Introduction
Marrow mesenchymal stem cells (MSCs) derived from
the bone marrow stromal system have different and distinct lineage
potential (1). Bone marrow MSCs may
be easily isolated and exhibit a multipotent nature, including the
ability to be greatly expanded in vitro and induced to
differentiate into multiple mesenchymal cell types (2). MSCs serve as precursors for various
mesoderm-type cells, including osteoblasts, chondroblasts and
adipocytes (3–5). As MSCs are easily obtained and have
strong osteogenic differentiation capabilities, they are widely
applied in cellular therapy, tissue repair and regenerative
medicine (6,7).
Differentiation of MSCs into osteoblasts is a
complex process that is regulated by the expression of various
transcription factors, predominantly vascular endothelial growth
factor (VEGF) and bone morphogenetic protein 2 (BMP-2), and
expression of osteoblast-specific proteins, including alkaline
phosphatase (ALP) and collagen type (Ct) II (8–10).
Dexamethasone and other factors, including transforming growth
factor β (TGFβ), cyclooxygenase 2 (COX2) and platelet lysate, as
pluralistic osteogenic inducers have attracted widespread attention
from researchers (11). Ahmad and
Shakoori (12) have illustrated that
dexamethasone treatment accelerated murine MSC proliferation and
induced early differentiation of osteoblasts. BMP-2 is the most
widely studied and is the most potent inducer of osteoblastic
differentiation (13,14). It has previously been reported that
BMP-2 enhanced TGFβ3-mediated chondrogenesis of human bone marrow
MSCs, which suggested that the combination of BMP-2 and TGFβ3 is
superior to promote MSC osteogenic differentiation using TGF-β
alone in MSCs chondrogenesis (15).
Multiple studies have demonstrated that
environmental and hormonal factors as well as factors secreted by
the cells themselves, serve an important role in the
differentiation of MSCs into osteoblasts (16,17).
Various approaches and methodologies have been utilized to study
the molecular mechanism of MSC differentiation into osteoblasts
(18–20). In the present study, MSCs were
isolated from bone marrow of rabbits and the differentiation
process of osteoblasts was studied using various cell culture
media. Prior to differentiation, the first, second and third
passages of cells were performed to observe cell morphology
alterations, and the positive rate of MSC antigen presentation of
cluster of differentiation CD44 and CD105 was measured for cell
identification. Following various cultures, the activity of ALP by
Alizarin Red S staining was determined, and Ct I and osteocalcin
activities were measured using immunohistochemical staining.
Reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and western blotting were used to evaluate the expression
of VEGF, BMP-2 and Ct II.
Materials and methods
Experimental animals
One male New Zealand white rabbit (age, 4 months;
weight, 2.5–3.0 kg) was obtained from Hubei Provincial Center for
Disease Control and Prevention (Wuhan, China). The rabbit was
housed in a cage at 25±3°C in a 12 h light/dark cycle with 50%
relative humidity and received dry pellets ad libitum with
the intermittent addition of green fodder. All animal experiments
were performed according to the Policies on the Use of Animals and
Humans in Neuroscience Research, revised and approved by the
Society for Neuroscience in 1995 (21). All protocols involving animal
specimens were approved by the Ethical Committee of Tongji
Hospital, Tongji Medical College, Huazhong University of Science
and Technology Institution (Wuhan, China).
Isolation and culture of rabbit
MSCs
A total of 10–15 ml femoral supracondylar bone
marrow of New Zealand rabbits was extracted by bone marrow cavity
puncture as previously described (12). MSCs were flushed out using Dulbecco's
modified Eagle's medium (DMEM; incomplete) (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). A single cell suspension was
obtained by passing the flushed-out cells through a 22-gauge needle
several times and counting the number of cells using a
hemocytometer. DMEM supplemented with 10% fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc.), glutamine 10 mmol/l,
100 U/ml penicillin and 100 µg/ml streptomycin was added to the
cell suspension in 12-well culture plates. The plates were
incubated at 37°C with 95% air and 5% CO2. After 96 h of
incubation, the cells were washed with PBS and transferred to
96-well culture plates and cultured at 37°C with 95% air and 5%
CO2. When cells reached 80% confluence, the attached
cells were trypsinized with 0.125% trypsin for subculture.
Flow cytometric identification
Cells at the fourth passage were digested using
0.125% trypsin and centrifuged at 500 × g for 10 min at room
temperature. Following centrifugation, the cells were washed with
PBS three times, and the cell concentration was adjusted to
1×106 cells/ml. The cells were incubated in 3% bovine
serum albumin (BSA; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
for 20 min at 4°C to block non-specific protein.
Fluorescence-labeled anti-CD44 (ab157107; 1:100 dilution) and
anti-CD105 (ab135528; 1:50 dilution) (both Abcam, Cambridge, UK)
antibodies were added to each tube and incubated for 20 min in
darkness at 4°C. The unbound antibodies were washed off using PBS.
The cells were subsequently incubated with fluorescein
isothiocyanate conjugated goat-anti-rabbit immunoglobulin (Ig) G
secondary antibodies (ab97050; 1:200; Abcam) for 40 min at room
temperature. Cell surface antigens were detected using a flow
cytometer (Cytomics FC 500; Beckman Coulter, Inc., Brea, CA, USA)
and the results analyzed by CXP 2.1 software (Beckman Coulter,
Inc.).
Differentiation of induced, cultured
MSCs into osteoblasts
For osteoblast induction, second passage MSCs
(inoculation concentration, 1×106 cells/ml) were
cultured in each well of 24-well plates and divided into six
experimental groups (n=3) and a control group (n=3). The six
experimental groups were created by the addition of one of the
following to the complete culture medium: Dexamethasone
1×10−8 mol/l dexamethasone (D4902) + 10 mmol/l sodium
β-glycerophosphate (G9422) + 50 µg/l vitamin C (A4544); BMP-2
(B1814); COX2 (C0858); platelet lysate (SCM141);
1,25-dihydroxyvitamin D3[1,25-(OH)2VD3] (H107) (all
Sigma-Aldrich; Merck KGaA); and TGFβ (14-8348-62; Thermo Fisher
Scientific, Inc.). The cells of the control group were cultured in
complete medium. Cells were incubated at 37°C with 95% air and 5%
CO2 and the culture medium in each group was changed
every 3 days.
ALP activity
ALP activity staining was measured in all
experimental groups and the control group after 7 days of culture.
For this purpose, MSC layers were washed twice with PBS, fixed with
4% paraformaldehyde for 10 min at 4°C, then, rinsed with PBS and
stained with nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl
phosphate for 2 h in darkness at room temperature. The chromogenic
reaction was stopped by washing twice with dH2O and
following drying the samples were observed using a light microscope
at magnification, ×100.
Alizarin Red S staining
Alizarin Red S staining was performed after growing
the cells for 21 days in induction media. MSCs were washed twice
with PBS and fixed with ice-cold 70% ethanol for 1 h at 4°C, and
then rinsed with dH2O twice. Alizarin Red S solution was
added to cover the cells and incubated at room temperature for 30
min. The wells were washed four times with dH2O and
images were taken using an inverted microscope at magnification,
×100.
Immunohistochemical staining
The cell pellet was fixed in 4% paraformaldehyde for
20 min at 4°C and washed twice with PBS. Cells were incubated in
0.5% Txiton X-100 for 20 min at room temperature, washed twice with
PBS, and then treated with 3% H2O2 for 15 min
for the quenching of endogenous peroxidase activity at room
temperature. The sections were washed twice using PBS for 2 min and
antigen retrieval was performed with 2 mg/ml protease at 37°C for
30 min. Sections were washed with PBS for 5 min and non-specific
staining was blocked with 5% goat serum (Sigma-Aldrich; Merck KGaA;
1:20 dilution with PBS containing 1% BSA) for 20 min at room
temperature, followed by incubation with specific antibody to Ct I
and osteocalcin. Following cell cultured induction for 7 days, Ct I
was detected. Sections were incubated with a mouse monoclonal
antibodies against Ct I (ab21286; 1:200 dilution; Abcam) overnight
at 4°C. The spatial localization of Ct I was visualized by
incubation with mouse IgG horseradish peroxidase (HRP)-conjugated
secondary antibodies (ab6728; 1:200 dilution; Abcam) for 1 h at
room temperature, followed by 3,3′-diaminobenzidine
tetrahydrochloride (Sigma-Aldrich; Merck KGaA) in the presence of
H2O2 for 5–10 min at room temperature.
