Introduction
Moyamoya disease (MMD) is a unique clinical entity
that is characterized by the progressive occlusion of the bilateral
supraclinoid internal carotid artery. Collateral formation at the
base of the brain occurs accordingly to compensate for the
insufficient cerebral perfusion. If new-formed collaterals are not
sufficient to maintain normal cerebral perfusion, ischemic events
occur, particularly in pediatric patients who require more blood
flow for cerebral development and maturation. Excessive hemodynamic
stress in the collateral circulation may also cause hemorrhages,
which mainly occurs in adult patients. The goal of treatment for
MMD is to improve cerebral blood flow; however, medical treatments
appear to be ineffective in preventing ischemic and hemorrhagic
events (1,2). Surgical revascularization represents an
optimal therapeutic option (3).
Surgical procedures for MMD may be basically classified into three
categories: Direct bypass, including superficial temporal
artery-middle cerebral artery (STA-MCA) and occipital artery-MCA,
indirect bypass, including encephalo-duroarterio-synangiosis,
encephalo-myo-synangiosis (EMS),
encephalo-duroarterio-myo-synangiosis and
encephalo-duro-myo-synangiosis (EDMS), and combined bypass.
However, the optimal revascularization strategy for symptomatic
adult moyamoya patients remains controversial (4). Direct bypass offers the advantage of
immediate revascularization, and indirect bypass induces in-growth
of collaterals over time. Combined procedures, owing to the
advantages of direct and indirect bypasses, appear reasonable, but
this requires to be further supported with additional clinical
evidence (5–8). To date, no large randomized clinical
trials have been performed, and the clinical surgical choice is
largely dependent on the preference and training experience of the
cerebrovascular neurosurgeons.
In the present study, 64 adult moyamoya patients (76
hemispheres) who underwent combined STA-MCA bypass and EDMS at
Nanjing Brain Hospital (Nanjing, China) from January 2010 to
December 2015 were reviewed. Clinical outcomes, post-operative
collateral formation and revascularization patterns, cerebral
perfusion and changes in donor vessel caliber were evaluated to
facilitate the choice of surgical procedure for MMD in adults.
Materials and methods
Patients and clinical data
The clinical data of 64 adult moyamoya patients (76
hemispheres) who underwent combined STA-MCA bypass and EDMS at
Nanjing Brain Hospital (Nanjing, China) from January 2010 to
December 2015 were reviewed. The present retrospective study was
approved by the ethics committee of the Brain Hospital affiliated
to Nanjing Medical University (Nanjing, China). Written informed
consent was provided by the patients or their guardians.
All of the patients had typical MMD as specified by
previously published criteria (2).
Patients with the characteristic moyamoya vasculopathy but no
associated risk factors were diagnosed with MMD, whereas those with
generally acknowledged risk factors, including head/neck
radiotherapy (particularly for optic gliomas, craniopharyngiomas or
pituitary tumors), Down's syndrome, neurofibromatosis type 1,
sickle cell anemia, Graves' disease, meningeal infections including
tuberculous meningitis were diagnosed with moyamoya syndrome. The
detailed medical history of all the patients enrolled in the
present study was recorded, based on which all of those patients
with well-recognized risks were excluded. The patient inclusion
criteria were as follows: i) Age, >18 years; ii) clinical
diagnosis of MMDs confirmed by DSA; iii) patients were symptomatic
or had evidence of cerebral hemodynamic compromise based on
computed tomography (CT) perfusion (CTP). Pediatric patients (age,
<18 years) were excluded.
Surgical procedures
The neurosurgeons at our department are experienced
in performing STA-MCA bypass and EDMS operations. After intravenous
anesthesia, the respective patient was placed in the supine
position with the head rotated 60–80° toward the opposite side of
the surgery. The courses of the STA trunk and branches were marked
on the skin, and a frontal-temporal-parietal incision was designed
accordingly. The skin flap was dissected between the galea
aponeurotica and temporal fascia. The temporal muscle was dissected
from the bone with a retrograde dissection technique (9,10), and
the deep temporal artery (DTA) was easily preserved. A heart-like
frontal-temporal bone flap was made along the superior temporal
line, and the middle meningeal artery (MMA) passing through the
sphenoidal crest was kept intact. The dura mater was incised along
the MMA and its major branches, resulting in dura mater strips with
a width of 0.5–1.0 cm. The remaining part of the dura mater was
separated by radial incisions and flipped over. During this
procedure, it is important to preserve the DTA and MMA, as they are
the major source for revascularization in EDMS. M4 branches on the
cortical surface with a diameter of >0.8 mm were suitable for
direct anastomosis. An adequate length of the STA branch was
dissected, trimmed and anastomosed end-to-side to the M4 cortical
artery with 10–12 interrupted sutures. The temporal muscle covered
the exposed cortex and was fixed on the margin of the bone window.
The bone flap was trimmed to an appropriate shape and fixed without
compressing the STA. Usually, the total operation time was ~4 h.
The average blocking time was ~20 min. Intraoperative blood loss
was ~300 ml. Anemic moyamoya patients received a blood transfusion
during the operation, usually a 2U red blood cell suspension, to
ensure adequate blood volume and avoid low cerebral perfusion.
The post-operative therapy for moyamoya patients at
our center includes anti-platelet, anti-oxyradical and blood volume
management. The patient is prescribed aspirin (100 mg, quaque
nocte) if no obvious intracranial hemorrhage is visible on routine
head CT. 2,500-3,000 ml intravenous fluid infusion per day was
necessary during the early stage after surgery. Semiliquid and soft
diets are preferred for the patients after surgery.
