Introduction
Ischemia-reperfusion injury (IRI) at least partly
contributes to the high morbidity and mortality rates of patients
with acute kidney injury (AKI) (1).
A number of conditions, including kidney transplantation, induce
renal IRI; this causes problems for many recipients of kidneys and
may therefore negatively impact postoperative consequences
(2,3). Ischemic kidneys receive a reduced blood
supply that does not meet metabolic demands, triggering severe
hypoxia associated with renal tubular dysfunction (4,5).
Paradoxically, subsequent reperfusion fails to restore normal
levels of perfusion; however, it induces further damage by
activating detrimental mechanisms, such as those associated with
oxidative stress (6).
Oxidative stress is a condition in which the
formation of reactive oxygen species (ROS) exceeds the capacity of
the endogenous antioxidant defense system (7,8). In
renal tissues, oxidative stress and associated sterile inflammation
serve a major role in the pathogenesis of AKI, which occurs by
complex mechanisms involving the ROS-mediated generation of
vasoconstrictive prostanoids, lipid peroxidation and activation of
pro-apoptotic factors (9–12). The increased accumulation of ROS and
oxidative stress markers is frequently observed in the kidneys of
animals with AKI (13). Under normal
conditions, nuclear factor-erythroid-2 (NF-E2)-related factor 2
(Nrf2) serves a important role in combatting oxidative stress and
the subsequent induction of >250 genes, including those encoding
antioxidant enzymes (14).
Attenuating the activation of Nrf2 may amplify cellular oxidative
stress and associated pathological events. By contrast, strategies
aimed at restoring Nrf2 activity may prevent the induction of
IRI-associated renal injury and inhibit progression to AKI.
Despite important advances in understanding the
pathological basis of AKI, clinical trials investigating the use of
synthetic drugs to treat patients with AKI have been limited thus
far (15). Considering the
pathological features of AKI, including its rapid progression and
involvement of multiple genes, it is understandable that
researchers aim to develop multi-target, drug-based preventative
strategies to treat this condition.
Traditional medicines often use multi-component
extracts of natural products and may be developed as therapeutic
strategies to treat AKI due to their multi-target potential and
established biosafety (16).
Phytochemicals from medicinal plants have attracted increased
attention, as they are able to scavenge ROS and inhibit its
formation, thereby reducing oxidative stress levels (17,18).
Rhus verniciflua Stokes (RVS) is a tree that belongs to the
Anacardiaceae family that consists of >250 species primarily
cultivated in Asian countries, including Korea and China (19). In Korea, RVS has been traditionally
been added to chicken soup and used to treat conditions, including
menstrual cycle irregularities, indigestion and high blood pressure
(20). Several studies using nuclear
magnetic resonance or liquid chromatography/mass spectrometry have
revealed that many bioactive compounds exhibiting antioxidant
activity are present in RVS, including gallic acid (GA),
protocatechuic acid, quercetin, fustin, fisetin, sulfuretin,
coumaric acid, kaempferol-3-O-glucoside, kaempferol and butein
(21–25). Of these, fisetin has been reported to
induce the expression of heme oxygenase-1 (HO-1), a major component
of cellular antioxidant enzymes, in human umbilical vein
endothelial cells by augmenting the nuclear translocation of Nrf2
(26). Furthermore, in a mouse model
of inflammatory bowel disease it was demonstrated that GA activates
and upregulates the expression of Nrf2 and associated antioxidant
enzymes, including superoxide dismutase and catalase (CAT), thereby
inducing a therapeutic effect (27).
However, to date, no studies have been conducted to identify
whether RVS itself can prevent the progression to AKI by activating
the Nrf2-mediated defense mechanism against oxidative stress.
The aim of the current study was to investigate
whether oral administration of RVS could prevent the progress of
AKI via modulation of the Nrf2/antioxidant enzyme pathway, using
in vivo and in vitro IRI-induced AKI models.
Materials and methods
Preparation of RVS extract
The RVS extract was supplied by Lifetree Biotech
Co., Ltd. (Suwon, South Korea). The extract was prepared using a
previously described protocol (28).
Briefly, RVS timber was harvested in Wonju, (Gangwon-do, South
Korea) and subsequently cut into pieces measuring 11×1×0.2 cm. The
pieces were mixed with water in a 1:10 w/v ratio and eluted with
boiling water at 90–110°C for 4 h. The extract was concentrated to
yield a solid content of 15%. Subsequently, the concentrated
extract was diluted with an equal volume of dextrin and
spray-dried. Urushiol, an allergenic constituent of RVS, was
extracted. The urushiol-free RVS extract was authenticated by the
Korea Advanced Food Research Institute (Seoul, South Korea).
Animals
A total of 32 8-week-old male C57BL/6 weighing 20–22
g were purchased from Samtako Bio Korea, Co., Ltd. (Osan, Korea).
Mice were housed in an environmentally controlled room at 23±2°C at
a relative humidity of 60±10% under a 12-h light/dark cycle. All
mice had ad libitum access to water and food. Experiments
were conducted in accordance with the ‘Guide for the Care and Use
of Laboratory Animals’ (National Institutes of Health publication,
8th Edition, 2011) (29). All
experiments involving mice were approved by the Institutional
Animal Care and Use Committee (approved protocol number:
P-16-10-A-02) of Konyang University (Daejeon, Korea).
Experimental design
Mice were randomly divided in 4 groups (all n=8): A
control group, an RVS-treated group (RVS), an IRI-operation group
(IRI) and an IRI-operation group that received pretreatment with
RVS (IRI+RVS). Whereas the control group was intraorally
administered with 500 µl of distilled water as a vehicle, the RVS
group was intraorally administered with RVS (20 mg/kg/day) diluted
in the same volume of vehicle over 14 days. For mice in the IRI+RVS
group, the same dose of RVS as that administered to mice in the RVS
group was applied prior to the IRI operation for the same duration.
For mice in the IRI group, the same volume of distilled water was
intraorally administered for 14 days prior to the IRI
operation.
Operation and tissue sampling
Mice in the IRI and IRI+RVS groups were anesthetized
with intraperitoneal injections of xylazine (10 mg/kg, Huons Global
Co., Ltd., Seongnam, Korea) and ketamine (80 mg/kg, Bayer AG,
Leverkusen, Germany). The abdominal area was sterilized with
Betadine (Mundipharma International Ltd., Seoul, Korea) and a
laparotomy was made by midline incision. To induce renal ischemia,
bilateral renal pedicles were clamped for 60 min with serrated
vascular clamps in a sterile operating field. Following removal of
the clamp to allow reperfusion, the color of kidney was inspected
to confirm the restoration of blood flow. The abdomen was then
sutured in two layers. Following surgery, 50 ml/kg normal saline
was administered intraperitoneally. Mice were allowed to recover in
their home cages until they were fully awake and active. Following
23 h reperfusion, all mice were euthanized. The left kidneys of
each group were removed and frozen for immunoblot assays and the
right kidneys were fixed with formalin prior to histological study.
Arterial blood was collected from the abdominal aorta for
serological assays prior to euthanasia.
Serological assay
Following the collection of arterial blood, sera
were obtained by centrifugation at a speed of 250 × g for 10 min at
4°C and stored at −70°C prior to assays. Levels of blood urea
nitrogen (BUN; cat. no. 9903040; Fujifilm, Tokyo, Japan),
creatinine (cat. no. 9903090; Fujifilm) and lactate dehydrogenase
(LDH; cat. no. 9903190; Fujifilm) were measured through ELISA using
a DRI-CHEM 3000 colorimetric analyzer (Fujifilm) at an excitation
wavelength of 625, 600 and 540 nm, respectively.
Histology
Right kidney tissues were excised, fixed in 10%
neutral buffered formalin for 48 h at 4°C and embedded in paraffin.
Paraffin blocks were sliced into sections 5-µm thick using the
Leica RM2255 microtome (Leica Microsystems GmbH, Wetzlar, Germany).
