Introduction
Cervical cancer is the third most common type of
cancer among women worldwide, and the majority of cases are
associated with infection caused by human papillomavirus (HPV)
(1). Radiotherapy, surgery and
chemotherapy are the current therapeutic options for the treatment
of cervical cancer. However, these treatments are limited by high
cost, high systemic toxicity, severity of side effects and drug
resistance (2). The mortality rate
for cervical cancer is relatively low in developed countries due to
cancer screening and the use of the HPV vaccine. However, it is the
major cause of cancer-associated mortality in women in developing
countries (3). Thus, more effective
and convenient anti-neoplastic treatments are required for cervical
cancer.
Numerous types of naturally derived phytochemicals
have been demonstrated to have anti-cancer effects on cervical
cancer cell lines (4–6). In a previous study, curcumin restored
p53, induced DNA damage and inhibited cell proliferation in HeLa
cells (7). Ellagic acid also
exhibited anti-tumor effects, since it arrested CaSki cells in
G0/G1 phase and induced apoptosis (8).
Isorhamnetin, an O-methylated flavonol, is mainly
extracted from sea buckthorn (Hippophae rhamnoides L.)
(9). Previous studies revealed that
isorhamnetin exerts multiple pharmacological functions, including
anti-inflammatory, antioxidant and anticancer activities (10–12).
Isorhamnetin has been reported to downregulate several inflammatory
proteins, including cyclooxygenase-2, prostaglandin E2, tumor
necrosis factor-α and nuclear factor κB (NF-κB) (11). Furthermore, isorhamnetin induced the
expression of NF-E2-related factor 2-dependent antioxidant genes,
resulting in reduced oxidative stress (13). In addition, isorhamnetin has been
demonstrated to upregulate p53, activate the expression of the
apoptotic factors B-cell lymphoma 2-associated X protein and
caspase-2, and induce apoptosis in lung cancer cells (14). Recently, it has been reported that
several isorhamnetin glycoside derivatives exhibit moderate
antitumor activity in cervical cancer (15,16).
However, to the best of our knowledge, the mechanism the antitumor
effect of isorhamnetin on cervical cancer cell lines has remained
elusive. Therefore, the present study investigated whether
isorhamnetin exerts anti-proliferative effects on the human
cervical cancer cell line HeLa.
Materials and methods
Isorhamnetin preparation
Isorhamnetin was from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). It was first dissolved in dimethyl sulfoxide
(DMSO) to generate a stock solution. For cell treatments, the stock
solution was further diluted in culture medium as required. The
final concentration of DMSO in the culture medium was <0.4%
(v/v).
Chemicals
Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS), propidium iodide and trypsin-EDTA were from
Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Bovine
serum albumin (BSA), DMSO and Trypan blue were from Sigma-Aldrich
(Merck KGaA). Penicillin and streptomycin were obtained from
M&C Gene Technology (Beijing, China).
Antibodies
The primary antibodies to checkpoint kinase (Chk) 1
(cat. no. 2360), Chk2 (cat. no. 3440) and call division cycle (Cdc)
2 (cat. no. 9116), Cdc25C (cat. no. 4688), phosphorylated (p)-Cdc2
(Tyr15; cat. no. 4539), p-Chk1 (Ser345; cat. no. 2348), p-Chk2
(Thr68; cat. no. 2197) and p-Cdc25C (Ser216; cat. no. 4901),
β-actin (cat. no. 4970) (all dilution 1:1,000) and cyclin B1 (cat.
no. 4135; dilution 1:2,000) were obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Primary antibodies for
α-tubulin (cat. no. ab7750) and β-tubulin (cat. no. ab70187) were
obtained from Abcam (Cambridge, MA, USA) and used at dilution
1:500. The secondary antibodies for goat anti-rabbit (cat. no.
A0208) and goat anti-mouse (cat. no. A0216) were purchased from
Beyotime Institute of Biotechnology (Haimen, China) and used at
dilution 1:1,000. For western blots, the antibodies were diluted in
0.5% blocking buffer (add 5 g BSA, 1.22 g Tris and 8.78 g NaCl to 1
l distilled water and adjust pH to 7.5).
Cell culture
HeLa cells were obtained from Bioleaf Company
(Shanghai, China) and cultured in DMEM supplemented with 10% FBS,
100 U/ml penicillin and 100 µg/ml streptomycin. The cells were
maintained in a humidified atmosphere of 5% CO2 at 37°C
and were passaged every 2–3 days.
Cell proliferation assay
The anti-proliferative activity of isorhamnetin was
measured using a Trypan blue dye exclusion assay (17). HeLa cells were seeded in a 96-well
plate at 5,000 cells/well. After 12 h, various concentrations of
isorhamnetin (0, 1, 10, 100 or 1,000 µmol/l) were applied to the
cells for 24, 48 or 72 h. The cells were then trypsinized and
re-suspended in PBS. Trypan blue dye solution (0.4%) was added to
the cell suspension. After 2 min, the number of colored (dead)
cells and unstained (viable) cells per mm2 was counted
under a phase contrast microscope. Results were expressed as the
mean ± standard deviation of six independent experiments. The
IC50 of isorhamnetin was determined using SPSS
Statistics version 19.0 (IBM Corp., Armonk, NY, USA).
