Introduction
Nasopharyngeal carcinoma (NPC) is endemic in
Southern China (1). It was the
eighth most common type of cancer in 2010, and accounted for 3.5%
of all new cancer cases worldwide (2). Genetic susceptibility and Epstein-Barr
virus infection are important etiological factors in NPC (3). For decades, the primary treatment
strategies used for NPC were chemotherapy and radiotherapy
(4,5). Recently, the platinum doublet of
fluorouracil plus cisplatin reigned as a first-line treatment
strategy for chemotherapy (2).
However, its efficacy remains controversial. Radiotherapy was the
mainstay treatment for NPC due to its relatively high
radiosensitivity and deep-seated anatomical position, which made
surgical resection challenging (6).
In addition, great technological advances have been made in
radiotherapy, including three-dimensional conformal radiotherapy
and intension-modulated radiotherapy (7,8).
However, due to irradiation resistance, some patients with NPC
present with metastases following radiotherapy (5). Hence, enhancing the radiosensitivity of
NPC may provide a novel treatment strategy for NPC.
Slug is a conserved zinc finger transcription
factor, which belongs to the Snail family and presents an
anti-apoptotic effect by regulating the transactivation of p53
upregulated modulator of apoptosis (PUMA) and the expression of B
cell lymphoma-2 (Bcl-2) and Bcl-2-associated X protein (9,10). It
has been demonstrated that Slug-deficient cells are radiosensitive
to DNA damage (11,12). Slug inhibition also increases the
radiosensitivity of HSC3 and HSC6 cells by upregulating PUMA in
oral squamous carcinoma (13). In
addition, Slug expression inhibits calcitriol-mediated sensitivity
to radiation in colorectal cancer (14) and upregulates radiation-induced PUMA
in cholangiocarcinoma (15).
However, whether Slug inhibition may enhance radiosensitivity of
NPC remains unknown.
Radio-gene therapy has been developed as a novel
strategy, which combines traditional radiotherapy with gene therapy
(16,17). Due to radiation resistance,
radiotherapy is sometimes ineffective, resulting in worse side
effects (18). Therefore, it is
necessary to understand the radiosensitive tumor targets and
mechanisms underlying the development of radioresistance.
Thus, the aim of the present study was to
investigate whether Slug inhibition may increase the
radiosensitivity of NPC cell line C666-1. Following combined
treatment of lentivirus-mediated Slug RNA interference (RNAi)
transfection with X-ray irradiation, the expression of Slug was
decreased, which resulted in increased irradiation (IR)-induced
G0/G1 arrest and cell apoptosis of C666-1.
These findings may offer novel insights into radiotherapy with gene
therapy in the treatment of NPC.
Materials and methods
Cell culture
Human NPC cell line C666-1 was obtained from the
Xiangya Central Experiment Laboratory (Central South University,
Hunan, China). Cells were cultured in RPMI-1640 media (GE
Healthcare Life Sciences, Logan, UT, USA) supplemented with 10%
fetal bovine serum (Hangzhou Sijiqing Biological Engineering
Materials Co., Ltd., Hangzhou, China), 50 U/ml penicillin G and 50
U/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) with 5% CO2 in a humidified atmosphere at 37°C.
The cells were passaged every 3 days.
Irradiation procedure
Irradiation was performed with a single dose of
X-rays ranging from 1–8 Gy using a linear accelerator (Trilogy,
Austin, TX, USA) with 6 MV photons/100 cm focus-surface distance at
room temperature. The dose rate was 4.0 Gy/min, which was
determined by Fricke's chemical dosimeter (19).
Construction of lentiviral
vectors
In order to produce lentivirus expressing RNAi
specific for the Slug gene, the RNAi sequence for human Slug
(GGAATATGTGAGCCTGGGC GCC) was identified using the BLOCK-iT RNAi
Designer program (Invitrogen; Thermo Fisher Scientific, Inc.) and
the negative control construct (control RNAi) was created using a
scrambled sequence (GAACCGTGTCTTCCTCAGTATC). The two sequences were
annealed and cloned into the AgeI and EcoRI enzyme sites of
pGCSIL-GFP vector (500 ng/µl; Shanghai Genechem Co., Ltd.,
Shanghai, China), respectively. Following confirmation of the
constructed plasmids by DNA sequencing, lentiviral vector DNA and
packaging vectors (1 µg/ml; pHelper 1.0 and pHelper 2.0; Shanghai
Genechem Co., Ltd.) were then transfected into 293T cells (Sangon
Biotech, Co., Ltd., Shanghai, China) using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). Following 48 h of
culture, supernatants containing lentiviruses, including
pGCSIL-Slug-shRNA-LV and pGCSIL-neg-shRNA-LV were harvested,
respectively. Purification was then performed at 1,000 × g and 4°C
for 2 min using ultracentrifugation (Himac CT15RE; Hitachi, Tokyo,
Japan) and the titer of lentivirus was determined. The lentiviruses
were stored for 1 week in −80°C and prepared for infection.