Alterations in the histological structures of the kidney were
examined by staining with hematoxylin and eosin (H-E). Two
microscopic fields of tissue sections taken from every mouse were
randomly selected. These fields were photographed at a
magnification of ×400 using a digital camera connected to a Leica
DM4 light microscope (Leica Microsystems GmbH) and examined by
three blinded observers. Renal tubular injury was scored by
estimating the percentage of tubules in the corticomedullary
junction and outer medulla that exhibited epithelial necrosis or
necrotic debris and determined as follows: 0, none; 1+, <10; 2+,
10–25; 3+, 26–45; 4+, 46–75; and 5+, >75%.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
Paraffin-embedded kidney sections were
deparaffinized in xylene and rehydrated in a graded series of
ethanol solutions. The TUNEL assay was performed using an In
Situ Cell Death Detection kit (Roche Diagnostics, Indianapolis,
IN, USA), following the manufacturer's protocol. Nuclei were
counterstained with DAPI. Among the resulting TUNEL-stained tissue
sections, two sections from each mouse were randomly selected and
captured using a digital camera connected to a Leica DMI6000
inverted microscope (Leica Microsystems) to detect fluorescence.
Images were visualized at a magnification of ×400. The number of
TUNEL-positive stained nuclei per high power field (HPF) was
counted and averaged by three blind observers.
Cell culture and in vitro hypoxia
Human kidney epithelial cells, HK-2 cells, were
obtained from the Korean Cell Line Bank (Seoul, Korea). Cells were
cultured for 72 h in 25-cm2 cell culture flasks
containing RPMI 1640 culture medium supplemented with 10% fetal
bovine serum, 100 U/ml penicillin and 100 mg/ml streptomycin at
37°C in a humidified atmosphere consisting of 5% CO2/95%
air. All of the aforementioned chemicals were purchased from Thermo
Fisher Scientific, Inc. (Waltham, MA, USA). For in vitro
hypoxia conditioning, cobalt chloride (CoCl2), a
chemical inducer of hypoxia-inducible factor-1, was used. To
determine the approximate values of the median lethal dose (LD50)
and the median lethal time (LT50) of CoCl2,
dose-dependent and time-dependent changes in the viability of HK-2
cells following treatment with different doses (0, 10, 100, 300,
600 and 900 µl) of CoCl2 for various incubation times
(0, 6, 12, 24 and 48 h) were analyzed. The LD50 and LT50 of
CoCl2 were 300 µM and 24 h, respectively; this dose of
CoCl2 and incubation time was used in all subsequent
experiments, apart from the ROS measurement, in which 30 min was
selected as the incubation time.
Cell viability assay
HK-2 cells were seeded in a 96-well plate at a
density of 1×104 cells/well. After incubation with RVS
(0, 40, 80, 120, 160 and 200 µg/ml) with or without 300 µM
CoCl2 for 24 h, cell viability was measured by
estimating the amount of reduced
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT),
a pale yellow substrate that is reduced by mitochondrial activity
in living cells, following exposure to different conditions. For
this, 100 µl culture medium was aspirated and 20 µl MTT
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) diluted in PBS at a
ratio of 5 mg/ml was added to the wells and incubated for 4 h at
37°C. The resulting formazan crystals were dissolved following the
addition of dimethyl sulfoxide. Using an Epoch microplate
spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA),
cell viability was estimated by detecting color intensity at an
excitation wavelength of 570 nm.
ROS measurement
HK-2 cells grown on glass coverslips were split into
the following 4 groups, with 5 samples per group (n=5): A control
group (cells treated with distilled water), an RVS group (cells
treated with 40 µg/ml RVS), a CoCl2 group (cells treated
with 300 µM CoCl2) and a CoCl2+RVS group
(cells treated with 40 µg/ml RVS and 300 µM CoCl2).
Distilled water and RVS were diluted in culture medium and applied
to the cells for 24 h. CoCl2 was subsequently added to
the culture medium for 30 min. Subsequently, the amount of cellular
ROS was measured using the OxiSelect™ Intracellular ROS
assay kit (Cell Biolabs Inc., San Diego, CA, USA) following the
manufacturer's protocol. 2′,7′-dichlorodihydrofluorescin diacetate
(DCFH-DA) is converted to 2′,7′-dichlorodihydrofluorescin (DCFH),
which is rapidly oxidized to fluorescent
2′,7′-dichlorodihydrofluorescein (DCF) by ROS. The fluorescence
intensity is proportional to ROS levels within the cell cytosol.
From each group, ≥5 HPFs were examined using a fluorescence
microscope connected to a digital camera at a magnification of
×400. The fluorescent intensities in each HPF were measured and
averaged using an image analysis system (ImageJ software; version,
1.49; National Institutes of Health, Bethesda, MD, USA).
Immunofluorescence
HK-2 cell groups (control, RVS, CoCl2 and
CoCl2+RVS) were grown on glass coverslips (n=5 per
group). Distilled water and RVS were diluted in culture medium and
applied to the cells for 24 h. CoCl2 was subsequently
added to the culture medium for 24 h. Coverslips were fixed with 4%
paraformaldehyde for 30 min at 4°C, blocked with 1% bovine serum
albumin (Sigma-Aldrich; Merck KGaA) diluted in PBS for 1 h at 24°C
and incubated with anti-Nrf2 rabbit polyclonal antibody (1:1,000,
cat. no., sc-722; Santa Cruz Biotechnology, Dallas, TX, USA) for 24
h at 4°C. Subsequently, cells were incubated with Alexa
Fluor® 488-conjugated goat anti-rabbit antibody
(1:2,000, cat. no., ab150077; Abcam, Cambridge, UK) at room
temperature for 1 h. Coverslips were then counterstained for 5 min
with DAPI and mounted with ProLong Gold mounting media (cat. no.,
8961; Cell Signaling Technology, Danvers, MA, USA) at 24°C and
examined using the LSM 700 laser scanning confocal microscope
(magnification, ×400; Zeiss AG, Oberkochen, Germany).
Western blot analysis
Total proteins were extracted from the HK-2 cell
groups. Total protein extraction and western blot analyses were
performed as previously described (15). Cells were harvested, transferred into
lysis buffer (Pro-Prep™; intron Biotechnology, Inc.,
Seoungnam, Korea) and homogenized. The total protein concentration
of the supernatant was determined using a BCA protein assay (Pierce
Biotechnology, Inc., Rockford, IL, USA). Subsequently, the protein
sample (30 µg/ml) was separated by 10% SDS-PAGE and transferred
onto a polyvinylidenedifluoride (PVDF) membrane (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), which was blocked with 5%
nonfat milk in Tris-buffered saline with 0.1% Tween 20 (TBS-T) for
2 h at room temperature. The membranes were incubated with rabbit
polyclonal antibodies against HO-1 (1:1,000, cat. no. sc-136960),
CAT (1:500, cat. no. sc-271803) and GAPDH (1:2,000, cat. no.
sc-25778) for 24 h at 4°C. Proteins were then incubated with
horseradish peroxide-conjugated anti-rabbit immunoglobulin G
(1:1,000, cat. no. sc-2030) for 1 h at 36°C. To quantify the
nuclear translocation of Nrf2, a subcellular protein fractionation
kit (cat. no. 87790; Thermo Fisher Scientific, Inc.) was used to
isolate nuclear fractions following the manufacturer's protocol.
Protocols for the determination of total protein concentration in
nuclear fractions, their SDS-PAGE separation and their transfer to
PVDF membranes were identical as those aforementioned. The
membranes were incubated with the Nrf2 rabbit polyclonal antibody
(1:1,000; cat. no. sc-722) for 24 h at 4°C. Proteins were then
incubated with horseradish peroxide-conjugated anti-rabbit
immunoglobulin G (1:1,000; cat. no., sc-2030) for 1 h at 36°C. The
quality of the isolation of nuclear fractions was confirmed by
immunoblots using Lamin B rabbit polyclonal antibody (1:1,000; cat.
no. sc-3755). All aforementioned antibodies were purchased from
Santa Cruz Biotechnology (Dallas, TX, USA). Following five washes
with PBS, proteins on the PVDF membranes were detected using a
chemiluminescence detection system (Amersham ECL Prime Western
Blotting Detection Reagent; GE Healthcare Life Sciences, Little
Chalfont, UK) following the manufacturer's protocol. The resulting
bands were photographed using a Davinch-Chemi imaging device
(Davinch-K, Seoul, Korea) and their intensities were quantified
using ImageJ (version, 1.49; National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
All data are presented as the mean ± standard error
of the mean. Statistical analysis was conducted using PASW version
18 (SPSS, Inc., Chicago, IL, USA). Comparisons of data from
different groups were performed with one-way analysis of variance
followed by a Tukey post-hoc test. P<0.05 was considered to
indicate a statistically significant difference. Each ‘n’ value
refers to the number of separate experiments conducted.
