Introduction
Thyroid cancer represents the most common cancer in
the endocrine system (1–4). Most thyroid cancers originate from
thyroid follicular cells (>90%), which can differentiate into
papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma
(FTC), while only less than 5% derive from C-cells (5). Of the PTC patients 40–60% are aged over
45 years, mainly middle aged and elderly people with lymphatic
metastasis (6). The most common
follicular tumors now are mostly benign hyperplastic tumors, and
PTC represents the most common thyroid cancer (approximately 90%)
(7). PTC and FTC may progress to a
poorly differentiated carcinoma or complete loss of differentiation
to produce anaplastic thyroid carcinoma (ATC) (8).
A large number of studies have been conducted to
screen candidate markers of thyroid cancer at present. The
differential expression of excessively large numbers of molecules
was screened out in thyroid carcinoma tissues (9–11). The
adenosine monophosphate deaminase (AMPD) encoded by AMPD1 gene has
the function of catalyzing adenosine monophosphate inosinemonpho
sphate (12,13) in purine nucleotide metabolism and
energy metabolism, especially skeletal muscle and cardiac muscle.
Currently, it is known that AMPD1 gene is generally localized on
the p13-21 region chromosome, with a length of 20 kb, consisting of
16 exons and 15 introns (14). In
most cases, the presence of the nonsense mutation of 34 at position
of AMPD1 and common polymorphism of C-T transformation contribute
to the emergence of premature termination codon; some related
metabolic muscle disorders are caused by the lack of AMPD activity
(15). It is proved that mutations
in the AMPD1 allele play a protective role in the onset of
congestive heart failure, which can also help (16) and relatively reduce aortic stiffness
and inflammation in patients with coronary artery disease (CAD)
(13). However, there are few
studies on AMPD1 in human malignant tumors; particularly, its
relationship with the occurrence and development of thyroid cancer,
and the expression level and prognosis of thyroid cancer are rarely
reported. This study aimed to further understand the expression of
AMPD1 and its relationship with cancer.
Materials and methods
Clinical data
A total of 157 patients with PTC diagnosed by
pathology admitted to the Department of Oncology in The Second
Affiliated Hospital of Soochow University from May 2011 to August
2016 were collected, including 75 males and 82 females, aged 43–77
years, with an average age of 62.15±10.61 years. According to the
eighth edition of tumor-node-metastasis (TNM) staging issued by
American Joint Committee on Cancer (AJCC), there were 44 cases in
stage I, 63 cases in stage II, 32 cases in stage III and 18 cases
in stage IV. One hundred normal people were enrolled as control
group. None of the patients had been treated with radiotherapy and
chemotherapy before operation. The study was approved by the Ethics
Committee of The Second Affiliated Hospital of Soochow University,
and patients signed the informed consent.
Main instruments and reagents
ABI StepOne Plus (Applied Biosystems, Foster City,
CA, USA) fluorescence quantitative polymerase chain reaction (PCR)
instrument, NanoDrop 2000 spectrophotometer, KH19A desktop
high-speed high-performance centrifuge (KAIDA); −80°C
low-temperature refrigerator (Thermo Fisher Scientific); AxyGen
Total RNA Extraction kit was purchased from Takara Biotechnology
Co., Ltd., Dalian, China; reverse transcription kit was from Thermo
Fisher Scientific; fluorescent quantitative PCR kit was from Takara
Biotechnology Co., Ltd.
Collection of samples
Peripheral blood (5 ml) was extracted from patients
with PTC and volunteers in normal control group before operation
and placed into an ethylenediaminetetraacetic acid-k (EDTA-k)
anticoagulant tube, followed by centrifugation at 3500 × g for 5
min. The supernatant was taken and centrifuged at 8000 × g at 4°C
for 10 min. The supernatant was collected and stored at −80°C.
Design and synthesis of primers
cDNA sequences of AMPD1 gene were synthesized using
primer design software Primer 5, and primers were synthesized by
Guangzhou Shangeng Biotechnology Co., Ltd. (Table I). PCR amplification reaction: 8 µl
ddH2O, 10 µl Fast qPCR Mix (Takara Biotechnology Co.,
Ltd.), 2 µl cDNA template, 0.2 µl upstream primer and 0.2 µl
downstream primer (10 µmol/l); PCR was amplified by Applied
Biosystems StepOne Plus: pre-denaturation at 95°C for 10 min,
denaturation at 95°C for 15 sec, annealing and extending at 60°C
for 30 sec, a total of 45 cycles. The relative expression level of
AMPD1 was calculated using 2−∆CT method.
 | Table I.Internal reference of AMPD1
primers. |
Table I.