Following induction for 14 days, osteocalcin was
detected. Sections were incubated with a mouse monoclonal antibody
to osteocalcin (1:200; Abcam) overnight at 4°C. The spatial
localization of osteocalcin was visualized by incubation with goat
IgG HRP-conjugated secondary antibody (ab6728; 1:200 dilution;
Abcam) for 1 h at room temperature, followed by
3,3′-diaminobenzidine tetrahydrochloride (Sigma, Missouri, USA) in
the presence of H2O2 for 5–10 min at room
temperature.
Following this, the sections were rinsed with PBS,
counterstained with hematoxylin for 3 min at room temperature,
dehydrated with graded ethanol (80% alcohol 1 min, 95% alcohol 1
min twice and dehydrated alcohol 1 min) and xylene for 1 min twice,
and mounted with Entellan® (KGaA). The sections were
observed by light microscopy (CX41; Olympus Corporation, Tokyo,
Japan) at magnification, ×200.
RNA extraction and RT-qPCR
Total RNA was extracted from cells in each group
with TRIzol reagent (Takara Biotechnology Co., Ltd., Dalian, China)
and detected by an ultraviolet spectrophotometer and 1% agarose
electrophoresis and visualized with ethidium bromide. For each
sample, 1 µg RNA was reverse transcribed to obtain first-strand
cDNA using a PrimeScript® RT reagent kit with gDNA
Eraser (Takara Biotechnology Co., Ltd.) according to the
manufacturer's protocol at 42°C for 2 min, 37°C for 15 min and 85°C
for 15 sec. Expression levels of target genes BMP2, VEGF and Ct II
were analyzed using RT-qPCR. Primer Premier 5.0 (PREMIER Biosoft
International, Palo Alto, CA, USA) was used to design the
fluorescent primers (Table I). Each
reaction (20 µl total volume) contained 10 µl 2X SYBR Premix Ex
Taq™ (Takara Biotechnology Co., Ltd.), 0.50 µmol/l each
primer and 0.2±0.02 µg cDNA template. The following three-step
RT-qPCR reaction was performed: Pre-denaturation at 95°C for 30
sec, followed by 40 cycles of denaturation at 95°C for 5 sec,
annealing at 60°C for 20 sec and elongation at 72°C for 20 sec. The
transcriptional levels of genes were calculated using the
2−ΔΔCq method (22). By
genome analysis, the results demonstrated that GAPDH was the most
stable gene among the three commonly used housekeeping genes GAPDH,
18s ribosomal RNA and β-actin in all cells. The threshold cycle
(Ct) was determined for each reaction by using the
2−ΔΔCq method, which generated Ct values for each gene
of interest normalized to the endogenous control gene (GAPDH). The
quantification of target and reference genes was evaluated using
standard curves, and the ratio between the target and reference
gene represented the relative expression levels of the target gene.
For each group, three samples were measured, and three technical
replicates of each measurement were obtained.
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction.
|
| Direction
(5′-3′) |
|---|
|
|
|
|---|
| Gene | Forward | Reverse |
|---|
| Bone morphogenetic
protein 2 |
GTGAGGATTAGCAGGTCTT |
CTGGATTTGAGGCGTTT |
| Vascular
endothelial growth factor |
GACATCTTCCAGGAGTACCC |
GAGGTTTGATCCGCATGAT |
| Collagen type
II |
AACACTGCCAACGTCCAGAT |
AGTGGATATGGCACGACGTC |
| GAPDH |
ACCCACTCCTCTACCTTCG |
CACCACCCTGTTGCTGT |
Western blot analysis
Western blot analysis was performed to determine the
expression of VEGF, BMP-2 and Ct II proteins in MSCs cultured in
different cell culture media. The cells were homogenized with RIPA
lysis buffer (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China) and centrifuged at 12,000 × g for 20 min at 4°C.
The protein concentration was determined using a bicinchoninic acid
protein assay kit (Bio-swamp; Wuhan Beinglay Biological Technology
Co., Ltd., Wuhan, China). Equal amounts of protein (30 µg) were
separated by 10% SDS-PAGE and subsequently transferred to a
polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA,
USA). The membranes were blocked for 2 h at room temperature with
5% skim milk in Tris-buffered saline (20 mmol/l Tris, 500 mmol/l
NaCl and 0.05% Tween 20). Subsequently, the membrane was incubated
with specific antibodies overnight at 4°C, including rabbit
anti-BMP-2 antibody (ab14933; 1:1,000; Abcam), rabbit anti-VEGF
antibody (ab32152; 1:4,000; Abcam) and human anti-Ct II antibody
(ab159157; 1:2,000; Abcam). Anti-GAPDH antibody (ab181602;
1:10,000; Abcam) was selected as an internal reference. Following
this, the membranes were washed with Tris-buffered saline and
incubated with goat anti-rabbit secondary antibody (PAB160009;
1:10,000; Bio-swamp; Wuhan Beinglay Biological Technology Co.,
Ltd.) for 2 h at room temperature. Immunoreactivity was visualized
by colorimetric reaction using an enhanced chemiluminescence
substrate buffer (EMD Millipore). Membranes were scanned with a Gel
Doz EZ imager and the bands were quantified using Quantity One 5.0
software (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
All data were expressed as the mean ± standard
deviation. Statistical differences were analyzed by one-way
analysis of variance using SPSS 18.0 (SPSS, Inc., Chicago, IL,
USA). Significant differences were detected by the Duncan's test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
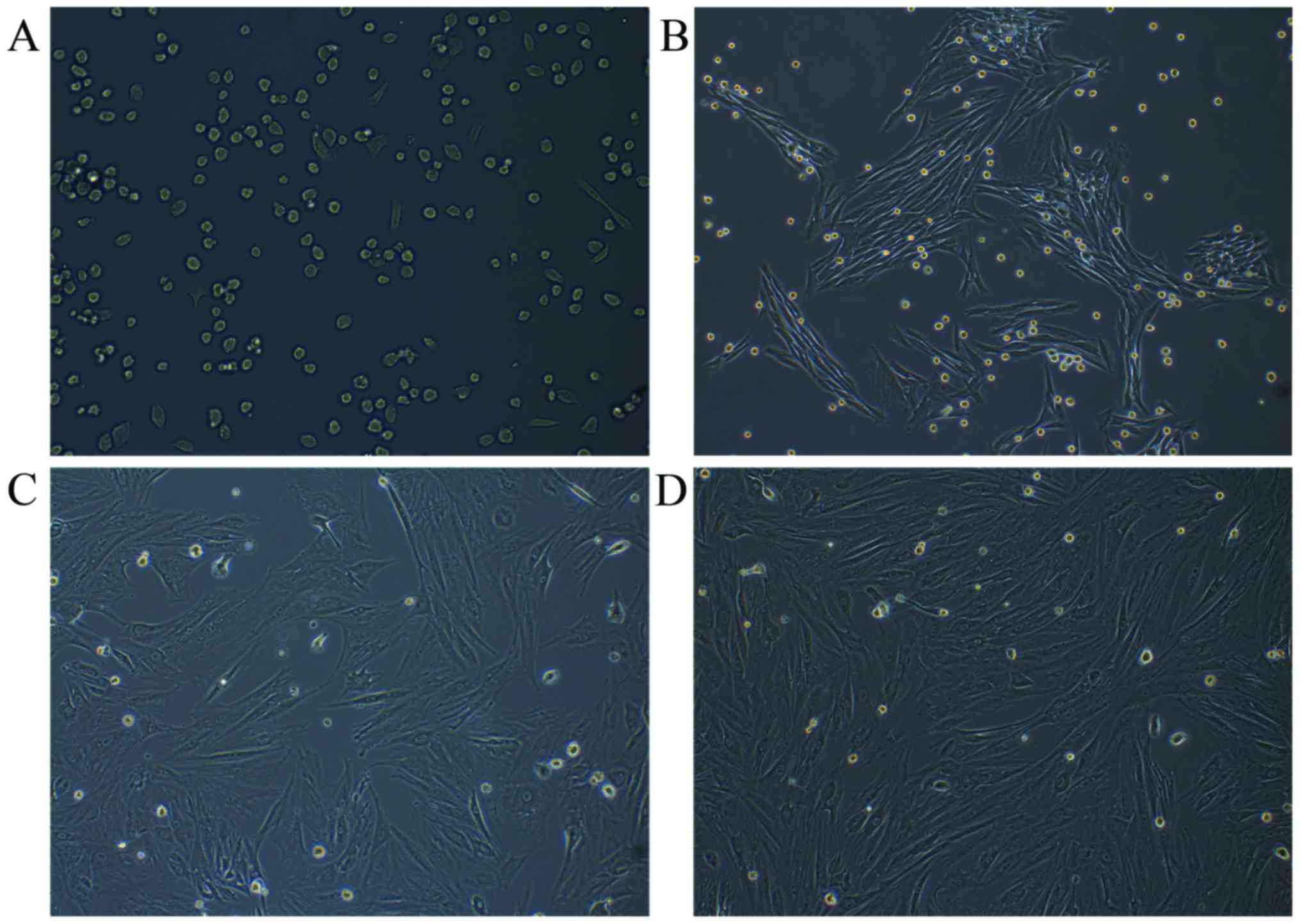
Observation and identification of
rabbit MSCs
Rabbit MSCs were successfully isolated following the
incubation of flushed-out cells from bone marrow. The MSCs were
attached to the surface of the plate and were predominantly
circular-shaped cells of uniform size after culture for 24 h
(Fig. 1A). At the first, second and
third passages, after 5 days, the cells were spindle-shaped
(fibroblast-like cells), which is typical morphology of MSCs
(Fig. 1B-D).
