Introduction
Fracture is a very common bone injury. The
occurrence of fracture in the forearms, hands, and feet of children
is high, particularly in the ankles, with a global incident rate of
187 per 100,000 people. However, incidence rates vary among
countries, ages, sexes and sites of injury (1–3). Due to
the specific characteristics of children's bones, delayed and/or
improper treatment of ankle fracture in children may cause bone
deformity and disability (4,5). The process of bone formation involves a
balance between osteoblast and osteoclast activity and the healing
of fractures requires a large number of osteoblasts (6). It has been demonstrated that there is a
link between osteoclasts and inflammatory cytokines: High numbers
of osteoclasts are associated with high numbers of inflammatory
cytokines, including TNF-α and IL-1, following bone fracture
(7).
Owing to the close association of chronic
inflammatory processes to endogenous prostaglandin production
(8), bradykinin (BK) stimulates bone
resorption in neonatal mouse calvariae, suggesting that kinins
should be regarded as candidates for osteoclastic activation in
inflammatory conditions (9,10). BK may stimulate bone resorption and
potentiate the bone resorptivity induced by interleukin (IL)-1
(11). Furthermore, receptor
activator of nuclear factor κ-B ligand (RANKL), a tumor necrosis
factor (TNF)-related cytokine, is an important factor affecting
bone resorption (12,13). RANKL may activate the cognate
receptor RANK on osteoclast progenitor cells and TNF
receptor-associated factors (TRAFs)/mitogen-activated protein
kinases, resulting in the differentiation of osteoclast progenitor
cells that may then fuse into multinucleated, bone-resorbing
osteoclasts (12–14).
N-methyl pyrrolidone (NMP), a small bioactive
molecule, enhances bone formation and inhibits osteoclast
differentiation (15,16). It has been demonstrated that NMP
inhibits inflammation by repressing the NF-kB pathway (17). This indicates that NMP may be used as
an adjuvant therapy alongside established methods of bone fracture
treatment. Therefore, the present study investigated the effects of
NMP on inflammatory process using MG-63 cells stimulated with BK as
an inflammatory process model. It was demonstrated that NMP reduced
the expression of iNOS/COX-2 and the increase in the expression of
inflammatory cytokines, including IL-1β, IL-6 and TNF-α, induced by
BK. Taken together, the results of the current study suggest that
NMP exerts its anti-inflammatory function by downregulating the
expression of phosphorylated (p)-c-Jun N-terminal kinases (JNK) and
p-p38, which may regulate osteoblast and osteoclast activity by
decreasing and increasing their numbers, respectively. This may
promote bone formation and thus help to relieve ankle fractures in
children.
Materials and methods
Patients
A total of 60 peripheral blood samples (2 ml per
individual) from 60 children with ankle fracture, as well as 60
peripheral blood samples from 60 healthy children were collected at
Children's Hospital Affiliated to Nanjing Medical University from
August 2015 to April 2016. The average age of patients and healthy
controls were 6.0±1.2 and 5.9±1.3 years old, respectively. The
ratio of the boys and girls was equal in the two groups of
children. Informed consent was obtained from the parents or
guardians of all children enrolled and the present study was
approved by the Ethics Committee of Children's Hospital Affiliated
to Nanjing Medical University (Nanjing, China).
Cell culture
The human osteoblastic osteosarcoma cell line MG-63,
which expresses osteoblastic phenotypes, was obtained from the
American Type Culture Collection (cat. no. CRL-1427; Manassas, VA,
USA) and was used in current study. Cells were seeded into 9.5
cm2 culture dishes at a concentration of 104
cells/cm2 and α-Minimum Essential medium (MEM)/10% fetal
calf serum (FCS; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) was added to each dish. Cells were cultured for 1–2 days
until they reached 80–90% confluence, then washed twice with
phosphate-buffered saline (PBS) twice and once with serum-free
α-MEM. Finally, cells were cultured for 48 h at 37°C in 10 nM NMP
(Selleck Chemicals, Houston, TX, USA) containing α-MEM/1% FCS.
Subsequently, 1 µM BK was added and cells were cultured for a
further 24 h at 37°C. Cells were divided into 4 different groups: A
control group (cells without any treatment), a 1 µM BK induced
group (cells were treated with 1 µM BK for 24 h at 37°C), a 1 µM BK
+ 5 mM NMP group (cells were treated with 5 mM NMP for 48 h at 37°C
and then 1 µM BK for 24 h at 37°C) and a 1 µM BK + 10 mM NMP group
(cells were treated with 10 mM NMP for 48 h at 37°C and then 1 µM
BK for 24 h at 37°C).
Cell viability assay
Cell viability was tested using a Water-soluble
tetrazolium-1 (WST-1) Cell Proliferation and Cytotoxicity assay kit
(cat. no. C0036; Beyotime Institute of Biotechnology, Haimen,
China), a colorimetric assay for nonradioactive quantification of
cellular proliferation, viability and cytotoxicity, according to
the manufacturer's protocol. Briefly, MG-63 cells were cultured in
96-well plates for 24 h and then incubated with different
concentrations of NMP (0, 5 or 10 mM) (17) for 48 h at 37°C. Following
stimulation, WST-1 (1/10 of total volume) was added to each well
and incubated for a further 2 h at 37°C in the dark. Absorbance at
450 nm in each well was evaluated using a microplate reader.