Flow cytometric analysis
The cell cycle distribution was assessed by flow
cytometry (18). HeLa cells were
first cultured at a density of 1×106 cells/100 mm for 24
h and then the medium was replaced with fresh medium containing
various concentrations of isorhamnetin. After incubation for 24 or
72 h, cells were stained with propidium iodide and analyzed using a
flow cytometer (BD Accuri™ C6; BD Biosciences, Franklin Lakes, NJ,
USA). Data analysis was performed using BD CellQuest™ cell cycle
analysis software version 5.1 (BD Biosciences). The experiments
were performed in triplicate.
Western blot analysis
HeLa cells were treated with different
concentrations of isorhamnetin for 24 h. Subsequently, the cells
were washed with PBS. For the extraction of total protein, cells
were collected by scraping and incubated for 1 h in sample buffer
[150 mmol/l sodium chloride, 20 mmol/l Tris (pH 7.5), 1 mmol/l
EDTA, 2.5 mmol/l sodium pyrophosphate, 1 mmol/l β-glycerophosphate,
1% Triton X-100, 1 mmol/l sodium orthovanadate, 1 mmol/l phenyl
methane sulfonyl fluoride, 0.5% sodium deoxycholate, 1% protease
inhibitor and 20 mmol/l sodium fluoride], and then centrifuged at
12,000 × g for 30 min at 4°C. The total protein concentration in
the clear supernatant was evaluated using the Bradford method.
Aliquots containing 30 µg protein were subjected to 15% SDS-PAGE.
The proteins were then electrophoretically transferred to a
nitrocellulose membrane (cat. no. HATF00010; EMD Millipore,
Billerica, MA, USA). Membranes were blocked for 1 h at room
temperature with a 3% blocking buffer (add 30 g BSA in
Tris-buffered saline (TBS) buffer to final volume 1 l). The
membranes were rinsed with TBS buffer (add 1.22 g Tris and 8.78 g
NaCl to 1 l distilled water and adjust pH to 7.5 with HCl) three
times, for 5 min each time. The primary antibodies were added in 10
ml 0.5% blocking buffer and incubated for 2 h at room temperature.
The membranes were washed twice for 10 min each time with Tween-20
TBS buffer (add 0.5 ml 0.05% Tween-20 to 1 l TBS buffer). The
secondary antibodies were added in a 5 ml 0.5% blocking buffer and
incubated for 1 h at room temperature. The membranes were washed
twice for 10 min with Tween-20 TBS buffer. Specific signals were
detected by enhanced chemiluminescence (Amersham International; GE
Healthcare, Little Chalfont, UK). The densitometry was performed
using ImageJ software (National Institute of Health, Bethesda,
Maryland, USA) and the density of each band was normalized against
β-actin. All experiments were performed in triplicate.
Statistical analysis
Differences between the control and treatment groups
were analyzed by analysis of variance, followed by Duncan's
multiple range test. SPSS Statistics version 19.0 was used for
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference. Values are expressed as the
mean ± standard deviation of at least three independent
experiments.
Results
Isorhamnetin inhibits HeLa cell
proliferation
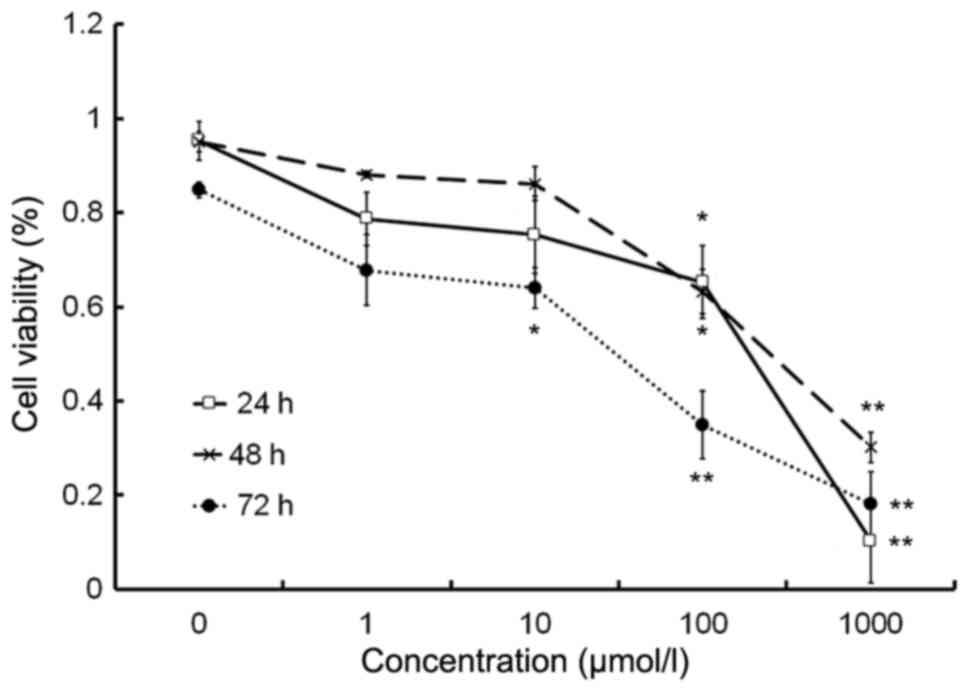
The cell proliferation assay demonstrated that
isorhamnetin inhibited the proliferation of HeLa cells at
concentrations of >10 µmol/l in a dose-dependent manner
(Fig. 1). The IC50 values
of isorhamnetin in HeLa cells were 100.03 µmol/l at 24 h, 304.15
µmol/l at 48 h and 54.79 µmol/l at 72 h.