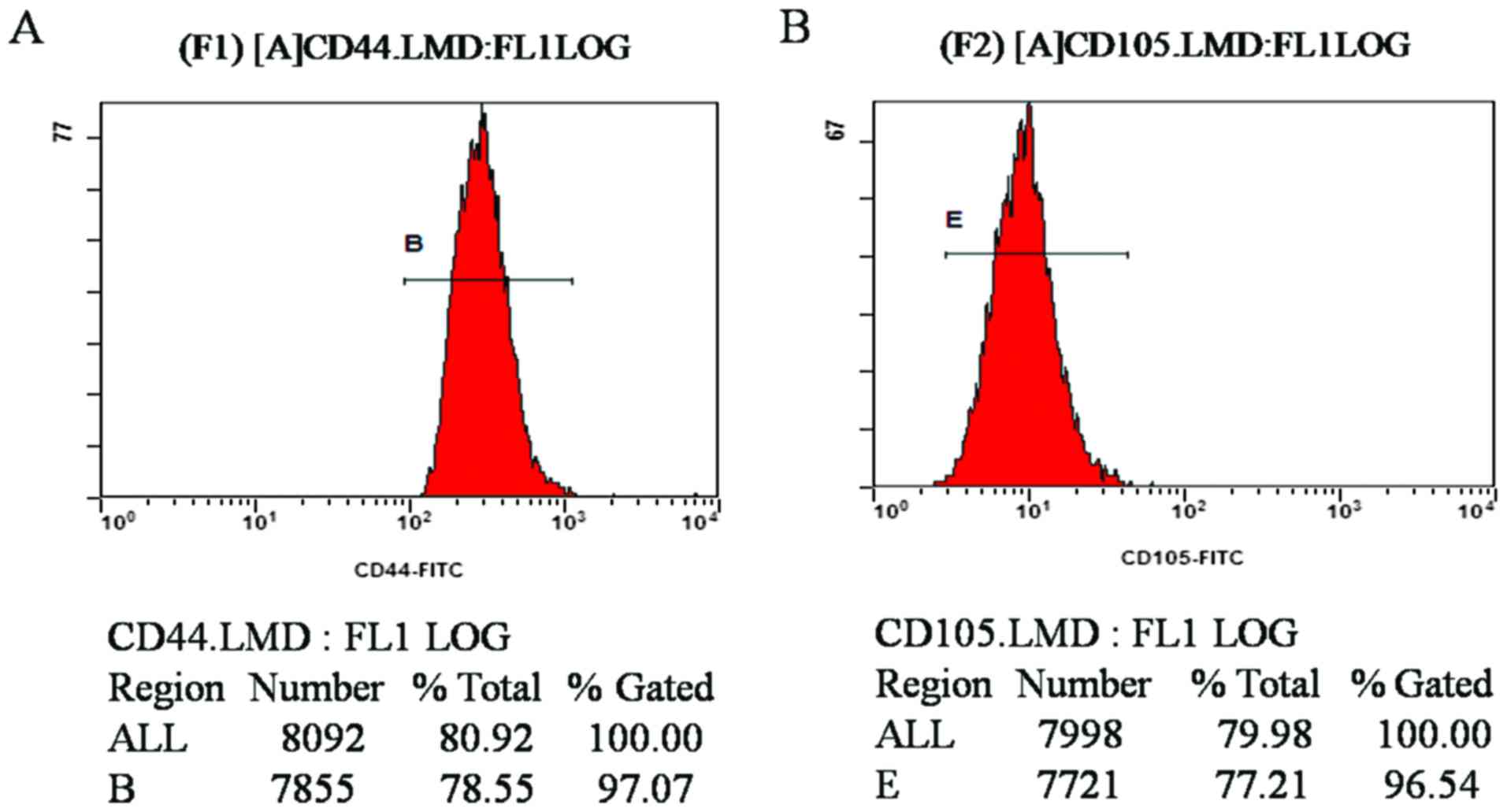
Detection of rabbit MSC surface
antigens
Flow cytometric results demonstrated that the CD44-
and CD105-positive rates of MSCs at the fourth passage were 97.07
and 96.54%, respectively (Fig. 2A and
B). The results revealed that the purity of the isolated and
cultured MSCs in the experiment was high, confirming that the cells
were derived from differentiated BMSCs following induction.
ALP staining
Following culture in osteogenic medium for 7 days,
ALP staining was positive. Cells demonstrated gray-black and black
granule precipitate in the cytoplasm precipitation (Fig. 3). Compared with the control group,
ALP activity was markedly increased in the dexamethasone group,
BMP-2 group and TGFβ group (Fig. 3A, B
and D). The ALP activity in the COX2 group was higher than that
in the 1,25-(OH)2VD3 group and platelet lysate group,
but the COX-2 group was similar to the control group (Fig. 3E). These results indicated that
treated with dexamethasone, BMP-2 or TGFβ notably increased ALP
activity.