Clinical follow-up and post-operative
angiographic evaluation
Post-operative complications were recorded in
detail, including motor and sensory deficits, epilepsy and
hemorrhage. Neurological deficits and recurrent cerebral vascular
events were evaluated by modified Rankin scale (mRS) scoring and
Kaplan-Meier analysis. CT, CT angiography (CTA), CTP and DSA were
routinely performed; cranial CT and CTA examinations were performed
on the first post-operative day and further CTP and DSA
examinations were required for all patients at 6 months after
surgery. However, not all patients were routinely available during
the follow-up period, certain patients underwent DSA on the 10th
day following surgery, 12 months following surgery or yearly
thereafter.
Pre-operative angiographic stages were classified
according to the standards proposed by Suzuki and Takaku (11). Post-operative collateral grading was
evaluated by reviewing the lateral view of the external carotid
artery angiography. The newly developed collateral circulation
through the combined revascularization was graded according to
angiographically visible supplied territory dimensions (12). In brief, scoring was as follows: 0,
No collaterals present in the target revascularization area; 1,
collaterals present in one-third or less of the MCA territory; 2,
in one to two thirds of the MCA territory; 3, in >two thirds of
the MCA territory. This grading method provides an overall
evaluation of the compensation area without considering the
collaterals derived from the direct bypass or the EDMS. Using these
scores, the patients were categorized into 4 patterns of
revascularization (13): Pattern I,
direct revascularization with a higher score than indirect
revascularization; pattern II, direct revascularization with a
similar score to indirect revascularization; pattern III, indirect
revascularization with a lower score than direct revascularization;
pattern IV, no obvious revascularization.
Indirect revascularization mainly depended on the
contribution of different donor arteries, including the MMA and
DTA. Direct revascularization mainly depended on the contribution
of the STA. DSA post-processing software (GE Healthcare Advantage
workstation 4.0; GE Healthcare Advantage workstation; GE
Healthcare, Little Chalfont, UK and Siemens Axiom Artis 3.0;
Siemens, Ag, Munich, Germany) were used to measure the inner
diameters of the STA trunk, its frontal and parietal branches, MMA
and DTA on the lateral angiographic projection of the external
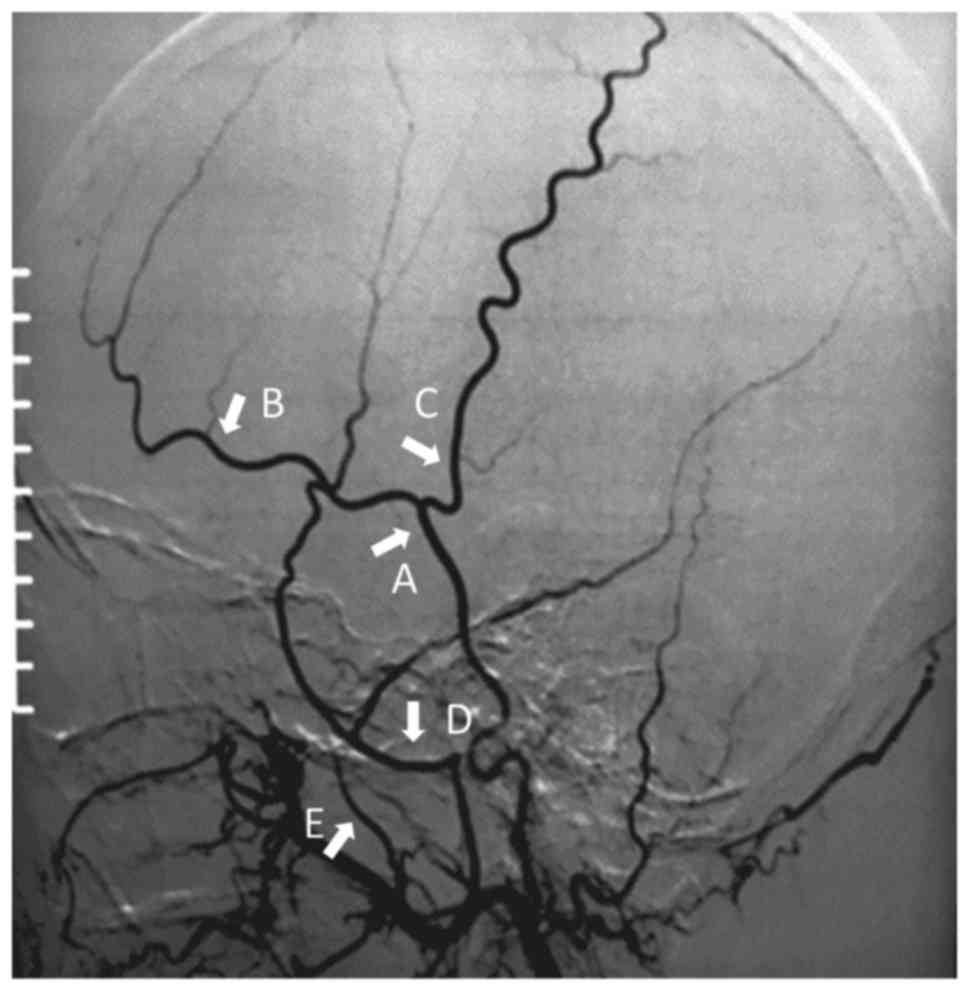
carotid artery. The measurement sites were marked as follows
(Fig. 1): STA trunk-proximal to its
bifurcation into frontal and parietal branches; frontal and
parietal branch-proximal to its first bifurcation; MMA-distal trunk
after the MMA passing through the spinous foramen, which appears as
a sharp angle on angiography; DTA-the trunk distal to the
origination from the maxillary artery. Measurements were performed
at similar locations on the follow-up angiograms at different
time-points (10 days, 6 months and 12 months after surgery). The
calculation work was performed by 2 blinded
neurointerventionalists.
Statistical analysis
SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) and
GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, USA) were
used for statistical analysis. Differences among different groups
were assessed using a Student's t-test (paired sample).