Isorhamnetin induces cell cycle arrest
in G2/M phase
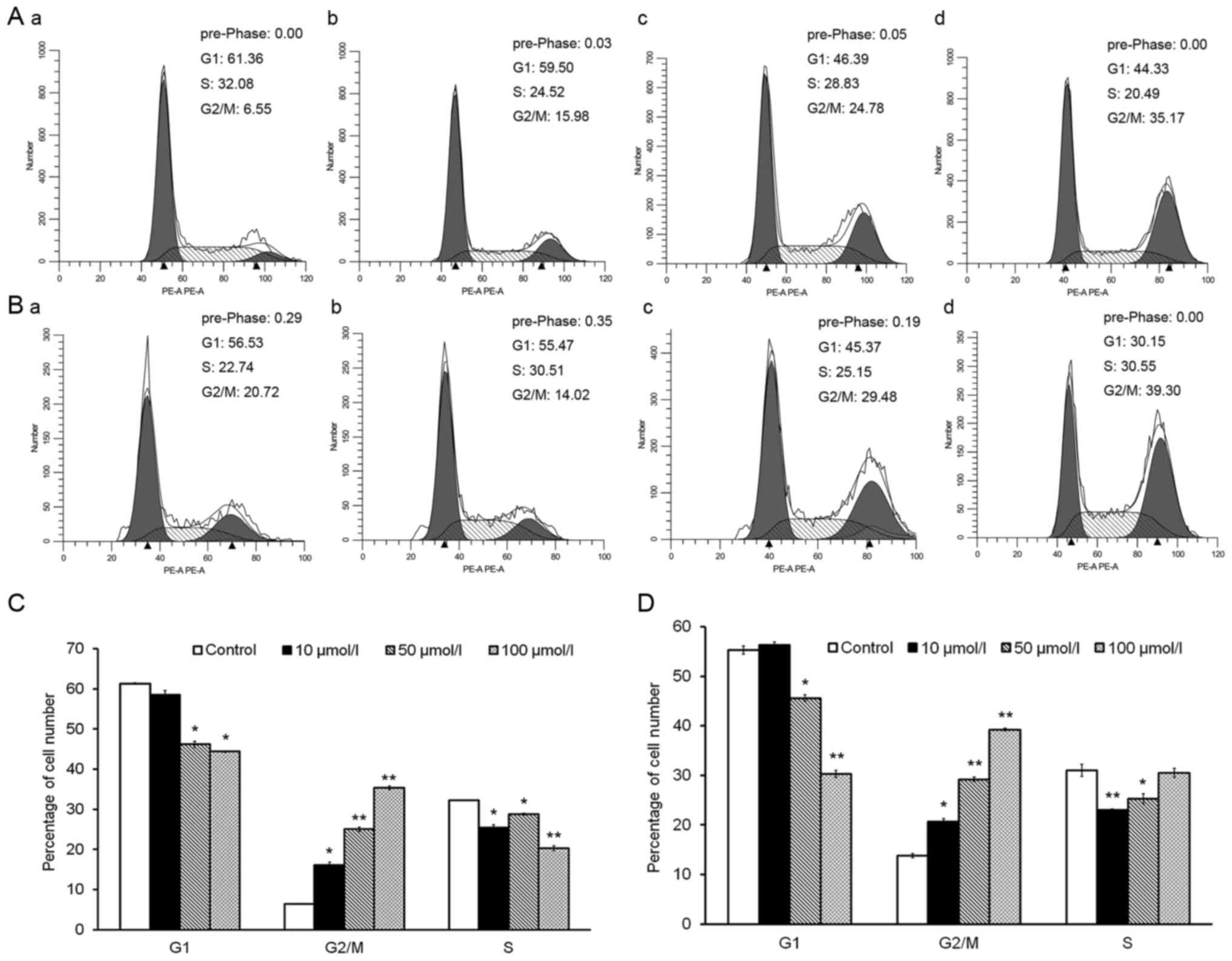
Cell cycle distribution analysis revealed that
treatment with isorhamnetin for 24 and 72 h significantly increased
the proportion of cells in G2/M phase (Fig. 2A and B). Upon treatment with 10, 50
or 100 µmol/l isorhamnetin for 24 h, the percentage of cells in
G2/M phase rose 2.5-, 3.8- and 5.5-fold, respectively, compared
with that in the control group (Fig.
2C). After treatment for 72 h, the same concentrations of
isorhamnetin increased the percentage of cells in G2/M phase by
1.5-, 2.1- and 2.8-fold, respectively, compared with those in the
control group (Fig. 2D). By
contrast, treatment with various concentrations of isorhamnetin for
24 and 72 h decreased the percentage of HeLa cells in G1 phase in a
dose-dependent manner (Fig. 2C and
D). These results indicated that isorhamnetin causes cell cycle
arrest at G2/M phase, accompanied by a decrease in the percentage
of cells in G1 phase, in a dose-dependent manner in HeLa cells.