ELISA assay
The supernatants were collected from the peripheral
blood of healthy controls and patients with ankle fracture via
centrifugation (3,000 × g at 4°C for 15 min). ELISA kits were then
used to detect TNF-α (cat. no. E-EL-H0109c), IL-1β (cat. no.
E-EL-H0149c) and IL-6 (cat. no. E-EL-H0102c) levels in the
peripheral blood of healthy controls and patients with ankle
fracture following the manufacturer's protocol. All ELISA kits were
purchased from Elabscience Biotechnology Co., Ltd (Wuhan, Hubei,
China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from MG-63 cells was isolated using TRIzol
(Invitrogen; Thermo Fisher Scientific Inc.), following the
manufacturer's protocol. A total of 1 µg RNA was reverse
transcribed into cDNA using the TaqMan microRNA Reverse
Transcription kit (Invitrogen) according to the manufacturer's
protocol. Analysis of mRNA levels was performed using the
TaqMan® Universal PCR Master Mix kit (Thermo Fisher
Scientific Inc.) on an ABI PRISM 7900 HT sequence-detection system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The
thermocycling conditions were as follows: 95°C for 10 min, followed
by 40 cycles of 95°C for 10 sec and 60°C for 60 sec. The primer
sequences used were as follows: IL-1β, forward,
5′-TGTGAAATGCCACCTTTTGA-3′ and reverse, 5′-TGAGTGATACTGCCTGCCTG-3′;
IL-6, forward, 5′-CCGGAGAGGAGACTTCACAG-3′ and reverse,
5′-CAGAATTGCCATTGCACA-3′; TNF-α, forward,
5′-GAACTGGCAGAAGAGGCACT-3′ and reverse, 5′-GGTCTGGGCCATAGAACTGA-3′;
cyclooxygenase (COX)-2, forward, 5′-TCCATTGACCAGAGCAGAGA-3′ and
reverse, 5′-TCTGGACGAGGTTTTTCCAC-3′; inducible nitric oxide
synthase (iNOS), forward, 5′-CACCTTGGAGTTCACCCAGT-3′ and reverse,
5′-ACCACTCGTACTTGGGATGC-3′, GAPDH, forward,
5′-GGCATTGCTCTCAATGACAA-3′ and reverse, 5′-TGTGAGGGAGATGCTCAGTG-3′.
Relative gene expression was quantified using the 2−ΔΔCq
method (18).
Western blotting
MG-63 cells were cultured to 80–90% confluence
monolayers in 60 cm2 petri dishes, washed twice in PBS
and once in serum free α-MEM, then cultured with α-MEM (without
serum) for 24 h.
Following incubation, MG-63 cells were washed with
PBS three times prior to addition of the lysis buffer (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China).
Protein concentrations of the cell lysates were measured using a
BCA assay (Thermo Fisher Scientific, Inc.), with bovine serum
albumin (Thermo Fisher Scientific, Inc.) acting as a standard. The
sample buffer was added to cell lysates and boiled for 3 min.
Samples (2 µg/lane) were loaded on 10% SDS-PAGE and blotted onto
PVDF membranes, which were blocked with 5% skim milk for 2 h at
room temperature. Membranes were subsequently incubated with
primary antibodies (all Cell Signaling Technology Inc., Danvers,
MA, USA) against TNF-α (1:1,000; cat. no. 3707), IL-1β (1:1,000;
cat. no. 12703), IL-6 (1:1,000; cat. no. 12153), p-p38 (1:1,000;
cat. no. 1170), p-ERK (1:1,000; cat. no. 4370), p-JNK (1:1,000;
cat. no. 4668), and β-actin (1:5,000; cat no. 4970) overnight at
4°C, washed 3 times for 10 min/wash in TBST and then incubated with
the anti-rabbit immunoglobulin G, horseradish peroxidase-linked
Antibody (1:5,000; cat. no. 7074; Cell Signaling Technology Inc.),
for 1 h at room temperature. Membranes were then washed with TBST
and developed using a chemiluminence detection kit (cat. no. 6883;
Cell Signaling Technology Inc.), in accordance with the
manufacturer's protocol.
Statistical analysis
All experiments were performed ≥3 times and results
were expressed as the mean ± standard deviation. Comparisons
between two groups were analyzed using the Student's t-test and
comparisons among multiple groups were assessed using one-way
analysis of variance followed by the Bonferroni post-hoc test.
P<0.05 was determined to indicate a statistically significant
difference.