Non-parametric data were analyzed by Fisher's exact test. Survival
evaluation was performed using Kaplan-Meier analysis. Values are
expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient demographics and clinical
presentation
Combined bypass surgery was applied to 76
hemispheres of 64 moyamoya patients from January 2010 to December
2015. The patient cohort comprised 31 males and 33 females aged
20–68 years. The follow-up duration was 6–70 months (mean, 23±14
months). Of the operated hemispheres, 21 were on the left, 29 on
the right and 13 were bilateral. The most common clinical
manifestation of MMD in this series was cerebral ischemia,
including transient ischemic attack (TIA) and infarction. A total
of 42 patients (65.6%) presented with ischemic symptoms (12 TIA and
30 cerebral infarctions), while 14 (21.9%) patients presented with
spontaneous intraventricular or intraparenchymal hemorrhage.
Another 8 patients (12.5%) complained of headache. All of the
patients received a definite diagnosis of MMD and underwent
evaluation of cerebral perfusion and vascular compensation prior to
surgical interventions by head CT, magnetic resonance scan, CTP and
DSA. According to the scale by Suzuki and Takaku (11), 26, 35, 12 and 3 hemispheres were
considered to be stage II, III, IV and V, respectively, while none
were stage I or VI. Surgical plans were largely dependent on the
clinical symptoms, signs and cerebral perfusion of MMD patients. In
detail, bypass was performed on the hemisphere with worse cerebral
perfusion and collateral compensation first, followed by the
contralateral side. If the cerebral perfusion and collateral
compensation of bilateral hemispheres were similar, the
non-dominant hemisphere was preferred, which was mostly the
right-hand side. For patients with hemorrhagic onset, the bleeding
hemisphere was prioritized. All 64 MMD patients received combined
bypass. The characteristics of enrolled patients are summarized in
Table I.
 | Table I.Clinical characteristics of the
enrolled 64 moyamoya patients undergoing combined STA-MCA and
EDMS. |
Table I.
Clinical characteristics of the
enrolled 64 moyamoya patients undergoing combined STA-MCA and
EDMS.
| Parameter | N |
|---|
| General
characteristics |
|
| No. of
patients (hemispheres) | 64 (76) |
| Mean age
± SD (years) | 42±11 |
| Gender
(male/female) | 31/33 |
| Initial
presentation |
|
| TIA | 12 |
|
Infarction | 30 |
|
Hemorrhage | 14 |
|
Headache | 8 |
| Suzuki and Takaku
stage |
|
|
I/II/III/IV/V/VI | 0/26/35/12/3/0 |
| Surgical
procedures |
|
|
Hemispheres
(left/right/bilateral) | 21/29/13 |
|
STA-MCA+EDMS | 76 |
| Postoperative
complications |
|
|
TIA/Hemodynamic disorders | 9 |
|
Infarction | 4 |
|
Epilepsy | 2 |
|
Hemorrhage | 1 |