Internal reference of AMPD1
primers.
| Gene | Primer sequence |
|---|
| AMPD1 | U:
5′-AAGGCCGCCCAGAGCTTATTCAT-3′ |
| primer | D:
5′-CTTCAGCAGGGTCCGAGGTATTC-3′ |
| U6 internal | U:
5′-CTCGCTTCGGCAGCACA-3′ |
| reference | D:
5′-AACGCTTCACGAATTTGCGT-3′ |
Statistical analysis
SPSS 22.0 (Version X; IBM, Armonk, NY, USA)
statistical software was used for statistical analysis. Measurement
data were expressed as mean ± SD, and t-test was applied for the
comparison of means between two samples. Pearson's correlation
analysis was utilized for analyzing the correlation. Furthermore,
the relationship between AMPD1 and 5-year survival rate was
analyzed by single-factor and multivariate Cox regression.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression levels of AMPD1 in PTC
patients and control group
The results of expression levels of AMPD1 in serum
of PTC patients and control group detected by real-time reverse
transcription polymerase chain reaction (RT-PCR) indicated that the
expression level of AMPD1 in serum of PTC patients was remarkably
reduced compared with that in normal control group (P<0.01)
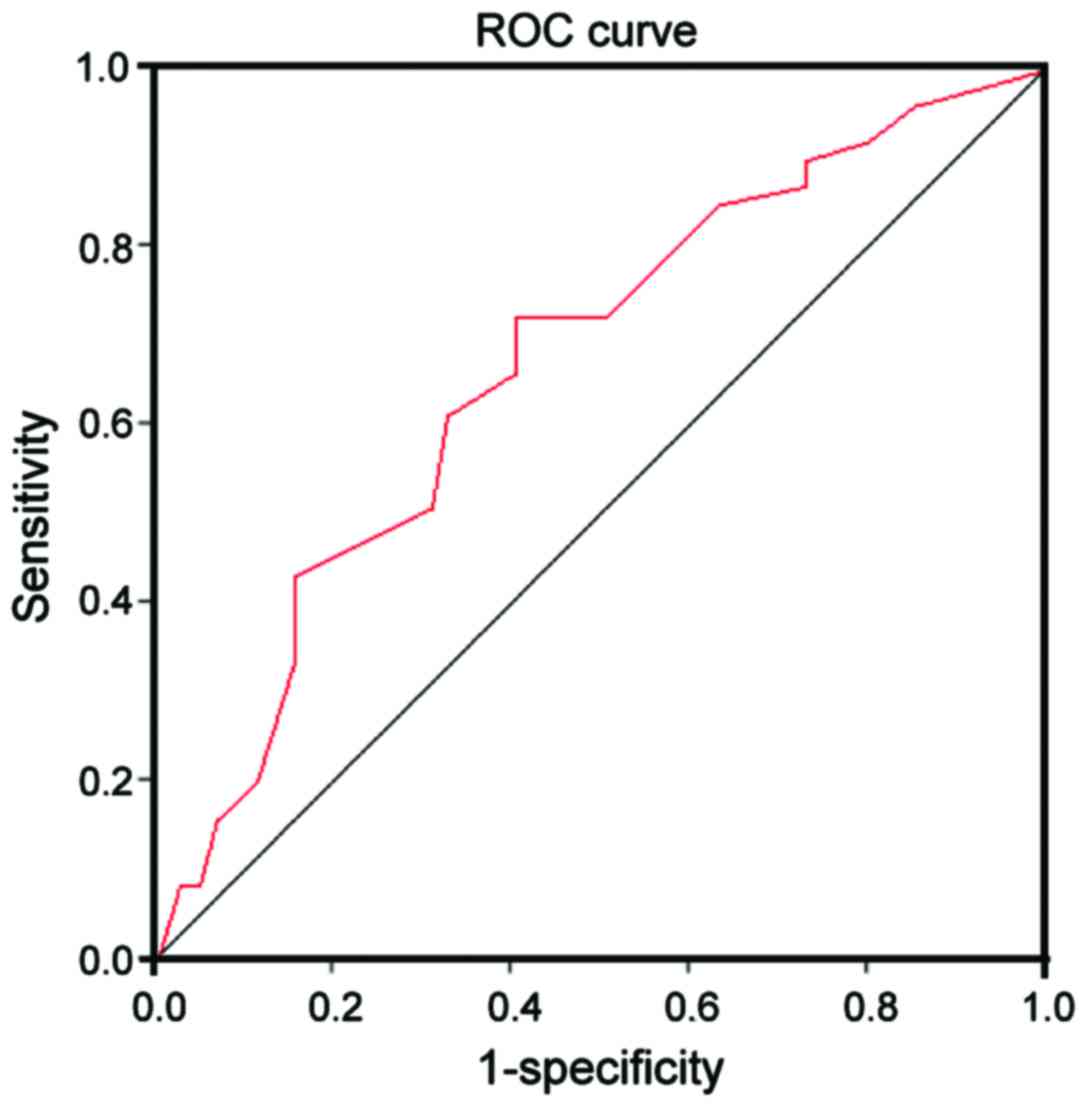
(Table II). The receiver operating
characteristic (ROC) curve analysis was further performed,
suggesting that the risk in PTC patients could be predicted by
AMPD1 with a certain value, the area under the curve (AUC) was
0.713, and 95% confidence interval (CI) was 0.692–0.797 (Fig. 1).