Results
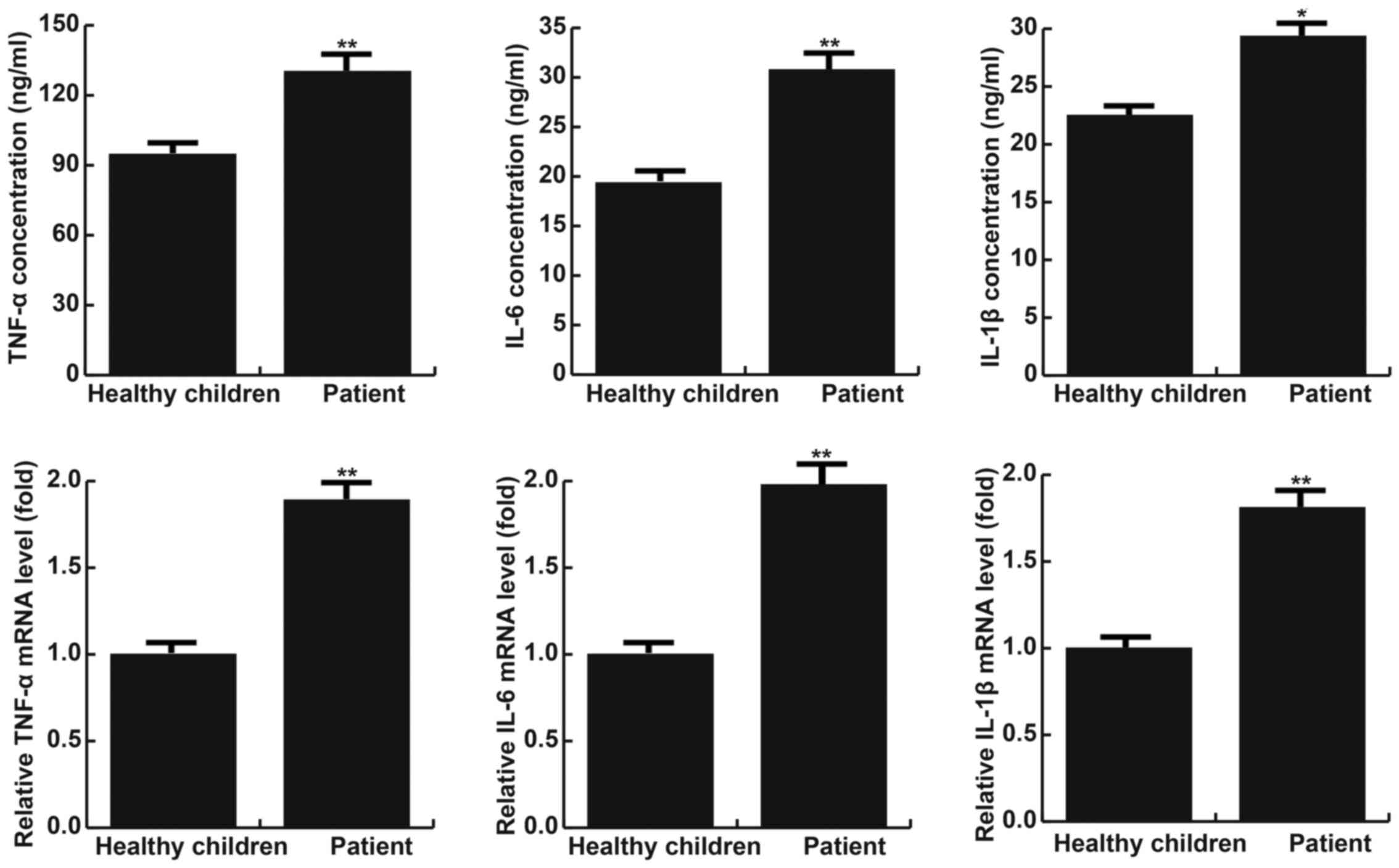
TNF-α, IL-1β and IL-6 levels are
higher in children with fractured ankles than in healthy
children
The results of RT-qPCR indicated that levels of
TNF-α, IL-1β and IL-6 mRNA were significantly increased in children
with ankle fractures compared with healthy children (all P<0.05;
Fig. 1). Furthermore, levels TNF-α,
IL-1β and IL-6 protein were measured using ELISA and it was
determined that they were significantly increased in patients with
ankle fractures compared with healthy children (all P<0.01;
Fig. 1).
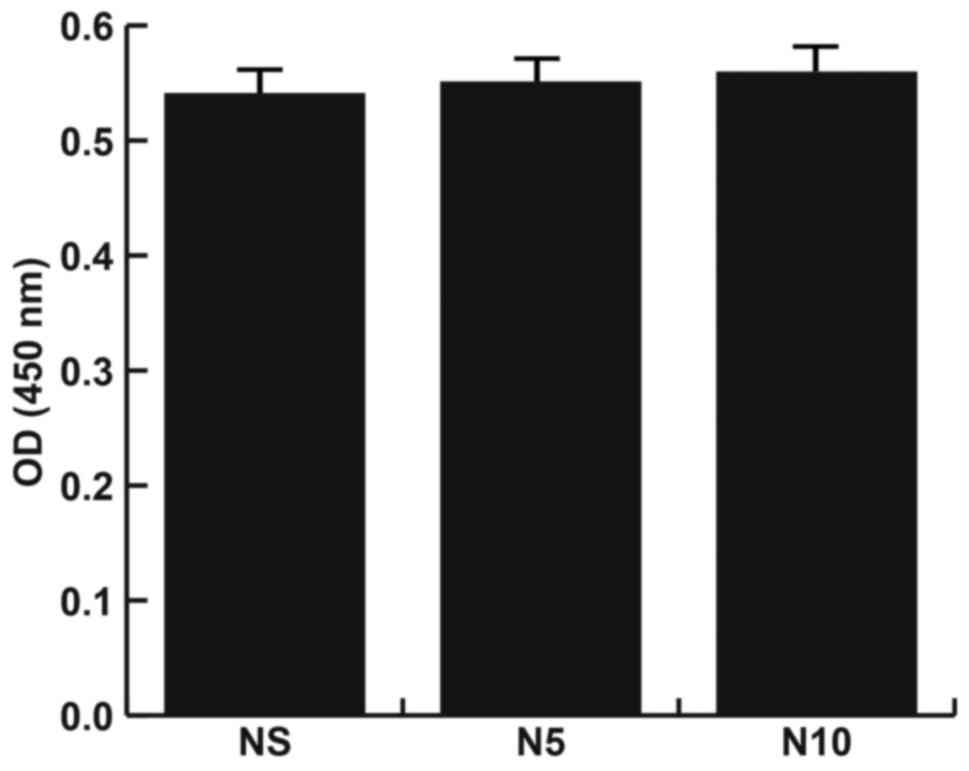
MG-63 cells treated with NMP do not
exhibit cytotoxicity
Cells were divided into 3 different groups: A
control group, a 5 nM NMP treatment group and a 10 mM NMP treatment
group. Cell viability in each group was analyzed. The results
demonstrated that there were no significant changes in absorbance
among the 3 different groups (Fig.