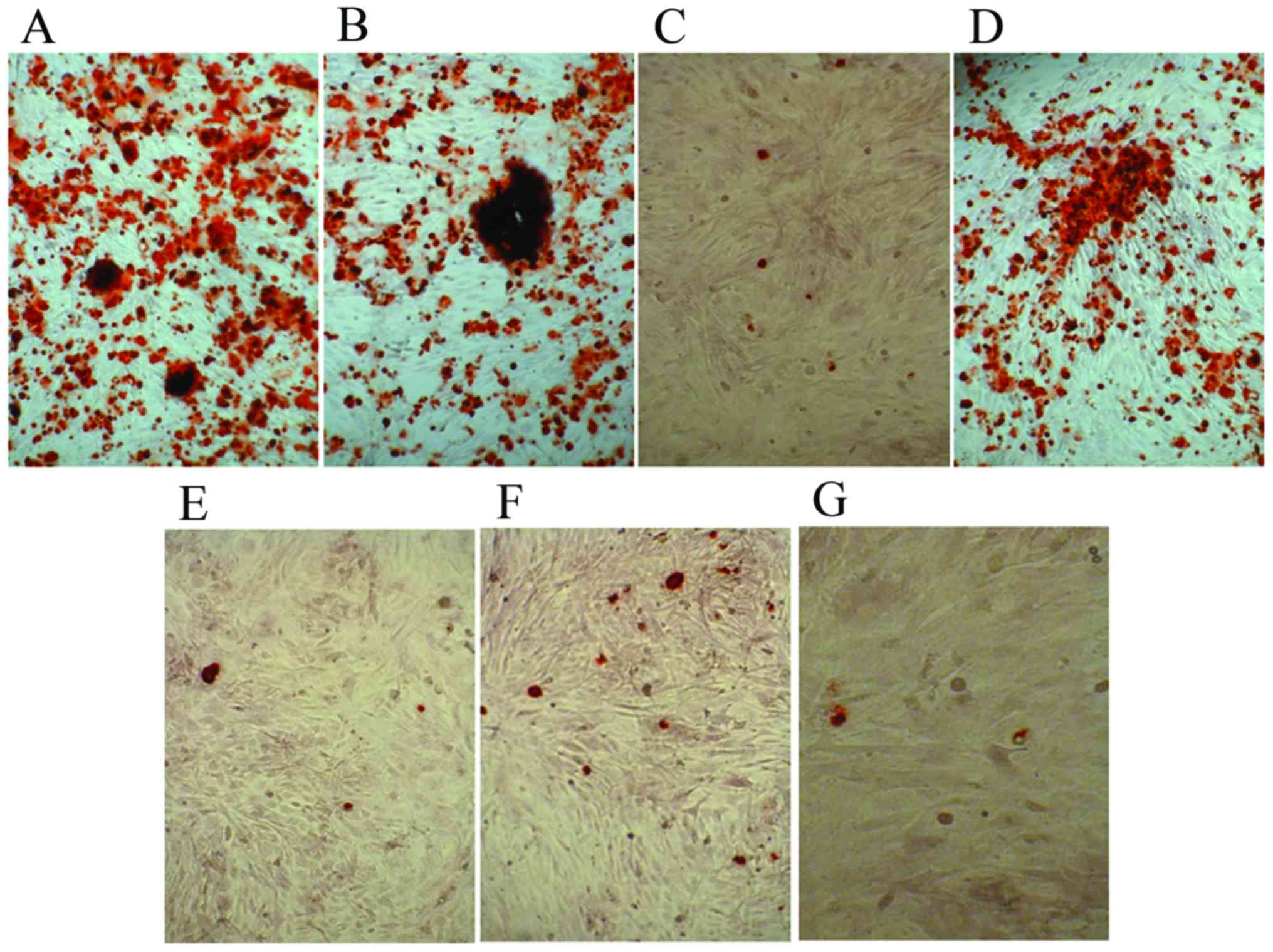
Alizarin Red S staining
The control cells and experimental groups with
various osteoblast induction media were stained with Alizarin Red S
following 21 days of differentiation. Alizarin Red S staining
indicated dark red precipitated calcium deposits (Fig. 4). The dexamethasone, BMP-2 and TGFβ
groups demonstrated markedly enhanced mineralization of MSCs
compared with that observed in the other groups (Fig. 4A, B and D). Whereas cells in
1,25-(OH)2VD3, platelet lysate, COX-2 and control media were
negative. The results demonstrated that dexamethasone, BMP-2 and
TGFβ promoted mineralization of MSCs.
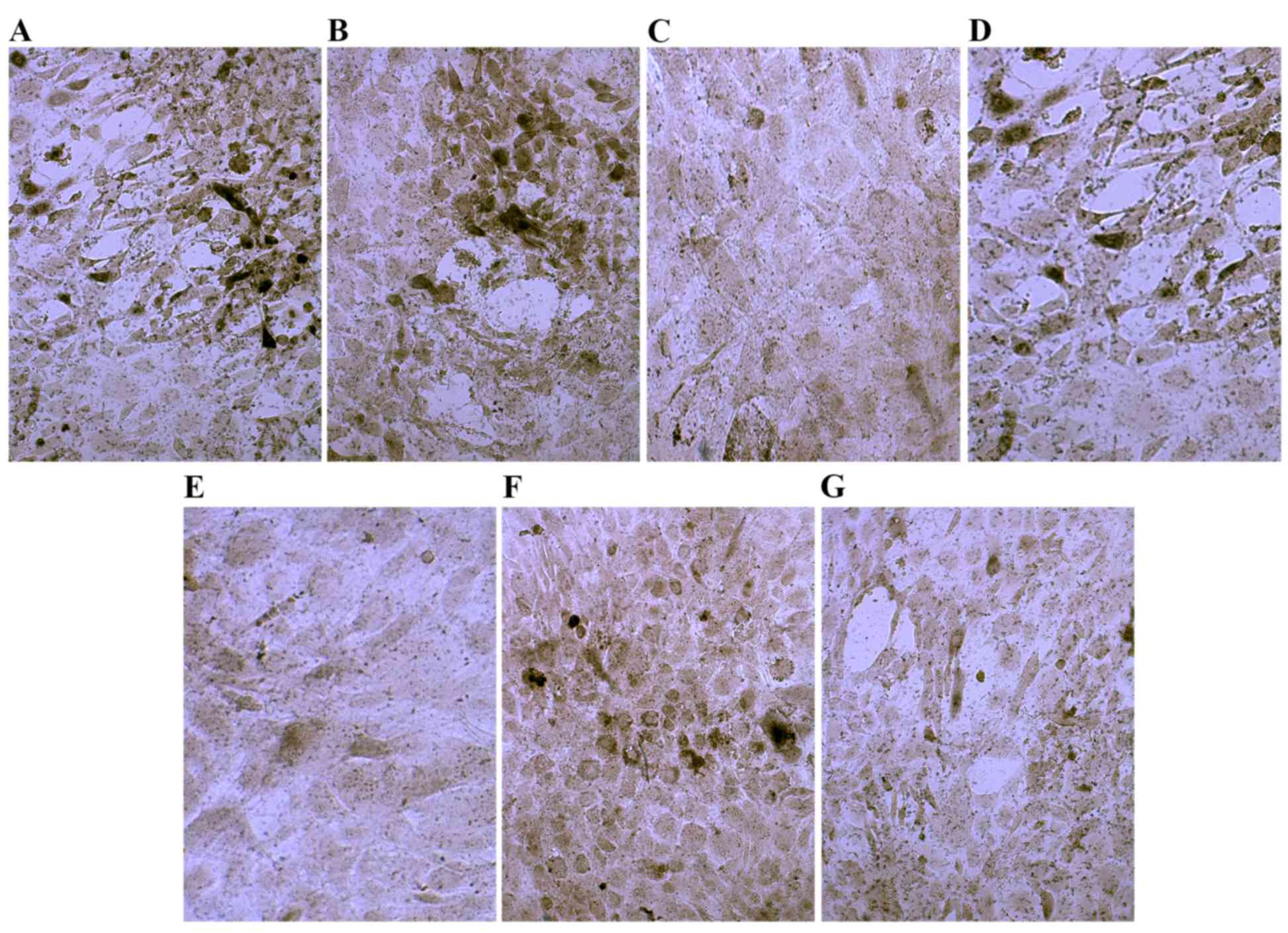