Isorhamnetin alters the levels and
activity of G2/M phase-regulatory proteins
Isorhamnetin affects Chk2
phosphorylation
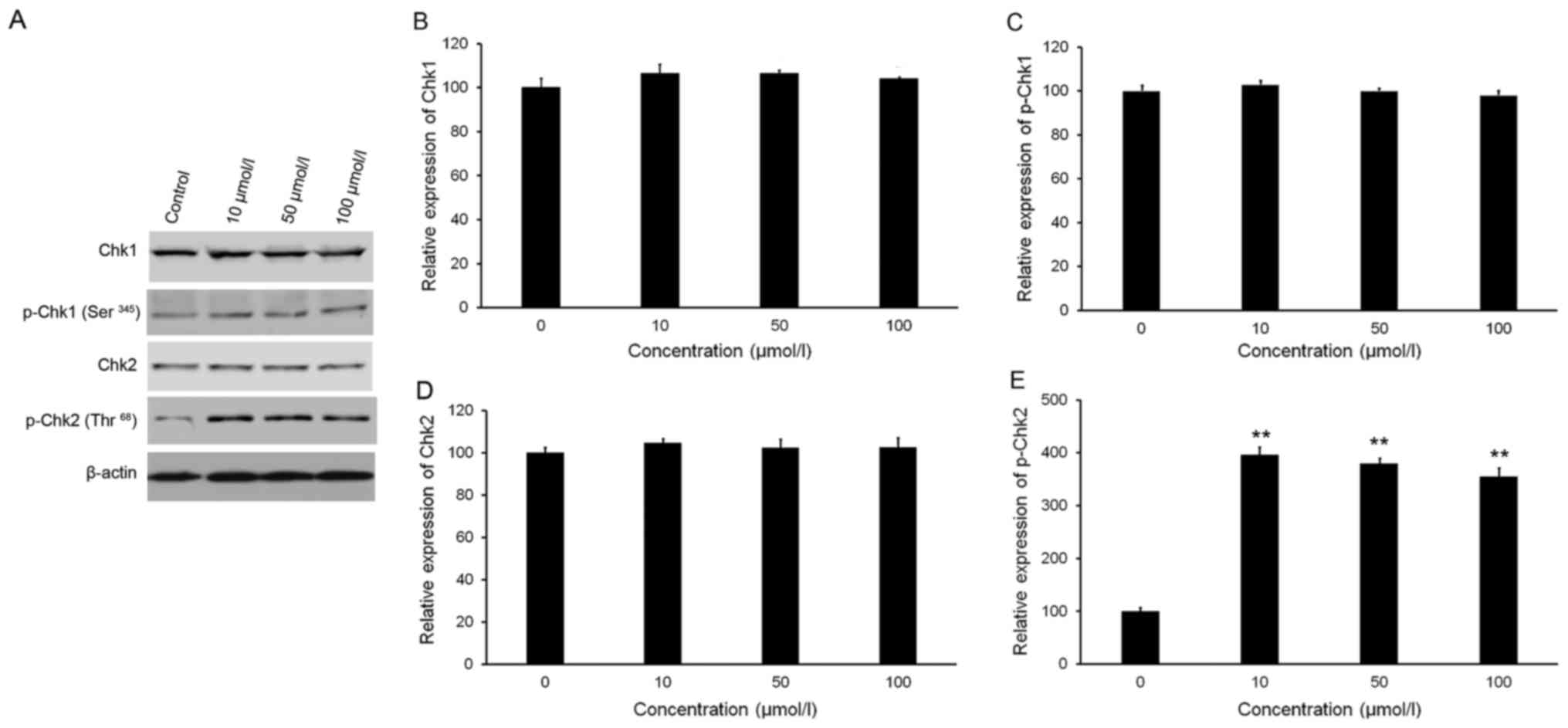
As presented in Fig.
3A-C, western blot analysis revealed no significant variations
in the phosphorylation and total protein levels of Chk1 following
treatment with isorhamnetin. Treatment with isorhamnetin markedly
increased the phosphorylation of Chk2 (Thr68), but with no
significant alteration in Chk2 total protein levels (Fig. 3A, D and E). Quantitative analysis
indicated that the level of p-Chk2 in HeLa cells treated with 10,
50 and 100 µmol/l isorhamnetin was increased by 4.0-, 3.8- and
3.5-fold, respectively, compared with that in the control group
(Fig. 3E).
Isorhamnetin affects the protein
levels of Cdc25C and p-Cdc25C
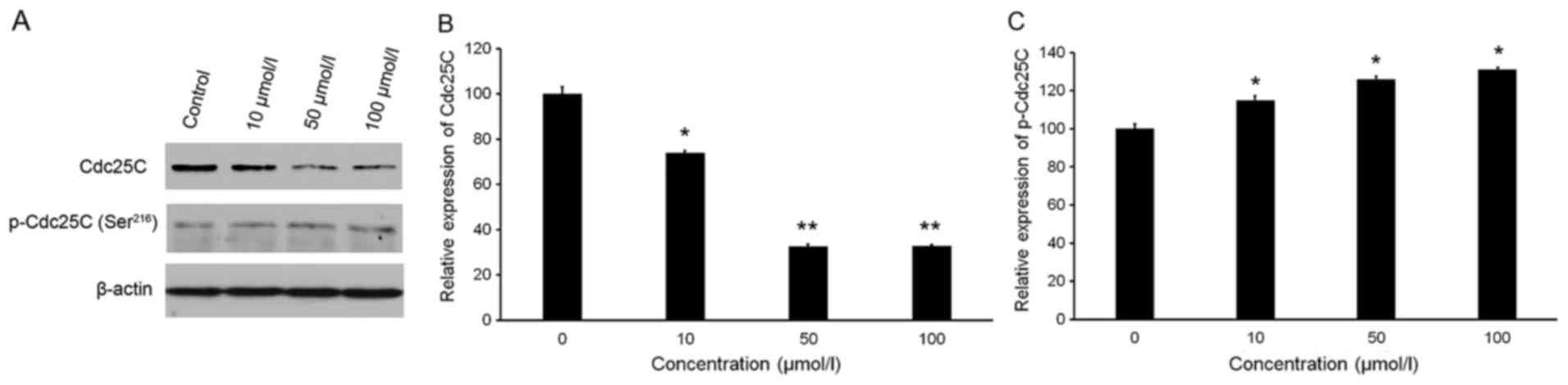
As presented in Fig. 4A
and B, isorhamnetin slightly increased the protein levels of
p-Cdc25C (Ser216). Conversely, isorhamnetin treatment significantly
inhibited the protein expression of Cdc25C (Fig. 4A and C). Treatment with 10, 50 and
100 µmol/l isorhamnetin caused 26.15, 67.57 and 67.25% inhibition
of Cdc25C levels, respectively, compared with the those in the
control group (Fig. 4C).