2), indicating that NMP does not induce cytotoxicity in MG-63
cells.
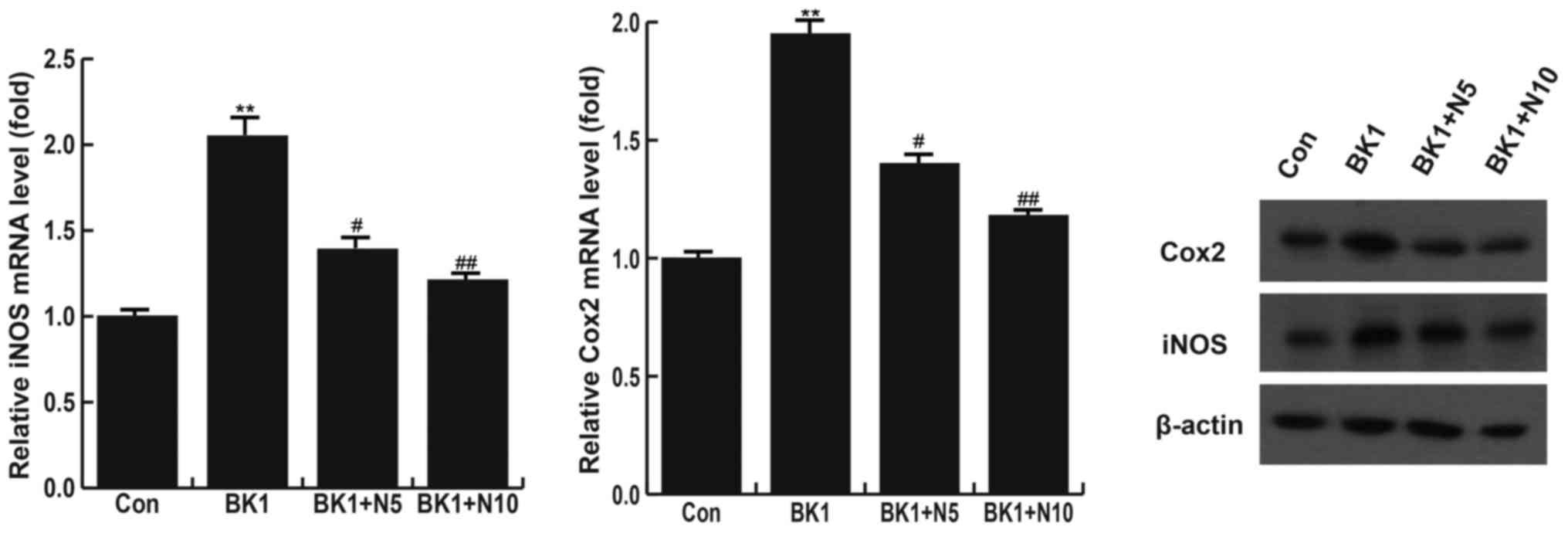
NMP significantly inhibits the
BK-induced increase of COX-2 and iNOS
Cells were divided into 4 different groups: A
control group, a 1 µM BK induced group, a 1 µM BK + 5 mM NMP group
and a 1 µM BK + 10 mM NMP group. The results indicated that,
compared with the control group, levels of COX-2 and iNOS mRNA were
significantly increased in the BK group (P<0.01). This increase
in COX-2 and iNOS levels was significantly reversed following
treatment with 5 mM (P<0.05) and 10 mM NMP (P<0.01; Fig. 3). Levels of COX-2 and iNOS proteins
were also assessed using western blotting and the results were
consistent with those of RT-qPCR (Fig.
3).