Ct I and osteocalcin are increased in
MSCs following culture in osteogenic induction media
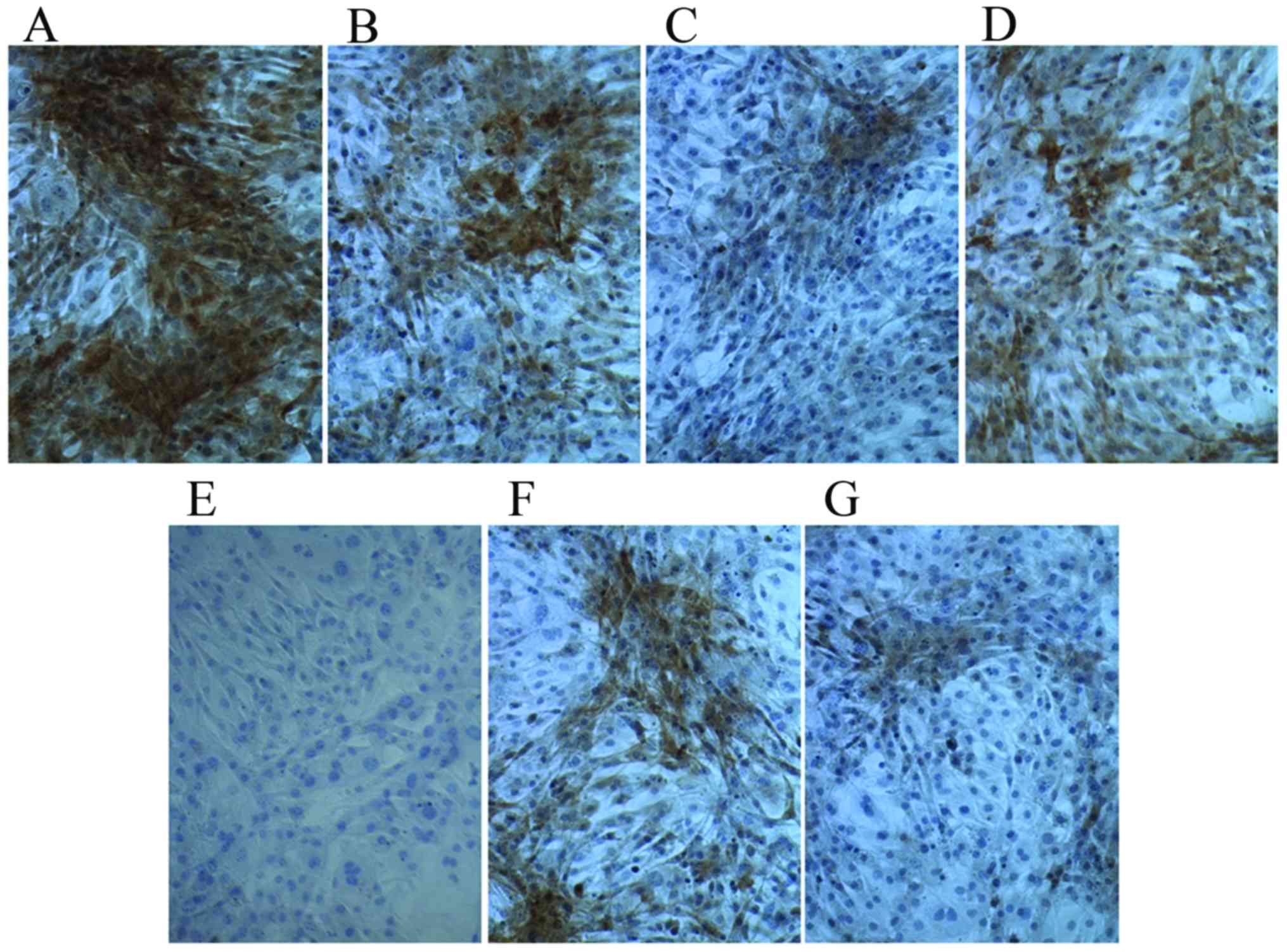
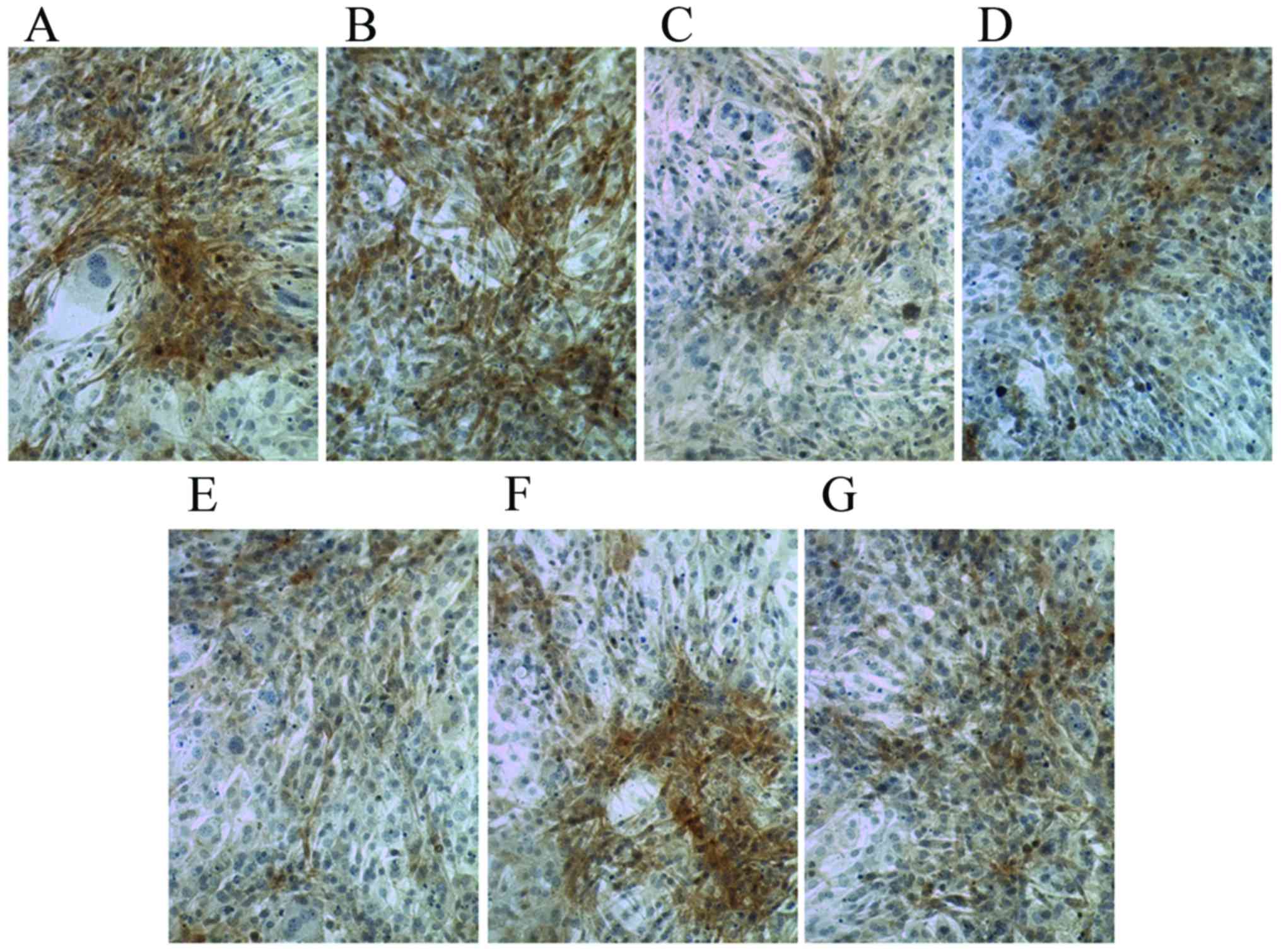
Following culture in osteogenic medium for 7 days,
compared with the control group, Ct I and osteocalcin levels were
markedly increased in the dexamethasone, BMP-2, TGFβ and COX2
groups (Figs. 5 and 6). However, compared with the control group
the Ct I and osteocalcin levels in the 1,25-(OH)2VD3 and platelet
lysate group revealed no notable difference. This suggests that
dexamethasone, BMP-2, TGFβ and COX2 may have induced the activity
of Ct I and osteocalcin in MSCs.