Results
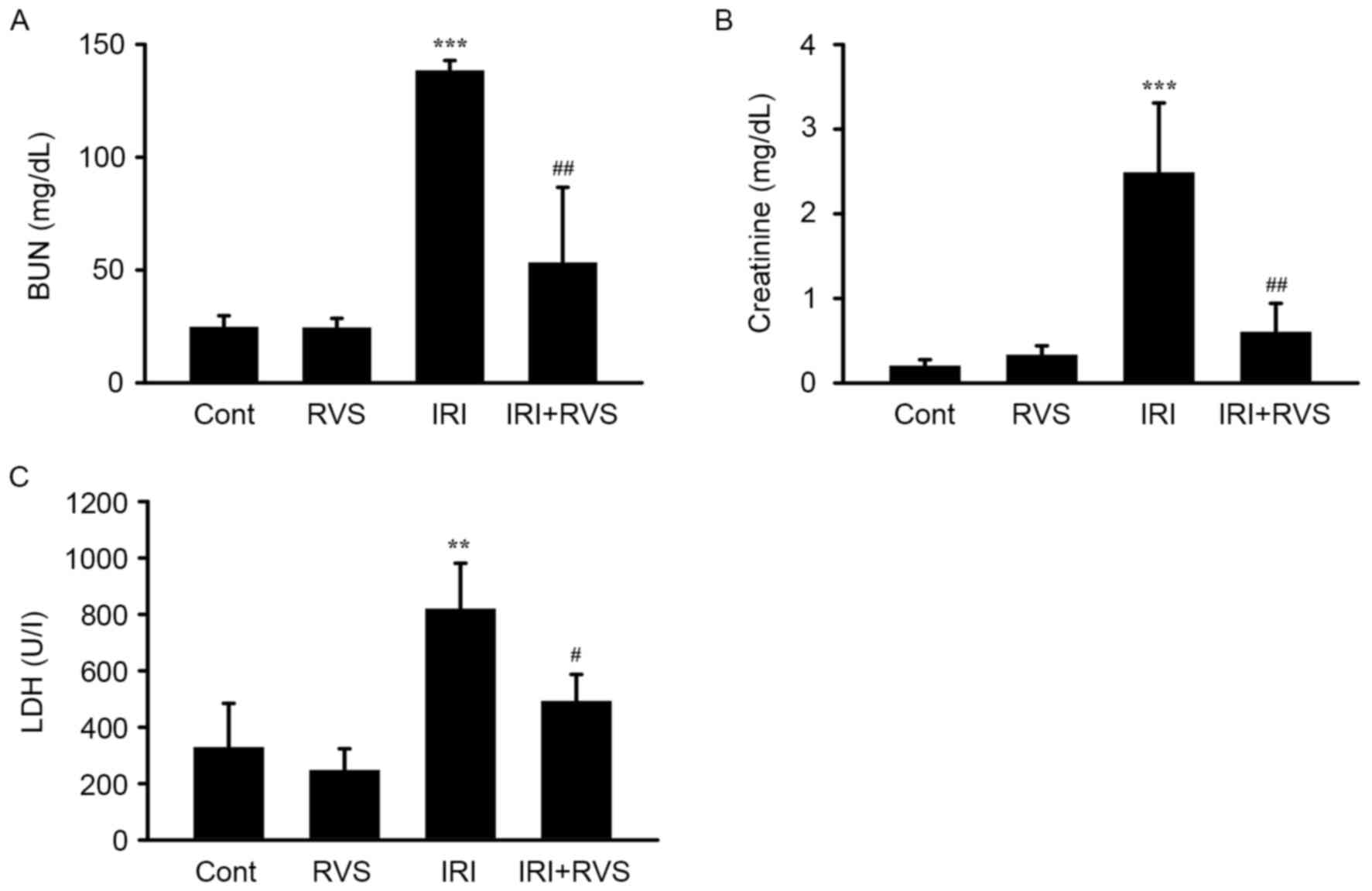
RVS attenuates the IRI-induced
decrease of renal function parameters
To clarify the role of RVS in renal IRI, C57BL/6
mice were subjected to IRI (1 h renal artery occlusion followed by
23 h reperfusion) with or without RVS pretreatment (14 days, 20
mg/kg/day). RVS alone did not alter renal function, as indicated by
the unchanged levels of serum BUN, creatinine and LDH in the RVS
group (Fig. 1). By contrast,
compared with the control group, the IRI group clearly exhibited
AKI and renal dysfunction, as indicated by significant increases in
levels of serum BUN (138.3±4.5 vs. 24.6±5.1, P<0.001),
creatinine (2.5±0.8 vs. 0.16±0.05, P<0.001) and LDH (818.8±162.5
vs. 317.8±129.5, P<0.01). However, there were significant
reductions in serum BUN (53.2±33.5 vs. 138.3±4.5, P<0.01),
creatinine (0.6±0.3 vs. 2.5±0.8, P<0.01) and LDH (491.7±95.2 vs.
818.8±162.5, P<0.05) levels compared with those in the IRI
group. These data indicate that RVS may prevent the IRI-induced
deterioration of renal function.
RVS attenuates IRI-induced
histological damage
Representative images of H-E stained tissue sections
of the corticomedullary junction (Fig.
2A) and outer medulla (Fig. 2B)
indicate that renal tissue from the control and RVS groups
exhibited normal morphology. Kidneys in the IRI group exhibited
acute tubular damage, indicated by tubular swelling, infiltrated
inflammatory cells (arrowhead) and loss of the brush border due to
apoptosis/necrosis of the tubular epithelium (arrow) in renal
tissue. However, RVS treatment markedly attenuated these
pathological features, suggesting that RVS protects the tubular
epithelium. This hypothesis is supported by the fact that kidney
injury scores were significantly lower in the IRI+RVS group
compared with the IRI group (3.3±0.7 vs. 2.3±0.7, P<0.001;
Fig. 2C).
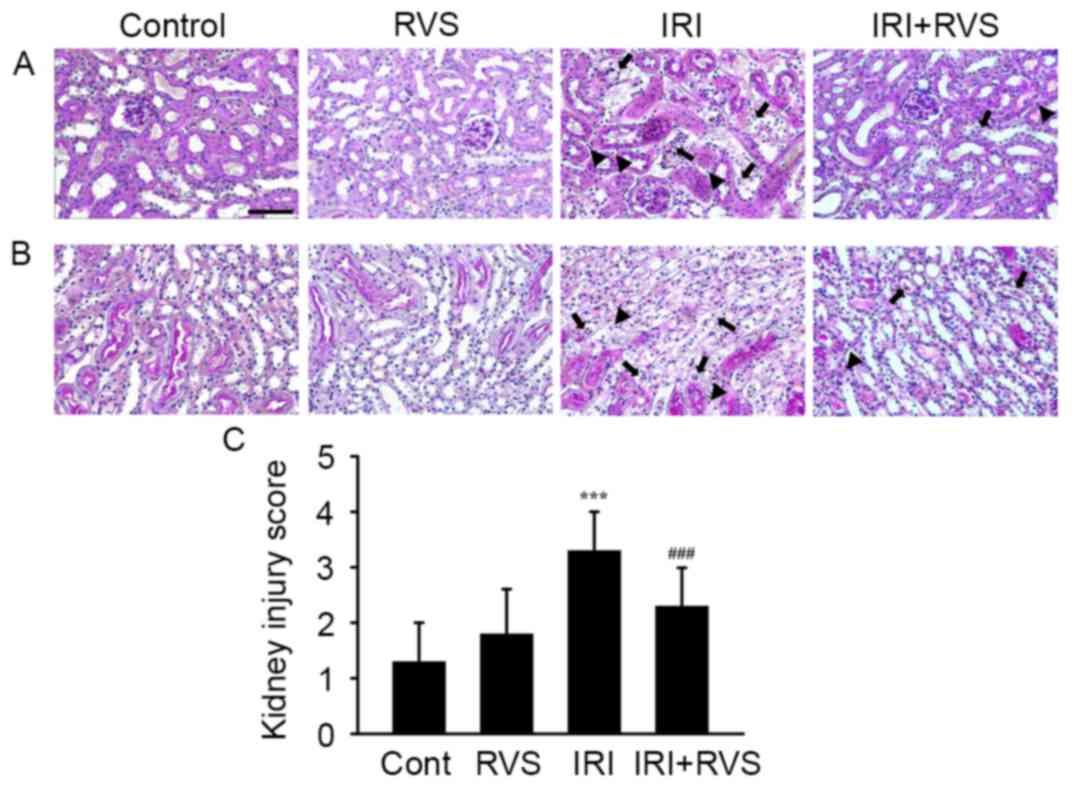 | Figure 2.Effect of RVS on IRI-induced
alterations on renal histoarchitecture. RVS (20 mg/kg/day) was
intraorally administered for 14 days to mice with or without IRI
and their kidneys were stained with hematoxylin and eosin for
histological examination. Representative images of (A) the
corticomedullary junction and (B) the outer medulla of Cont, RVS,
IRI and IRI+RVS groups (n=7 per group) are presented. Black
arrowheads and black arrows indicate an infiltrated inflammatory