NMP treatment significantly inhibits
the BK-induced increase of TNF-α, IL-1β and IL-6
In the BK group, levels of TNF-α, IL-1β and IL-6
mRNA were significantly higher compared with the control group
(P<0.01); however, these increases were attenuated following
treatment with 10 nM NMP (P<0.05; Fig. 4). Treatment with 5 nM NMP did not
significantly reverse the increase in TNF-α and IL-1β mRNA
expression but did significantly reverse the increase in IL-6 mRNA
expression induced by BK (P<0.05; Fig. 4). The results of western blotting
demonstrated that changes in the levels of TNF-α, IL-1β and IL-6
protein were in line with the changes in mRNA levels (Fig. 4).
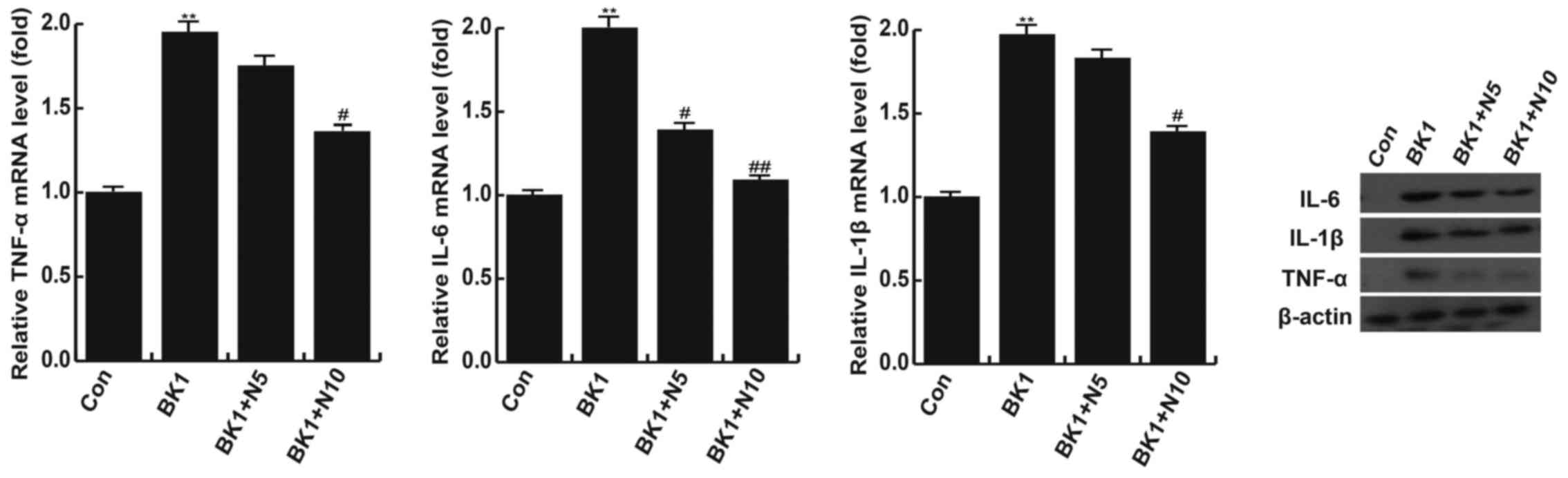 | Figure 4.NMP significantly inhibits the
BK-induced increase of TNF-α, IL-1β and IL-6. Following stimulation
with BK, levels of TNF-α, IL-1β and IL-6 mRNA were significantly
higher compared with the control group. mRNA expression of TNF-α,
IL-1β and IL-6 were inhibited following treatment with NMP. Changes
in the expression of TNF-α, IL-1β and IL-6 protein were similar to
those of mRNA. **P<0.01 vs. Con; #P<0.05 and
##P<0.01 vs. BK1. Con, control group; BK1, 1 µM BK
treatment group; BK1 + N5, 1 µM BK + 5 mM NMP treatment group; BK1
+ N10, 1 µM BK + 10 mM NMP treatment group. BK, bradykinin; NMP,
N-methyl pyrrolidone; TNF-α, tumor necrosis factor α; IL-1β,
interleukin 1β; IL-6, interleukin 6. |
NMP markedly inhibits the BK-induced
overactivation of JNK and p38
Compared with the control group, the expression of
phosphorylated (p)-extracellular signal-regulated kinase (ERK),
p-JNK and p-p38 were all markedly upregulated in the BK group.
Treatment with 10 nM NMP markedly inhibited the BK-induced
upregulation of p-JNK and p-p38 expression. However, it did not
decrease the expression of p-ERK (Fig.
5).