Infection of lentivirus
C666-1 cells were cultured in 60-mm dishes with
5×105 cells in each plate. Cells were cultured in
complete medium with lentiviruses at a multiplicity of infection of
10 for 24 h at 37°C. Fresh culture medium was then used to
substitute the old media. Total protein was isolated and the
expression of Slug was detected by western blotting. Three groups
of C666-1 cells were used in subsequent assays: CON group, a blank
control group with no infection; NC group, a negative control group
infected with pGCSIL-neg-shRNA-LV; and KD group, a Slug RNAi group
infected with pGCSIL-Slug-shRNA-LV.
Western blot analysis
Cell extracts were prepared in
radioimmunoprecipitation (RIPA) assay buffer (150 mM NaCl, 0.1%
SDS, 0.5% sodium deoxycholate, 1% Nonidet P-40 and 50 mM Tris, pH
8.0), with the addition of 2 mM phenylmethylsulfonyl fluoride. RIPA
lysis buffer was freshly prepared and added to infected cells in
6-well plates (100 µl/well) on ice, which were then incubated for
10 min. Protein concentrations were determined by BCA method with a
protein assay kit. Equal amounts of proteins (10 µg/condition) were
boiled for 10 min in loading buffer (Thermo Fisher Scientific,
Inc.) prior to being separated on 15% SDS-PAGE. Separated proteins
were then transferred to polyvinylidene difluoride membranes at 100
V for 1 h prior to membrane blocking in PBS with 5% skim milk
powder and Tween-20 at room temperature for 2 h. Membranes were
then incubated with anti-Slug primary antibody (cat. no. 9585;
1:5,000) and anti-GAPDH primary antibody (cat. no. 2118L; 1:3,000)
(both from Cell Signaling Technology, Inc., Danvers, MA, USA) at
4°C overnight. Goat anti-rabbit horse radish peroxidase-conjugated
secondary antibodies (cat. no. ab205718; Abcam, Cambridge, UK) were
diluted 1:1,000 with PBST buffer and membranes were incubated for
60 min at room temperature. Membranes were washed three times using
PBST prior to each step. Protein bands were visualized using an
enhanced chemiluminescence system (Pierce; Thermo Fisher
Scientific, Inc.). The images were captured and analyzed using
ImageJ software 1.51e (National Institutes of Health, Bethesda, MD,
USA).
Clonogenic cell survival
Cells (5×105) were seeded in culture
dishes with Dulbecco's modified Eagle's medium (10% fetal bovine
serum; Thermo Fisher Scientific, Inc.) and irradiated the next day
at the 4 Gy dose according to the reference (20). A total of 24 h following irradiation,
cells were trypsinized, plated in 60-mm dishes and incubated for 14
days at 37°C to allow colony growth. Colonies were fixed with 10%
methanol for 8 min stained with Giemsa for 5–10 min at room
temperature and colonies containing >50 cells were counted using
a light microscope and ×200 magnification. The survival fraction
(SF) was calculated as: [(Mean colonies counted)/(cells plated) ×
(plating efficiency)]; plating efficiency was defined as: [(Mean
colonies counted)/(cells plated)] for unirradiated controls (0 Gy).
The data were fit into the multi-target single-hit mathematical
model (21) and survival curves for
the three groups were created. Radiobiological parameters,
including the SF at 2 Gy (SF2), mean lethal dose (D0),
quasi-threshold dose (Dq) and the extrapolate number (N) were
calculated according to the survival curves.
Cell cycle analysis
A total of 24 h following irradiation with 4 Gy
X-rays, cells were washed twice with PBS and harvested by
trypsinization. Cells were then fixed with 70% ethanol at −20°C and
resuspended in 20 µg/ml propidium iodide (PI; Abcam) for 30 min at
4°C. DNA content was detected using a flow cytometer (FACSCalibur™;
BD Biosciences, Franklin Lakes, NJ, USA). The relative proportion
of cells in the individual cell cycle phase fraction was determined
by the flow cytometry data using FCSExpress 3.0 (De Novo Software,
Glendale, CA, USA).