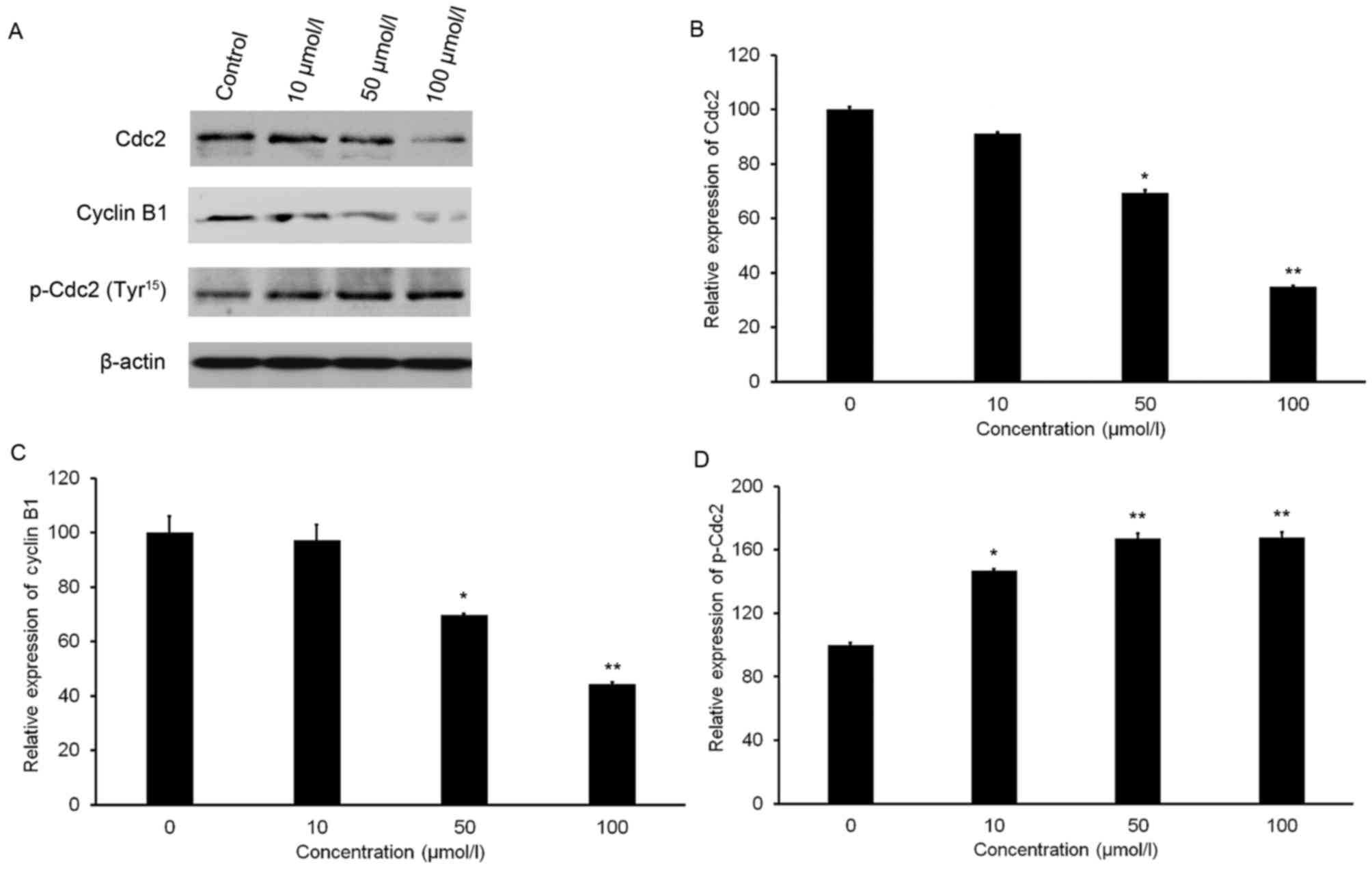
Isorhamnetin affects the protein
levels of Cdc2, p-Cdc2 and cyclin B1
A slight increase in the protein level of p-Cdc2
(Tyr15) was observed after isorhamnetin treatment (Fig. 5A and B). Furthermore, isorhamnetin
treatment resulted in a significant decrease in Cdc2 and cyclin B1
expression in a dose-dependent manner (Fig. 5A). Treatment with 100 µmol/l
isorhamnetin resulted in 65.28 and 55.81% decreases in Cdc2 and
cyclin B1 expression, respectively, compared with that in the
control group (Fig. 5C and D).