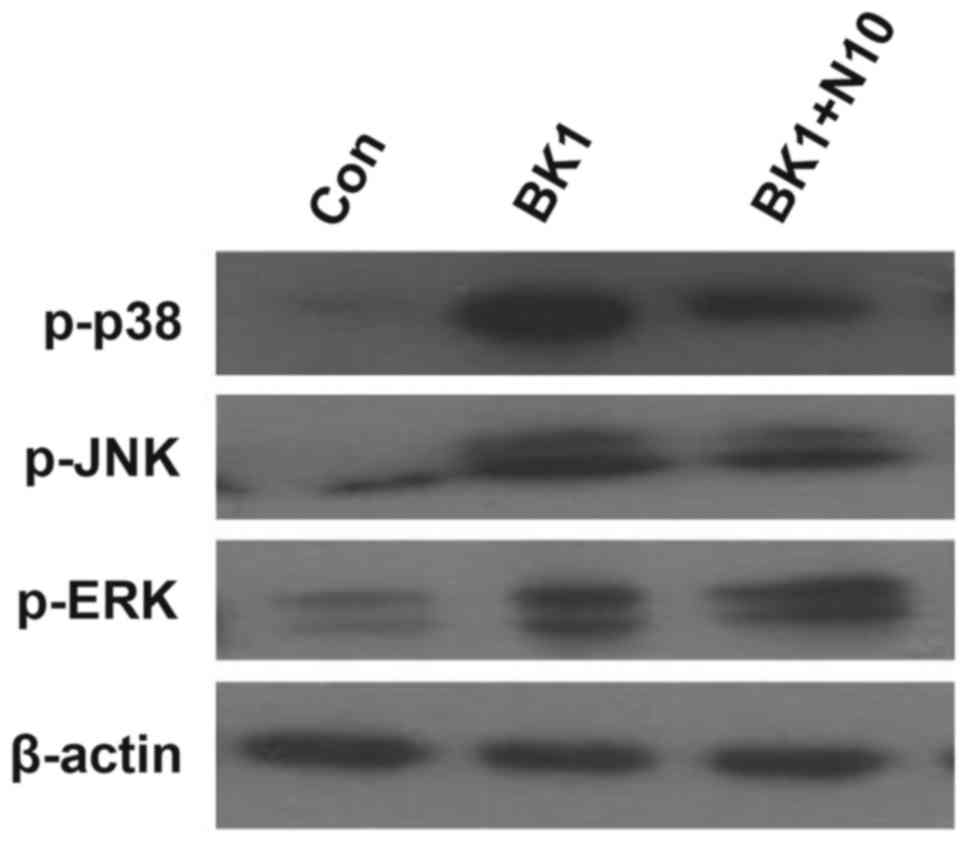 | Figure 5.NMP significantly inhibits the
BK-induced overactivation of JNK and p38. BK induced the
upregulation of p-ERK, p-JNK and p-p38. NMP treatment markedly
inhibited the BK-induced upregulation of p-JNK and p-p38; however,
NMP treatment induced no significant effect on p-ERK. Con, control
group; BK1, 1 µM BK treatment group; BK1 + N5, 1 µM BK + 5 mM NMP
treatment group; BK1 + N10, 1 µM BK + 10 mM NMP treatment group;
p-, phosphorylated; NMP, N-methyl pyrrolidone; BK, bradykinin; JNK,
c-Jun NH2-terminal kinase; ERK, extracellular signal-related
kinase. |
Discussion
Taken together, the results of the present study
indicate that, compared with healthy children, levels of TNF-α,
IL-1β and IL-6 were upregulated in children with ankle fracture.
NMP dose-dependently reversed the BK-induced upregulation of TNF-α,
IL-1β, IL-6, iNOS and COX-2 mRNA and protein, and also inhibited
the BK-induced activation of JNK and p38 pathways in MG-63 cells.
The anti-inflammatory function of NMP may reduce osteoclast and
increase osteoblast levels, thus promoting bone formation.
Therefore, the current study identified a potential novel method of
treating ankle fracture.
It has been demonstrated that persistent
inflammation directly affects osteogenesis in trauma-induced
fracture; however, the precise molecular mechanism of action
remains unclear and further research into the association between
inflammation and osteoblasts/osteoclasts is required (19). A normal physiological inflammatory
response is a type of defensive reaction, which is beneficial to
fracture healing; however, the long-term chronic inflammatory
response caused by infection (20)
or other elements, including diabetes mellitus, smoking and
alcohol, (21) inhibits fracture
healing. It has been reported that TNF-α expression is
significantly higher in the blood of patients with rheumatoid
arthritis (22). Furthermore,
multiple inflammatory factors including TNF-α, IL-1β and IL-6
stimulate the differentiation and formation of osteoclasts
(23–26). Therefore, the current study
investigated whether TNF-α, IL-1β and IL-6 levels differed between
healthy children and those with ankle fracture. The results of
RT-qPCR and western blotting demonstrated that levels of
inflammatory factors were all significantly higher in children with
ankle fracture than in healthy children. The current study aimed to
identify novel effective methods of treating ankle fracture.
The human osteoblastic osteosarcoma cell line MG-63
was used in the present study. BK was reported to stimulate bone
resorption in rats (11); thus 1 µM
BK was used to generate an inflammatory model in the present study.
Previous studies demonstrated that NMP enhances bone formation and
inhibits osteoclast differentiation (15,16).
Therefore, the present study investigated the effects of NMP on the
BK-stimulated inflammatory response in MG-63 cells.
The current study investigated whether NMP induced
cytotoxicity in MG-63 cells. Cell viability was evaluated and the
results demonstrated that NMP did not induce cytotoxicity in MG-63
cells. Therefore, two different concentrations of NMP were used to
assess the effect of NMP on BK-induced inflammation in MG-63
cells.
COX-2 is an enzyme encoded by the prostaglandin
endoperoxide synthase 2 gene (27).
In the majority of cells, COX-2 is not expressed or underexpressed
in normal physiological conditions but overexpressed in
inflammatory conditions (28).
iNOS is calcium-insensitive and the gene that codes
for iNOS is located on chromosome 17 (29). The activation of the NF-κB-dependent
iNOS promoter supports the inflammation-mediated stimulation of
iNOS transcription (30).
