Australian
Schizophrenia Research Bank, : Genome-wide supported variant MIR137
and severe negative symptoms predict membership of an impaired
cognitive subtype of schizophrenia. Mol Psychiatry. 18:774–780.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takeda K, Kermani P, Anastasia A, Obinata
Y, Hempstead BL and Kurihara H: BDNF protects human vascular
endothelial cells from TNFα-induced apoptosis. Biochem Cell Biol.
91:341–349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dong J, Wang Y, Wang Y, Wei W, Min H, Song
B, Xi Q, Teng W and Chen J: Iodine deficiency increases apoptosis
and decreases synaptotagmin-1 and PSD-95 in rat hippocampus. Nutr
Neurosci. 16:135–141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu R, Xing L, Kong D, Jiang J, Shang L
and Hao W: Bisphenol A inhibits proliferation and induces apoptosis
in micromass cultures of rat embryonic midbrain cells through the
JNK, CREB and p53 signaling pathways. Food Chemical Toxicol.
52:76–82. 2013. View Article : Google Scholar
|
|
13
|
Zuo H, Lin T, Wang D, Peng R, Wang S, Gao
Y, Xu X, Zhao L, Wang S and Su Z: RKIP regulates neural cell
apoptosis induced by exposure to microwave radiation partly through
the MEK/ERK/CREB pathway. Mol Neurobiol. 51:1520–1529. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Welch C, Chen Y and Stallings R:
MicroRNA-34a functions as a potential tumor suppressor by inducing
apoptosis in neuroblastoma cells. Oncogene. 26:5017–5022. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao L, Gu H, Chang J, Wu J, Wang D, Chen
S, Yang X and Qian B: MicroRNA-383 regulates the apoptosis of tumor
cells through targeting Gadd45g. PLoS One. 9:e1104722014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu G, Wang T, Wang T, Song J and Zhou Z:
Effects of apoptosis-related proteins caspase-3, Bax and Bcl-2 on
cerebral ischemia rats. Biomed Rep. 1:861–867. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liang K, Ye Y, Wang Y, Zhang J and Li C:
Formononetin mediates neuroprotection against cerebral
ischemia/reperfusion in rats via downregulation of the Bax/Bcl-2
ratio and upregulation PI3K/Akt signaling pathway. J Neurol Sci.
344:100–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Malla R, Gopinath S, Alapati K, Gondi CS,
Gujrati M, Dinh DH, Mohanam S and Rao JS: Correction:
Downregulation of uPAR and cathepsin B induces apoptosis via
regulation of Bcl-2 and Bax and inhibition of the PI3K/Akt pathway
in gliomas. PLoS One. 9:2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yao D, Zhang WR, He X, Wang JH, Jiang KW
and Zhao ZY: The expression of PI3K/Akt signaling pathway and PTEN
in hippocampus of the brain and the correlation with cognitive
impairment after neonatal hypoxic ischemic brain damage in rats.
Int J Clin Exp Med. 9:9044–9053. 2016.
|
|
20
|
Shu Y, Zhang H, Kang T, Zhang JJ, Yang Y,
Liu H and Zhang L: PI3K/Akt signal pathway involved in the
cognitive impairment caused by chronic cerebral hypoperfusion in
rats. PLoS One. 8:e819012013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Wu C, Han B, Xu F, Mao M, Guo X
and Wang J: Dexmedetomidine attenuates repeated propofol
exposure-induced hippocampal apoptosis, PI3K/Akt/Gsk-3β signaling
disruption, and juvenile cognitive deficits in neonatal rats. Mol
Med Rep. 14:769–775. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li KK, Pang JC, Lau KM, Zhou L, Mao Y,
Wang Y, Poon WS and Ng HK: MiR-383 is downregulated in
medulloblastoma and targets peroxiredoxin 3 (PRDX3). Brain Pathol.