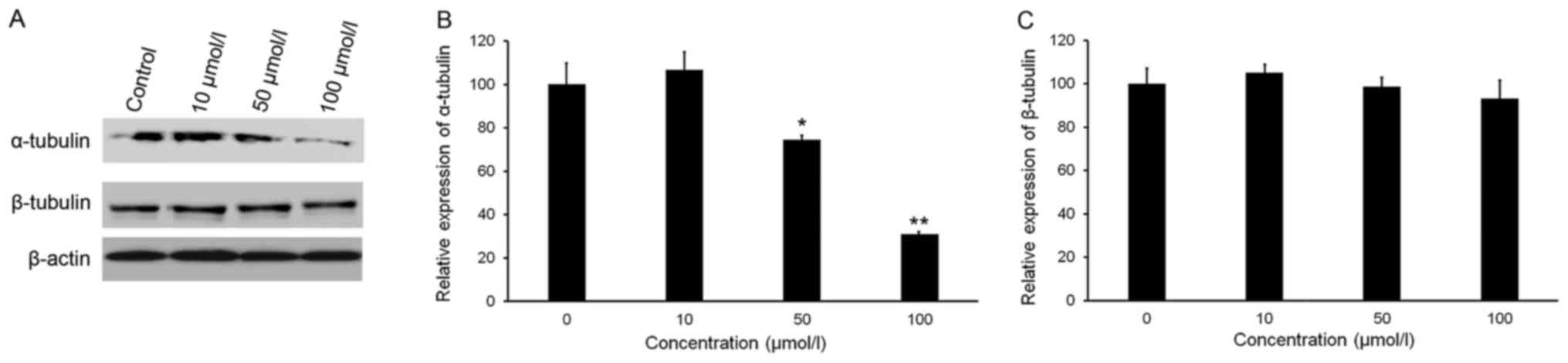
Isorhamnetin reduces the protein
levels of tubulins in HeLa cells
Maintenance of a critical threshold of tubulin
protein is essential for the function of microtubule networks.
Analysis of tubulin protein levels indicated that isorhamnetin
downregulated the expression of α-tubulin in a dose-dependent
manner, but did not affect the expression of β-tubulin (Fig. 6A-C). The relative expression of
α-tubulin in HeLa cells treated with 50 and 100 µmol/l isorhamnetin
decreased by 25.6 and 69.0%, respectively, compared with that in
the control group (Fig. 6C).
Discussion
The occurrence and progression of cervical carcinoma
is associated with HPV infection as well as a wide range of
cellular, epigenetic, genetic, immunological and environmental
factors (19). The present study was
the first to report the anti-proliferative function of isorhamnetin
in HeLa cervical cancer cells.
Initially, the anti-proliferative effect of
isorhamnetin on HeLa cells was assessed by the mitochondrial
respiration-dependent MTT reduction method. However, as
isorhamnetin has light absorption properties, it interfered with
the result of the MTT assay. Therefore, the trypan blue exclusion
method was used. The results indicated that isorhamnetin inhibited
the proliferation of HeLa cells in a dose-dependent manner at 24,
48 and 72 h. Of note, the IC50 of isorhamnetin after 48
h of treatment was nearly three times greater than that after 24 h
of treatment. As an underlying cause, it is possible that a repair
system was activated in cells treated with isorhamnetin for 48 h,
which will be investigated in further experiments.
The cell cycle determines cell proliferation and
regulates complex processes that determine cell growth and
division. All signaling pathways affecting the cell cycle must be
precisely regulated in order to determine the fate of the cell. In
almost all cancer cell types, a number of different mechanisms
influence the normal cell cycle (20). To date, numerous anticancer drugs
have been demonstrated to arrest the cell cycle at a certain phase
(18,21). In the present study, the results
indicated that isorhamnetin caused cell cycle arrest of HeLa cells
at G2/M phase in a dose-dependent manner. Therefore, isorhamnetin
may have potential as a treatment to prevent cancer growth.
Ataxia telangiectasia mutated (ATM) and ataxia
telangiectasia and Rad3-related (ATR) proteins are kinases that are
activated in the presence of cell damage signals, and induce either
cell cycle arrest or apoptosis. Certain antineoplastic drugs have
been demonstrated to activate these two checkpoint proteins
(22,23). Activated ATM and ATR phosphorylate
and activate two further checkpoint effector kinases, Chk1 and Chk2
(24), which phosphorylate a series
of functionally associated cellular substrates (25). Chk1 is thought to be activated
through phosphorylation of its Ser345 residue by ATR, and Chk2 is
activated through phosphorylation of Thr68 by ATM (26,27). In
the present study, treatment with isorhamnetin markedly increased
p-Chk2 (Thr68), with no significant change in Chk2 total protein
expression. However, no obvious alteration was observed in the
protein level of p-Chk1 (Ser345). The results implied that
isorhamnetin may cause cell cycle arrest at the G2/M phase via
activation of the ATM-Chk2 pathway.