Furthermore, iNOS produces large quantities of NO following
stimulation by IL-1 and TNF-α (31).
It has been reported that BK stimulates the
expression of COX-2 mRNA in calvarial bones (32). LPS may also induce the expression of
iNOS and COX-2 in RAW264.7 macrophages (17). Therefore, the current study evaluated
whether BK induces the upregulation of iNOS and COX-2, and whether
NMP significantly reverses BK-induced upregulation in MG-63 cells.
Compared with the control group, BK significantly increased levels
of COX-2 and iNOS mRNA and protein, which were significantly
inhibited by treatment with 10 nM NMP.
A link between osteoclast levels and inflammatory
cytokines, including TNF-α and IL-1, has been identified (7). BK stimulates bone resorption and
potentiates the bone resorptivity of IL-1 (11). Additionally, RANKL participates in
bone resorption (12,13) and activates TRAFs (12–14).
Therefore, the current study aimed to identify the effect of NMP on
the expression of inflammatory cytokines. The results demonstrated
that BK significantly increased levels of TNF-α, IL-1β and IL-6
mRNA and protein compared with the control group. These increases
were reversed by treatment with 10 nM NMP.
Previous studies have suggested that NMP may enhance
bone formation and inhibit osteoclast differentiation (15,16).
However, the molecular mechanism by which NMP exerts its
anti-inflammatory function in MG-63 cells remains unresolved.
p38 and JNK, but not ERK, are involved in important
signal-transducing pathways in the interactions between kinins and
IL-1β that lead to the upregulation of COX-2 expression (32). Western blotting was performed to
determine the activation levels of ERK, JNK and p38. The results
indicated that, compared with the control group, the expression of
p-ERK, p-JNK and p-p38 were all significantly upregulated following
induction with BK. Furthermore, NMP treatment significantly
inhibited the BK-induced upregulation of p-JNK and p-p38, but not
p-ERK.
Taken together, these results indicate that NMP
represses inflammation in MG-63 cells that is induced following
activation of the JNK and p38 pathways. The anti-inflammatory
function of NMP may therefore promote the healing of ankle fracture
by enhancing bone formation. Thus, it may be utilized in
conjugation with other established methods of fracture treatment in
the future; however, the clinical effects of NMP on children with
ankle fracture remains unclear.
Ankle injury is a common pediatric injury that
causes persistent pain, movement limitation, swelling and stiffness
(33). Timely diagnosis, treatment
and nursing care are very important for the reduction of pain and
the healing of fractures. The Ottawa Ankle Rules (OAR) (34–36) that
were developed by Ottawa emergency physicians were adopted to
assist in determining if radiography should be used to determine
whether radiography would be appropriate to diagnose ankle fracture
in a patient experiencing ankle pain. It has been demonstrated that
when pediatric emergency department (ED) nurses accurately apply
and interpret OAR, children in the hospital ED were treated with a
nursing collaborative practice protocol (CPP) to minimize
throughput time and expedite patient care (37). This suggests that CPP may be a novel
treatment in children with fractured ankles and NMP may be used as
a part of CPP. However, the clinical application of NMP still
requires further research to confirm this proposition. Future
studies should be undertaken to investigate the effect of NMP on
fractures and novel therapeutic strategies, potentially including
CPP, for the treatment of children with ankle fractures.
References
|
1
|
MacIntyre NJ and Dewan N: Epidemiology of
distal radius fractures and factors predicting risk and prognosis.
J Hand Ther. 29:136–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moon RJ, Harvey NC, Curtis EM, de Vries F,
van Staa T and Cooper C: Ethnic and geographic variations in the
epidemiology of childhood fractures in the United Kingdom. Bone.
85:9–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hedström EM, Svensson O, Bergström U and
Michno P: Epidemiology of fractures in children and adolescents.
Acta Orthop. 81:148–153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morris MWJ and Bell MJ: The
socio-economical impact of paediatric fracture clinic appointments.
Injury. 37:395–397. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chevalley T, Bonjour JP, van Rietbergen B,
Rizzoli R and Ferrari S: Fractures in healthy females followed from
childhood to early adulthood are associated with later menarcheal
age and with impaired bone microstructure at peak bone mass. J Clin
Endocrinol Metab. 97:4174–4181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He Z, Selvamurugan N, Warshaw J and
Partridge NC: Pulsed electromagnetic fields inhibit human
osteoclast formation and gene expression via osteoblasts. Bone.
106:194–203. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang H, Zhao N, Xu X, Xu Y, Li S, Zhang J
and Yang P: Dose-specific effects of tumor necrosis factor alpha on
osteogenic differentiation of mesenchymal stem cells. Cell Prolif.
44:420–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Selwyn BM, Figueroa CD, Fink E, Swan A,
Dieppe PA and Bhoola KD: A tissue kallikrein in the synovial fluid
of patients with rheumatoid arthritis. Ann Rheum Dis. 48:128–133.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lerner UH, Jones IL and Gustavsson GT:
Bradykinin, a new potential mediator of inflammation-induced bone
resorption: Studies of the effects on mouse calvarial bones and
articular cartilage in vitro. Arthritis Rheum. 30:530–540. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lerner UH and Lundberg P: Kinins and
neuro-osteogenic factors. Bilezikian JP, Raisz LG and Rodan GA:
Principles of bone biology. 2nd. San Diego: Academic Press; pp.