Apoptosis assay
A total of 24 h following exposure to 4 Gy X-rays,
cells were stained with Annexin V-allphycocyanin (APC)/PI (Abcam)
for 15 min at room temperature to measure cell apoptosis. A total
of 1.0×106 cells were washed twice with ice-cold PBS and incubated
for 30 min in binding buffer at room temperature.
Fluorescence-activated cell sorting analysis for Annexin V-APC/PI
staining was performed by flow cytometry (FACSCalibur™; BD
Biosciences) with FCS Express 3.0 software. Cells that stained
positive for Annexin V and negative for PI were undergoing
apoptosis. Cells that stained positive for Annexin V and PI were
either in the end of stage of apoptosis, undergoing necrosis or
were already dead. Cells that stained negative for Annexin V and PI
were alive and not undergoing measurable apoptosis.
Statistical analysis
All experiments were performed in triplicate and
data were expressed as the mean ± standard deviation. Statistically
significant differences between groups were determined using one
way analysis of variance followed by the Tukey's post hoc multiple
comparison test using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference. The survival curve was drawn using Sigma Plot 12.0
(Systat Software, Inc., San Jose, CA, USA).
Results
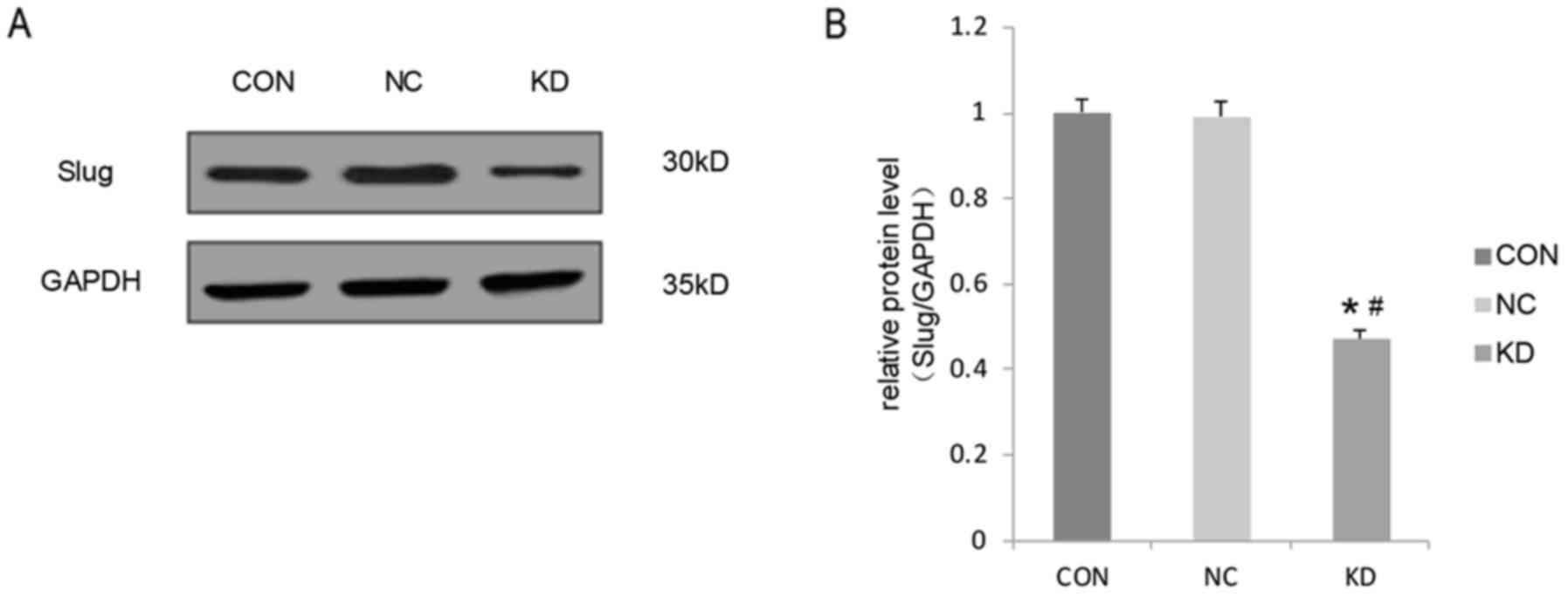
Slug is downregulated by
lentivirus-mediated RNAi in C666-1 cells
A lentivirus vector system derived from HIV-1 was
used to express short hairpin RNA (shRNA) directed against Slug to
downregulate its expression in C666-1 cells. The effect of
lentivirus-mediated RNAi knockdown of Slug on its expression was
determined using western blot analysis. The expression of Slug in
the KD group was significantly decreased compared with that in the
CON and NC groups (Fig. 1). The
difference in Slug expression between the CON group and the NC
group was not significant. Thus, these results confirmed the
downregulation of Slug in the C666-1-transfected cell line.