Activation of Chk1 or Chk2 leads to the
phosphorylation of a variety of cell cycle regulatory proteins,
including Cdc25C (28). Furthermore,
p-Cdc25C (Ser216) provides a binding site for 14-3-3 family
proteins, resulting in inhibition of Cdc25C-mediated G2/M
transition (29). In the present
study, western blot analysis demonstrated that Cdc25C protein
expression was decreased, while its inactive form, p-Cdc25C
(Ser216), was slightly elevated, indicating the inactivation of
Cdc25C.
Cdc2 [also known as cyclin D kinase 1] and cyclin B1
constitute a complex called mitosis-promoting factor. This complex
is involved in regulating G2/M transition (30). As cells enter into S and
G2 phases, complexes of Cdc2 with B-type cyclins are in
an inactive state due to Tyr15 phosphorylation of Cdc2. However, at
the onset of the M phase, Cdc25 tyrosine phosphatases
dephosphorylate Cdc2 at Tyr15. The Cdc2-cyclin B complexes trigger
numerous events associated with the M phase (31,32). In
order to elucidate the mechanism of cell cycle arrest upon
isorhamnetin treatment, the protein levels of p-Cdc2 (Tyr15), Cdc2
and cyclin B1 were compared. Treatment with isorhamnetin
dose-dependently decreased Cdc2 and cyclin B1, with a slight
increase in p-Cdc2 (Tyr15). These changes may be associated with
cell cycle arrest of HeLa cells in G2/M phase.
Microtubules are a component of the cytoskeleton. A
large and diverse group of anticancer drugs derived from natural
products target microtubules in cancer cells. Microtubules are
composed of tubulin proteins (α-tubulin and β-tubulin), and are
assembled dynamically through polymerization and de-polymerization.
The normal regulation of microtubule assembly serves a key function
in cells during M phase (33). Thus,
in the present study, α-tubulin and β-tubulin expression were
determined after treatment with isorhamnetin. Western blot analysis
demonstrated that the expression of α-tubulin was reduced in
isorhamnetin-treated cells, but there were no significant
differences in β-tubulin expression. In summary, these results
indicated that isorhamnetin disrupted tubulin polymerization,
causing cell cycle arrest at G2/M phase.
In conclusion, the present study demonstrated that
isorhamnetin significantly inhibits the proliferation of HeLa cells
in vitro. The mechanism of the anti-proliferative effect of
isorhamnetin was closely associated with cell cycle arrest at the
G2/M phase through activation of the ATM-Chk2 pathway and
disruption of microtubule function. The present study provides a
basis for the development of isorhamnetin as a potential
therapeutic drug for cervical cancer. Regarding the reproducibility
of the results, it is required to test the effects of isorhamnetin
on further cervical cancer cell lines e.g., SiHa and CaSki, in the
future.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Projects of
International Cooperation and Exchanges of the Ministry of Science
and Technology in China (grant no. 2014DFR31230) and the National
Natural Science Foundation of China (grant no. 31360382).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW conceived and designed the study, performed the
majority of the experiments and wrote the paper. HS performed the
flow cytometric analysis. YB helped to design the study, reviewed
and edited the manuscript and approved the final version to be
published. JL performed the cell culture. LF helped to analyze the
data. WS helped to analyze the data and was a major contributor in
writing the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Arbyn M, Castellsagué X, de Sanjosé S,
Bruni L, Saraiya M and Bray F: Worldwide burden of cervical cancer
in 2008. Ann Oncol. 22:2675–2686. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Farrand L, Oh SW, Song YS and Tsang BK:
Phytochemicals: A multitargeted approach to gynecologic cancer
therapy. Biomed Res Int. 2014:8901412014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kumar D, Basu S, Parija L, Rout D, Manna
S, Dandapat J and Debata PR: Curcumin and ellagic acid
synergistically induce ROS generation, DNA damage, p53 accumulation
and apoptosis in HeLa cervical carcinoma cells. Biomed
Pharmacother. 81:31–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Debata PR, Castellanos MR, Fata JE,
Baggett S, Rajupet S, Szerszen A, Begum S, Mata A, Murty VV, Opitz
LM and Banerjee P: A novel curcumin-based vaginal cream Vacurin
selectively eliminates apposed human cervical cancer cells. Gynecol
Oncol. 129:145–153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thacker PC and Karunagaran D: Curcumin and
emodin down-regulate TGF-β signaling pathway in human cervical
cancer cells. PLoS One. 10:e01200452015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maher DM, Bell MC, O'Donnell EA, Gupta BK,
Jaggi M and Chauhan SC: Curcumin suppresses human papillomavirus
oncoproteins, restores p53, Rb, and PTPN13 proteins and inhibits
benzo[a]pyrene-induced upregulation of HPV E7. Mol Carcinog.
50:47–57. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Narayanan BA, Geoffroy O, Willingham MC,
Re GG and Nixon DW: p53/p21(WAF1/CIP1) expression and its possible
role in G1 arrest and apoptosis in ellagic acid treated cancer
cells. Cancer Lett. 136:215–221. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y, Chi G, Shen B, Tian Y and Feng H:
Isorhamnetin ameliorates LPS-induced inflammatory response through
downregulation of NF-κB signaling. Inflammation. 39:1291–1301.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang JS, Shih CM, Wang SH, Chen TT, Lin
CN and Ko WC: Mechanisms of suppression of nitric oxide production
by 3-O-methylquercetin in RAW 264.7 cells. J Ethnopharmacol.