cells and necrotic tubules, respectively. Scale bar=200 µm. (C)
Renal tubular injury was scored by estimating the percentage of
tubules in the corticomedullary junction and outer medulla that
exhibited epithelial necrosis or had necrotic debris and were cast
as follows: 0, none; 1+, <10; 2+, 10–25; 3+, 26–45; 4+, 46–75;
and 5+, >75%. Values are expressed as the mean ± standard error
of the mean. ***P<0.001 vs. Cont group; ###P<0.001
vs. IRI group. RVS, Rhus verniciflua Stokes; IRI,
ischemia-reperfusion injury; Cont, control. |
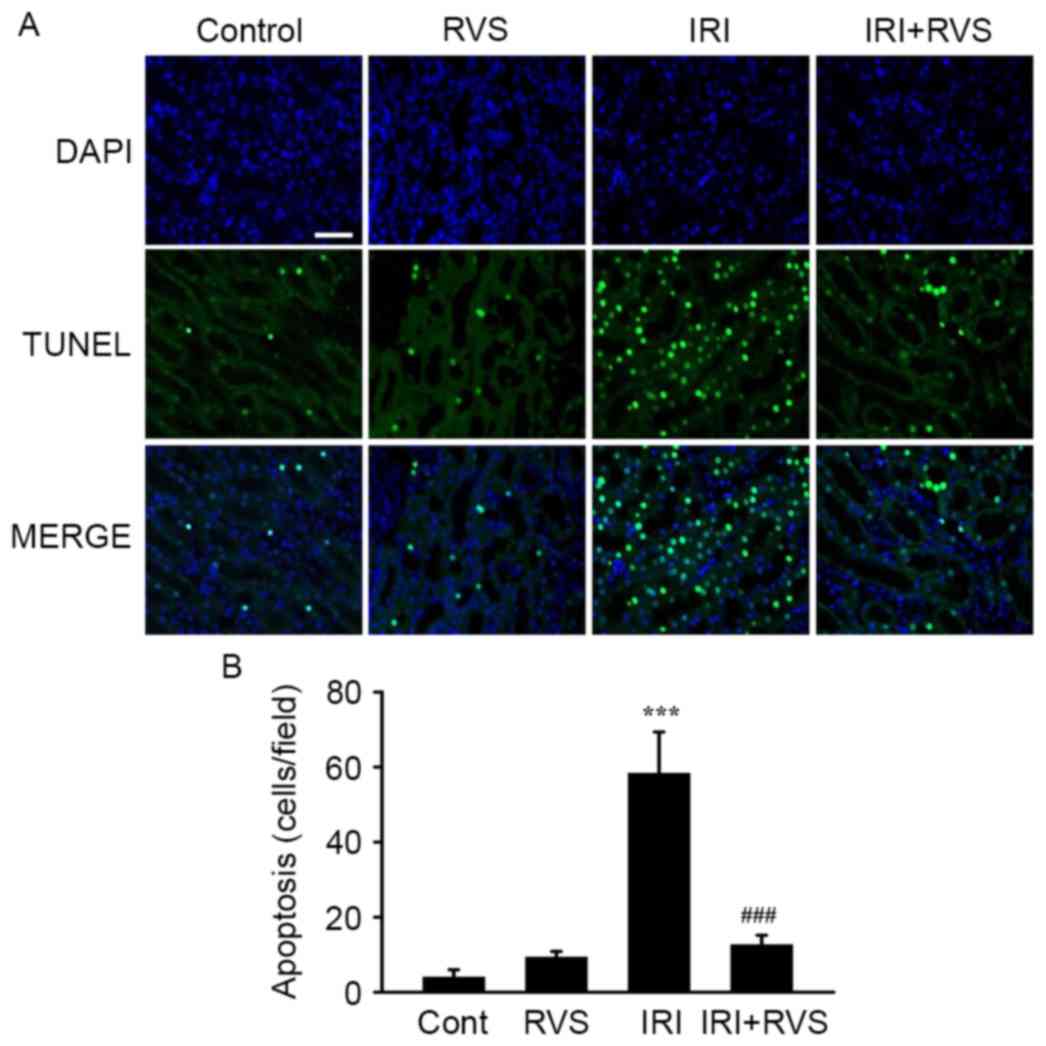
RVS inhibits renal tubular cell
apoptosis
Tubular apoptosis normally precedes/accompanies
tubular cell death; therefore, the number of apoptotic cells in the
kidney was quantified using a TUNEL assay. As presented in a
representative image (Fig. 3A) and
in a graph presenting quantified results (Fig. 3B), the number of TUNEL-positive cells
was significantly increased in the IRI group compared with the
control group (58.3±11.0 vs. 4.0±2.0, P<0.001). However,
significantly fewer TUNEL-positive cells were visible in the
IRI+RVS group than in the IRI group (12.7±2.5 vs. 58.3±11.0,
P<0.001). As expected, the number of TUNEL-positive cells in the
RVS group did not differ significantly compared with the control
group. Collectively, these data suggest that RVS protects the
kidney from the histological deterioration of renal tubules and
that this effect was due to the inhibition of apoptosis in the
renal tubular epithelium.
RVS protects HK-2 cells from
CoCl2-induced damage
To confirm the protective effect of RVS on cell
damage caused by IRI, hypoxia was chemically induced by the
addition of CoCl2, a well-known inducer of in
vitro IRI, in the human kidney epithelial cell line HK-2. The
approximate LD50 and LT50 values of CoCl2 were
identified as 300 µM and 24 h, respectively (rectangular boxes;
Fig. 4A and B). There was no
significant decrease in cell viability of HK-2 cells incubated with
different doses (0–200 µg/ml) of RVS, indicating that RVS does not
induce toxicity in this cell line (Fig.
4C). Notably, incubation with 40–160 RVS µg/ml significantly
increased the viability of HK-2 cells treated with 300 µM
CoCl2 for 24 h (P<0.01; Fig. 4D). Collectively, these results
demonstrate that pre-treatment with RVS inhibits the cytotoxic
effect of CoCl2 on HK-2 cells.