 | Table II.Expression level of AMPD1 in serum of
PTC patients and control group. |
Table II.
Expression level of AMPD1 in serum of
PTC patients and control group.
| Group | n | Expression level of
AMPD1 | P-value | t value |
|---|
| Patient | 157 |
2.14±2.45 |
|
|
| Control | 100 |
4.67±1.91 | <0.01 | 5.925 |
Relationships between expression level
of AMPD1 and clinicopathological features in PTC patients
According to the differential expression of AMPD1 in
PTC patients and combination with clinical pathological data, it
was found that the expression of AMPD1 in serum of PTC patients was
not significantly different from the clinicopathological features
such as sex, age, lymph node metastasis and the number of lesions
(P>0.05); there were distinct differences between its expression
and TNM staging and tumor diameter (P<0.05) (Table III).
 | Table III.Relationships between expression level
of AMPD1 and clinicopathological features in PTC patients. |
Table III.
Relationships between expression level
of AMPD1 and clinicopathological features in PTC patients.
| Clinical pathological
features | Expression level of
AMPD1 | P-value |
|---|
| Age/years |
| 0.059 |
| ≥45 | 2.15 (1.65–3.03) |
|
|
<45 | 3.81 (1.25–2.96) |
|
| Sex |
| 0.751 |
| Male | 3.67 (1.86–2.55) |
|
|
Female | 3.11 (1.57–2.67) |
|
| Tumor diameter |
| 0.045 |
| ≥20
mm | 2.01 (1.29–2.59) |
|
| <20
mm | 3.54 (1.89–3.09) |
|
| Lymph node
metastasis |
| 0.135 |
| Yes | 2.39 (1.48–3.21) |
|
| No | 3.18 (1.80–2.67) |
|
| TNM staging |
| 0.017 |
| I–II | 3.44 (1.36–3.54) |
|
|