| Clinical
follow-up |
|
| Mean
follow-up period ± SD (months) | 23±14 |
| Symptoms
(improved/no change/aggravated) | 57/3/4 |
|
Re-hemorrhage, n | 2 |
| Postoperative
angiographic grading |
|
| <1/3
MCA territory | 6 |
| 1/3-2/3
MCA territory | 11 |
| >2/3
MCA territory | 59 |
Clinical outcomes for MMD patients
Peri-operative complications
Post-operative complications frequently occurred
during the first two weeks after the operation. Within the cohort,
new clinical symptoms in the ipsilateral and contralateral
hemisphere appeared in 16/64 patients (25%). Symptomatic cerebral
hyperperfusion syndrome or TIA was observed in nine patients
(14.1%), who experienced transient numbness of the limbs and/or
inarticulate speech without any new parenchymal lesions. A total of
four patients (6.25%) experienced new-onset cerebral infarction.
Transient seizure occurred in two patients (3.13%), and one patient
(1.56%) developed slight cerebral hemorrhage in the ipsilateral
basal ganglia.
Neurological improvements during
follow-up
Three patients continued to complain of dizziness,
headache and limb numbness, respectively. Two patients suffered a
TIA and one patient had a huge hematoma in the contralateral
hemisphere at 18 months after surgery as a result of sudden
unconsciousness. This patient received emergent hematoma removal,
decompressive craniectomy and EDMS, and remained in a state of
severe disability during the follow-up period. Another patient
developed a headache due to slight intraventricular hemorrhage at
the 30th post-operative month. No mortalities occurred until the
end of the follow up period. mRS scoring was performed to evaluate
neurological deficits pre-operatively and at 6 months after the
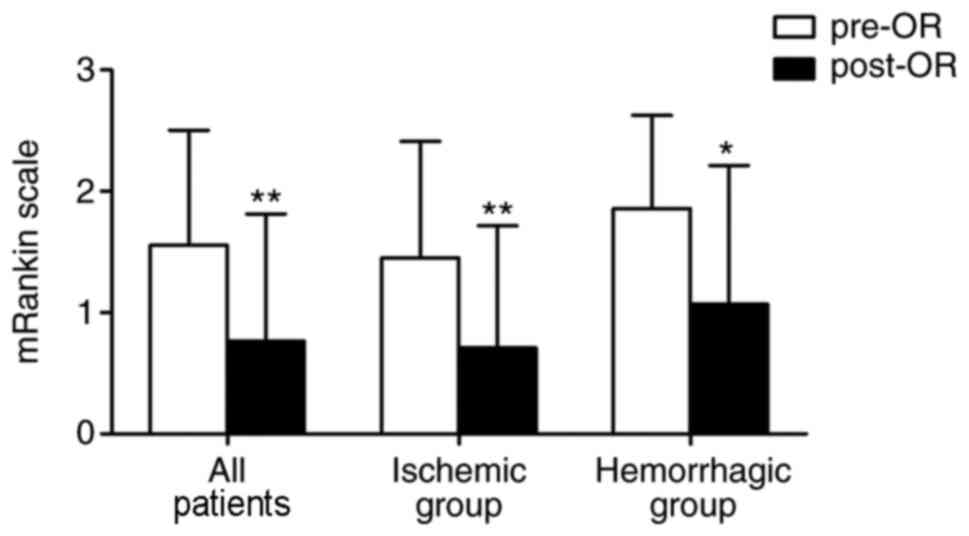
operation. As presented in Fig. 2,
the mRS score was significantly decreased after the operation not
only in all patients (1.55±0.95 pre-operation vs. 0.76±1.05
post-operation, P<0.001), but also in the ischemic subgroup
(1.45±0.96 pre-operation vs. 0.71±1.01 post-operation, P<0.01)
and hemorrhagic subgroup (1.86±0.77 pre-operation vs. 1.07±1.14
post-operation, P<0.05). However, no statistically significant
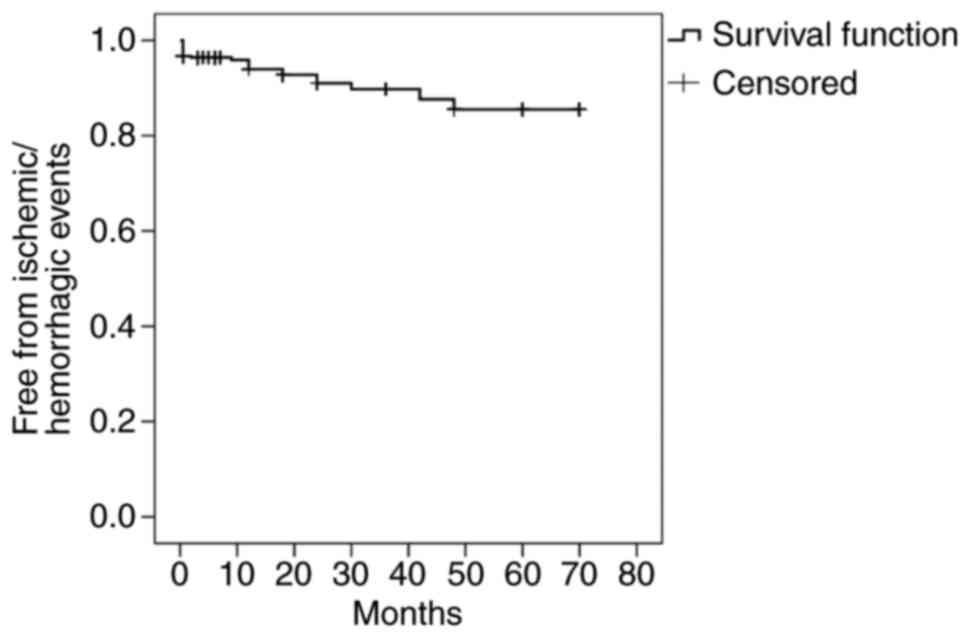
difference was present between these two subgroups. Kaplan-Meier
analysis was performed, considering the time-point of ischemic or
hemorrhagic events as the end-point. When no such event occurred
over the follow-up period, the time recorded was the last available
clinical follow-up time (Fig. 3).
The overall rate of cerebral ischemia and hemorrhage was 19.7 and
3.9% respectively.
Angiographic outcomes
Vascular remodeling
characterizations
All of the patients received DSA examination at 6
months after the operation. A total of 3 STAs were occluded.