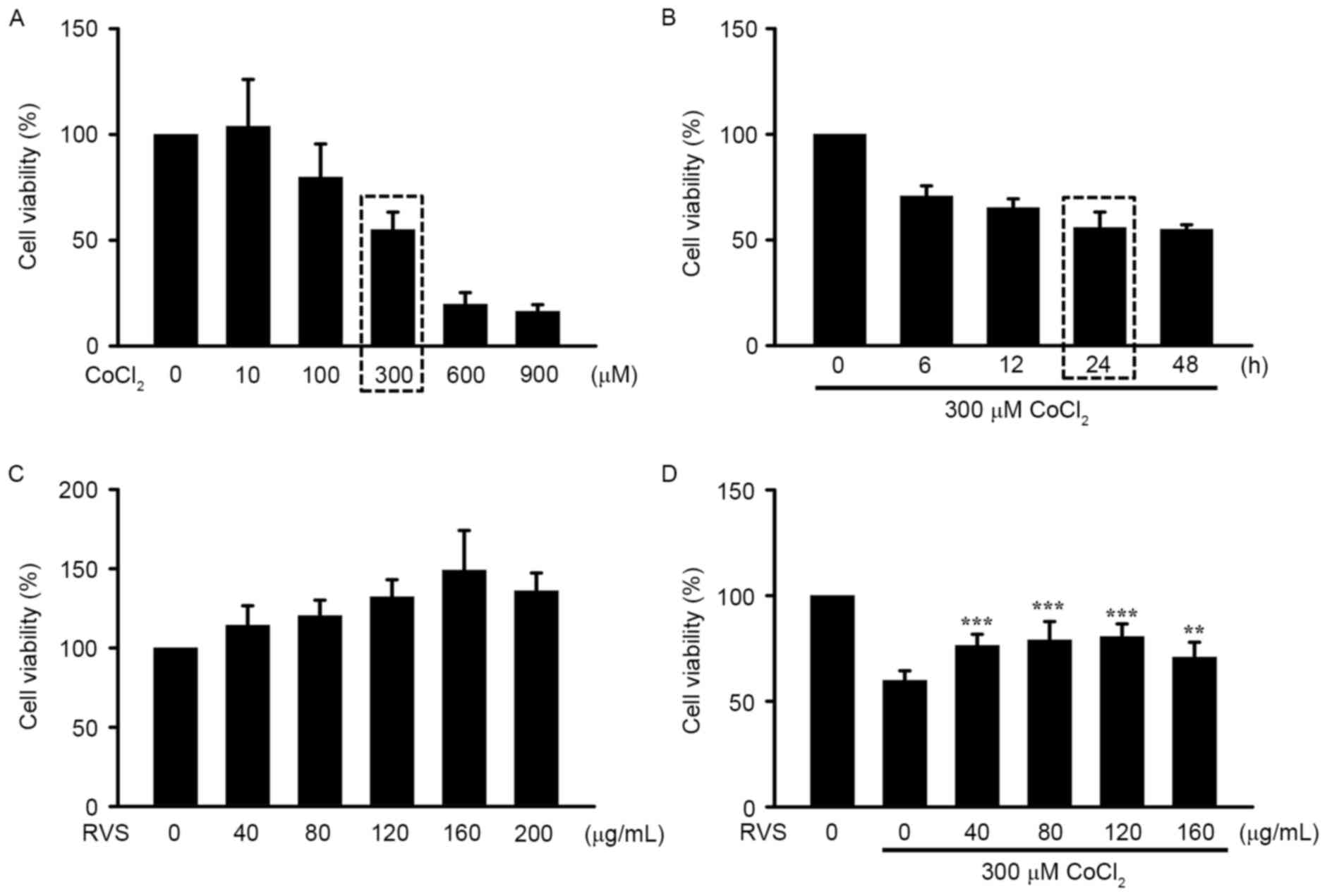 | Figure 4.Effects of RVS on HK-2 cell viability
in vitro following IRI. HK-2 cells were pretreated with or
without RVS for 24 h and then challenged with CoCl2, a
chemical known to imitate hypoxic injury. Cell viability was
determined using an MTT assay. (A) Dose-dependent cell viability
following incubation with varying concentrations of
CoCl2 (0, 10, 100, 300, 600 and 900 µM) and (B)
time-dependent cell viability following incubation with 300 µM
CoCl2 for different durations (0, 6, 12, 24 and 48 h).
(C) Identification of the safe concentration range of RVS by
incubating cells with different concentrations of RVS (0, 40, 80,
120, 160 and 200 µg/ml). (D) The effects of 24 h incubation with
RVS (0, 40, 80, 120 and 160 µg/ml) and on CoCl2 (300
µM)-induced alteration of HK-2 cell viability were assessed. Values
are presented as the mean ± standard error of the mean. **P<0.01
and ***P<0.001 vs. cells incubated with 300 µM CoCl2
alone. RVS, Rhus verniciflua Stokes; IRI,
ischemia-reperfusion injury. |
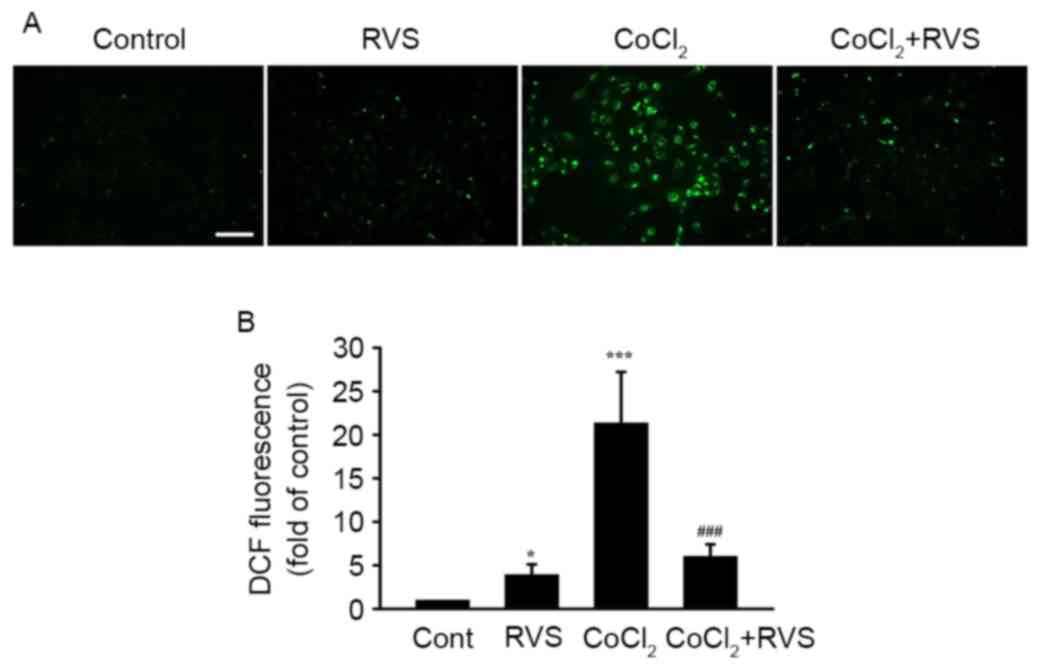
RVS inhibits CoCl2-induced
ROS generation in HK-2 cells
To determine whether antioxidant properties are
involved in the protective effect of RVS against
CoCl2-induced damage in HK-2 cells, intracellular ROS
levels in different cell groups was detected using DCFH-DA. As RVS
significantly elevated cell viability even at a dose of 40 µg/ml,
this dose was used in the current study. As indicated in a
representative fluorescence image (Fig.
5A) and a quantitative graph (Fig.
5B), cells treated with 40 µg/ml RVS for 24 h exhibited
significantly higher ROS intensity compared with those in the
control group (3.9±1.3 vs. 1.0±0, P<0.05). Cells incubated with
300 µM CoCl2 for 30 min exhibited an even higher ROS
intensity compared with the control (21.4±5.9, P<0.001 vs.
control). However, cells treated with 40 µg/ml RVS for 24 h and
then incubated with 300 µM CoCl2 for 30 min exhibited
significantly lower ROS intensity than the CoCl2 group
(6.0±1.4 vs. 21.4±5.9, P<0.001). These data suggest that the
RVS-mediated protection of HK-2 cells from CoCl2 is at
least partly due to the inhibition of ROS generation.