23:413–425. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lian J, Tian H, Liu L, Zhang XS, Li WQ,
Deng YM, Yao GD, Yin MM and Sun F: Downregulation of microRNA-383
is associated with male infertility and promotes testicular
embryonal carcinoma cell proliferation by targeting IRF1. Cell
Death Dis. 1:e942010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He Z, Cen D, Luo X, Li D, Li P, Liang L
and Meng Z: Downregulation of miR-383 promotes glioma cell invasion
by targeting insulin-like growth factor 1 receptor. Med Oncol.
30:5572013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cazzalini O, Scovassi AI, Savio M, Stivala
LA and Prosperi E: Multiple roles of the cell cycle inhibitor
p21(CDKN1A) in the DNA damage response. Mutat Res. 704:12–20. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chakraborty C, Doss CG, Bandyopadhyay S
and Agoramoorthy G: Influence of miRNA in insulin signaling pathway
and insulin resistance: Micro-molecules with a major role in type-2
diabetes. Wiley Interdiscip Rev RNA. 5:697–712. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Method. 25:402–408. 2001.
View Article : Google Scholar
|
|
28
|
Smith CC, Guévremont D, Williams JM and
Napper RM: Apoptotic cell death and temporal expression of
apoptotic proteins Bcl-2 and Bax in the hippocampus, following
binge ethanol in the neonatal rat model. Alcohol Clin Exp Res.
39:36–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cao J, Chen J, Wang J, Jia R, Xue W, Luo Y
and Gan X: Effects of fluoride on liver apoptosis and Bcl-2, Bax
protein expression in freshwater teleost, Cyprinus carpio.
Chemosphere. 91:1203–1212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bustos FJ, Varela-Nallar L, Campos M,
Henriquez B, Phillips M, Opazo C, Aguayo LG, Montecino M,
Constantine-Paton M, Inestrosa NC and van Zundert B: PSD95
suppresses dendritic arbor development in mature hippocampal
neurons by occluding the clustering of NR2B-NMDA receptors. PLoS
One. 9:e940372014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bell KF, Bent RJ, Meese-Tamuri S, Ali A,
Forder JP and Aarts MM: Calmodulin Kinase IV-dependent CREB
activation is required for neuroprotection via NMDA receptor-PSD95
disruption. J Neurochem. 126:274–287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen K, Li G, Geng F, Zhang Z, Li J, Yang
M, Dong L and Gao F: Berberine reduces ischemia/reperfusion-induced
myocardial apoptosis via activating AMPK and PI3K-Akt signaling in
diabetic rats. Apoptosis. 19:946–957. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Roy R, Singh SK, Chauhan LK, Das M,
Tripathi A and Dwivedi PD: Zinc oxide nanoparticles induce
apoptosis by enhancement of autophagy via PI3K/Akt/mTOR inhibition.