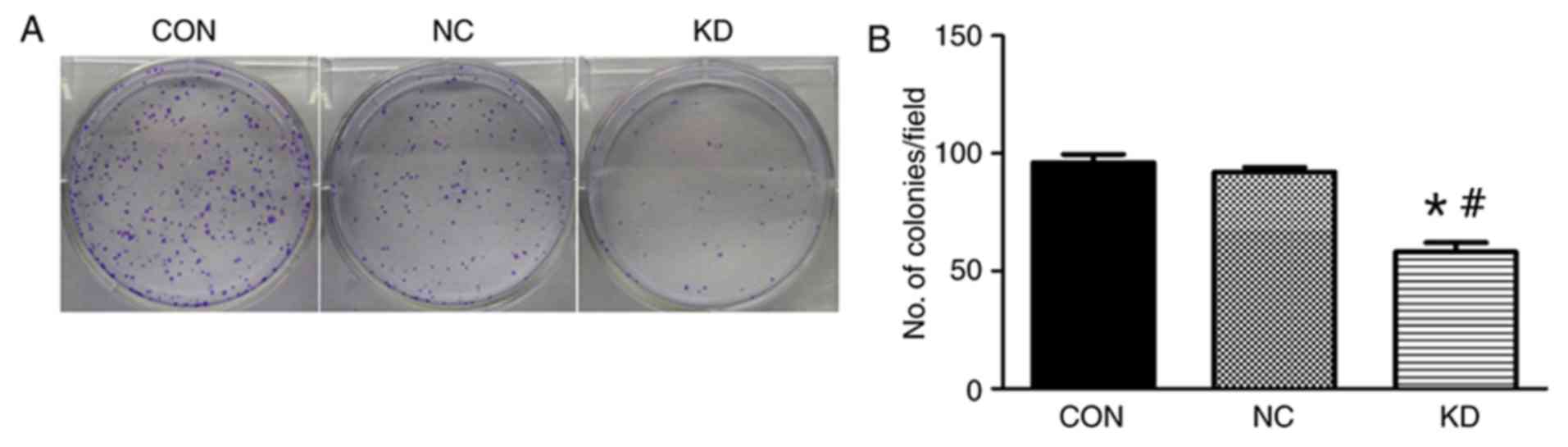
Slug inhibition increases
radiosensitivity and decreases cell survival of C666-1
The effects of Slug inhibition in combination with
X-ray irradiation on C666-1 cell survival was investigated using a
clonogenic survival assay. The results indicated that Slug
inhibition significantly increased cell sensitivity to X-ray
irradiation compared with the level of sensitivity observed in the
NC group and CON group (Fig. 2). The
values of SF2, D0, Dq and N for the KD group were all decreased
significantly compared with those in the CON and NC groups
(Table I). SF2 was reduced to 57.6%
in the KD group from 85.5% in the CON group, and the sensitization
enhancement ratio was 1.48 (data now shown). In addition, the
results indicated that cell colony forming efficiency was decreased
in the KD group compared with that observed in the NC group
following irradiation treatment (Fig.
2). There were few visible colonies that were able to be easily
seen with the naked eye that were irradiated with 4 Gy in the KD
group in comparison with the CON and NC groups (Fig. 2).
 | Table I.Survival curve parameters fitting the
data into a multi-target single-hit model. |
Table I.