103:281–287. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang JH, Kim SC, Shin BY, Jin SH, Jo MJ,
Jegal KH, Kim YW, Lee JR, Ku SK, Cho IJ and Ki SH: O-Methylated
flavonol isorhamnetin prevents acute inflammation through blocking
of NF-κB activation. Food Chem Toxicol. 59:362–372. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saud SM, Young MR, Jones-Hall YL, Ileva L,
Evbuomwan MO, Wise J, Colburn NH, Kim YS and Bobe G:
Chemopreventive activity of plant flavonoid isorhamnetin in
colorectal cancer is mediated by oncogenic Src and β-catenin.
Cancer Res. 73:5473–5484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang JH, Shin BY, Han JY, Kim MG, Wi JE,
Kim YW, Cho IJ, Kim SC, Shin SM and Ki SH: Isorhamnetin protects
against oxidative stress by activating Nrf2 and inducing the
expression of its target genes. Toxicol Appl Pharmacol.
274:293–301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Q, Ren FQ, Yang CL, Zhou LM, Liu YY,
Xiao J, Zhu L and Wang ZG: Anti-proliferation effects of
isorhamnetin on lung cancer cells in vitro and in vivo. Asian Pac J
Cancer Prev. 16:3035–3042. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sobral F, Calhelha RC, Barros L, Dueñas M,
Tomás A, Santos-Buelga C, Vilas-Boas M and Ferreira IC: Flavonoid
composition and antitumor activity of bee bread collected in
northeast Portugal. Molecules. 22(pii): E2482017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi X, Liu D, Zhang J, Hu P, Shen W, Fan
B, Ma Q and Wang X: Extraction and purification of total flavonoids
from pine needles of Cedrus deodara contribute to anti-tumor in
vitro. BMC Complement Altern Med. 16:2452016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Strober W: Trypan blue exclusion test of
cell viability. Curr Protoc Immunol. 111:A3.B.1–3. 2015. View Article : Google Scholar
|
|
18
|
Lv TZ and Wang GS: Antiproliferation
potential of withaferin A on human osteosarcoma cells via the
inhibition of G2/M checkpoint proteins. Exp Ther Med. 10:323–329.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Burd EM: Human papillomavirus and cervical
cancer. Clin Microbiol Rev. 16:1–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miao R, Wei J, Lv M, Cai Y, Du Y, Hui X
and Wang Q: Conjugation of substituted ferrocenyl to thiadiazine as
apoptosis-inducing agents targeting the Bax/Bcl-2 pathway. Eur J
Med Chem. 46:5000–5009. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu Y, Ching YP, Zhou Y, Chiu JF, Chen F
and He QY: Multiple pathways were involved in tubeimoside-1-induced
cytotoxicity of HeLa cells. J Proteomics. 75:491–501. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meyn MS: Ataxia-telangiectasia and
cellular responses to DNA damage. Cancer Res. 55:5991–6001.
1995.PubMed/NCBI
|
|
23
|
Shiloh Y: Ataxia-telangiectasia: Closer to
unraveling the mystery. Eur J Hum Genet. 3:116–138. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Khanna KK and Jackson SP: DNA
double-strand breaks: Signaling, repair and the cancer connection.
Nat Genet. 27:247–254. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
O'Neill T, Giarratani L, Chen P, Iyer L,
Lee CH, Bobiak M, Kanai F, Zhou BB, Chung JH and Rathbun GA:
Determination of substrate motifs for human Chk1 and hCds1/Chk2 by
the oriented peptide library approach. J Biol Chem.
277:16102–16115. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao H and Piwnica-Worms H: ATR-mediated
checkpoint pathways regulate phosphorylation and activation of
human Chk1. Mol Cell Biol. 21:4129–4139. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ahn JY, Schwarz JK, Piwnica-Worms H and
Canman CE: Threonine 68 phosphorylation by ataxia telangiectasia
mutated is required for efficient activation of Chk2 in response to
ionizing radiation. Cancer Res. 60:5934–5936. 2000.PubMed/NCBI
|
|
28
|
Hirao A, Cheung A, Duncan G, Girard PM,
Elia AJ, Wakeham A, Okada H, Sarkissian T, Wong JA, Sakai T, et al:
Chk2 is a tumor suppressor that regulates apoptosis in both an
ataxia telangiectasia mutated (ATM)-dependent and an
ATM-independent manner. Mol Cell Biol. 22:6521–6532. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Agarwal C, Tyagi A and Agarwal R: Gallic
acid causes inactivating phosphorylation of cdc25A/cdc25C-cdc2 via
ATM-Chk2 activation, leading to cell cycle arrest, and induces
apoptosis in human prostate carcinoma DU145 cells. Mol Cancer Ther.
5:3294–3302. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pines J and Hunter T: Human cyclin A is
adenovirus E1A-associated protein p60 and behaves differently from
cyclin B. Nature. 346:760–763. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Russell P and Nurse P: The mitotic inducer
nim1+ functions in a regulatory network of protein
kinase homologs controlling the initiation of mitosis. Cell.
49:569–576. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kumagai A and Dunphy WG: The cdc25 protein
controls tyrosine dephosphorylation of the cdc2 protein in a
cell-free system. Cell. 64:903–914. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jordan MA: Mechanism of action of
antitumor drugs that interact with microtubules and tubulin. Curr
Med Chem Anticancer Agents. 2:1–17. 2002. View Article : Google Scholar : PubMed/NCBI
|