Generally, good revascularization was established in most patients.
On post-operative collateral grading, 92.1% (70/76) of sides were
scored as 2 and 77.6% (59/76) of sides were scored as 3. Poor
revascularization with sparse vessels was obtained in 6 sides (6
MMD patients), whose distribution area was less than 1/3 of the MCA
territory. At 12 months after combined bypass, 40 patients received
DSA re-examination. A total of 35 sides (87.5%) were scored as 2 or
3. The collateralization area of the direct and indirect bypass was
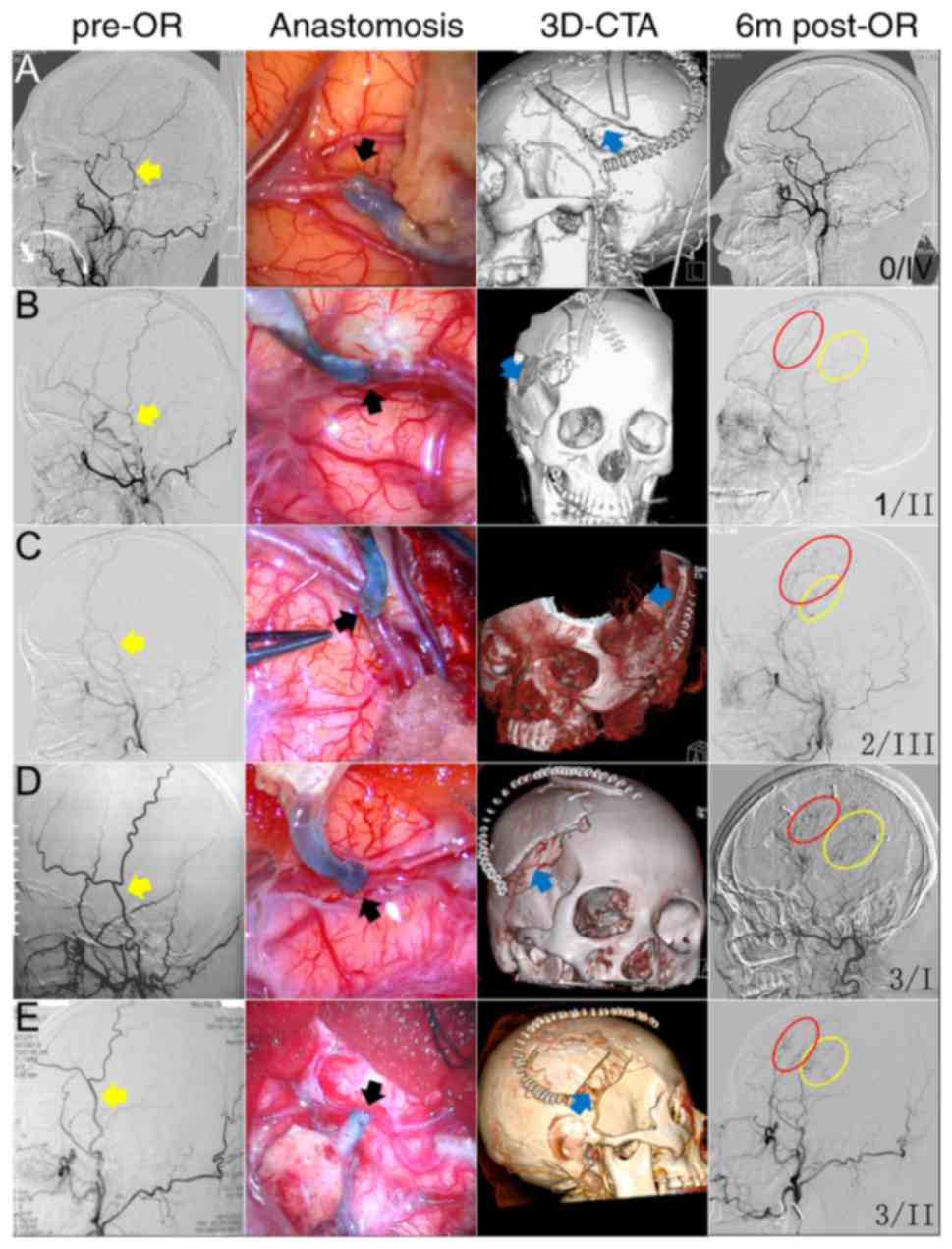
then compared. At 6 months after the operation, 19 hemispheres
demonstrated that STA-MCA anastomosis provided a greater collateral
territory than EDMS (STA-MCA anastomosis >EDMS, pattern I),
while the opposite (STA-MCA anastomosis <EDMS, pattern III) was
observed in 31 hemispheres. An equivalent contribution to
revascularization (STA-MCA anastomosis=EDMS, pattern II) was
present in 25 hemispheres. Pattern IV vascular remodeling was
observed in only one hemisphere (Fig.
4).
Morphological alterations of donor
vessels
Compared with the pre-operative caliber (2.60±0.65
mm), the caliber of the major trunk of the STA increased profoundly
at post-operative day 10 (3.32±1.05 mm, P<0.05 vs.
pre-operation), and shrank back to the pre-operative caliber at 6
months (2.20±1.01 mm, P>0.05) and 12 months (2.36±0.73 mm,
P>0.05). On DSA, STA shrinkage occurred in parallel with the
development of prominent collaterals from indirect bypass at 6
months post operation, indicating that the STA may have a dominant
role through providing immediate flow augmentation in the early
phase after surgery and may slowly regress due to indirect
neoangiogenesis and revascularization. Longitudinal changes
(pre-operation vs. 10 days vs. 6 months post-surgery) of the
frontal and parietal branches of the STA were similar to those of
the STA trunk; however, they did not achieve statistical
significance (frontal branch: 1.48±0.47, 2.01±0.59 and 1.31±0.44
mm; parietal branch: 1.24±0.47, 1.87±1.10 and 1.50±0.75 mm). The
MMA and DTA are the major donor vessels in EDMS. Over the
observation period, the diameter of MMA was obviously enlarged at
10 days (1.72±0.54 mm, P<0.05 vs. pre-operation), 6 months
(1.92±0.79 mm, P<0.05) and 12 months (1.87±0.69 mm, P<0.05)
in comparison to the pre-operative diameter (1.30±0.46 mm). Similar
morphological changes were observed in the DTA whose diameter
significantly increased from 1.11±0.25 mm (pre-operation) to
2.02±0.47 mm at post-operative day 10, 2.13±0.52 mm at 6 months and
2.11±1.16 mm at 12 months (P<0.05 vs. pre-operation for each).
This compensative enlargement was probably due to increased blood
flow after indirect bypass.