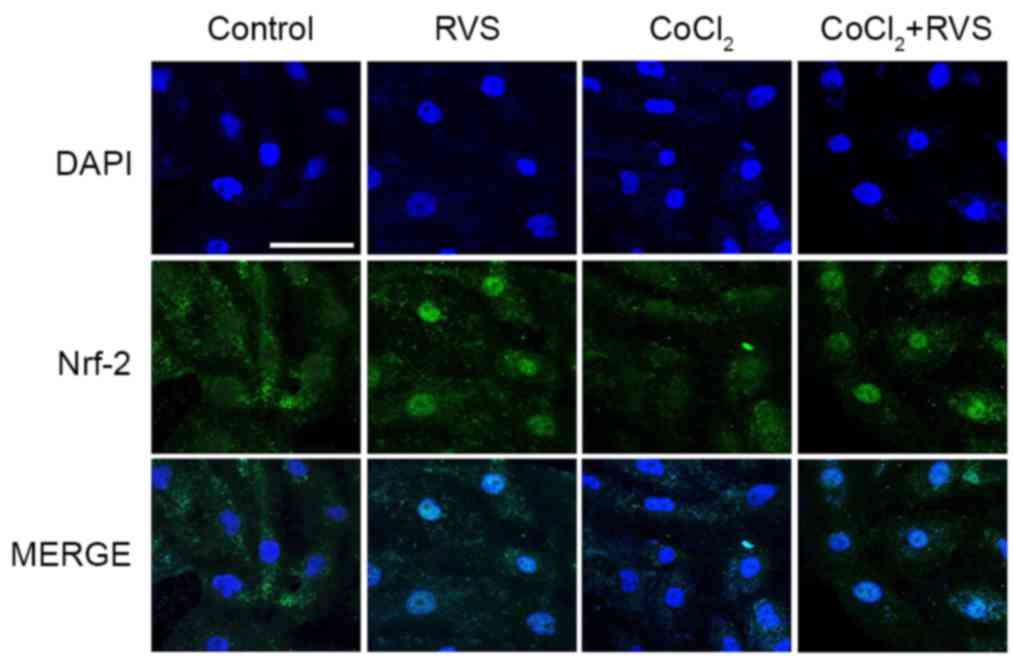
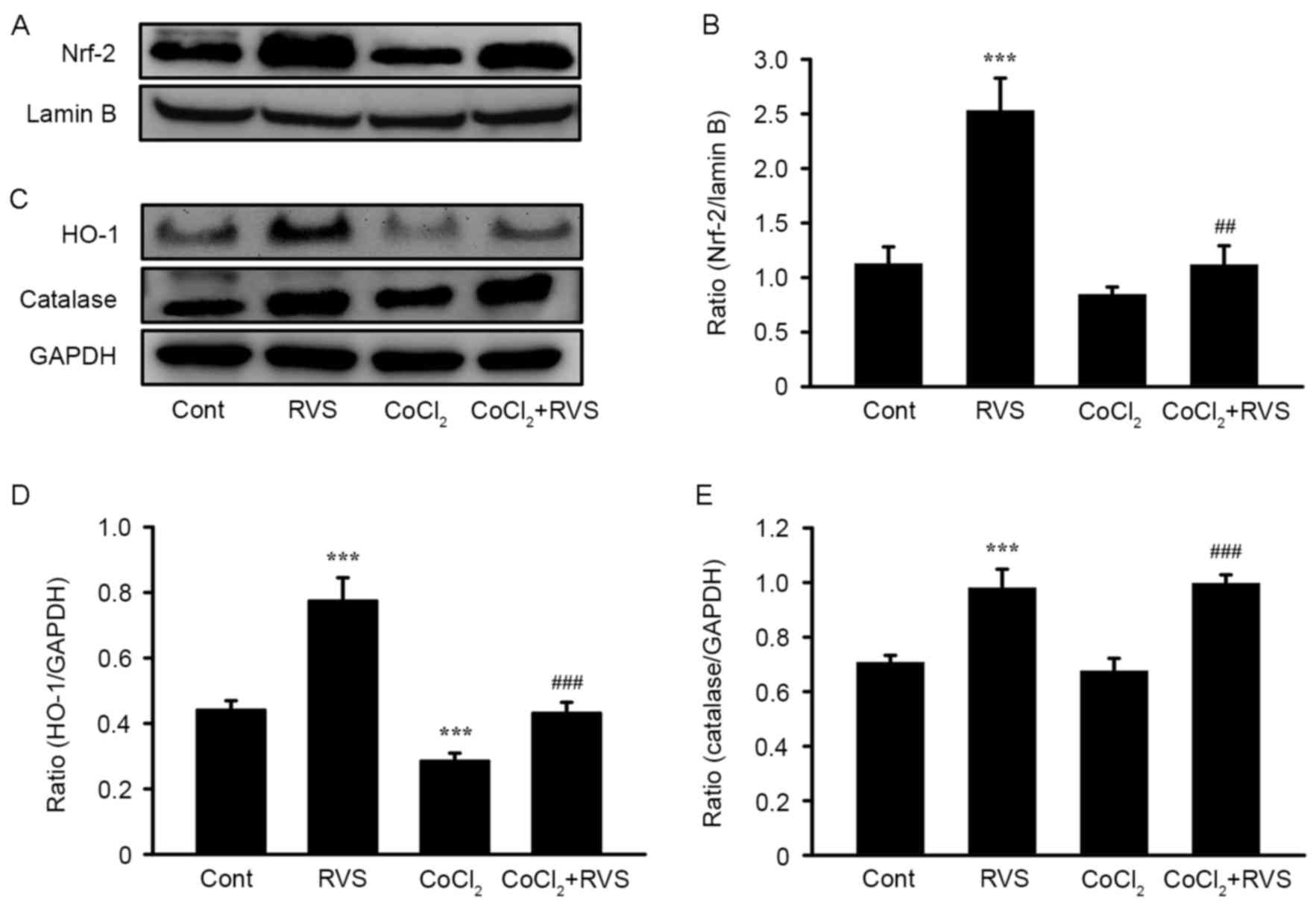
RVS increases the nuclear
translocation of Nrf2 and expression of HO-1 and CAT
To determine the upstream signaling pathway involved
in the RVS-mediated inhibition of ROS generation, the nuclear
translocation of Nrf2 and the expression of cellular antioxidant
enzymes HO-1 and CAT were assessed, which are encoded by Nrf2
target genes. The translocation of Nrf2 from the cytosol into the
nucleus was assessed using confocal microscopy. The results
indicated that Nrf2 proteins (green) were primarily distributed in
the cytoplasm of the control group (Fig.
6). In the RVS group (40 µg/ml, 24 h), the majority of the
cytoplasmic Nrf2 was translocated into the nucleus, as indicated by
intense nuclear Nrf2 staining in the immunofluorescence assay. In
the CoCl2 group (300 µM, 30 min), Nrf2 fluorescence was
decreased compared with that of control group and fluorescence was
mostly cytosolic. However, robust nuclear translocation of Nrf2 was
detected in the CoCl2+RVS group.
To confirm the translocation of Nrf2 into the
nucleus following RVS treatment with or without CoCl2,
cell lysates were separated into cytosolic and nucleic fractions
and the expression of Nrf2 in each fraction was measured using
western blot analysis. Representative band images (Fig. 7A) and quantification of the results
(Fig. 7B) demonstrated that Nrf2
expression was significantly increased in the nuclear fraction of
the RVS group compared with the control group (2.5±0.3 vs. 1.1±0.2,
P<0.001). As expected, compared with the control group, the
CoCl2 group exhibited a reduction in the expression of
nuclear Nrf2 (0.8±0.1 vs. 1.1±0.2, P<0.01). However, Nrf2
expression was significantly increased in the CoCl2+RVS
group compared with the CoCl2 group (1.2±0.3 vs.
0.8±0.1, P<0.01).
Subsequently, the expression of HO-1 and CAT in the
cell homogenates from each treatment group was measured. These are
major cellular antioxidant enzymes that are upregulated following
Nrf2 translocation. As presented in the representative band images
(Fig. 7C) and quantification graphs
for HO-1 (Fig. 7D) and CAT (Fig. 7E), levels of HO-1 and CAT were
significantly increased in cell homogenates from the RVS group
compared with those of the control group (0.8±0.1 vs. 0.44±0.03 and
1.0±0.06 vs. 0.7±0.02, respectively, both P<0.001). In the
CoCl2 group, levels of HO-1 were significantly decreased
(P<0.001) but levels of CAT were unchanged, compared with the
control group. However, levels of HO-1 and CAT were significantly
increased in the CoCl2+RVS group compared with the
CoCl2 group (0.43±0.03 vs. 0.28±0.02 and 1.0±0.03 vs.
0.7±0.04, respectively, both P<0.001). Collectively, these data
suggest that RVS exerts antioxidant effects by increasing the
nuclear translocation of Nrf2 and thereby upregulating the
expression of cellular antioxidant enzymes, including HO-1 and
CAT.
Discussion
ROS are continuously generated in live cells as a
natural product of oxidative metabolism and act as messengers for
signal transduction in various cellular processes, including
mitosis, gene expression and proliferation (30–33).
However, excess ROS are implicated in various pathologies including
ageing, cancer, inflammation and renal disease (34–37).
Consequently, antioxidants with the potential to reduce ROS may
prevent or treat diseases associated with oxidative injury
(38). Therefore, various natural
products with ROS-scavenging properties may be developed as
promising therapeutic strategies to treat oxidative damage-related
conditions. Exposure to certain natural products elevates
intracellular ROS levels and alleviates mild oxidative stress. Such
mild oxidative stress may initiate the signal transduction
responsible for the induction and activation of antioxidant enzymes
(39). In accordance with this
hypothesis, the results of the current study indicate that
treatment with RVS induces a significant, elevation of ROS in HK-2
cells. Thus, RVS may increase the expression of HO-1 and CAT by
inducing mild oxidative stress.