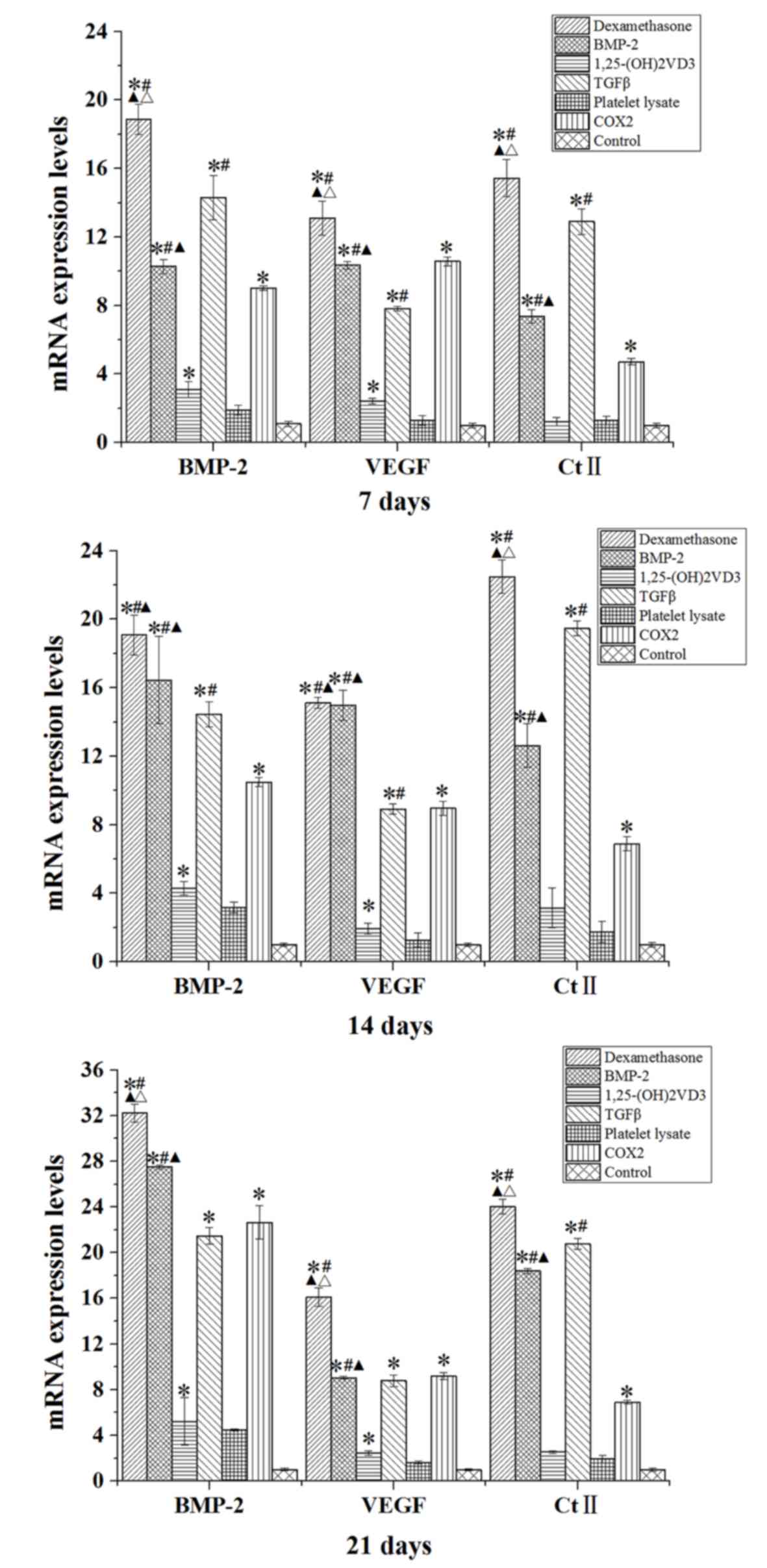
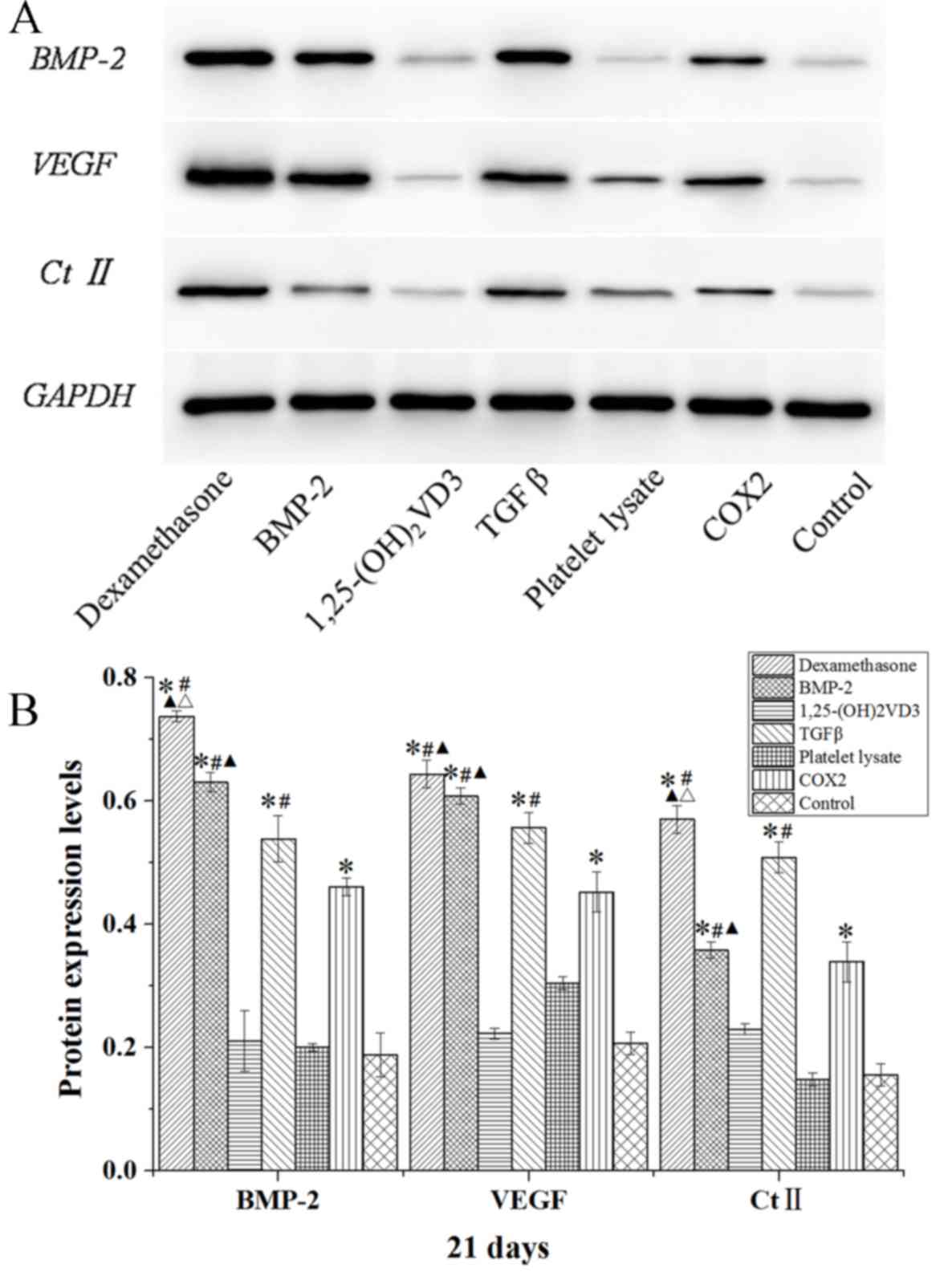
BMP-2, VEGF and Ct II mRNA and protein
expression levels are increased in MSCs following culture in
osteogenic induction media
Compared with the control group, rabbit MSCs
cultured in osteogenic medium (dexamethasone, BMP-2, TGFβ and COX2
groups) for 7, 14 and 21 days demonstrated significantly increased
mRNA expression levels of BMP-2, VEGF and Ct II (P<0.05;
Fig. 7). The expression level of
BMP-2 was also significantly increased in the
1,25-(OH)2VD3 group compared with the level in the
control group at days 7, 14 and 21 (P<0.05; Fig. 7).
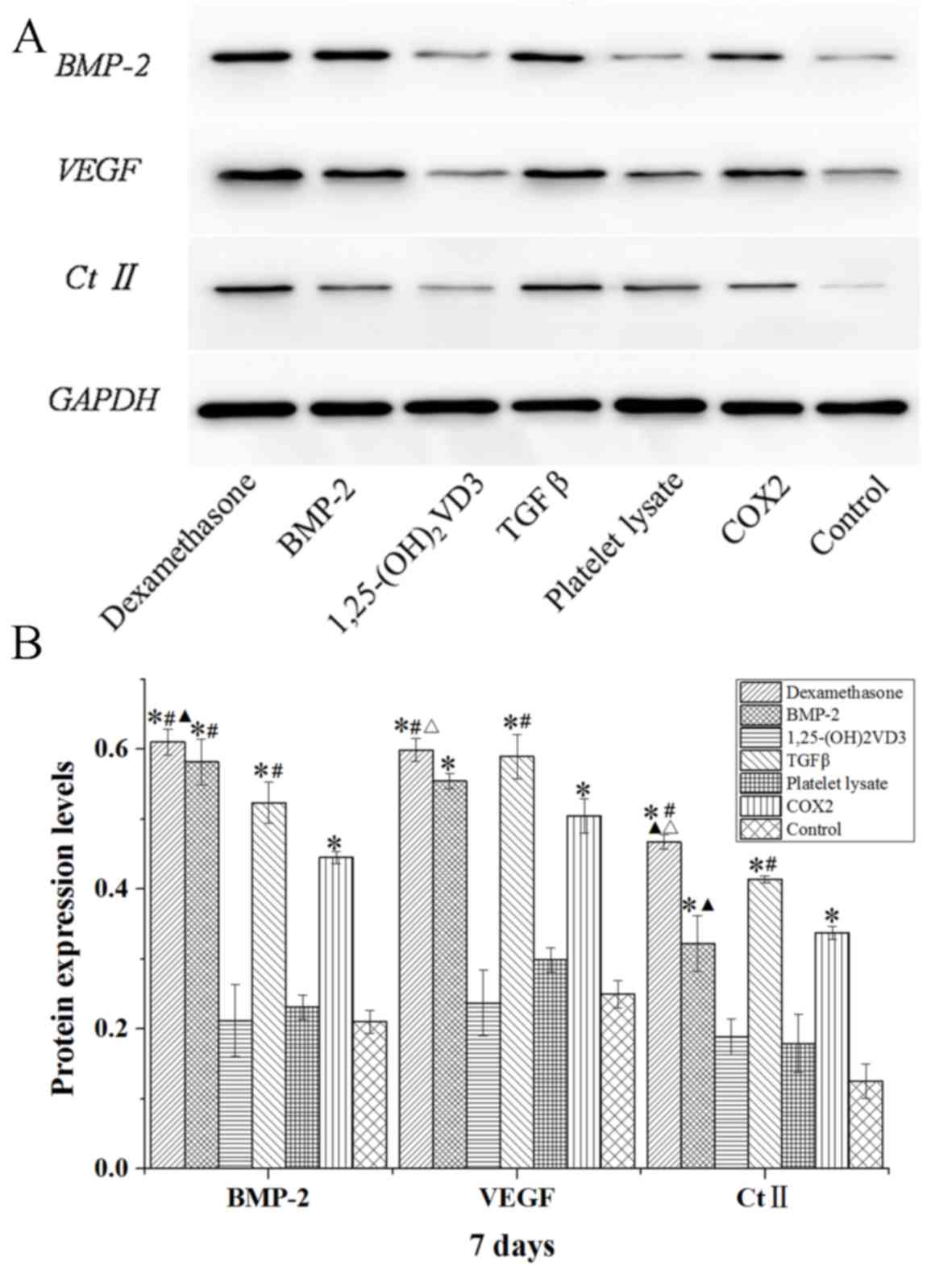
The protein expression levels of BMP-2, VEGF and Ct
II in rabbit MSCs are demonstrated in Figs. 8–10.