Survival curve parameters fitting the
data into a multi-target single-hit model.
|
| Groups |
|---|
|
|
|
|---|
| Parameter | CON | NC | KD |
|---|
| D0 | 2.269 | 2.226 | 1.988a |
| Dq | 2.128 | 2.078 | 1.338b |
| N | 3.724 | 3.596 | 1.916c |
| SF2 | 0.855 | 0.823 | 0.576d |
Effect of Slug inhibition combined
with X-ray irradiation on the C666-1 cell cycle
The impact of Slug inhibition and X-ray irradiation
on the cell cycle was also investigated. Analysis indicated that
Slug inhibition and X-ray irradiation induced a significant
increase in the G0/G1 phase (56.09±1.07% NC
group vs. 72.21±1.38% KD group) and decrease in the S phase in the
proportion of cells (32.13±1.14% NC group vs. 15.17±1.16% KD group)
in the KD group compared with the proportion in the NC group
(Fig. 3). The frequency of cells
with a downregulated expression of Slug in the S phase was
significantly decreased in the KD group compared with that in the
CON group and NC group. These results indicate that Slug inhibition
combined with X-ray irradiation increased the number of cells in
the G0/G1 phase of the cell cycle.
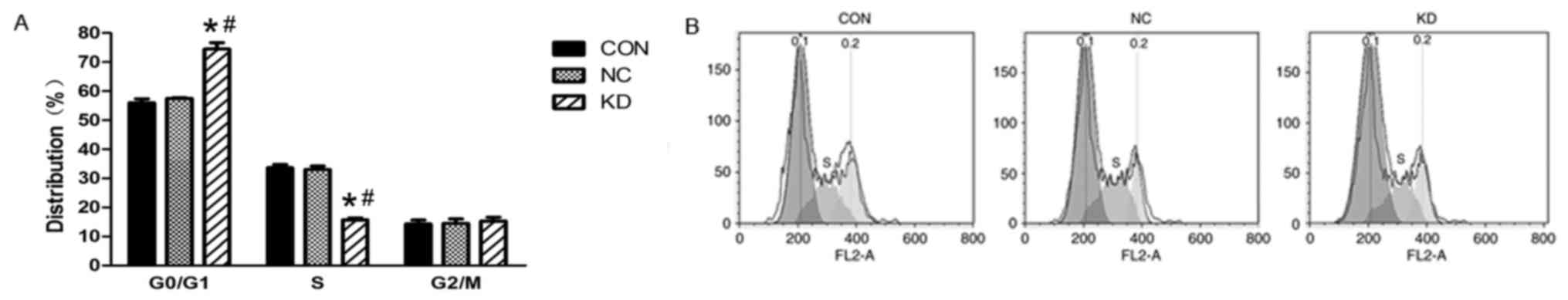 | Figure 3.Changes in the cell cycle following
irradiation at 4 Gy in C666-1 cells treated with Slug RNAi. (A)
Proportion of cells in the G0/G1 and S phases
of the cell cycle in the NC, CON and KD groups. (B) C666-1 cell
cycles were detected using flow cytometry analysis in the different
groups. Values are expressed as the mean ± standard deviation. All
experiments were performed in triplicate. *P<0.05 vs. NC group;
#P<0.05 vs. CON group. RNAi, RNA interference; CON,
control group consisting of untreated C666-1 cells; NC, negative
control consisting of C666-1 cells infected with
pGCSIL-neg-shRNA-LV; KD, Slug RNAi group consisting of C666-1 cells
infected with pGCSIL-Slug-shRNA-LV; G0, resting phase;
G1, gap 1 phase; S, synthesis phase; G2, gap
2 phase; M, mitotic phase. |
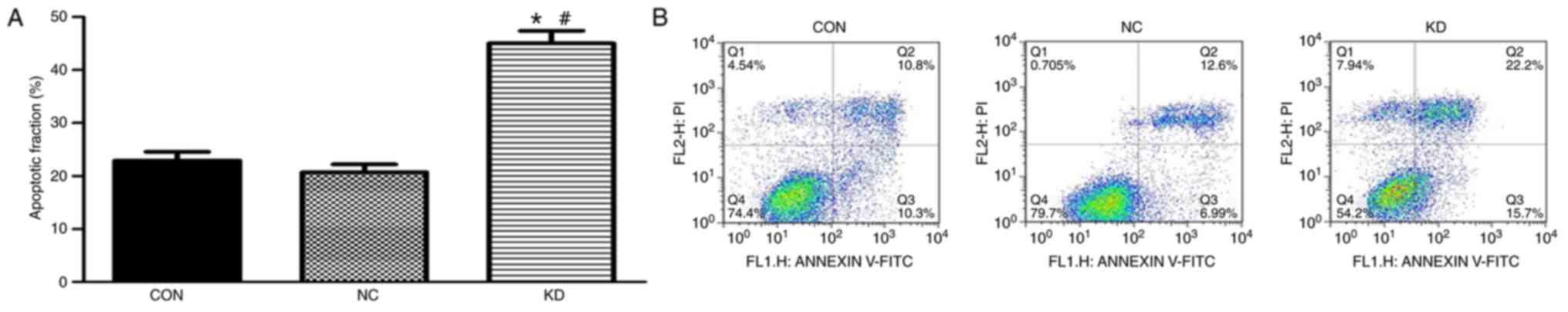
Slug inhibition combined with X-ray
irradiation increases apoptosis of C666-1 cells
The impact of Slug inhibition combined with X-ray
irradiation treatment on C666-1 cell apoptosis was evaluated using
flow cytometry analysis. The percentage of apoptotic cells in the
KD group was significantly increased compared with that in the NC
and CON groups (Fig. 4). The
percentage of apoptotic cells in the CON and NC groups were
22.91±0.41 and 20.61±0.38% respectively, while the percentage of
apoptotic cells in the KD group was 45.12±0.65%. These results
suggest that apoptosis was increased in cells with downregulated
expression of Slug following irradiation compared with that
observed in the control groups.
Discussion
NPC is an epithelial malignancy arising in the head
and neck region of the body (22).
It is prevalent in Southern China with an annual incidence of
20/100,000 people (23). The ‘gold
standard’ of treatment for NPC is radiotherapy, which has a cure