III–IV | 1.87 (1.22–2.57) |
|
| Number of
lesions |
| 0.074 |
|
Single | 2.16 (1.69–3.49) |
|
|
Multiple | 1.54 (2.19–3.56) |
|
Analysis of prognosis-related factors
in PTC patients
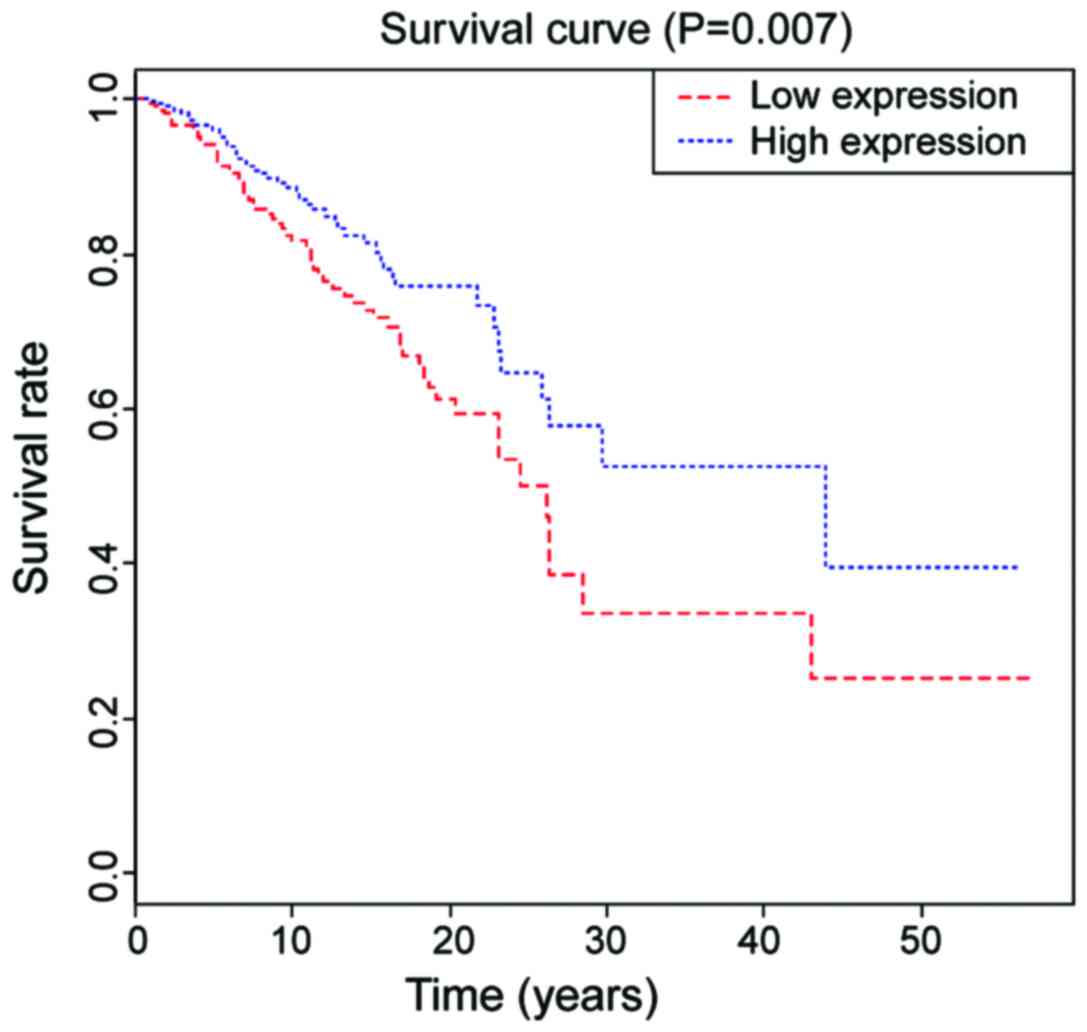
The 5-year survival rate in PTC patients with high
expression of AMPD1 was 62.4, which was 45.5 in the group with low
expression of AMPD1, and the difference was statistically
significant (P=0.007, Fig. 2). The
single factor Cox analysis revealed that sex, age, number of
lesions, TNM staging and the occurrence of lymph node metastasis
were significantly correlated with the prognosis of patients
(P<0.05) (Table IV).
Multivariate Cox analysis showed that TNM staging hazard ratio
(HR)=2.93, 95% CI: 1.52–7.04, P=0.015 was an independent prognostic
factor in PTC patients (Table
V).
 | Table IV.Single factor analysis for PTC
patients. |
Table IV.
Single factor analysis for PTC
patients.
| Influencing
factors | n | 5-year survival rate
(%) | HR | P-value |
|---|
| Sex |
|
| 0.77 | 0.036 |
| Male | 75 | 60.6 |
|
|
|
Female | 82 | 69.4 |
|
|
| Age |
|
| 0.84 | 0.574 |
| ≥45 | 69 | 64.7 |
|
|
|
<45 | 88 | 60.1 |
|
|
| Tumor diameter |
|
| 1.91 | 0.617 |
| ≥20
mm | 61 | 50.3 |
|
|
| <20
mm | 96 | 63.4 |
|
|
| Lymph node
metastasis |
|
| 0.67 | 0.007 |
| Yes | 49 | 55.6 |
|
|
| No | 108 | 67.4 |
|
|
| TNM staging |
|
| 0.52 | 0.057 |
| I–II | 85 | 68.1 |
|
|
|
III–IV | 72 | 50.2 |
|
|
| Number of
lesions |
|
|
| 0.011 |
|
Single | 79 | 67.4 |
|
|
|
Multiple | 78 | 52.7 |
|
|
 | Table V.Multivariate analysis for prognosis of
PTC patients. |
Table V.