The current study focused on the modulatory effects
of RVS on Nrf2, a redox-sensitive transcription factor, as an
upstream regulator of HO-1 and CAT (40). Nrf2 is a member of the NF-E2 family
of basic leucine zippers and is able to deactivate ROS (41). Under normal conditions, Nrf2 is
located in the cytoplasm and is sequestered by Kelch-like
ECH-associated protein 1 (Keap1), which disturbs the nuclear
translocation of Nrf2 (42).
Following activation of the Nrf2/antioxidant responsive element
(ARE) pathway, the expression of various antioxidant enzymes,
including HO-1 and CAT, are induced (43). Thus, there is substantial interest in
therapeutic candidates that may augment this pathway. To date,
in vitro and in vivo experiments have proven that
phytochemicals, including curcumin, epigallocatechin-3-gallate,
resveratrol and quercetin enhance the Nrf2-ARE pathway, thereby
inducing antioxidant enzymes (44–47).
Certain constituents of RVS, including sulfuretin and butein, may
stimulate this pathway and this has been demonstrated in a human
neuronal cell line damaged by amyloid-β (48) and a human dental pulp cell line
challenged with hydrogen peroxide (49). However, to the best of our knowledge,
it has not yet been reported that RVS or its constituents exert
therapeutic effects on IRI-mediated AKI by modulating the Nrf2/ARE
pathway.
The results of the current study determined that the
activation of Nrf2/ARE-mediated antioxidant enzymes was involved in
protecting against AKI. The present study indicates that RVS
exhibits protective properties against AKI, for example, in the
activation of the antioxidant defence system. However, it should be
considered that RVS may exert its therapeutic action using
alternate mechanisms, including via anti-inflammatory effects. In
fact, many factors that may be possible targets of the
anti-inflammatory property of RVS are present during the
pathogenesis of IRI-induced AKI. During the development of AKI,
resident macrophages in the kidney activate and secrete various
chemokines and cytokines (50).
Furthermore, lymphocytes infiltrate the damaged kidney and
contribute to the inflammatory process (51). Thus, owing to its well-known
anti-inflammatory effects (52,53), it
was assumed that RVS exerts beneficial effects on AKI through this
mechanism.
The present study did not determine the upstream
machineries of RVS-mediated Nrf2 mobilization, which should be
considered a limitation. As aforementioned, Nrf2 is tightly bound
to Keap1 and is anchored in the cytoplasm, resulting in its
ubiquitination and subsequent degradation under normal conditions.
However, when exposed to sub-lethal oxidative damage or treatment
with certain pharmaceuticals, the Nrf2-Keap1 protein-protein
interaction (PPI) is inhibited, which may liberate Nrf2 from Keap1,
leading to its translocation into the nucleus (54–56).
Therefore, using individual compounds within RVS, including, GA,
fustin, fisetin, sulfuretin and butein and additional approaches,
including a binding activity assay for the identification of
Nrf2-Keap1 PPI inhibitory roles in the context of AKI are required
to establish the precise action of RVS on AKI.
In conclusion, the present study indicated that the
intraoral administration of RVS induces a therapeutic effect on
IRI-induced AKD. These effects may be due to the attenuation of ROS
production via the upregulation of the antioxidant defense system
in renal tubular cells. Using the crude extract of RVS, as well as
individual compounds within RVS, novel approaches for unveiling
multifunctional therapeutic mechanisms and specific molecular
targets may be required to determine the protective effects of RVS
on AKI.
Acknowledgements
The current study was supported by the Development
of Forest Science and Technology (grant no. S111414L030100) and the
Korea Research Foundation (grant nos. NRF-2014R1A1A403005726 and
NRF-2016R1C1B2012351).
References
|
1
|
Qiao X, Li RS, Li H, Zhu GZ, Huang XG,
Shao S and Bai B: Intermedin protects against renal
ischemia-reperfusion injury by inhibition of oxidative stress. Am J
Physiol Renal Physiol. 304:F112–F119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin M, Li L, Li L, Pokhrel G, Qi G, Rong R
and Zhu T: The protective effect of baicalin against renal
ischemia-reperfusion injury through inhibition of inflammation and
apoptosis. BMC Complement Altern Med. 14:192014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ponticelli C: Ischaemia-reperfusion
injury: A major protagonist in kidney transplantation. Nephrol Dial
Transplant. 29:1134–1140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bonventre JV and Yang L: Cellular
pathophysiology of ischemic acute kidney injury. J Clin Invest.
121:4210–4221. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Munshi R, Hsu C and Himmelfarb J: Advances
in understanding ischemic acute kidney injury. BMC Med. 9:112011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yellon DM and Hausenloy DJ: Myocardial
reperfusion injury. N Engl J Med. 357:1121–1135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Walker LM, York JL, Imam SZ, Ali SF,
Muldrew KL and Mayeux PR: Oxidative stress and reactive nitrogen
species generation during renal ischemia. Toxicol Sci. 63:143–148.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cutrín JC, Zingaro B, Camandola S, Boveris
A, Pompella A and Poli G: Contribution of gamma glutamyl
transpeptidase to oxidative damage of ischemic rat kidney. Kidney
Int. 57:526–533. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moss NG, Vogel PA, Kopple TE and
Arendshorst WJ: Thromboxane-induced renal vasoconstriction is
mediated by the ADP-ribosyl cyclase CD38 and superoxide anion. Am J
Physiol Renal Physiol. 305:F830–F838. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ugochukwu NH and Cobourne MK: Modification
of renal oxidative stress and lipid peroxidation in
streptozotocin-induced diabetic rats treated with extracts from
Gongronema latifolium leaves. Clin Chim Acta. 336:73–81. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kandemir FM, Ozkaraca M, Yildirim BA,
Hanedan B, Kirbas A, Kilic K, Aktas E and Benzer F: Rutin
attenuates gentamicin-induced renal damage by reducing oxidative
stress, inflammation, apoptosis, and autophagy in rats. Renal
Failure. 37:518–525. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aragno M, Cutrin JC, Mastrocola R,
Perrelli MG, Restivo F, Poli G, Danni O and Boccuzzi G: Oxidative
stress and kidney dysfunction due to ischemia/reperfusion in rat:
Attenuation by dehydroepiandrosterone. Kidney Int. 64:836–843.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ozkok A and Edelstein CL: Pathophysiology
of cisplatin-induced acute kidney injury. Biomed Res Int.
2014:9678262014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kobayashi A, Kang MI, Watai Y, Tong KI,
Shibata T, Uchida K and Yamamoto M: Oxidative and electrophilic
stresses activate Nrf2 through inhibition of ubiquitination
activity of Keap1. Mol Cell Biol. 26:221–229. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen J, Wang W, Zhang Q, Li F, Lei T, Luo
D, Zhou H and Yang B: Low molecular weight fucoidan against renal
ischemia/reperfusion injury via inhibition of the MAPK signaling
pathway. PLoS One. 8:e562242013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhong Y, Deng Y, Chen Y, Chuang PY and
Cijiang He J: Therapeutic use of traditional Chinese herbal
medications for chronic kidney diseases. Kidney Int. 84:1108–1118.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen W, Jia Z, Pan MH and Anandh Babu PV:
Natural products for the prevention of oxidative Stress-related
diseases: Mechanisms and strategies. Oxid Med Cell Longev.
2016:46285022016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Su ZY, Shu L, Khor TO, Lee JH, Fuentes F
and Kong AN: A perspective on dietary phytochemicals and cancer
chemoprevention: Oxidative stress, Nrf2, and epigenomics. Top Curr
Chem. 329:133–162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kitts DD and Lim KT: Antitumorigenic and
cytotoxic properties of an ethanol extract from Rhus
verniciflua Stokes (RVS). J Toxicol Environ Health A.
64:357–371. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim SA, Kim SH, Kim IS, Lee D, Dong MS, Na
CS, Nhiem X and Yoo HH: Simultaneous determination of bioactive
phenolic compounds in the stem extract of Rhus verniciflua
stokes by high performance liquid chromatography. Food Chem.
141:3813–3819. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Badhani B, Sharma N and Kakkar R: Gallic
acid: A versatile antioxidant with promising therapeutic and
industrial applications. RSC Advances. 5:27540–27557. 2015.
View Article : Google Scholar
|
|
22
|
Choi YJ, Do GM, Shin JH, Kim JY and Kwon
O: Standardized Rhus verniciflua stokes extract and its
major flavonoid fustin attenuate oxidative stress induced by
tert-butyl hydroperoxide via activation of nuclear factor erythroid
2-related factor. J Korean Soc Appl Biol Chem. 57:27–30. 2014.