rate of >90% in patients with early-stage NPC (24). However, due to radiation resistance,
the efficacy of radiotherapy is limited, especially in advanced
stages of NPC. Hence, decreasing the radiation resistance and
increasing radiosensitivity is a method that may improve the
efficacy of radiotherapy in patients with NPC.
The Slug protein belongs to the Snail superfamily of
zinc finger transcription factors, which is associated with
embryonic development, regulation of carcinogenesis in various
cancer types and anti-apoptosis (25–29). It
has been demonstrated that Slug may be involved in the
radioresistance of different types of cancer, including colorectal
cancer, cholangiocarcinoma and ovarian cancer (14,15,30,31).
However, there have been few studies on the function of Slug in
association with radioresistance in NPC. Therefore, the present
study investigated the effect of Slug inhibition on the
radiosensitivity of NPC cell line C666-1.
The effect of Slug inhibition on the
radiosensitivity of NPC cell line C666-1 was investigated by
downregulating the expression of Slug by infecting the cells with
Slug-specific RNAi-expressing lentivirus. Following downregulation,
the expression of Slug was significantly decreased. A clonogenic
survival assay was then performed, which suggested that the
downregulation of Slug decreased clonogenic survival. In addition,
Slug inhibition increased the number of cells in the
G0/G1 phase, while the number in the S phase
was decreased. Furthermore, a cell apoptosis assay was applied to
measure cell apoptosis following X-ray irradiation in combination
with Slug downregulation. The results indicated that Slug
inhibition induced cell apoptosis following irradiation. Slug
inhibition may therefore, increase the radiosensitivity of NPC via
the induction of apoptosis and cell cycle arrest.
It has been demonstrated that Slug serves a role in
the radioresistance of several types of cancer (31,32).
However, the mechanisms underlying the radioresistance of cancer
remain unclear. PUMA is a pivotal protein in apoptosis and it has
been suggested that PUMA may increase sensitivity to
radiation-induced apoptosis in different types of cancer (15,33,34).
Slug is a suppressor of PUMA transcription, which inhibits the
expression of PUMA resulting in cell survival (35). Therefore, it may be hypothesized that
Slug, as a radioprotection agent, may serve an important role in
decreasing radiosensitivity by inhibiting the expression of PUMA.
Following the downregulation of Slug, it has been demonstrated that
PUMA increases cell apoptosis and sensitivity to radiation
(13). In future studies, further
investigation of the pathways involved in the development of NPC is
required.
In conclusion, the results of the current study
indicated that Slug may be a potential target of radio-gene therapy
to increase the radiosensitivity of NPC.
Glossary
Abbreviation
Abbreviations:
|
NPC
|
nasopharyngeal carcinoma
|
References
|
1
|
Cancer incidence in five continents.