Multivariate analysis for prognosis of
PTC patients.
| Influencing
factor | HR | P-value | 95% CI |
|---|
| Sex | 0.69 | 0.547 | 1.27–5.17 |
| Lymph node
metastasis | 0.81 | 0.095 | 1.47–4.94 |
| TNM staging | 2.93 | 0.015 | 1.52–7.04 |
| Number of
lesions | 1.27 | 0.184 | 1.38–6.14 |
Discussion
Thyroid cancer (THCA) is a common malignant tumor in
the endocrine system, which mainly derives from thyroid epithelial
cells, accounting for 1% of the systemic malignancy. Currently, its
incidence is increasing year by year. Papillary thyroid carcinoma
(PTC) is the most common pathological type of THCA, and the
cervical lymph node metastasis may appear in the early stage of the
disease. Thus, the early diagnosis and surgical treatment of PTC
are conducive to improving the prognosis of patients (17).
The adenosine monophosphate deaminase (AMPD) encoded
by AMPD1 gene has the function of catalyzing adenosine
monophosphate inosinemonophosphate (8,9) in
purine nucleotide metabolism and energy metabolism, especially
skeletal muscle and cardiac muscle. At present, AMPD1 gene has been
studied in diabetes mellitus (18)
and cardiovascular disease (19).
The purpose of this study is to identify new tumor markers in THCA
thus controlling the disease through better diagnosis and
treatment.
The study revealed that the expression level of
AMPD1 in serum of PTC patients was significantly lower than that of
normal human serum; by combining with clinical analysis, it showed
that AMPD1 expression was positively related with age, tumor
diameter and TNM staging of patients; the higher the age was, the
lower the expression level of AMPD1 would be; the larger the tumor
diameter was, the lower the expression level of AMPD1 would be; the
higher the TNM staging was, the lower the expression level of AMPD1
would be. The above results indicated that AMPD1 may be involved in
the pathological development of PTC.
This study revealed that the risk of PTC can be
predicted by AMPD1 expression, which is correlated with
pathological staging, TNM grading, distant metastasis and lymph
node metastasis of patients. Importantly, the low expression of
AMPD1 is associated with low overall survival rate, which is an
independent prognostic factor for PTC patients. Clinically, it is
difficult to diagnose papillary thyroid microcarcinoma (PTMC) with
diameter less than 10 mm before surgery due to the small size,
unobvious onset and lack of specific clinical manifestations and it
is only found when thyroidectomy or progressive lymph node
metastasis is required due to other causes (20).
In conclusion, this study indicated that AMPD1 is
closely linked to TNM staging. In the following study, we will
further expand the sample size to analyze the expression of AMPD1
in PTC, so as to provide a basis for the diagnosis of PTC. In
summary, AMPD1 expression in serum of PTC is downregulated and
plays a role in the occurrence of PTC, which is able to guide the
diagnosis and treatment in clinical practice.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TZ contributed to the conception and design of the
study and drafted the study. HW was responsible for acquisition and
analysis of data and revised the manuscript. Both authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Second Affiliated Hospital of Soochow University (Suzhou,
China). Signed written informed consents were obtained from the
patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Valmasoni M, Pierobon ES, Ruol A, De
Pasqual CA, Zanchettin G, Moletta L, Salvador R, Costantini M and
Merigliano S: Endoscopic tumor length should be reincluded in the
esophageal cancer staging system: Analyses of 662 consecutive
patients. PLoS One. 11:e01530682016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mazzaferri EL and Young RL: Papillary
thyroid carcinoma: A 10 year follow-up report of the impact of
therapy in 576 patients. Am J Med. 70:511–518. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jazdzewski K, Murray EL, Franssila K,
Jarzab B, Schoenberg DR and de la Chapelle A: Common SNP in
pre-miR-146a decreases mature miR expression and predisposes to
papillary thyroid carcinoma. Proc Natl Acad Sci USA. 105:pp.
7269–7274. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang Y, Prasad M, Lemon WJ, Hampel H,
Wright FA, Kornacker K, LiVolsi V, Frankel W, Kloos RT, Eng C, et
al: Gene expression in papillary thyroid carcinoma reveals highly
consistent profiles. Proc Natl Acad Sci USA. 98:pp. 15044–15049.
2001; View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu Y, Wang H, Chen E, Xu Z, Chen B and Lu
G: Candidate microRNAs as biomarkers of thyroid carcinoma: A
systematic review, meta-analysis, and experimental validation.