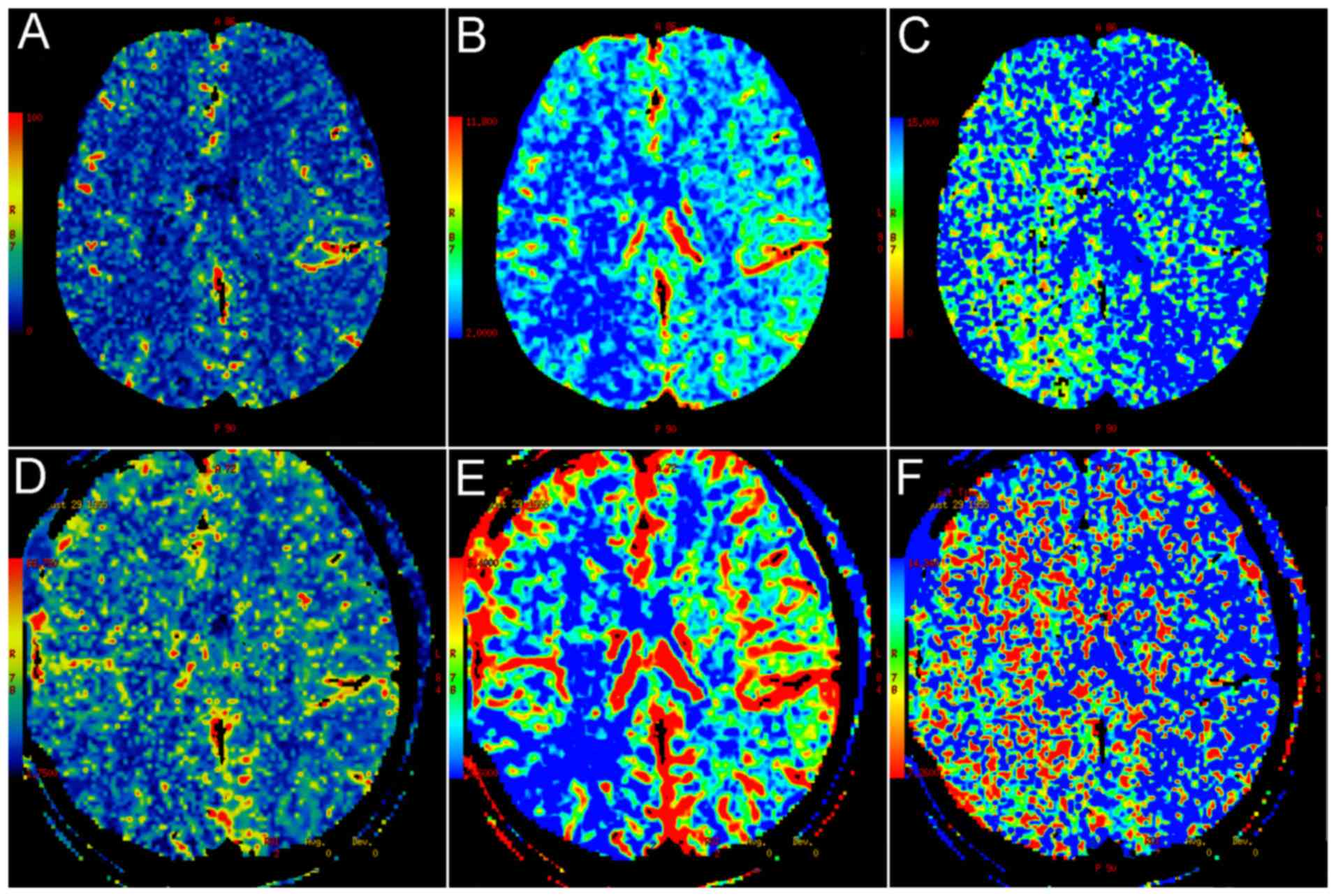
Cerebral perfusion after surgery
CTP was applied to evaluate cerebral blood supply on
admission and at 6 months after the operation. At 6 months, 70
hemispheres (92.1%; 58 MMD patients) demonstrated increased
cerebral blood flow compared with the pre-operative one. As
presented in Fig. 5, the mean blood
flow and mean blood volume were enhanced in the operated side
compared with that in the other side, while the mean transit time
was shortened after the operation.
Vascular reconstruction discrepancies
between ischemic and hemorrhagic patients
Of the enrolled patients, 42 (65.6%, 49 hemispheres)
presented with ischemic symptoms, while 14 patients (21.9%, 17
hemispheres) presented with hemorrhage. At 6 months, in the
ischemic group, the number of patients with an angiographic
collateralization score of 0, 1, 2 and 3 was 0, 3, 6 and 40
respectively, which is similar to that in the hemorrhagic group (0,
1, 3 and 13) according to Fisher's exact test. In addition, the
contributions from EDMS or STA-MCA anastomosis to
re-vascularization were similar (Table
II). Thus, patients in the ischemic and hemorrhagic group had
similar angiographic collateralization score and vascular
reconstruction patterns.
 | Table II.Vascular reconstruction discrepancies
at the postoperative 6-month evaluation between ischemic and
hemorrhagic moyamoya patients. |
Table II.
Vascular reconstruction discrepancies
at the postoperative 6-month evaluation between ischemic and
hemorrhagic moyamoya patients.
|
|
| Collateral grading on
DSA | Vascular
reconstruction pattern |
|---|
|
|
|
|
|
|---|
| Group | Hemispheres
(n) | Score 0–1 | Score 2–3 | Type I | Type II | Type III |
|---|
| Ischemic | 49 | 3 (6.12) | 46 (93.88) | 12 (24.49) | 17 (34.69) | 20 (40.82) |
| Hemorrhagic | 17 | 1 (5.88) | 16 (94.12) | 4 (23.53) | 6 (35.29) | 7 (41.18) |
| P-value |
| 0.729 |
| 1.000 |
|
|
Discussion
Within the cohort of the present study, most
moyamoya patients recovered well after receiving combined STA-MCA
and EDMS with no recurrent acute cerebrovascular events during the
follow-up period. Angiographic evaluation indicated good
revascularization and increased cerebral blood flow in most
patients subjected to combined bypass. Collateral revascularization
originating from EDMS was more predominant than that from STA-MCA
anastomosis at the 6-month post-operative evaluation. The diameter
of the major trunk of the STA, as well as its frontal and parietal
branches increased profoundly at post-operative day 10 and had
shrunk back to pre-operative size at 6 and 12 months, while the MMA
and DTA demonstrated continuous enlargement during the follow-up
time. No collateral discrepancies were observed between ischemic
and hemorrhagic groups. The present study indicated that combined
STA-MCA bypass and EDMS was a good option for treating MMD.
Intracranial and extracranial vascular
reconstruction is the mainstay for MMD treatment (1,2).