View Article : Google Scholar
|
|
23
|
Lee DS, Kim KS, Ko W, Li B, Jeong GS, Jang
JH, Oh H and Kim YC: The cytoprotective effect of sulfuretin
against tert-Butyl hydroperoxide-induced hepatotoxicity through
Nrf2/ARE and JNK/ERK MAPK-mediated heme oxygenase-1 expression. Int
J Mol Sci. 15:8863–8877. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Serobatse KRN and Kabanda MM: Antioxidant
and antimalarial properties of butein and homobutein based on their
ability to chelate iron (II and III) cations: A DFT study in vacuo
and in solution. Eur Food Res Technol. 242:71–90. 2016. View Article : Google Scholar
|
|
25
|
Kiliç I and Yeşiloğlu Y: Spectroscopic
studies on the antioxidant activity of p-coumaric acid. Spectrochim
Acta A Mol Biomol Spectrosc. 115:719–724. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee SE, Jeong SI, Yang H, Park CS, Jin YH
and Park YS: Fisetin induces Nrf2-mediated HO-1 expression through
PKC-δ and p38 in human umbilical vein endothelial cells. J Cell
Biochem. 112:2352–2360. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pandurangan AK, Mohebali N, Norhaizan ME
and Looi CY: Gallic acid attenuates dextran sulfate sodium-induced
experimental colitis in BALB/c mice. Drug Des Devel Ther.
9:3923–3934. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gil MN, Choi DR, Yu KS, Jeong JH, Bak DH,
Kim DK, Lee NS, Lee JH, Jeong YG, Na CS, et al: Rhus
verniciflua Stokes attenuates cholestatic liver
cirrhosis-induced interstitial fibrosis via Smad3 down-regulation
and Smad7 up-regulation. Anat Cell Biol. 49:189–198. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Institute for Laboratory Animal Research
(ILAR): Guide for the care and use of laboratory animals. National
Academy Press; Washington, DC: 2011
|
|
30
|
Turpaev KT: Reactive oxygen species and
regulation of gene expression. Biochemistry (Mosc). 67:281–292.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Day RM and Suzuki YJ: Cell proliferation,
reactive oxygen and cellular glutathione. Dose Response. 3:425–442.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dixit R and Cyr R: Cell damage and
reactive oxygen species production induced by fluorescence
microscopy: Effect on mitosis and guidelines for non-invasive
fluorescence microscopy. Plant J. 36:280–290. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sauer H, Wartenberg M and Hescheler J:
Reactive oxygen species as intracellular messengers during cell
growth and differentiation. Cell Physiol Biochem. 11:173–186. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nath KA and Norby SM: Reactive oxygen
species and acute renal failure. Am J Med. 109:665–678. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liou GY and Storz P: Reactive oxygen
species in cancer. Free Radic Res.
44:2010.10.3109/10715761003667554. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mittal M, Siddiqui MR, Tran K, Reddy SP
and Malik AB: Reactive oxygen species in inflammation and tissue
injury. Antioxid Redox Signal. 20:1126–1167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pham-Huy LA, He H and Pham-Huy C: Free
radicals, antioxidants in disease and health. Int J Biomed Sci.
4:89–96. 2008.PubMed/NCBI
|
|
38
|
Kelly FJ: Use of antioxidants in the
prevention and treatment of disease. J Int Fed Clin Chem. 10:21–23.
1998.PubMed/NCBI
|
|
39
|
Hu R and Kong AN: Activation of MAP
kinases, apoptosis and nutrigenomics of gene expression elicited by
dietary cancer-prevention compounds. Nutrition. 20:83–88. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chang SY, Chen YW, Zhao XP, Chenier I,
Tran S, Sauvé A, Ingelfinger JR and Zhang SL: Catalase prevents
maternal diabetes-induced perinatal programming via the Nrf2-HO-1
defense system. Diabetes. 61:2565–2574. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Motohashi H, Kimura M, Fujita R, Inoue A,
Pan X, Takayama M, Katsuoka F, Aburatani H, Bresnick EH and
Yamamoto M: NF-E2 domination over Nrf2 promotes ROS accumulation
and megakaryocytic maturation. Blood. 115:677–686. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Johnson JA, Johnson DA, Kraft AD, Calkins
MJ, Jakel RJ, Vargas MR and Chen PC: The Nrf2-ARE pathway: An
indicator and modulator of oxidative stress in neurodegeneration.
Ann N Y Acad Sci. 1147:61–69. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Petri S, Körner S and Kiaei M: Nrf2/ARE
signaling pathway: Key mediator in oxidative stress and potential
therapeutic target in ALS. Neurol Res Int. 2012:8780302012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang C, Zhang X, Fan H and Liu Y: Curcumin
upregulates transcription factor Nrf2, HO-1 expression and protects
rat brains against focal ischemia. Brain Res. 1282:133–141. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Krajka-Kuźniak V, Szaefer H, Stefański T,
Sobiak S, Cichocki M and Baer-Dubowska W: The effect of resveratrol
and its methylthio-derivatives on the Nrf2-ARE pathway in mouse
epidermis and HaCaT keratinocytes. Cell Mol Biol Lett. 19:500–516.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Schadich E, Hlaváč J, Volná T, Varanasi L,
Hajdúch M and Džubák P: Effects of ginger phenylpropanoids and
quercetin on Nrf2-ARE pathway in human BJ fibroblasts and HaCaT
keratinocytes. Biomed Res Int. 2016:21732752016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sahin K, Tuzcu M, Gencoglu H, Dogukan A,
Timurkan M, Sahin N, Aslan A and Kucuk O:
Epigallocatechin-3-gallate activates Nrf2/HO-1 signaling pathway in
cisplatin-induced nephrotoxicity in rats. Life Sci. 87:240–245.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kwon SH, Ma SX, Hwang JY, Lee SY and Jang
CG: Involvement of the Nrf2/HO-1 signaling pathway in
sulfuretin-induced protection against amyloid beta25-35
neurotoxicity. Neuroscience. 304:14–28. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lee DS, Li B, Kim KS, Jeong GS, Kim EC and
Kim YC: Butein protects human dental pulp cells from hydrogen
peroxide-induced oxidative toxicity via Nrf2 pathway-dependent heme
oxygenase-1 expressions. Toxicol In Vitro. 27:874–881. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ramesh G and Reeves WB: TNF-alpha mediates
chemokine and cytokine expression and renal injury in cisplatin
nephrotoxicity. J Clin Invest. 110:835–842. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Okyay GU, İnal S, Öneç K, Er RE, Paşaoğlu
O, Paşaoğlu H, Derici U and Erten Y: Neutrophil to lymphocyte ratio
in evaluation of inflammation in patients with chronic kidney
disease. Ren Fail. 35:29–36. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ip WKE, Hoshi N, Shouval DS, Snapper S and
Medzhitov R: Anti-inflammatory effect of IL-10 mediated by
metabolic reprogramming of macrophages. Science. 356:513–519. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Flaishon L, Hart G, Zelman E, Moussion C,
Grabovsky V, Lapidot Tal G, Feigelson S, Margalit R, Harmelin A,
Avin-Wittenberg T, et al: Anti-inflammatory effects of an
inflammatory chemokine: CCL2 inhibits lymphocyte homing by
modulation of CCL21-triggered integrin-mediated adhesions. Blood.
112:5016–5025. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Itoh K, Wakabayashi N, Katoh Y, Ishii T,
Igarashi K, Engel JD and Yamamoto M: Keap1 represses nuclear
activation of antioxidant responsive elements by Nrf2 through
binding to the amino-terminal Neh2 domain. Genes Dev. 13:76–86.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Satoh T, Okamoto SI, Cui J, Watanabe Y,
Furuta K, Suzuki M, Tohyama K and Lipton SA: Activation of the
Keap1/Nrf2 pathway for neuroprotection by electrophillic
(correction of electrophillic) phase II inducers. Proc Natl Acad
Sci USA. 103:768–773. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Itoh K, Wakabayashi N, Katoh Y, Ishii T,
O'Connor T and Yamamoto M: Keap1 regulates both cytoplasmic-nuclear
shuttling and degradation of Nrf2 in response to electrophiles.
Genes Cells. 8:379–391. 2003. View Article : Google Scholar : PubMed/NCBI
|