Cancer Med. 5:2602–2614. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Agrawal N, Akbani R, Aksoy BA, Ally A,
Arachchi H, Asa SL, Auman JT, Balasundaram M, Balu S, Baylin SB, et
al Cancer Genome Atlas Research Network, : Integrated genomic
characterization of papillary thyroid carcinoma. Cell. 159:676–690.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fu XM, Guo W, Li N, Liu HZ, Liu J, Qiu SQ,
Zhang Q, Wang LC, Li F and Li CL: The expression and function of
long noncoding RNA lncRNA-ATB in papillary thyroid cancer. Eur Rev
Med Pharmacol Sci. 21:3239–3246. 2017.PubMed/NCBI
|
|
8
|
Ragazzi M, Ciarrocchi A, Sancisi V,
Gandolfi G, Bisagni A and Piana S: Update on anaplastic thyroid
carcinoma: Morphological, molecular, and genetic features of the
most aggressive thyroid cancer. Int J Endocrinol. 2014:7908342014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wei WJ, Lu ZW, Wang Y, Zhu YX, Wang YL and
Ji QH: Clinical significance of papillary thyroid cancer risk loci
identified by genome-wide association studies. Cancer Genet.
208:68–75. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu X, Qu S, Liu R, Sheng C, Shi X, Zhu G,
Murugan AK, Guan H, Yu H, Wang Y, et al: TERT promoter mutations
and their association with BRAF V600E mutation and aggressive
clinicopathological characteristics of thyroid cancer. J Clin
Endocrinol Metab. 99:E1130–E1136. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kunstman JW, Juhlin CC, Goh G, Brown TC,
Stenman A, Healy JM, Rubinstein JC, Choi M, Kiss N, Nelson-Williams
C, et al: Characterization of the mutational landscape of
anaplastic thyroid cancer via whole-exome sequencing. Hum Mol
Genet. 24:2318–2329. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang L, Mo X, Xu Y, Zuo B, Lei M, Li F,
Jiang S, Deng C and Xiong Y: Molecular characterization and
expression patterns of AMP deaminase1 (AMPD1) in porcine skeletal
muscle. Comp Biochem Physiol B Biochem Mol Biol. 151:159–166. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tousoulis D, Kioufis S, Siasos G,
Oikonomou E, Zaromitidou M, Maniatis K, Kokkou E, Mazaris S,
Zakynthinos G, Konsola T, et al: The impact of AMPD1 gene
polymorphism on vascular function and inflammation in patients with
coronary artery disease. Int J Cardiol. 172:e516–e518. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sabina RL, Morisaki T, Clarke P, Eddy R,
Shows TB, Morton CC and Holmes EW: Characterization of the human
and rat myoadenylate deaminase genes. J Biol Chem. 265:9423–9433.
1990.PubMed/NCBI
|
|
15
|
Fischer S, Drenckhahn C, Wolf C, Eschrich
K, Kellermann S, Froster UG and Schober R: Clinical significance
and neuropathology of primary MADD in C34-T and G468-T mutations of
the AMPD1 gene. Clin Neuropathol. 24:77–85. 2005.PubMed/NCBI
|
|
16
|
Loh E, Rebbeck TR, Mahoney PD, DeNofrio D,
Swain JL and Holmes EW: Common variant in AMPD1 gene predicts
improved clinical outcome in patients with heart failure.
Circulation. 99:1422–1425. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li J, Yang T, Zhao T, Liang J and Lin YS:
Clinical outcome of radioiodine therapy in low-intermediate risk
papillary thyroid carcinoma with BRAF(V600E) mutation. Zhongguo Yi
Xue Ke Xue Yuan Xue Bao. 38:346–350. 2016.(In Chinese). PubMed/NCBI
|
|
18
|
Cheng J, Morisaki H, Toyama K, Sugimoto N,
Shintani T, Tandelilin A, Hirase T, Holmes EW and Morisaki T:
AMPD1: A novel therapeutic target for reversing insulin resistance.
BMC Endocr Disord. 14:962014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feng AF, Liu ZH, Zhou SL, Zhao SY, Zhu YX
and Wang HX: Effects of AMPD1 gene C34T polymorphism on cardiac
index, blood pressure and prognosis in patients with cardiovascular
diseases: A meta-analysis. BMC Cardiovasc Disord. 17:1742017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hay ID, Bergstralh EJ, Goellner JR,
Ebersold JR and Grant CS: Predicting outcome in papillary thyroid
carcinoma: Development of a reliable prognostic scoring system in a
cohort of 1779 patients surgically treated at one institution
during 1940 through 1989. Surgery. 114:1050–1058. 1993.PubMed/NCBI
|