Intracranial and extracranial vascular reconstruction surgery
mainly includes direct and indirect revascularization. Direct
revascularization increases the cerebral blood flow and instantly
improves cerebral metabolism (14,15), but
the drawback is that STA or MCA branches of moyamoya patients may
be fragile and easily occluded; thus, the long-term effect of
direct revascularization remains elusive. In addition, patients
with direct revascularization are prone to developing cerebral
hemodynamic disturbances and perioperative complications (16). In addition, recipient vessels of MMD
patients always have a small diameter and thin wall, which are
difficult to be anastomosed by inexperienced surgeons or hospitals
(17,18). By contrast, indirect
revascularization facilitates the formation of new blood vessels on
the surface of the brain through the STA, the dura, the temporal
muscle and the periosteum anastomosis (19–21). It
has several merits, including safe and simple operation, as well as
no damage to the already formed collateral circulation. However,
certain studies reported that indirect revascularization is
effective for children with MMD, but less effective for adults
(22,23). However, certain studies indicate that
MMA and DTA may develop good neovasculature after indirect bypass,
which is beneficial for alleviating clinical symptoms and stroke
prevention (24,25). In the present study, the reasons for
poor collateral formation from indirect bypass may include the
following: i) The bone flap is too small to make full use of the
temporal muscle; ii) failure to maintain the integrity of the deep
temporal artery when stripping the temporalis from the skull; iii)
damage of the branches of the middle meningeal branch when milling
of the bone flap or cutting of the dura mater. Therefore, in order
to make full use of the DTA and MMA, a large frontal-temporal bone
flap was generated to ensure that the temporal muscle was
sufficiently attached to the surface of the brain. In addition, the
arachnoid was fully opened to facilitate the contact between
temporal muscle and cerebral cortex, as well as stimulate
angiogenesis. Reverse temporal muscle separation technology was
applied in our group to ensure the integrity of the deep temporal
artery network, which allowed for maximum temporal muscle and
cerebral cortex formation after anastomosis. The integrity of the
MMA and its major branches was maintained without destroying
already formed anastomosis between the MMA and cerebral cortex. The
dura was reversed and stuck onto the surface of the cortex, which
made the spontaneous anastomosis extend from the existing bone
window. Overall, several surgical modifications may help moyamoya
patients to form good collateral and develop optimal clinical
outcomes.
In the present study, STA-MCA anastomosis was the
treatment providing the most collateral formation in 19
hemispheres, which was less than the number of hemispheres in which
EDMS had the predominant effect (n=31). An equivalent contribution
to revascularization by STA-MCA and EDMS was present in 25
hemispheres. These data suggested that EDMS and STA-MCA may
contribute to revascularization corporately. According to the
histopathological concepts, revascularization may include two
types: Arteriogenesis and angiogenesis. Arteriogenesis is
designated as the remodeling of pre-existing arterioarteriolar
anastomoses, which may have a critical role in direct anastomosis
(13,26). Furthermore, the pressure gradient
from the STA to the recipient artery is a key factor for promoting
satisfactory arteriogenesis. Angiogenesis usually occurs in the
fibrous coat developing between the transplanted muscle and the
arachnoid membrane (13,27), which is assumed to participate in
indirect anastomosis. To this point, there also exits a reciprocal
mechanism to develop completed collaterations between direct
STA-MCA bypass and indirect EDMS.
Previous studies reported that STA-MCA bypass
increases STA and MCA blood flow during the early vascular
remodeling period (14,15). The increase of STA blood flow may be
associated with the low resistance of the intracranial vascular
bed, while the increase of MCA blood flow may be due to the support
of the STA. Consistent with this, the present study observed that
the STA was enlarged during the early post-operative phase and
shrank gradually, while the MMA and DTA demonstrated continuous
widening during the follow-up period. With the development of
indirect revascularization and enlargement of MMA and DTA, the
blood flow demand from the STA-MCA declines (28,29),
which is a possible reason for the thinning and even occlusion of
the STA. These alterations suggested that direct STA-MCA bypass
provides early augmentation of cerebral perfusion, whereas indirect
EDMS provides a more durable long-term revascularization.
Therefore, the present study suggests that combining
direct and indirect bypass in a suitable effort to take advantage
of the immediate revascularization provided by direct arterial
bypass, but also to maximize the eventual cerebral
revascularization.
Acknowledgements
The authors would like to thank Dr Meijuan Zhang
(Department of Neurology, The Affiliated Drum Tower Hospital of
Nanjing University, Nanjing, China) for professional language
editing of the manuscript. This study was supported by the National
Natural Science Foundation of China (grant no. 81301049), the
Natural Science Foundation of Jiangsu Province of China (grant no.
BK20130085) and the Project supported by the Medical Science and
Technology Development Foundation, Nanjing Department of Health
(grant no. YKK14100). Part of this study was previously presented
at the International Stroke Conference, February 2017, poster no.
TP449.
Glossary
Abbreviations
Abbreviations:
|
CTP
|
computed tomography perfusion
|
|
DSA
|
digital subtraction angiography
|
|
DTA
|
deep temporal artery
|
|
EDMS
|
encephalo-duro-myo-synangiosis
|
|
MMA
|
middle meningeal artery
|
|
MMD
|
moyamoya disease
|
|
mRS
|
modified Rankin scale
|
|
STA-MCA
|
superficial temporal artery-middle
cerebral artery
|
References
|
1
|
Pandey P and Steinberg GK: Neurosurgical
advances in the treatment of moyamoya disease. Stroke.
42:3304–3310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scott RM and Smith ER: Moyamoya disease
and moyamoya syndrome. N Engl J Med. 360:1226–1237. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arias EJ, Derdeyn CP, Dacey RG Jr and
Zipfel GJ: Advances and surgical considerations in the treatment of
moyamoya disease. Neurosurgery. 74 Suppl 1:S116–S125. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baaj AA, Agazzi S, Sayed ZA, Toledo M,
Spetzler RF and van Loveren H: Surgical management of moyamoya
disease: A review. Neurosurg Focus. 26:E72009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Houkin K, Ishikawa T, Yoshimoto T and Abe
H: Direct and indirect revascularization for moyamoya disease
surgical techniques and peri-operative complications. Clin Neurol
Neurosurg. 99 Suppl 2:S142–S145. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim DS, Huh PW, Kim HS, Kim IS, Choi S,
Mok JH and Huh CW: Surgical treatment of moyamoya disease in
adults: Combined direct and indirect vs. indirect bypass surgery.