In the dexamethasone, BMP-2, TGFβ and COX2 groups, the BMP-2, VEGF
and Ct II protein expression levels were significantly higher than
those in the control group in rabbit MSCs at 7, 14 and 21 days
(P<0.05). The protein expression levels of BMP-2, VEGF and Ct II
were consistent with the results of their gene expression. These
results indicated that dexamethasone, BMP-2, TGFβ and COX2 induced
the expression of BMP-2, VEGF and Ct II in rabbit MSCs.
Discussion
MSCs may be induced to differentiate into multiple
cell types, such as osteoblasts (23). In the present study the
differentiation of rabbit MSCs was studied and markers of
differentiation were analyzed. Additionally, MSCs were
differentiated in different culture media, including media
containing dexamethasone, BMP-2, COX2, TGFβ, platelet lysate and
1,25-(OH)2VD3, respectively, and its effect was analyzed
by measuring ALP activity, mineralization and expression of related
genes and proteins. There are various factors that affect the
osteogenic differentiation of MSCs (24). In the present study, common
osteogenic induction factors were selected to explore their ability
to induce osteoblasts, which laid a foundation for subsequent
osteogenic induction.
Several methods are used for isolation of MSCs,
including negative and positive selection of cells (25), cell sorting (26) and applications of cytotoxic materials
(27); however, these methods have
varying impacts on MSC proliferation and differentiation. In the
present report, a simple method was utilized for the isolation of
MSCs. Cells were successfully isolated and were attached to the
surface of plates. Mostly circular-shaped cells of uniform size
were observed after culture for 24 h and, following additional days
of culture, the cells demonstrated spindle-shaped morphology.
The increase of ALP activity is an important marker
of MSC differentiation into osteoblasts. ALP is highly expressed at
an early stage of osteogenic differentiation to promote cell
maturation and calcification, which is therefore considered as an
important indicator for early osteogenesis (28). Mineralized nodules are typically
observed as a marker for terminal differentiation, and MSCs may be
stained for mineral deposition by Alizarin Red S (29). As indicated in the present study, the
expression level of ALP was markedly higher in differentiating
cells that were cultured in dexamethasone, BMP-2 and TGFβ media.
The present study demonstrated the positive impact of
dexamethasone, BMP-2 and TGFβ on the development of mineralized
nodules by detecting the calcium influx. Previous studies have
revealed that dexamethasone suppressed the proliferation and
accelerated the differentiation process of the MSCs (12,30).
BMP-2 has emerged as a key regulator of stem cell commitment and
serves an essential role in osteogenic differentiation (31). Several studies have applied BMP-2 to
MSCs for the repair of cartilage tissue (32,33).
TGFβ is also known to promote chondrogenic differentiation
(34). In the present study,
immunohistochemical staining of Ct I and osteocalcin also
demonstrated that the osteogenic potential of dexamethasone, BMP-2
and TGFβ was more profound than that of 1,25-(OH)2VD3,
platelet lysate and COX2.
The expression of osteogenesis-inducing genes and
proteins (BMP-2, VEGF and Ct II) in the present study were detected
by RT-qPCR and western blotting. The results indicated that the
mRNA and proteins expression levels of BMP-2, VEGF and Ct II were
significantly increased in rabbit MSCs induced by dexamethasone,
BMP-2, TGFβ and COX2. These results suggest that dexamethasone,
BMP-2, TGFβ and COX2 promote the expression of
osteogenesis-inducing genes and proteins during MSC chondrogenic
differentiation. The osteogenic differentiation of MSCs involves a
complex process orchestrated by multiple regulatory factors and
proteins. BMPs are master regulators of osteoblast differentiation
(35). It has been reported that
increased levels of BMP-2 promote the chondrogenic effect, as
indicated by increased expression levels of Ct II and expression of
chondrogenic markers (36). VEGF was
initially recognized as an endothelial-specific growth factor,
which increased vascular permeability and angiogenesis, and it is
now apparent that this cytokine regulates multiple biological
functions in the endochondral ossification of mandibular condylar
growth, as well as long bone formation (37). A study by Peng et al (38) reported that the addition of a
specific antagonist of VEGF significantly inhibited BMP-2-induced
bone formation and the associated angiogenesis indicated that
endogenous VEGF activity is important for bone formation. BMP-2,
VEGF and Ct II are important regulators that promote osteogenic
differentiation of MSCs. Accordingly, it may be inferred from the
present study that dexamethasone, TGFβ and COX2 may have the
potential to increase the expression of osteogenesis-inducing genes
and proteins to accelerate the osteogenic differentiation of
MSCs.
In conclusion, rabbit MSCs were successfully
isolated from bone marrow using a simple procedure and
differentiated into osteoblast-like cells as indicated by raised
ALP, Ct I and osteocalcin activities, mineralization and increased
expression of osteogenesis-inducing genes and proteins. The present
study also revealed that dexamethasone, BMP-2 and TGFβ have a
positive effect on MSC differentiation. These results may provide a
basis for the development of sequential delivery systems for
multiple growth factors in bone engineering.