Toxicol Lett. 227:29–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luo T, Yin S, Shi R, Xu C, Wang Y, Cai J,
Yue Y and Wu A: miRNA expression profile and involvement of
Let-7d-APP in aged rats with isoflurane-induced learning and memory
impairment. PLoS One. 10:e01193362015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu W, Liu C, Zhu J, Shu P, Yin B, Gong Y,
Qiang B, Yuan J and Peng X: MicroRNA-16 targets amyloid precursor
protein to potentially modulate Alzheimer's-associated pathogenesis
in SAMP8 mice. Neurobiol Aging. 33:522–534. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu Y, Wang XJ, Wang N, Cui CL and Wu LZ:
Electroacupuncture Ameliorates propofol-induced cognitive
impairment via an opioid receptor-independent mechanism. Am J Chin
Med. 44:705–719. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang B, Liang G, Khojasteh S, Wu Z, Yang
W, Joseph D and Wei H: Comparison of neurodegeneration and
cognitive impairment in neonatal mice exposed to propofol or
isoflurane. PLoS One. 9:e991712014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Luo J, Min S, Wei K, Li P, Dong J and Liu
YF: Propofol protects against impairment of learning-memory and
imbalance of hippocampal Glu/GABA induced by electroconvulsive
shock in depressed rats. J Anesth. 25:657–665. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schoen J, Husemann L, Tiemeyer C, Lueloh
A, Sedemund-Adib B, Berger KU, Hueppe M and Heringlake M: Cognitive
function after sevoflurane-vs propofol-based anaesthesia for
on-pump cardiac surgery: A randomized controlled trial. Br J
Anaesth. 106:840–850. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Butterfield NN, Graf P, Macleod BA, Ries
CR and Zis AP: Propofol reduces cognitive impairment after
electroconvulsive therapy. J ECT. 20:3–9. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pan HC, Jiang Q, Yu Y, Mei JP, Cui YK and
Zhao WJ: Quercetin promotes cell apoptosis and inhibits the
expression of MMP-9 and fibronectin via the AKT and ERK signalling
pathways in human glioma cells. Neurochem Int. 80:60–71. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Romanov V, Whyard TC, Waltzer WC, Grollman
AP and Rosenquist T: Aristolochic acid-induced apoptosis and G2
cell cycle arrest depends on ROS generation and MAP kinases
activation. Arch Toxicol. 89:47–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Choe JY, Park KY and Kim SK: Oxidative
stress by monosodium urate crystals promotes renal cell apoptosis
through mitochondrial caspase-dependent pathway in human embryonic
kidney 293 cells: Mechanism for urate-induced nephropathy.
Apoptosis. 20:38–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang Y, Han L, Qi W, Cheng D, Ma X, Hou
L, Cao X and Wang C: Eicosapentaenoic acid (EPA) induced apoptosis
in HepG2 cells through ROS-Ca (2+)-JNK mitochondrial pathways.
Biochem Biophys Res Commun. 456:926–932. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang D, Zhou XH, Zhang J, Zhou YX, Ying
J, Wu GQ and Qian JH: Propofol promotes cell apoptosis via
inhibiting HOTAIR mediated mTOR pathway in cervical cancer. Biochem
Biophys Res Commun. 468:561–567. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li XM, Zhou MT, Wang XM, Ji MH, Zhou ZQ
and Yang JJ: Resveratrol pretreatment attenuates the
isoflurane-induced cognitive impairment through its
anti-inflammation and-apoptosis actions in aged mice. J Mol
Neurosci. 52:286–293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yap JL, Cao X, Vanommeslaeghe K, Jung KY,
Peddaboina C, Wilder PT, Nan A, MacKerell AD Jr, Smythe WR and
Fletcher S: Relaxation of the rigid backbone of an
oligoamide-foldamer-based α-helix mimetic: Identification of potent
Bcl-x L inhibitors. Org Biomol Chem. 10:2928–2933. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yue J, Ben Messaoud N and López JM:
Hyperosmotic shock engages two positive feedback loops through
Caspase-3-dependent Proteolysis of JNK1-2 and Bid. J Biol Chem.
290:30375–30389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Das S, Cordis GA, Maulik N and Das DK:
Pharmacological preconditioning with resveratrol: Role of
CREB-dependent Bcl-2 signaling via adenosine A3 receptor
activation. Am J Physiol Heart Circ Physiol. 288:H328–H335. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fujii M, Sherchan P, Soejima Y, Hasegawa
Y, Flores J, Doycheva D and Zhang JH: Cannabinoid receptor type 2
agonist attenuates apoptosis by activation of phosphorylated
CREB-Bcl-2 pathway after subarachnoid hemorrhage in rats. Exp
Neurol. 261:396–403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yoshii A and Constantine-Paton M:
Postsynaptic localization of PSD-95 is regulated by all three
pathways downstream of TrkB signaling. Front Synaptic Neurosci.
6:62014. View Article : Google Scholar : PubMed/NCBI
|