Neurol Med Chir. 52:333–338. 2012. View Article : Google Scholar
|
|
7
|
Czabanka M, Peña-Tapia P, Scharf J,
Schubert GA, Munch E, Horn P, Schmiedek P and Vajkoczy P:
Characterization of direct and indirect cerebral revascularization
for the treatment of european patients with moyamoya disease.
Cerebrovasc Dis. 32:361–369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rashad S, Fujimura M, Niizuma K, Endo H
and Tominaga T: Long-term follow-up of pediatric moyamoya disease
treated by combined direct-indirect revascularization surgery:
Single institute experience with surgical and perioperative
management. Neurosurg Rev. 39:615–623. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oikawa S, Mizuno M, Muraoka S and
Kobayashi S: Retrograde dissection of the temporalis muscle
preventing muscle atrophy for pterional craniotomy. Technical note.
J Neurosurg. 84:297–299. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kadri PA and Al-Mefty O: The anatomical
basis for surgical preservation of temporal muscle. J Neurosurg.
100:517–522. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suzuki J and Takaku A: Cerebrovascular
‘moyamoya’ disease. Disease showing abnormal net-like vessels in
base of brain. Arch Neurol. 20:288–299. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsushima T, Inoue T, Suzuki SO, Fujii K,
Fukui M and Hasuo K: Surgical treatment of moyamoya disease in
pediatric patients-comparison between the results of indirect and
direct revascularization procedures. Neurosurgery. 31:401–405.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saito N and Imai H: Insights on the
revascularization mechanism for treatment of moyamoya disease based
on the histopathologic concept of angiogenesis and arteriogenesis.
World Neurosurg. 75:204–205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee M, Guzman R, Bell-Stephens T and
Steinberg GK: Intraoperative blood flow analysis of direct
revascularization procedures in patients with moyamoya disease. J
Cereb Blood Flow Metab. 31:262–274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arikan F, Vilalta J, Torne R, Noguer M,
Lorenzo-Bosquet C and Sahuquillo J: Rapid resolution of brain
ischemic hypoxia after cerebral revascularization in moyamoya
disease. Neurosurgery. 76:302–312. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu J, Shi L, Guo Y, Xu B and Xu K:
Progress on complications of direct bypass for moyamoya disease.
Int J Med Sci. 13:578–587. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takagi Y, Hermanto Y, Takahashi JC, Funaki
T, Kikuchi T, Mineharu Y, Yoshida K and Miyamoto S:
Histopathological characteristics of distal middle cerebral artery
in adult and pediatric patients with moyamoya disease. Neurol Med
Chir (Tokyo). 56:345–349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin R, Xie Z, Zhang J, Xu H, Su H, Tan X,
Tian D and Su M: Clinical and immunopathological features of
moyamoya disease. PLoS One. 7:e363862012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuroda S and Houkin K: Moyamoya disease:
Current concepts and future perspectives. Lancet Neurol.
7:1056–1066. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scott RM: Surgery for moyamoya syndrome?
Arch Neurol. 58:128–129. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zipfel GJ, Fox DJ Jr and Rivet DJ:
Moyamoya disease in adults: The role of cerebral revascularization.
Skull Base. 15:27–41. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Macyszyn L, Attiah M, Ma TS, Ali Z, Faught
R, Hossain A, Man K, Patel H, Sobota R, Zager EL and Stein SC:
Direct versus indirect revascularization procedures for moyamoya
disease: A comparative effectiveness study. J Neurosurg.
126:1523–1529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mizoi K, Kayama T, Yoshimoto T and
Nagamine Y: Indirect revascularization for moyamoya disease: Is
there a beneficial effect for adult patients? Surg Neurol.
45:541–549. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kinugasa K, Mandai S, Kamata I, Sugiu K
and Ohmoto T: Surgical treatment of moyamoya disease: Operative
technique for encephalo-duro-arterio-myo-synangiosis, its
follow-up, clinical results, and angiograms. Neurosurgery.
32:527–531. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uchino H, Kim JH, Fujima N, Kazumata K,
Ito M, Nakayama N, Kuroda S and Houkin K: Synergistic interactions
between direct and indirect bypasses in combined procedures: The
significance of indirect bypasses in moyamoya disease.
Neurosurgery. 80:201–209. 2017.PubMed/NCBI
|
|
26
|
Heil M and Schaper W: Insights into
pathways of arteriogenesis. Curr Pharm Biotechnol. 8:35–42. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakamura M, Imai H, Konno K, Kubota C,
Seki K, Puentes S, Faried A, Yokoo H, Hata H, Yoshimoto Y and Saito
N: Experimental investigation of encephalomyosynangiosis using
gyrencephalic brain of the miniature pig: Histopathological
evaluation of dynamic reconstruction of vessels for functional
anastomosis. Laboratory investigation. J Neurosurg Pediatr.
3:488–495. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
King JA, Armstrong D, Vachhrajani S and
Dirks PB: Relative contributions of the middle meningeal artery and
superficial temporal artery in revascularization surgery for
moyamoya syndrome in children: The results of superselective
angiography. J Neurosurg Pediatr. 5:184–189. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Amin-Hanjani S, Singh A, Rifai H, Thulborn
KR, Alaraj A, Aletich V and Charbel FT: Combined direct and
indirect bypass for moyamoya: Quantitative assessment of direct
bypass flow over time. Neurosurgery. 73:962–967. 2013. View Article : Google Scholar : PubMed/NCBI
|