773–799. 2002
|
|
11
|
Lerner UH: Bradykinin synergistically
potentiates interleukin-1 induced bone resorption and prostanoid
biosynthesis in neonatal mouse calvarial bones. Biochem Biophys Res
Commun. 175:775–783. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Teitelbaum SL and Ross FP: Genetic
regulation of osteoclast development and function. Nat Rev Genet.
4:638–649. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lerner UH: New molecules in the tumor
necrosis factor ligand and receptor superfamilies with importance
for physiological and pathological bone resorption. Crit Rev Oral
Biol Med. 15:64–81. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ghayor C, Correro RM, Lange K,
Karfeld-Sulzer LS, Grätz KW and Weber FE: Inhibition of osteoclast
differentiation and bone resorption by N-methylpyrrolidone. J Biol
Chem. 286:24458–24466. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miguel BS, Ghayor C, Ehrbar M, Jung RE,
Zwahlen RA, Hortschansky P, Schmoekel HG and Weber FE: N-methyl
pyrrolidone as a potent bone morphogenetic protein enhancer for
bone tissue regeneration. Tissue Eng Part A. 15:2955–2963. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ghayor C, Gjoksi B, Siegenthaler B and
Weber FE: N-methyl pyrrolidone (NMP) inhibits
lipopolysaccharide-induced inflammation by suppressing NF-kB
signaling. Inflamm Res. 64:527–536. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Auer J, Berent B, Weber T and Eber B:
Clinical significance of pleiotropic effects of statins: Lipid
reduction and beyond. Curr Med Chem. 9:1831–1850. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baud V and Karin M: Signal transduction by
tumor necrosis factor and its relatives. Trends Cell Biol.
11:372–377. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gaston MS and Simpson AH: Inhibition of
fracture healing. J Bone Joint Surg Br. 89:1553–1560. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bauer DC: HMG CoA reductase inhibitors and
the skeleton: A comprehensive review. Osteoporos Int. 14:273–282.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bellosta S, Ferri N, Bernini F, Paoletti R
and Corsini A: Non-liPid-related effects of statins. Ann Med.
32:164–176. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Casey PJ and Seabra MC: Protein
prenyltransferases. J Biol Chem. 271:5289–5292. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Celeste AJ, Iannazzi JA, Taylor RC, Hewick
RM, Rosen V, Wang EA and Wozney JM: Identification of transforming
growth factor beta family members present in bone-inductive protein
purified from bovine bone. Proc Natl Acad Sci USA. 87:pp.
9843–9847. 1990; View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen G and Goeddel DV: TNF-R1 signaling: A
beautiful pathway. Science. 296:1634–1635. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hla T and Neilson K: Human
cyclooxygenase-2 cDNA. Proc Natl Acad Sci USA. 89:pp. 7384–7388.
1992; View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kurumbail RG, Kiefer JR and Marnett LJ:
Cyclooxygenase enzymes: Catalysis and inhibition. Curr Opin Struct
Biol. 11:752–760. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Knowles RG and Moncada S: Nitric oxide
synthases in mammals. Biochem J. 298:249–258. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Calegari-Silva TC, Pereira RM, De-Melo LD,
Saraiva EM, Soares DC, Bellio M and Lopes UG: NF-kappaB-mediated
repression of iNOS expression in Leishmania amazonensis macrophage
infection. Immunol Lett. 127:19–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Green SJ, Scheller LF, Marletta MA, Seguin
MC, Klotz FW, Slayter M, Nelson BJ and Nacy CA: Nitric oxide:
Cytokine-regulation of nitric oxide in host resistance to
intracellular pathogens. Immunol Lett. 43:87–94. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brechter AB and Lerner UH: Bradykinin
potentiates cytokine-induced prostaglandin biosynthesis in
osteoblasts by enhanced expression of cyclooxygenase 2, resulting
in increased RANKL expression. Arthritis Rheum. 56:910–923. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zwipp Z, Hoffmann R, Thermann H and
Wippermann BW: Rupture of the ankle ligaments. Int Orthop.
15:245–249. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stiell IG, Greenberg GH, McKnight RD, Nair
RC, McDowell I and Worthington JR: A study to develop clinical
decision rules for the use of radiography in acute ankle injuries.
Ann Emerg Med. 21:384–390. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stiell IG, McKnight RD, Greenberg GH,
McDowell I, Nair RC, Wells GA, Johns C and Worthington JR:
Implementation of the Ottawa ankle rules. JAMA. 271:827–832. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stiell IG, Greenberg GH, McKnight RD, Nair
RC, McDowell I, Reardon M, Stewart JP and Maloney J: Decision rules
for the use of radiography in acute ankle injuries. Refinement and
prospective validation. JAMA. 269:1127–1132. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Karpas A, Hennes H and Walsh-Kelly CM:
Utilization of the Ottawa ankle rules by nurses in a pediatric
emergency department. Acad Emerg Med. 9:130–133. 2002. View Article : Google Scholar : PubMed/NCBI
|