Volume VIII. IARC Sci Publ. 1–781. 2002.PubMed/NCBI
|
|
2
|
Chua MLK, Wee JTS, Hui EP and Chan ATC:
Nasopharyngeal carcinoma. Lancet. 387:1012–1024. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cui Q, Feng FT, Xu M, Liu WS, Yao YY, Xie
SH, Li XZ, Ye ZL, Feng QS, Chen LZ, et al: Nasopharyngeal carcinoma
risk prediction via salivary detection of host and Epstein-Barr
virus genetic variants. Oncotarget. 8:95066–95074. 2016.PubMed/NCBI
|
|
4
|
Chua ML and Chan AT: Gemcitabine: A game
changer in nasopharyngeal carcinoma. Lancet. 388:1853–1854. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xiao WW, Han F, Lu TX, Chen CY, Huang Y
and Zhao C: Treatment outcomes after radiotherapy alone for
patients with early-stage nasopharyngeal carcinoma. Int J Radiat
Oncol Biol Phys. 74:1070–1076. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brennan B: Nasopharyngeal carcinoma.
Orphanet J Rare Dis. 1:232006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Waldron J, Tin MM, Keller A, Lum C, Japp
B, Sellmann S, van Prooijen M, Gitterman L, Blend R, Payne D, et
al: Limitation of conventional two dimensional radiation therapy
planning in nasopharyngeal carcinoma. Radiother Oncol. 68:153–161.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Uzel EK, Karaçam S, Eliçin O and Uzel O:
Comparison of two different IMRT planning techniques in the
treatment of nasopharyngeal carcinoma. Effect on parotid gland
radiation doses. Strahlenther Onkol. 189:552–558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu T, Fan B, Lv C and Xiao D: Slug
mediates nasopharyngeal carcinoma radioresistance via
downregulation of PUMA in a p53-dependent and -independent manner.
Oncol Rep. 33:2631–2638. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee SH, Kim DY, Jing F, Kim H, Yun CO, Han
DJ and Choi EY: Del-1 overexpression potentiates lung cancer cell
proliferation and invasion. Biochem Biophys Res Commun. 468:92–98.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pérez-Losada J, Sánchez-Martín M,
Pérez-Caro M, Pérez-Mancera PA and Sánchez-García I: The
radioresistance biological function of the SCF/kit signaling
pathway is mediated by the zinc-finger transcription factor Slug.
Oncogene. 22:4205–4211. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pérez-Caro M, Bermejo-Rodríguez C,
González-Herrero I, Sánchez-Beato M, Piris MA and Sánchez-García I:
Transcriptomal profiling of the cellular response to DNA damage
mediated by Slug (Snai2). Br J Cancer. 98:480–488. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang F, Zhou L, Wei C, Zhao W and Yu D:
Slug inhibition increases radiosensitivity of oral squamous cell
carcinoma cells by upregulating PUMA. Int J Oncol. 49:709–719.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Findlay VJ, Moretz RE, Wang C, Vaena SG,
Bandurraga SG, Ashenafi M, Marshall DT, Watson DK and Camp ER: Slug
expression inhibits calcitriol-mediated sensitivity to radiation in
colorectal cancer. Mol Carcinog. 53 Suppl 1:E130–E139. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang K, Zhang B, Lu Y, Sun C, Zhao W,
Jiao X, Hu J, Mu P, Lu H and Zhou C: Slug inhibition upregulates
radiation-induced PUMA activity leading to apoptosis in
cholangiocarcinomas. Med Oncol. 28 Suppl 1:S301–S309. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao Y, Li Z, Sheng W, Miao J and Yang J:
Radiosensitivity by ING4-IL-24 bicistronic adenovirus-mediated gene
cotransfer on human breast cancer cells. Cancer Gene Ther.
20:38–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Finnon P, Kabacik S, MacKay A, Raffy C,
A'Hern R, Owen R, Badie C, Yarnold J and Bouffler S: Correlation of
in vitro lymphocyte radiosensitivity and gene expression
with late normal tissue reactions following curative radiotherapy
for breast cancer. Radiother Oncol. 105:329–336. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Markant SL, Esparza LA, Sun J, Barton KL,
McCoig LM, Grant GA, Crawford JR, Levy ML, Northcott PA, Shih D, et
al: Targeting sonic hedgehog-associated medulloblastoma through
inhibition of Aurora and Polo-like kinases. Cancer Res.