Acknowledgements
The present work was supported by the Programs of
Wuhan Municipal Science and Technology Bureau for Science and
Technology Development (grant no. 201260523187-1) and the Project
of Health and Family Planning Commission of Wuhan Municipality
(grant no. WX12B13).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cai M, Shen R, Song L, Lu M, Wang J, Zhao
S, Tang Y, Meng X, Li Z and He ZX: Bone marrow mesenchymal stem
cells (BM-MSCs) improve heart function in swine myocardial
infarction model through paracrine effects. Sci Rep. 6:282502016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baena RRY, Rizzo S, Graziano A and Lupi
SM: Bone regeneration in implant dentistry: Role of mesenchymal
stem cells. A Textbook of Advanced Oral and Maxillofacial Surgery
3. Motamedi MHK (ed). InTech; London: pp. 269–291. 2016
|
|
3
|
Gerstenfeld LC, Barnes GL, Shea CM and
Einhorn TA: Osteogenic differentiation is selectively promoted by
morphogenetic signals from chondrocytes and synergized by a
nutrient rich growth environment. Connec Tissue Res. 44 Suppl
1:S85–S91. 2003. View Article : Google Scholar
|
|
4
|
Davidson D, Blanc A, Filion D, Wang H,
Plut P, Pfeffer G, Buschmann MD and Henderson JE: Fibroblast growth
factor (FGF) 18 signals through FGF receptor 3 to promote
chondrogenesis. J Biol Chem. 280:20509–20515. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Janderová L, Mcneil M, Murrell AN, Mynatt
RL and Smith SR: Human mesenchymal stem cells as an in vitro model
for human adipogenesis. Obes Res. 11:65–74. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Studeny M, Marini FC, Champlin RE,
Zompetta C, Fidler IJ and Andreeff M: Bone marrow-derived
mesenchymal stem cells as vehicles for interferon-beta delivery
into tumors. Cancer Res. 62:3603–3608. 2002.PubMed/NCBI
|
|
7
|
Potapova I, Plotnikov A, Lu Z, Danilo P
Jr, Valiunas V, Qu J, Doronin S, Zuckerman J, Shlapakova IN, Gao J,
et al: Human mesenchymal stem cells as a gene delivery system to
create cardiac pacemakers. Circ Res. 94:952–959. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang W, Zhang X, Ling J, Wei X and Jian
Y: Osteo-/odontogenic differentiation of BMP2 and VEGF
gene-co-transfected human stem cells from apical papilla. Mol Med
Rep. 13:3747–3754. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rui YF, Lui PP, Ni M, Chan LS, Lee YW and
Chan KM: Mechanical loading increased BMP-2 expression which
promoted osteogenic differentiation of tendon-derived stem cells. J
Orthop Res. 29:390–396. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamasaki A, Itabashi M, Sakai Y, Ito H,
Ishiwari Y, Nagatsuka H and Nagai N: Expression of type I, type II,
and type X collagen genes during altered endochondral ossification
in the femoral epiphysis of osteosclerotic (oc/oc) mice. Calcif
Tissue Int. 68:53–60. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Atmani H, Audrain C, Mercier L, Chappard D
and Basle MF: Phenotypic effects of continuous or discontinuous
treatment with dexamethasone and/or calcitriol on osteoblasts
differentiated from rat bone marrow stromal cells. J Cell Biochem.
85:640–650. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ahmad A and Shakoori AR: Isolation and
differentiation of murine mesenchymal stem cells into osteoblasts
in the presence and absence of Dexamethasone. Pak J Zool.
44:1417–1422. 2012.
|
|
13
|
Kim S, Kang Y, Krueger CA, Sen M, Holcomb
JB, Chen D, Wenke JC and Yang Y: Sequential delivery of BMP-2 and
IGF-1 using a chitosan gel with gelatin microspheres enhances early
osteoblastic differentiation. Acta Biomater. 8:1768–1777. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song I, Kim BS, Kim CS and Im GI: Effects
of BMP-2 and vitamin D 3, on the osteogenic differentiation of
adipose stem cells. Biochem Biophys Res Commun. 408:126–131. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Majumdar HK, Banks V, Peluso D and Morris
EA: Isolation, characterization and chondrogenic potential of human
bone marrow-derived stromal cells. J Cell Physiol. 185:98–106.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marie PJ and Fromigué O: Osteogenic
differentiation of human marrow-derived mesenchymal stem cells.
Regen Med. 1:539–548. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou Y, Wu Y, Jiang X, Zhang X, Xia L, Lin
K and Xu Y: The effect of Quercetin on the osteogenesic
differentiation and angiogenic factor expression of bone
marrow-derived mesenchymal stem cells. PLoS One. 10:e01296052015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeng Q, Li X, Beck G, Balian G and Shen
FH: Growth and differentiation factor-5 (GDF-5) stimulates
osteogenic differentiation and increases vascular endothelial
growth factor (VEGF) levels in fat-derived stromal cells in vitro.
Bone. 40:374–381. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ghilzon R, Mcculloch CA and Zohar R:
Stromal mesenchymal progenitor cells. Leuk Lymphoma. 32:211–221.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Toquet J, Rohanizadeh R, Guicheux J,
Couillaud S, Passuti N, Daculsi G and Heymann D: Osteogenic
potential in vitro of human bone marrow cells cultured on
macroporous biphasic calcium phosphate ceramic. J Biomed Mater Res.
44:98–108. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Society for Neuroscience. Policies on the
use of animals and humans in research. Society for
Neuroscience.
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao RT, Zhan LP, Meng C, Zhang N, Chang
SM, Yao R and Li C: Homeobox B7 promotes the osteogenic
differentiation potential of mesenchymal stem cells by activating
RUNX2 and transcript of BSP. Int J Clin Exp Med. 8:10459–10470.
2015.PubMed/NCBI
|
|
24
|
Hu Y, Tang XX and He HY: Gene expression
during induced differentiation of bone marrow mesenchymal stem
cells into osteoblasts. Genet Mol Res. 12:6527–6534. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nadri S and Soleimani M: Isolation murine
mesenchymal stem cells by positive selection. In Vitro Cell Dev
Biol Anim. 43:276–82. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Van Vlasselaer P, Falla N, Snoeck H and
Mathieu E: Characterization and purification of osteogenic cells
from murine bone marrow by two-color cell sorting using anti-Sca-1
monoclonal antibody and wheat germ agglutinin. Blood. 84:753–763.
1994.PubMed/NCBI
|
|
27
|
Modderman WE, Vrijheid-Lammers T, Löwik CW
and Nijweide PJ: Removal of hematopoietic cells and macrophages
from mouse bone marrow cultures: Isolation of fibroblastlike
stromal cells. Exp Hematol. 22:194–201. 1994.PubMed/NCBI
|
|
28
|
Hu H, Chen M, Dai G, Du G, Wang X, He J,
Zhao Y, Han D, Cao Y, Zheng Y and Ding D: An Inhibitory Role of
Osthole in Rat MSCs Osteogenic Differentiation and Proliferation
via Wnt/β-Catenin and Erk1/2-MAPK Pathways. Cell Physiol Biochem.
38:2375–2388. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yun HM, Park KR, Quang TH, Oh H, Hong JT,
Kim YC and Kim EC: 2,4,5-Trimethoxyldalbergiquinol promotes
osteoblastic differentiation and mineralization via the BMP and
Wnt/β-catenin pathway. Cell Death Dis. 6:e18192015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Walsh S, Jordan GR, Jefferiss C, Stewart K
and Beresford JN: High concentrations of dexamethasone suppress the
proliferation but not the differentiation or further maturation of
human osteoblast precursors in vitro: Relevance to
glucocorticoid-induced osteoporosis. Rheumatology (Oxford).
40:74–83. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rui YF, Lin DU, Wang Y, Wang Y, Lui PP,
Tang TT, Chan KM and Dai KR: Bone morphogenetic protein 2 promotes
transforming growth factor β3-induced chondrogenesis of human
osteoarthritic synovium-derived stem cells. Chin Med J (Engl).
123:3040–3048. 2010.PubMed/NCBI
|
|
32
|
Sekiya I, Larson BL, Vuoristo JT, Reger RL
and Prockop DJ: Comparison of effect of BMP-2, −4, and −6 on in
vitro cartilage formation of human adult stem cells from bone
marrow stroma. Cell Tissue Res. 320:269–276. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kurth T, Hedbom E, Shintani N, Sugimoto M,
Chen FH, Haspl M, Martinovic S and Hunziker EB: Chondrogenic
potential of human synovial mesenchymal stem cells in alginate.
Osteoarthritis Cartilage. 15:1178–1189. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Barry F, Boynton RE, Liu B and Murphy JM:
Chondrogenic differentiation of mesenchymal stem cells from bone
marrow: Differentiation-dependent gene expression of matrix
components. Exp Cell Res. 268:189–200. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lo YC, Chang YH, Wei BL, Huang YL and
Chiou WF: Betulinic acid stimulates the differentiation and
mineralization of osteoblastic MC3T3-E1 cells: Involvement of
BMP/Runx2 and β-catenin signals. J Agric Food Chem. 58:6643–6649.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shirasawa S, Sekiya I, Sakaguchi Y,
Yagishita K, Ichinose S and Muneta T: In vitro chondrogenesis of
human synovium-derived mesenchymal stem cells: Optimal condition
and comparison with bone marrow-derived cells. J Cell Biochem.
97:84–97. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dai J and Rabie AB: VEGF: An essential
mediator of both angiogenesis and endochondral ossification. J Dent
Res. 86:937–950. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Peng H, Usas A, Olshanski A, Ho AM,
Gearhart B, Cooper GM and Huard J: VEGF improves, whereas sFlt1
inhibits, BMP2-induced bone formation and bone healing through
modulation of angiogenesis. J Bone Miner Res. 20:2017–2027. 2005.
View Article : Google Scholar : PubMed/NCBI
|