73:6310–6322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meesat R, Sanguanmith S, Meesungnoen J,
Lepage M, Khalil A and Jay-Gerin JP: Utilization of the ferrous
sulfate (Fricke) dosimeter for evaluating the radioprotective
potential of cystamine: Experiment and Monte Carlo simulation.
Radiat Res. 177:813–826. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang SX, Qiu QH, Chen WB, Liang CH and
Huang B: Celecoxib enhances radiosensitivity via induction of G2-M
phase arrest and apoptosis in nasopharyngeal carcinoma. Cell
Physiol Biochem. 33:1484–1497. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Spring E and Holmberg P: Evaluation of
experimental irradiation fractionation with the single-hit,
multi-target model. Acta Radiol Ther Phys Biol. 7:297–306. 1968.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma BB, Hui EP and Chan AT: Systemic
approach to improving treatment outcome in nasopharyngeal
carcinoma: Current and future directions. Cancer Sci. 99:1311–1318.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Razak AR, Siu LL, Liu FF, Ito E,
O'Sullivan B and Chan K: Nasopharyngeal carcinoma: The next
challenges. Eur J Cancer. 46:1967–1978. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chan AT: Current treatment of
nasopharyngeal carcinoma. Eur J Cancer. 47 Suppl 3:S302–S303. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nieto MA: The snail superfamily of
zinc-finger transcription factors. Nat Rev Mol Cell Biol.
3:155–166. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ros MA, Sefton M and Nieto MA: Slug, a
zinc finger gene previously implicated in the early patterning of
the mesoderm and the neural crest, is also involved in chick limb
development. Development. 124:1821–1829. 1997.PubMed/NCBI
|
|
27
|
Shih JY and Yang PC: The EMT regulator
slug and lung carcinogenesis. Carcinogenesis. 32:1299–1304. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu WS, Heinrichs S, Xu D, Garrison SP,
Zambetti GP, Adams JM and Look AT: Slug antagonizes p53-mediated
apoptosis of hematopoietic progenitors by repressing puma. Cell.
123:641–653. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Inukai T, Inoue A, Kurosawa H, Goi K,
Shinjyo T, Ozawa K, Mao M, Inaba T and Look AT: SLUG, a
ces-1-related zinc finger transcription factor gene with
antiapoptotic activity, is a downstream target of the E2A-HLF
oncoprotein. Mol Cell. 4:343–352. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arienti C, Tesei A, Carloni S, Ulivi P,
Romeo A, Ghigi G, Menghi E, Sarnelli A, Parisi E, Silvestrini R and
Zoli W: SLUG silencing increases radiosensitivity of melanoma cells
in vitro. Cell Oncol (Dordr). 36:131–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kurrey NK, Jalgaonkar SP, Joglekar AV,
Ghanate AD, Chaskar PD, Doiphode RY and Bapat SA: Snail and slug
mediate radioresistance and chemoresistance by antagonizing
p53-mediated apoptosis and acquiring a stem-like phenotype in
ovarian cancer cells. Stem Cells. 27:2059–2068. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang P, Liu H, Xia F, Zhang QW, Zhang YY,
Zhao Q, Chao ZH, Jiang ZW and Jiang CC: Epithelial-mesenchymal
transition is necessary for acquired resistance to cisplatin and
increases the metastatic potential of nasopharyngeal carcinoma
cells. Int J Mol Med. 33:151–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuribayashi K, Finnberg N, Jeffers JR,
Zambetti GP and El-Deiry WS: The relative contribution of
pro-apoptotic p53-target genes in the triggering of apoptosis
following DNA damage in vitro and in vivo. Cell Cycle.
10:2380–2389. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang R, Wang X, Li B, Lin F, Dong K, Gao P
and Zhang HZ: Tumor-specific adenovirus-mediated PUMA gene transfer
using the survivin promoter enhances radiosensitivity of breast
cancer cells in vitro and in vivo. Breast Cancer Res Treat.
117:45–54. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim S, Yao J, Suyama K, Qian X, Qian BZ,
Bandyopadhyay S, Loudig O, De Leon-Rodriguez C, Zhou ZN, Segall J,
et al: Slug promotes survival during metastasis through suppression
of Puma-mediated apoptosis. Cancer Res. 74:3695–3706. 2014.
View Article : Google Scholar : PubMed/NCBI
|